Root Illumination Promotes Seedling Growth and Inhibits Gossypol Biosynthesis in Upland Cotton
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Root Illumination
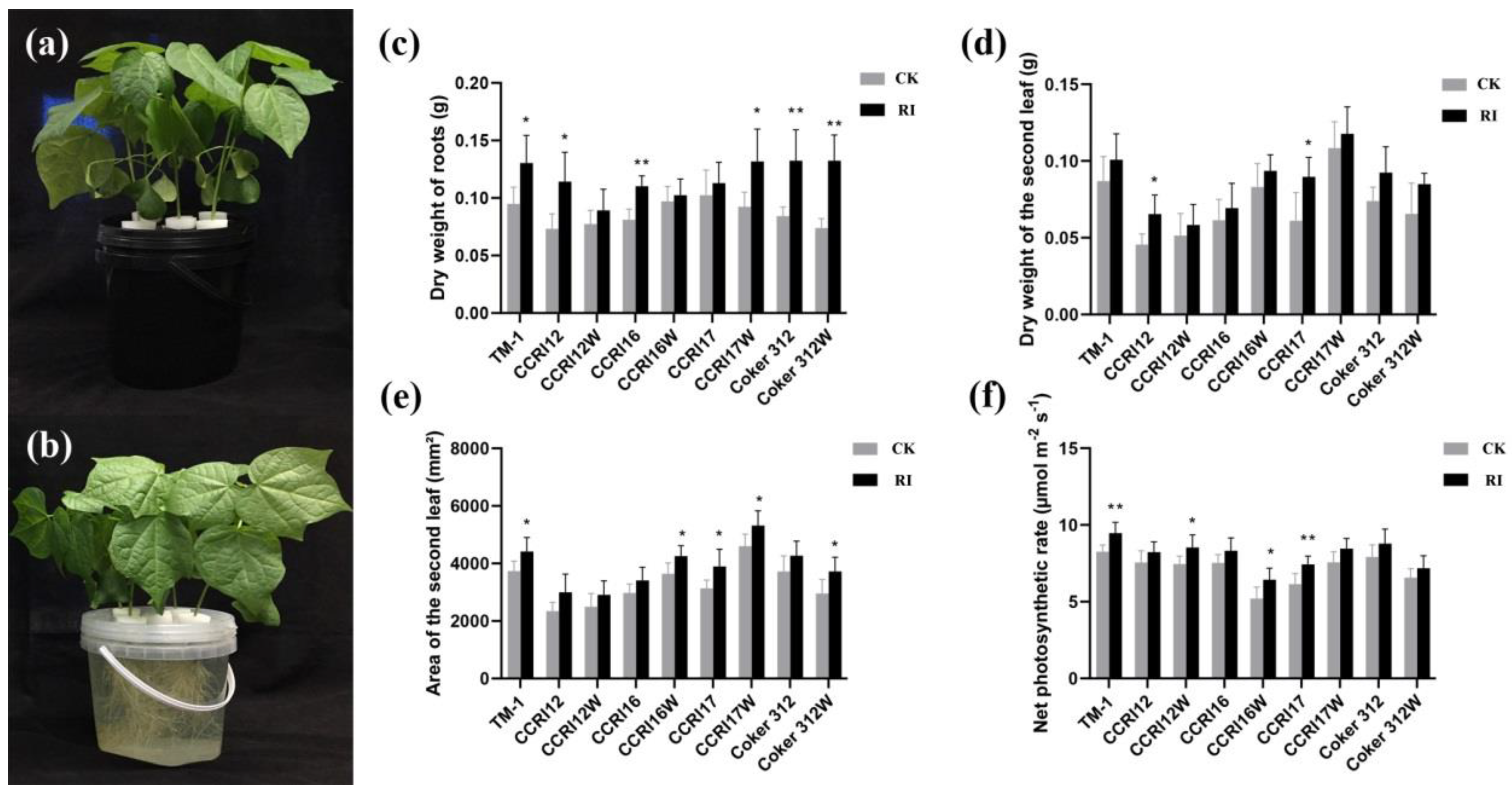
2.3. Determination of Biomass, Leaf Area and Photosynthesis
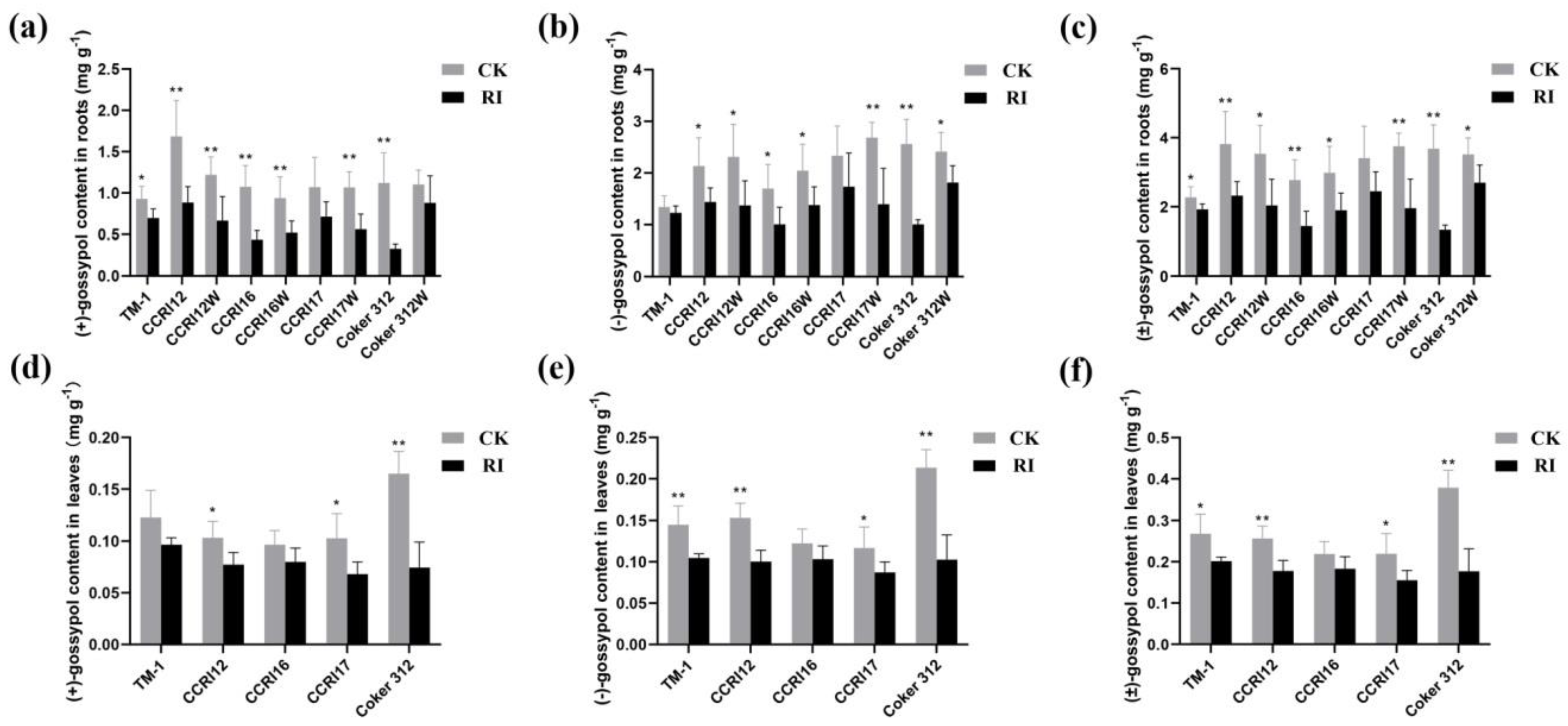
2.4. Extraction and Determination of Gossypol
2.5. Transcriptome Sequencing and Analysis
2.6. Statistical Analysis
3. Results
3.1. The Effect of RI on Growth of Cotton Seedlings
3.2. The Effect of RI on the Gossypol Content of Cotton Seedlings
3.3. Transcriptome Analysis in Roots of Cotton Seedlings
3.3.1. Sequencing Quality
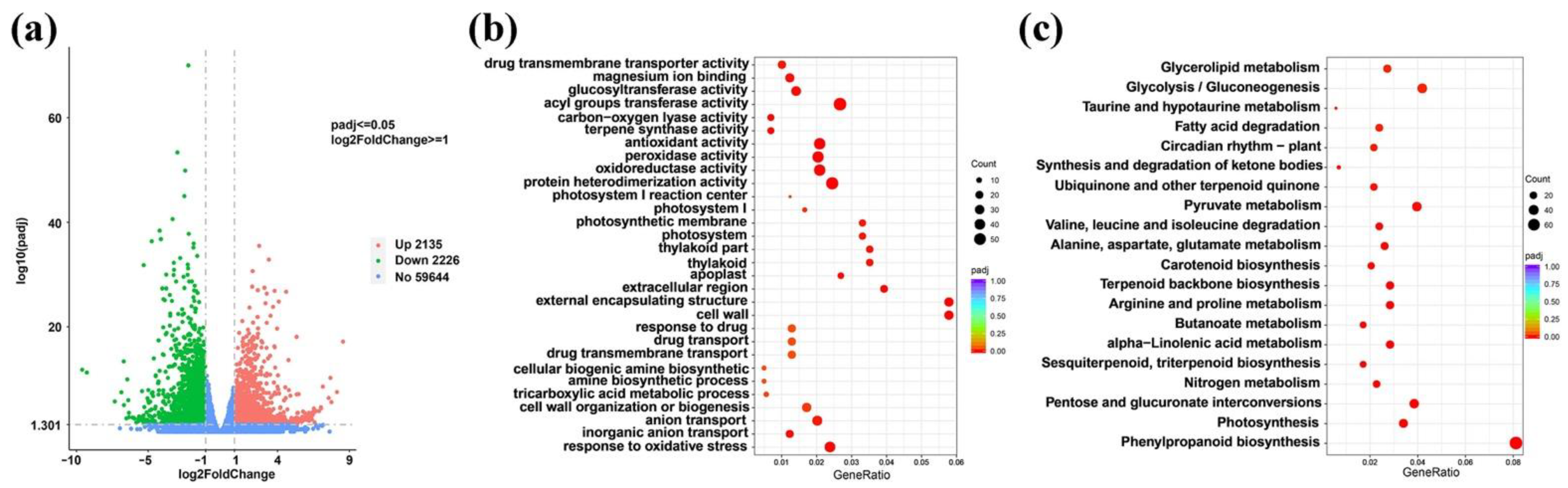
3.3.2. Analysis of Differentially Expressed Genes
3.3.3. GO Enrichment Analysis of Differentially Expressed Genes
3.3.4. KEGG Enrichment Analysis of Differentially Expressed Genes
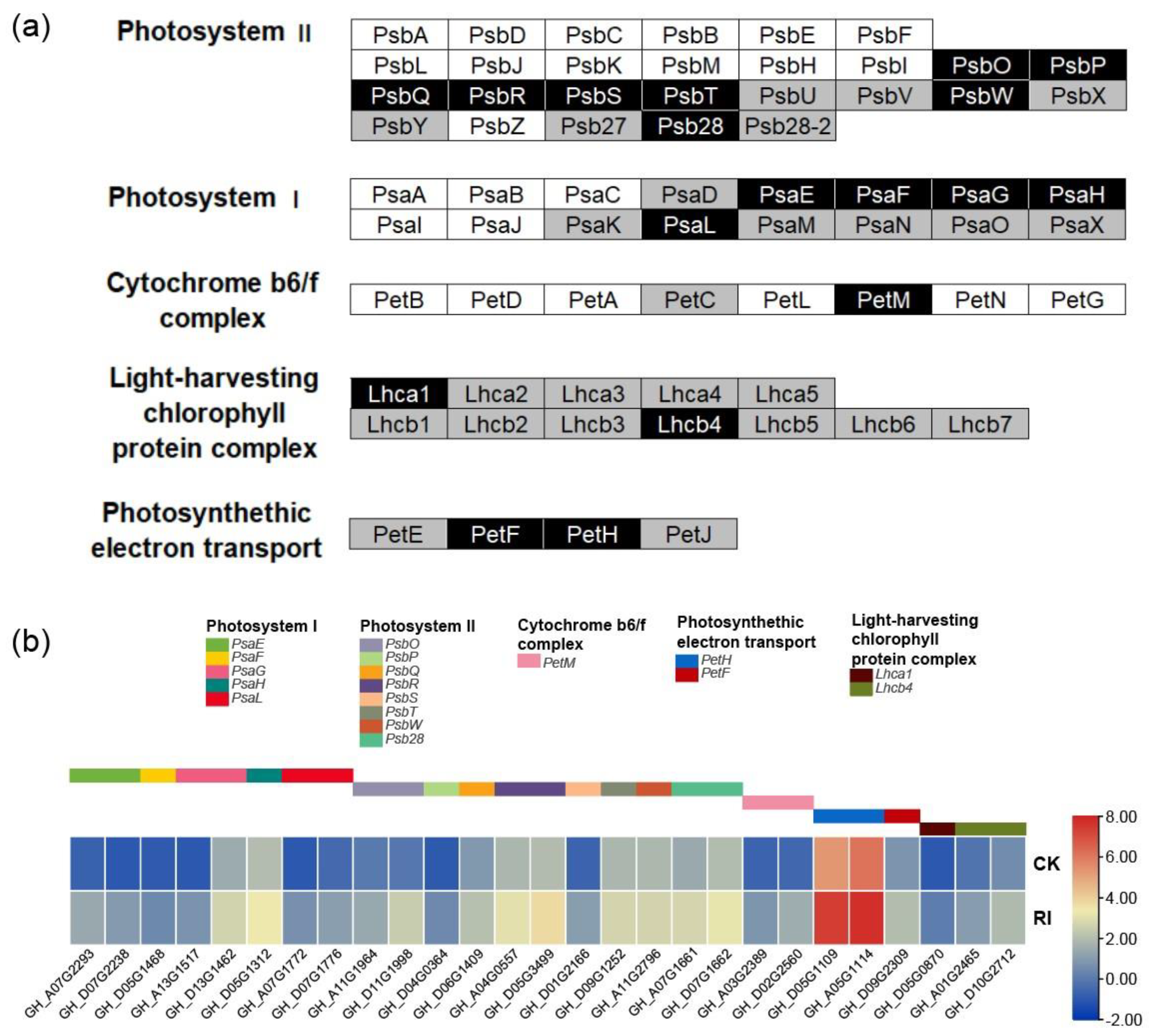
3.4. Photosynthesis Pathway Response to RI
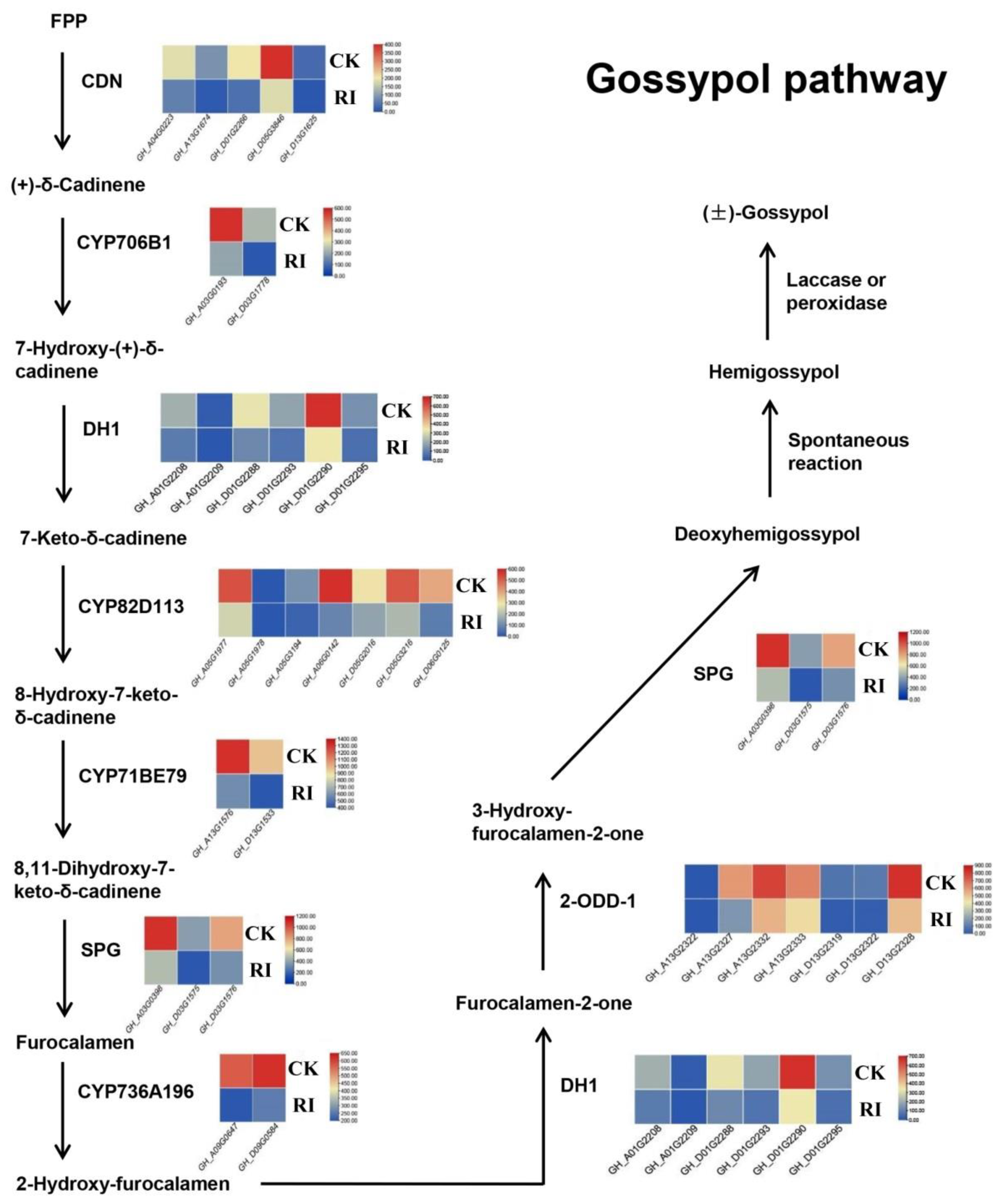
3.5. Gossypol Biosynthesis Pathway Response to RI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palle, S.R.; Campbell, L.M.; Pandeya, D.; Puckhaber, L.; Tollack, L.K.; Marcel, S.; Sundaram, S.; Stipanovic, R.D.; Wedegaertner, T.C.; Hinze, L.; et al. RNAi-mediated Ultra-low Gossypol Cottonseed Trait: Performance of Transgenic Lines under Field Conditions. Plant Biotechnol. J. 2013, 11, 296–304. [Google Scholar] [CrossRef]
- Sunilkumar, G.; Campbell, L.M.; Puckhaber, L.; Stipanovic, R.D.; Rathore, K.S. Engineering Cottonseed for Use in Human Nutrition by Tissue-specific Reduction of Toxic Gossypol. Proc. Natl. Acad. Sci. USA 2006, 103, 18054–18059. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Zhang, C.; Wu, Y.; Tang, Y. Metabolic Engineering of Gossypol in Cotton. Appl. Microbiol. Biotechnol. 2013, 97, 6159–6165. [Google Scholar] [CrossRef]
- Stipanovic, R.D.; Puckhaber, L.S.; Bell, A.A. Ratios of (+)- and (−)-Gossypol in Leaves, Stems, and Roots of Selected Accessions of Gossypium hirsutum var. Marie Galante (Watt) Hutchinson. J. Agric. Food Chem. 2006, 54, 1633–1637. [Google Scholar] [CrossRef]
- Stipanovic, R.D.; López, J.D.; Dowd, M.K.; Puckhaber, L.S.; Duke, S.E. Effect of Racemic, (+)- and (−)-Gossypol on Survival and Development of Heliothis Virescens Larvae. Environ. Entomol. 2008, 37, 1081–1085. [Google Scholar] [CrossRef]
- Puckhaber, L.S.; Dowd, M.K.; Stipanovic, R.D.; Howell, C.R. Toxicity of (+)- and (−)-Gossypol to the Plant Pathogen, Rhizoctonia Solani. J. Agric. Food Chem. 2002, 50, 7017–7021. [Google Scholar] [CrossRef]
- Abraham, K.J.; Margaret, L.P.; Margaret, E. The Phytoalexins Desoxyhemigossypol and Hemigossypol are Elicited by Xanthomonas in Gossypium Cotyledons. Phytochemistry 1999, 52, 829–836. [Google Scholar] [CrossRef]
- Li, C.; He, Q.; Zhang, F.; Yu, J.; Li, C.; Zhao, T.; Zhang, Y.; Xie, Q.; Su, B.; Mei, L.; et al. Melatonin Enhances Cotton Immunity to Verticillium Wilt via Manipulating Lignin and Gossypol Biosynthesis. Plant J. 2019, 100, 784–800. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Kulp, S.K.; Sugimoto, Y.; Jiang, J.; Lin, Y.C. The (−)-Enantiomer of Gossypol Possesses Higher Anticancer Potency than Racemic Gossypol in Human Breast Cancer. Anticancer Res. 2002, 22, 33–38. [Google Scholar]
- Keith, G.W.; Steven, J.W.; Bradley, S.H.; Shaomeng, W.; Kent, A.G.; Bhavna, K.; Jianyong, C.; Thomas, E.C.; Carol, R.B.; D’Silva, N.J. (−)-Gossypol Inhibits Growth and Promotes Apoptosis of Human Head and Neck Squamous Cell Carcinoma in Vivo. Neoplasia 2006, 8, 163–172. [Google Scholar]
- Kline, M.P.; Rajkumar, S.V.; Timm, M.M.; Kimlinger, T.K.; Haug, J.L.; Lust, J.A.; Greipp, P.R.; Kumar, S. R-(-)-gossypol (AT-101) Activates Programmed Cell Death in Multiple Myeloma Cells. Exp. Hematol. 2008, 36, 568–576. [Google Scholar] [CrossRef] [Green Version]
- Coyle, T.; Levante, S.; Shetler, M.; Winfield, J. In Vitro and in Vivo Cytotoxicity of Gossypol against Central Nervous System Tumor Cell Lines. J. Neuro-Oncol. 1994, 19, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Chang, H.L.; Wang, L.S.; Huang, Y.W.; Shu, S.; Dowd, M.K.; Wan, P.J.; Sugimoto, Y.; Lin, Y.C. Modulation of Multidrug Resistance Gene Expression in Human Breast Cancer Cells by (−)-Gossypol-enriched Cottonseed Oil. Anticancer Res. 2007, 27, 107–116. [Google Scholar]
- Coutinho, E.M. Gossypol: A contraceptive for men. Contraception 2002, 65, 259–263. [Google Scholar] [CrossRef]
- Lopez, L.M.; Grimes, D.A.; Schulz, K.F. Nonhormonal Drugs for Contraception in Men: A Systematic Review. Obstet. Gynecol. Surv. 2005, 60, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.H. Biosynthesis of Gossypol by Excised Cotton Roots. Nature 1961, 192, 888–889. [Google Scholar] [CrossRef]
- Zhao, T.; Xie, Q.; Li, C.; Li, C.; Mei, L.; Yu, J.Z.; Chen, J.; Zhu, S. Cotton Roots are the Major Source of Gossypol Biosynthesis and Accumulation. BMC Plant Biol. 2020, 20, 88. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Huang, J.; Chen, X.; Zhu, Y. Recent Advances and Future Perspectives in Cotton Research. Annu. Rev. Plant Biol. 2021, 72, 437–462. [Google Scholar] [CrossRef]
- Tian, X.; Ruan, J.; Huang, J.; Yang, C.; Fang, X.; Chen, Z.; Hong, H.; Wang, L.; Mao, Y.; Lu, S. Characterization of Gossypol Biosynthetic Pathway. Proc. Nat. Acad. Sci. USA 2018, 115, e5410–e5418. [Google Scholar] [CrossRef] [Green Version]
- Shawrang, P.; Mansouri, M.H.; Sadeghi, A.A.; Ziaie, F. Evaluation and Comparison of Gamma- and Electron Beam Irradiation Effects on Total and Free Gossypol of Cottonseed Meal. Radiat. Phys. Chem. 2011, 80, 761–762. [Google Scholar] [CrossRef]
- Nomeir, A.A.; Abou-Donia, M.B. Photodecomposition of Gossypol by Ultraviolet Irradiation. J. Am. Oil Chem. Soc. 1985, 62, 87–89. [Google Scholar] [CrossRef]
- Li, J.; Terzaghi, W.; Deng, X.Y. Genomic Basis for Light Control of Plant Development. Protein Cell 2012, 3, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Deng, X.W. Dissecting the Phytochrome A-dependent Signaling Network in Higher Plants. Trends Plant Sci. 2003, 8, 172–178. [Google Scholar] [CrossRef]
- Jiao, Y.; Lau, O.S.; Deng, X.W. Light-regulated Transcriptional Networks in Higher Plants. Nat. Rev. Genet. 2007, 8, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Lacek, J.; GarcíaGonzález, J.; Weckwerth, W.; Retzer, K. Lessons Learned from the Studies of Roots Shaded from Direct Root Illumination. Int. J. Mol. Sci. 2021, 22, 12784. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and Quantifying Mammalian Transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, J.; Fang, L.; Zhang, Z.; Ma, W.; Niu, Y.; Ju, L.; Deng, J.; Zhao, T.; Lian, J.; et al. Gossypium Barbadense and Gossypium Hirsutum Genomes Provide Insights into the Origin and Evolution of Allotetraploid Cotton. Nat. Genet. 2019, 51, 739–748. [Google Scholar] [CrossRef] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, G.; Wang, L.; Han, Y.; He, Q. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Grierson, C.; Nielsen, E.; Ketelaarc, T.; Schiefelbein, J. Root Hairs. Arabidopsis Book 2014, 12, e0172. [Google Scholar] [CrossRef] [Green Version]
- Bin, C.; Anne, M.J.; Ramsey, S.L.; Ralph, E.D.; Lowell, P.B. (R)-nicotine Biosynthesis, Metabolism and Translocation in Tobacco as Determined by Nicotine Demethylase Mutants. Phytochemistry 2013, 95, 188–196. [Google Scholar]
- Cheng, T.; Hu, L.; Wang, P.; Yang, X.; Peng, Y.; Lu, Y.; Chen, J.; Shi, J. Monoxide Potentiates High Temperature-Induced Nicotine Biosynthesis in Tobacco. Int. J. Mol. Sci. 2018, 19, 188. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.; Sasaki, D.; Noguchi, K.; Fujinuma, D.; Komatsu, H.; Kobayashi, M.; Sato, M.; Toyooka, K.; Sugimoto, K.; Niyogi, K.K.; et al. Photosynthesis of Root Chloroplasts Developed in Arabidopsis Lines Overexpressing GOLDEN2-LIKE Transcription Factors. Plant Cell Physiol. 2013, 54, 1365–1377. [Google Scholar] [CrossRef] [Green Version]
- Kumari, S.; Panigrahi, K.C.S. Light and Auxin Signaling Cross-talk Programme Root Development in Plants. J. Biosci. 2019, 44, 26. [Google Scholar] [CrossRef] [PubMed]
- Mieke, D.W.; Vinicius, C.G.; Christian, F. Light-Mediated Hormonal Regulation of Plant Growth and Development. Ann. Rev. Plant Biol. 2016, 67, 513–537. [Google Scholar]
- Tian, X.; Ruan, J.; Huang, J.; Fang, X.; Mao, Y.; Wang, L.; Chen, X.; Yang, C. Gossypol: Phytoalexin of Cotton. Sci. China Life Sci. 2016, 59, 122–129. [Google Scholar] [CrossRef] [Green Version]
| Sample | Raw Reads (bp) | Clean Reads (bp) | Q30 (%) | GC Content (%) | Mapped Rate (%) | Uniquely Mapped Rate (%) |
|---|---|---|---|---|---|---|
| CK_1 | 49,818,318 | 47,615,882 | 93.66 | 42.44 | 97.08 | 92.09 |
| CK_2 | 46,293,350 | 43,363,002 | 93.87 | 42.52 | 97.05 | 91.81 |
| CK_3 | 49,280,916 | 48,100,220 | 93.1 | 43.2 | 96.88 | 92.38 |
| RI_1 | 48,019,602 | 46,918,760 | 93.56 | 43.85 | 94.14 | 89.96 |
| RI_2 | 45,340,188 | 44,252,532 | 93.72 | 44.13 | 92.11 | 88.08 |
| RI_3 | 47,859,738 | 46,815,136 | 93.91 | 43.76 | 93.91 | 89.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhao, T.; Sheng, K.; Sun, Y.; Han, Y.; Chen, Y.; E, Z.; Zhu, S.; Chen, J. Root Illumination Promotes Seedling Growth and Inhibits Gossypol Biosynthesis in Upland Cotton. Plants 2022, 11, 728. https://doi.org/10.3390/plants11060728
Zhang J, Zhao T, Sheng K, Sun Y, Han Y, Chen Y, E Z, Zhu S, Chen J. Root Illumination Promotes Seedling Growth and Inhibits Gossypol Biosynthesis in Upland Cotton. Plants. 2022; 11(6):728. https://doi.org/10.3390/plants11060728
Chicago/Turabian StyleZhang, Jiayi, Tianlun Zhao, Kuang Sheng, Yue Sun, Yifei Han, Yiran Chen, Zhiying E, Shuijin Zhu, and Jinhong Chen. 2022. "Root Illumination Promotes Seedling Growth and Inhibits Gossypol Biosynthesis in Upland Cotton" Plants 11, no. 6: 728. https://doi.org/10.3390/plants11060728
APA StyleZhang, J., Zhao, T., Sheng, K., Sun, Y., Han, Y., Chen, Y., E, Z., Zhu, S., & Chen, J. (2022). Root Illumination Promotes Seedling Growth and Inhibits Gossypol Biosynthesis in Upland Cotton. Plants, 11(6), 728. https://doi.org/10.3390/plants11060728







