Genetic Analysis of Mutagenesis That Induces the Photoperiod Insensitivity of Wild Cotton Gossypium hirsutum Subsp. purpurascens
Abstract
1. Introduction
2. Results
2.1. Phenotypic Characterization
2.2. Cytological Observation
2.3. SSR Genotyping
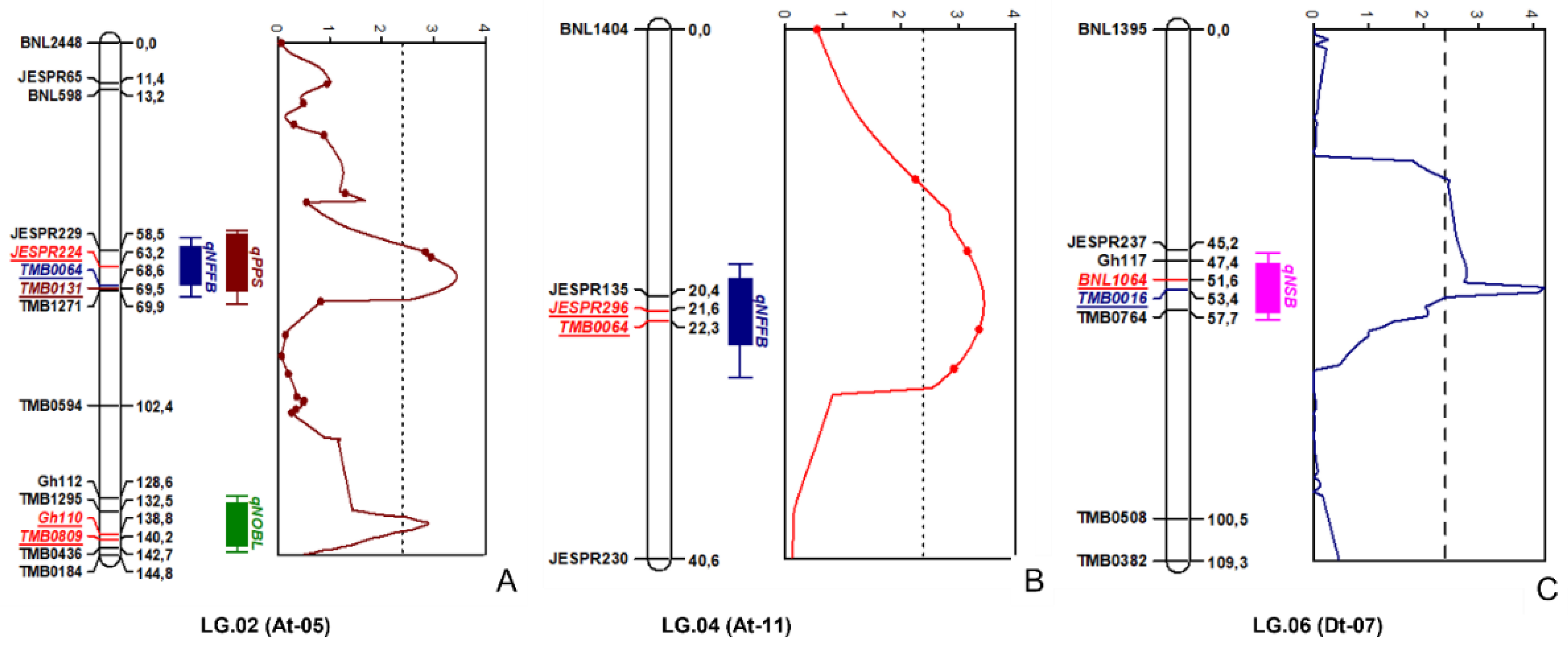
2.4. Genetic Linkage Groups
2.5. QTL Mapping
2.6. Candidate Gene Annotation
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Phenotypic Observation
4.3. Heritability Calculation
4.4. Cytological Analysis
4.5. DNA Isolation and SSR Analysis
4.6. Linkage Mapping and QTL Analysis
4.7. In Silico Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fryxell, P.A. A Redefinition of the Tribe Gossypieae. Bot. Gaz. 1968, 129, 296–308. [Google Scholar] [CrossRef]
- Fryxell, P.A. The Natural History of the Cotton Tribe; Texas A&M University Press: College Station, TX, USA, 1979. [Google Scholar] [CrossRef]
- Mauer, F.M. Origin and systematics of cotton. In Cotton, 1st ed.; AS Uz: Tashkent, Uzbekistan, 1954; pp. 1–384. [Google Scholar]
- Grover, C.E.; Gallagher, J.P.; Jareczek, J.J.; Page, J.T.; Udall, J.A.; Gore, M.A.; Wendel, J.F. Re-evaluating the phylogeny of allopolyploid Gossypium L. Mol. Phylogenet. Evol. 2015, 92, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.P.; Grover, C.E.; Rex, K.; Moran, M.; Wendel, J.F. A New Species of Cotton from Wake Atoll, Gossypium stephensii (Malvaceae). Syst. Bot. 2017, 42, 115–123. [Google Scholar] [CrossRef]
- Kohel, R.J. and Yu, J.Z. Molecular characterization of Gossypium germplasm for cotton improvement. In Managing Plant Genetic Diversity; Engels, J.M.M., Ramanatha Rao, V., Brown, A.H.D., Jackson, M.T., Eds.; CABI Publishing: Wallingford, UK, 2002; pp. 67–76. [Google Scholar]
- Wendel, J.F.; Grover, C.E. Taxonomy and Evolution of the Cotton Genus, Gossypium. In Cotton; Fang, D.D., Percy, R.G., Eds.; American Society of Agronomy Inc.: Madison, WI, USA, 2015; pp. 25–44. [Google Scholar] [CrossRef]
- Aslam, S.; Khan, S.H.; Ahmed, A.; Dandekar, A.M. The Tale of Cotton Plant: From Wild Type to Domestication, Leading to Its Improvement by Genetic Transformation. Am. J. Mol. Biol. 2020, 10, 91–127. [Google Scholar] [CrossRef]
- Huang, G.; Wu, Z.; Percy, R.G.; Bai, M.; Li, Y.; Frelichowski, J.E.; Hu, J.; Wang, K.; Yu, J.Z.; Zhu, Y. Genome sequence of Gossypium herbaceum and genome updates of Gossypium arboreum and Gossypium hirsutum provide insights into cotton A-genome evolution. Nat. Genet. 2020, 52, 516–524. [Google Scholar] [CrossRef]
- Watt, S.G. The Wild and Cultivated Cotton of the World; Longmans, Green, and Company: London, UK, 1907; p. 406. [Google Scholar]
- Hutchinson, J.B.; Stephens, S.G. Note on the “French” or “small-seeded” cotton grown in the West Indies in the eighteenth century. Trop Agr T’dad. 1944, 21, 123. [Google Scholar]
- Nazir, M.F.; He, S.; Ahmed, H.; Sarfraz, Z.; Jia, Y.; Li, H.; Sun, G.; Iqbal, M.S.; Pan, Z.; Du, X. Genomic insight into the biogeographic history, divergence, and adaptive potential of G. purpurascens, a forgotten landrace of G. hirsutum. J. Genet. Genom. 2021, 48, 473–484. [Google Scholar] [CrossRef]
- Liu, S.; Cantrell, R.G.; McCarty, J.C.; Stewart, J.M. Simple Sequence Repeat–Based Assessment of Genetic Diversity in Cotton Race Stock Accessions. Crop Sci. 2000, 40, 1459–1469. [Google Scholar] [CrossRef]
- Mccarty, J.C.; Wu, J.; Jenkins, J.N. Genetic diversity for agronomic and fiber traits in day-neutral accessions derived from primitive cotton germplasm. Euphytica 2006, 148, 283–293. [Google Scholar] [CrossRef]
- Abdurakhmonov, I.Y.; Kushanov, F.; Djaniqulov, F.; Buriev, Z.T.; Pepper, A.E.; Fayzieva, N.; Mavlonov, G.T.; Saha, S.; Jenkins, J.N.; Abdukarimov, A. The Role of Induced Mutation in Conversion of Photoperiod Dependence in Cotton. J. Hered. 2007, 98, 258–266. [Google Scholar] [CrossRef]
- Kushanov, F.; Buriev, Z.T.; Shermatov, S.E.; Turaev, O.S.; Norov, T.M.; Pepper, A.E.; Saha, S.; Ulloa, M.; Yu, J.Z.; Jenkins, J.N.; et al. QTL mapping for flowering-time and photoperiod insensitivity of cotton Gossypium darwinii Watt. PLoS ONE 2017, 12, e0186240. [Google Scholar] [CrossRef] [PubMed]
- Kushanov, F.N.; Pepper, A.E.; Yu, J.Z.; Buriev, Z.T.; Shermatov, S.E.; Saha, S.; Ulloa, M.; Jenkins, J.N.; Abdukarimov, A.; Abdurakhmonov, I.Y. Development, genetic mapping and QTL association of cotton PHYA, PHYB, and HY5-specific CAPS and dCAPS markers. BMC Genet. 2016, 17, 141. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Z.; Kohel, R.J.; Fang, D.D.; Cho, J.; Van Deynze, A.; Ulloa, M.; Hoffman, S.M.; Pepper, A.E.; Stelly, D.M.; Jenkins, J.N.; et al. A High-Density Simple Sequence Repeat and Single Nucleotide Polymorphism Genetic Map of the Tetraploid Cotton Genome. G3 Genes|Genomes|Genet. 2012, 2, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Kushanov, F.N.; Turaev, O.S.; Ernazarova, D.K.; Gapparov, B.M.; Oripova, B.B.; Kudratova, M.K.; Rafieva, F.U.; Khalikov, K.K.; Erjigitov, D.S.; Khidirov, M.T.; et al. Genetic Diversity, QTL Mapping, and Marker-Assisted Selection Technology in Cotton (Gossypium spp.). Front. Plant Sci. 2021, 12, 779386. [Google Scholar] [CrossRef]
- Wang, B.; Zhuang, Z.; Zhang, Z.; Draye, X.; Shuang, L.-S.; Shehzad, T.; Lubbers, E.L.; Jones, D.; May, O.L.; Paterson, A.H.; et al. Advanced Backcross QTL Analysis of Fiber Strength and Fineness in a Cross between Gossypium hirsutum and G. mustelinum. Front. Plant Sci. 2017, 8, 1848. [Google Scholar] [CrossRef]
- Ulloa, M.; Hutmacher, R.B.; Roberts, P.A.; Wright, S.D.; Nichols, R.L.; Davis, R.M. Inheritance and QTL mapping of Fusarium wilt race 4 resistance in cotton. Theor. Appl. Genet. 2013, 126, 1405–1418. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, A.; Li, J.; Zhang, J.; Li, S.; Zhang, J.; Ma, L.; He, R.; Song, W.; Guo, L.; et al. Examining two sets of introgression lines across multiple environments reveals background-independent and stably expressed quantitative trait loci of fiber quality in cotton. Theor. Appl. Genet. 2020, 133, 2075–2093. [Google Scholar] [CrossRef]
- Li, C.-Q.; Song, L.; Zhao, H.-H.; Wang, Q.-L.; Fu, Y.-Z. Identification of quantitative trait loci with main and epistatic effects for plant architecture traits in Upland cotton (Gossypium hirsutum L.). Plant Breed. 2014, 133, 390–400. [Google Scholar] [CrossRef]
- Sun, F.-D.; Zhang, J.-H.; Wang, S.-F.; Gong, W.-K.; Shi, Y.-Z.; Liu, A.-Y.; Li, J.-W.; Gong, J.-W.; Shang, H.-H.; Yuan, Y.-L. QTL mapping for fiber quality traits across multiple generations and environments in upland cotton. Mol. Breed. 2011, 30, 569–582. [Google Scholar] [CrossRef]
- Yu, J.; Jung, S.; Cheng, C.-H.; Lee, T.; Zheng, P.; Buble, K.; Crabb, J.; Humann, J.; Hough, H.; Jones, D.; et al. CottonGen: The Community Database for Cotton Genomics, Genetics, and Breeding Research. Plants 2021, 10, 2805. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Li, R.; Guo, Y. Arabidopsis aspartic proteases A36 and A39 play roles in plant reproduction. Plant Signal. Behav. 2017, 12, e1304343. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jiang, S.-C.; Mei, C.; Liang, S.; Yu, Y.-T.; Lu, K.; Wu, Z.; Wang, X.-F.; Zhang, D.-P. Crucial roles of the pentatricopeptide repeat protein SOAR1 in Arabidopsis response to drought, salt and cold stresses. Plant Mol. Biol. 2015, 88, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.; Rocheta, M.; Galego, L. Genetic control of flower shape in Antirrhinum majus. Development 1997, 124, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chen, C.; Wang, H.; Niu, E.; Zhao, P.; Fang, S.; Zhu, G.; Shang, X.; Guo, W. Cotton Fiber Development Requires the Pentatricopeptide Repeat Protein GhIm for Splicing of Mitochondrial nad7 mRNA. Genetics 2020, 217, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Toyokura, K.; Watanabe, K.; Oiwaka, A.; Kusano, M.; Tameshige, T.; Tatematsu, K.; Matsumoto, N.; Tsugeki, R.; Saito, K.; Okada, K. Succinic Semialdehyde Dehydrogenase is Involved in the Robust Patterning of Arabidopsis Leaves along the Adaxial–Abaxial Axis. Plant Cell Physiol. 2011, 52, 1340–1353. [Google Scholar] [CrossRef]
- Mukherjee, K.; Brocchieri, L.; Bürglin, T.R. A Comprehensive Classification and Evolutionary Analysis of Plant Homeobox Genes. Mol. Biol. Evol. 2009, 26, 2775–2794. [Google Scholar] [CrossRef]
- Cui, D.; Zhao, J.; Jing, Y.; Fan, M.; Liu, J.; Wang, Z.; Xin, W.; Hu, Y. The Arabidopsis IDD14, IDD15, and IDD16 Cooperatively Regulate Lateral Organ Morphogenesis and Gravitropism by Promoting Auxin Biosynthesis and Transport. PLoS Genet. 2013, 9, e1003759. [Google Scholar] [CrossRef]
- Djaniqulov, F. Radioactive mutagenesis of wild cotton species and its utilization in breeding. In Doctor of Science Dissertation; Institute of Experimental Biology, Academy of Sciences of Uzbekistan: Tashkent, Uzbekistan, 1992. [Google Scholar]
- Djaniqulov, F. About relation between radio-sensitivity and mutability of wild and tropical cultivated cotton forms. Proc. Russ. Acad. Agric. Sci. 2002, 2, 19–22. [Google Scholar]
- Singh, M.; Ceccarelli, S.; Hamblin, J. Estimation of heritability from varietal trials data. Theor. Appl. Genet. 1993, 86, 437–441. [Google Scholar] [CrossRef]
- Sanamyan, M.F.; Bobohujayev, S.U.; Abdukarimov, S.S.; Makamov, A.K.; Silkova, O.G. Features of Chromosome Introgression from Gossypium barbadense L. into G. hirsutum L. during the Development of Alien Substitution Lines. Plants 2022, 11, 542. [Google Scholar] [CrossRef]
- Paterson, A.H.; Brubaker, C.L.; Wendel, J.F. A rapid method for extraction of cotton (Gossypium spp.) genomic DNA suitable for RFLP or PCR analysis. Plant Mol. Biol. Rep. 1993, 11, 122–127. [Google Scholar] [CrossRef]
- Voorrips, R.E. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Z.; Ulloa, M.; Hoffman, S.M.; Kohel, R.J.; Pepper, A.E.; Fang, D.D.; Percy, R.G.; Burke, J.J. Mapping genomic loci for cotton plant architecture, yield components, and fiber properties in an interspecific (Gossypium hirsutum L. × G. barbadense L.) RIL population. Mol Genet Genom. 2014, 289, 1347–1367. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Basten, C.; Zeng, Z.; Windows QTL cartographer 2.5. Department of Statistics, North Carolina State University, Raleigh NCwebsite. 2015. Available online: https://statgen.ncsu.edu/qtlcart/WQTLCart.htm (accessed on 20 March 2015).
- Kosambi, D.D. The estimation of map distances from recombination values. Ann. Eugen. 1943, 12, 172–175. [Google Scholar] [CrossRef]
- Nelson, J.C. QGENE: Software for marker-based genomic analysis and breeding. Mol. Breed. 1997, 3, 239–245. [Google Scholar] [CrossRef]
- Okonechnikov, K.; Golosova, O.; Fursov, M.; The UGENE Team. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef]
- Li, F.; Fan, G.; Lu, C.; Xiao, G.; Zou, C.; Kohel, R.J.; Ma, Z.; Shang, H.; Ma, X.; Wu, J.; et al. Genome sequence of cultivated Upland cotton (Gossypium hirsutum TM-1) provides insights into genome evolution. Nat. Biotechnol. 2015, 33, 524–530. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2015, 44, D457–D462. [Google Scholar] [CrossRef]

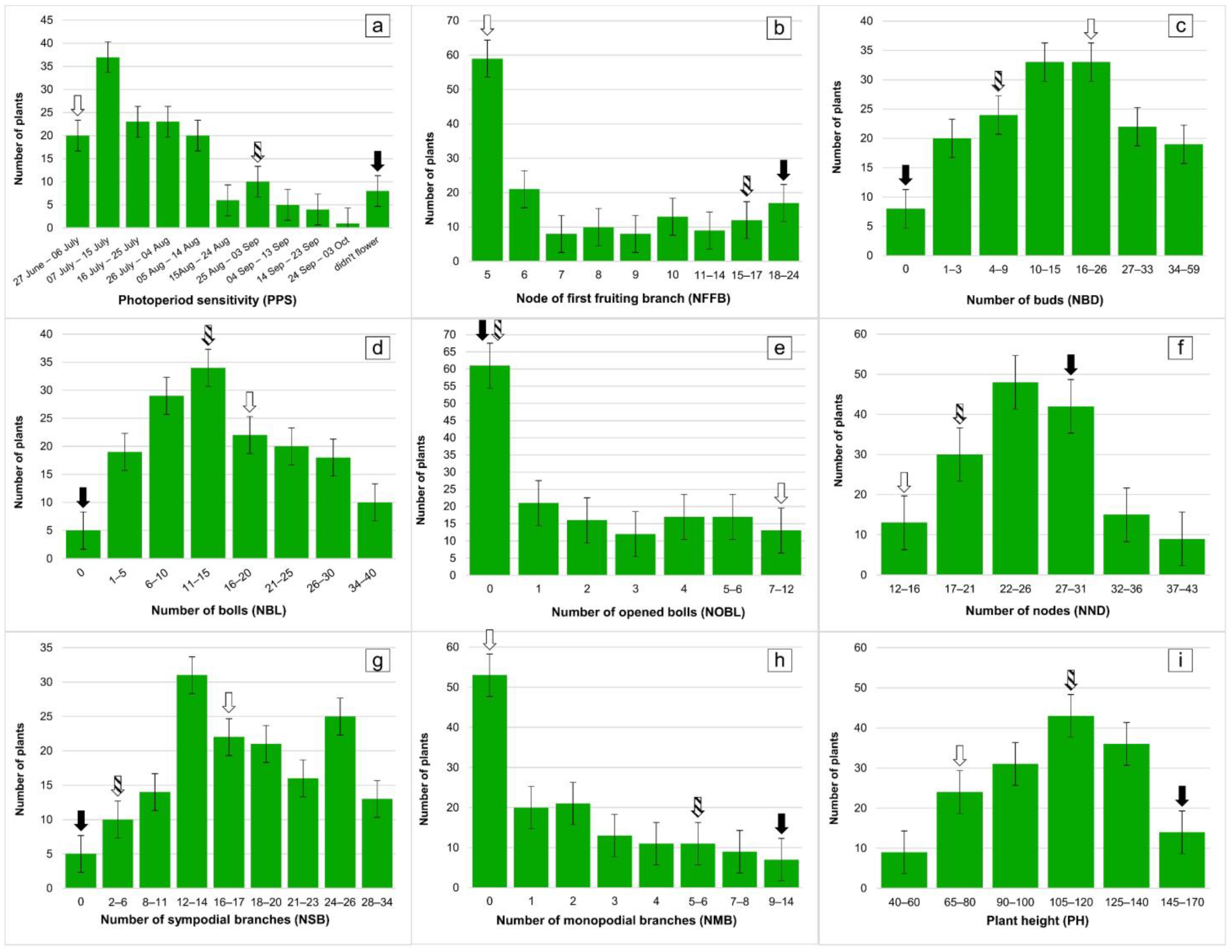

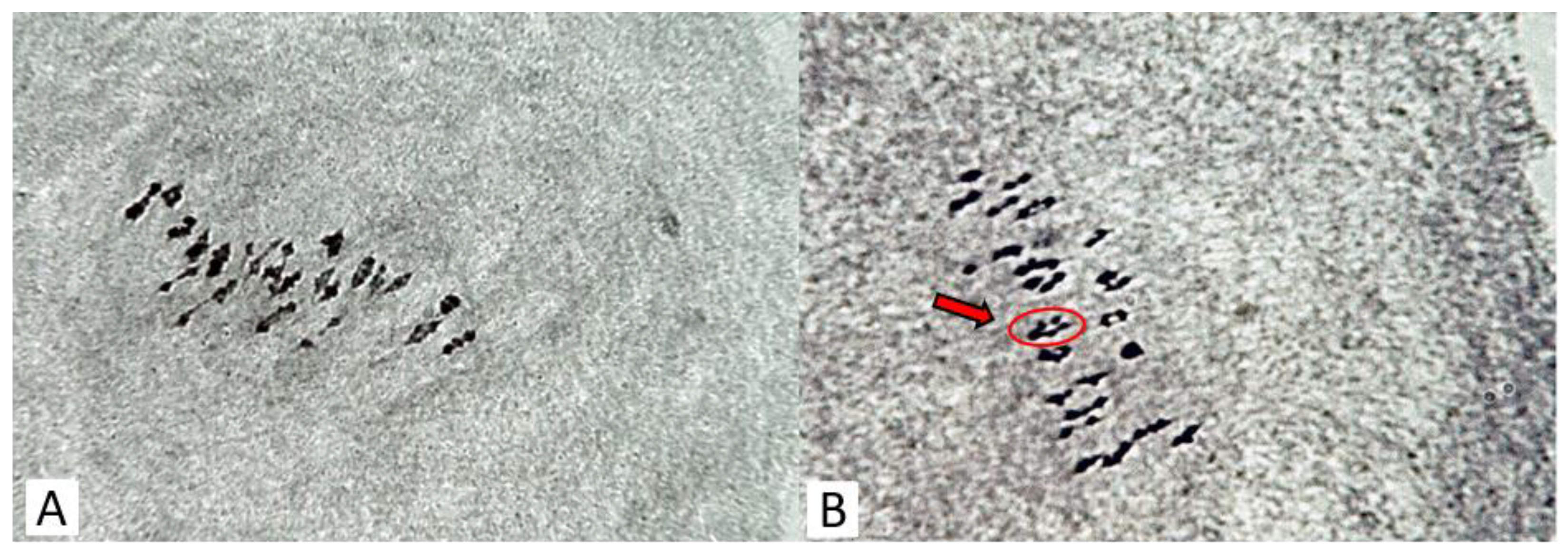
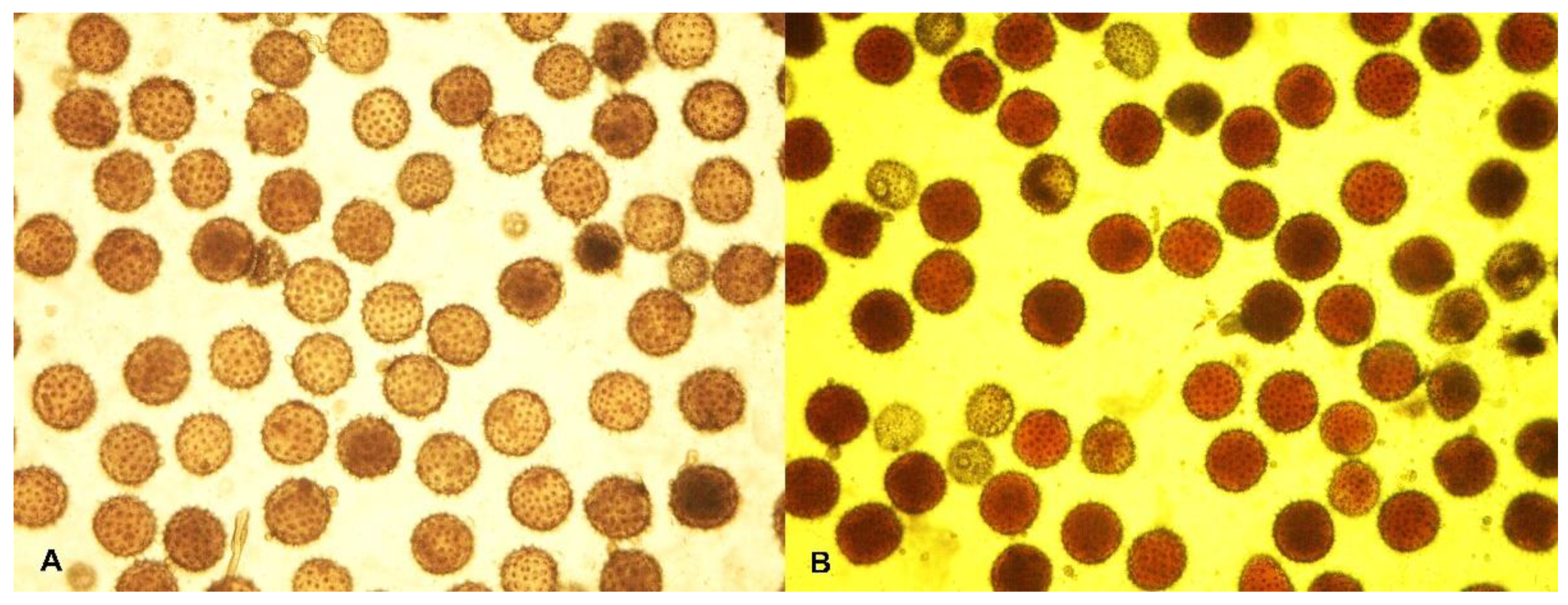
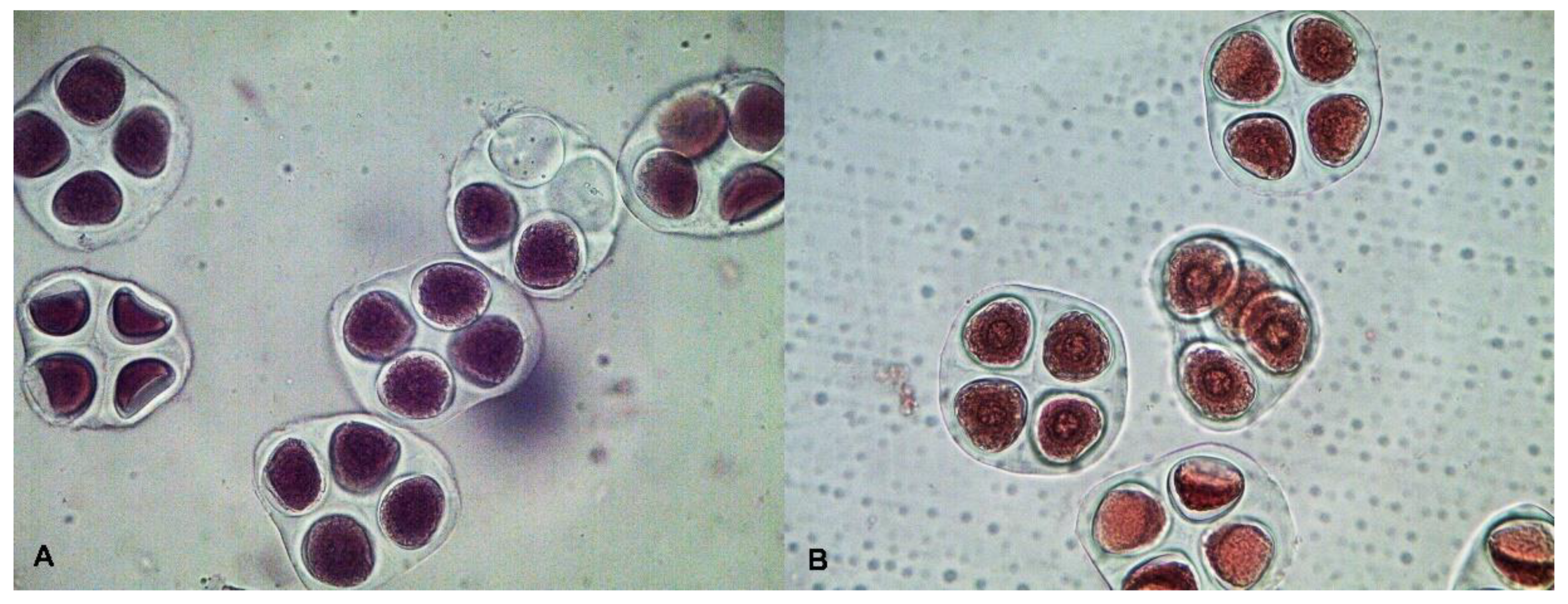
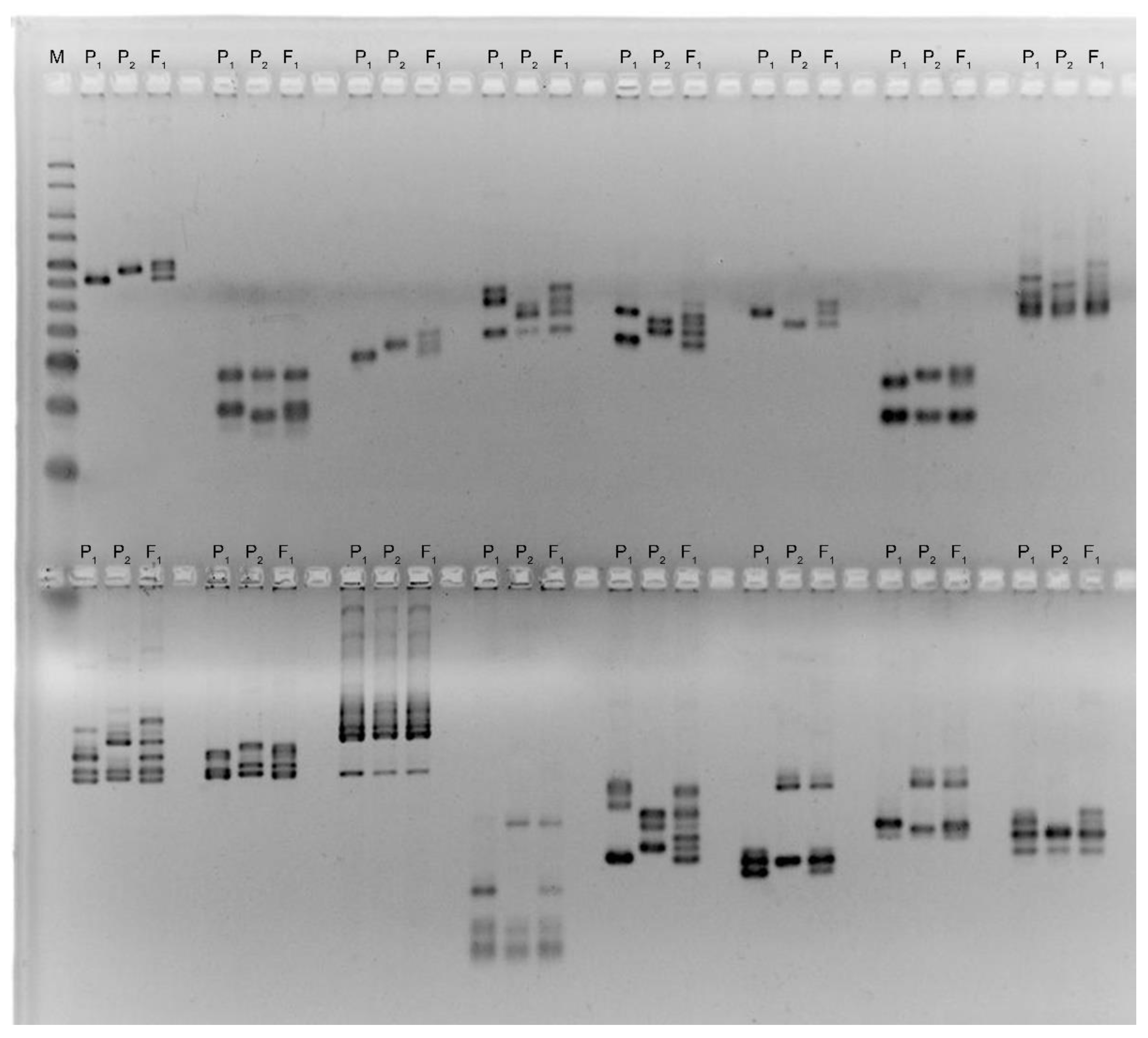
| Traits | Mutant Line—Kupaysin | Wild Type (El-Salvador) | F1 Generation | F2 Generation | ||||
|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SD * | SE ** | ||||
| Plant height (cm) | 80 | 175 | 120 | 40 | 170 | 109.33 | 26.96 | 2.15 |
| Number of monopodial branches | 0 | 7 | 5 | 1 | 14 | 3.93 | 3.16 | 0.33 |
| Number of sympodial branches | 15 | 5 | 5 | 2 | 34 | 17.70 | 6.99 | 0.58 |
| Number of nodes | 18 | 26 | 21 | 12 | 43 | 25.06 | 5.58 | 0.46 |
| Shape of bolls | ovoid | ovoid | ovoid | 23-cone-oval, 1-round, 112-ovoid | ||||
| Number of bolls per plant | 15 | 0 | 13 | 1 | 40 | 15.50 | 8.90 | 0.74 |
| Number of opened bolls per plant | 7 | 0 | 0 | 0 | 12 | 2.17 | 2.63 | 0.22 |
| Anthocyanin | Strong | Strong | Strong | Strong | ||||
| Bush shape | Compact | Spreading | Spreading | Compact—12.3%, Spreading—87.7% | ||||
| Node of first fruiting branch | 4 | 13–14 | 17 | 5 | 24 | 8.48 | 4.88 | 0.41 |
| PPS | PH | NFFB | NMB | NSB | NBL | NOBL | |
|---|---|---|---|---|---|---|---|
| P1 | 0.00 | 8.41 | 2.56 | 2.45 | 2.11 | 0.00 | 0.00 |
| P2 | 4.50 | 8.23 | 0.71 | 1.12 | 2.32 | 2.61 | 2.72 |
| F2 | 22.80 | 38.41 | 4.85 | 3.68 | 8.53 | 11.08 | 1.47 |
| Vg | 20.55 | 25.89 | 1.94 | 0.67 | 5.26 | 9.78 | 0.11 |
| H2 | 0.90 | 0.67 | 0.40 | 0.18 | 0.62 | 0.88 | 0.07 |
| SSR Marker Collection | Number of Mapped Markers | Number of Unmapped Markers |
|---|---|---|
| BNL SSR | 19 | 2 |
| GH SSR | 11 | 1 |
| JESPR SSR | 9 | 1 |
| TMB SSR | 24 | 2 |
| Total: | 63 | 6 |
| Linkage Groups | Chromosome | Number of Mapped Loci | Total Map Length (cM) | Mean Map Distance/Marker |
|---|---|---|---|---|
| LG01 | At-03 | 8 | 121.7 | 15.2 |
| LG02 | At-05 | 15 | 144.8 | 9.7 |
| LG03 | At-07 | 4 | 88.3 | 22.1 |
| LG04 | At-11 | 5 | 40.6 | 8.1 |
| LG05 | At-12 | 6 | 59.9 | 10.0 |
| LG06 | Dt-07 | 8 | 109.3 | 13.7 |
| LG07 | Dt-05 | 12 | 129.8 | 10.8 |
| LG08 | Dt-09a | 7 | 108.6 | 15.5 |
| LG09 | Dt-09b | 4 | 54.2 | 13.6 |
| Total: | 63 | 857.2 | 13.6 |
| # | QTL | Linkage Group | Chromosomal Location | Linked Markers | Position (cM) | LOD | Literature Reports | |
|---|---|---|---|---|---|---|---|---|
| Association of Markers with Other Traits | Reference | |||||||
| 1 | qNFFB | LG02 | At-05 | JESPR224_175-TMB0064_180 | 60.20–69.72 | 3.64 | Fiber micronaire (Dt-06); | [20] |
| Fiber strength (Dt-06) | ||||||||
| FOV race 4 resistances (Dt-06) | [21] | |||||||
| Flowering time (At-11) | [19] | |||||||
| Fiber length (At-11) | [22] | |||||||
| 2 | qNOBL | LG02 | At-05 | GH110_130-TMB0809_205 | 135.90–141.20 | 3.24 | Total fruit nodes; | [23] |
| Fruit branch angle (Dt-10). | ||||||||
| 3 | qPPS | LG02 | At-05 | JESPR224_175-TMB0131_240 | 58.90–69.91 | 3.64 | Fiber micronaire (Dt-06); | [20] |
| Fiber strength (Dt-06) | ||||||||
| FOV race 4 resistances (Dt-06) | [21] | |||||||
| 4 | qNFFB | LG04 | At-11 | JESPR296_135-TMB0064_200 | 21.20–22.40 | 3.71 | Fiber micronaire (Dt-06) | [20] |
| Flowering time (At-11) | [16] | |||||||
| Fiber length (At-11) | [22] | |||||||
| 5 | qNSB | LG06 | Dt-07 | BNL1064_150-TMB0016_250 | 48.30–56.40 | 4.2 | Fiber uniformity (Dt-06). | [24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kushanov, F.N.; Komilov, D.J.; Turaev, O.S.; Ernazarova, D.K.; Amanboyeva, R.S.; Gapparov, B.M.; Yu, J.Z. Genetic Analysis of Mutagenesis That Induces the Photoperiod Insensitivity of Wild Cotton Gossypium hirsutum Subsp. purpurascens. Plants 2022, 11, 3012. https://doi.org/10.3390/plants11223012
Kushanov FN, Komilov DJ, Turaev OS, Ernazarova DK, Amanboyeva RS, Gapparov BM, Yu JZ. Genetic Analysis of Mutagenesis That Induces the Photoperiod Insensitivity of Wild Cotton Gossypium hirsutum Subsp. purpurascens. Plants. 2022; 11(22):3012. https://doi.org/10.3390/plants11223012
Chicago/Turabian StyleKushanov, Fakhriddin N., Doniyor J. Komilov, Ozod S. Turaev, Dilrabo K. Ernazarova, Roza S. Amanboyeva, Bunyod M. Gapparov, and John Z. Yu. 2022. "Genetic Analysis of Mutagenesis That Induces the Photoperiod Insensitivity of Wild Cotton Gossypium hirsutum Subsp. purpurascens" Plants 11, no. 22: 3012. https://doi.org/10.3390/plants11223012
APA StyleKushanov, F. N., Komilov, D. J., Turaev, O. S., Ernazarova, D. K., Amanboyeva, R. S., Gapparov, B. M., & Yu, J. Z. (2022). Genetic Analysis of Mutagenesis That Induces the Photoperiod Insensitivity of Wild Cotton Gossypium hirsutum Subsp. purpurascens. Plants, 11(22), 3012. https://doi.org/10.3390/plants11223012









