Genome-Wide Identification of Cotton (Gossypium spp.) Glycerol-3-Phosphate Dehydrogenase (GPDH) Family Members and the Role of GhGPDH5 in Response to Drought Stress
Abstract
1. Introduction
2. Results
2.1. Identification and Fundamental Analysis of GPDH Genes from Diploid and Tetraploid Cotton
2.2. Structural Characterizations and Conserved Motif Analyses of GPDH Genes
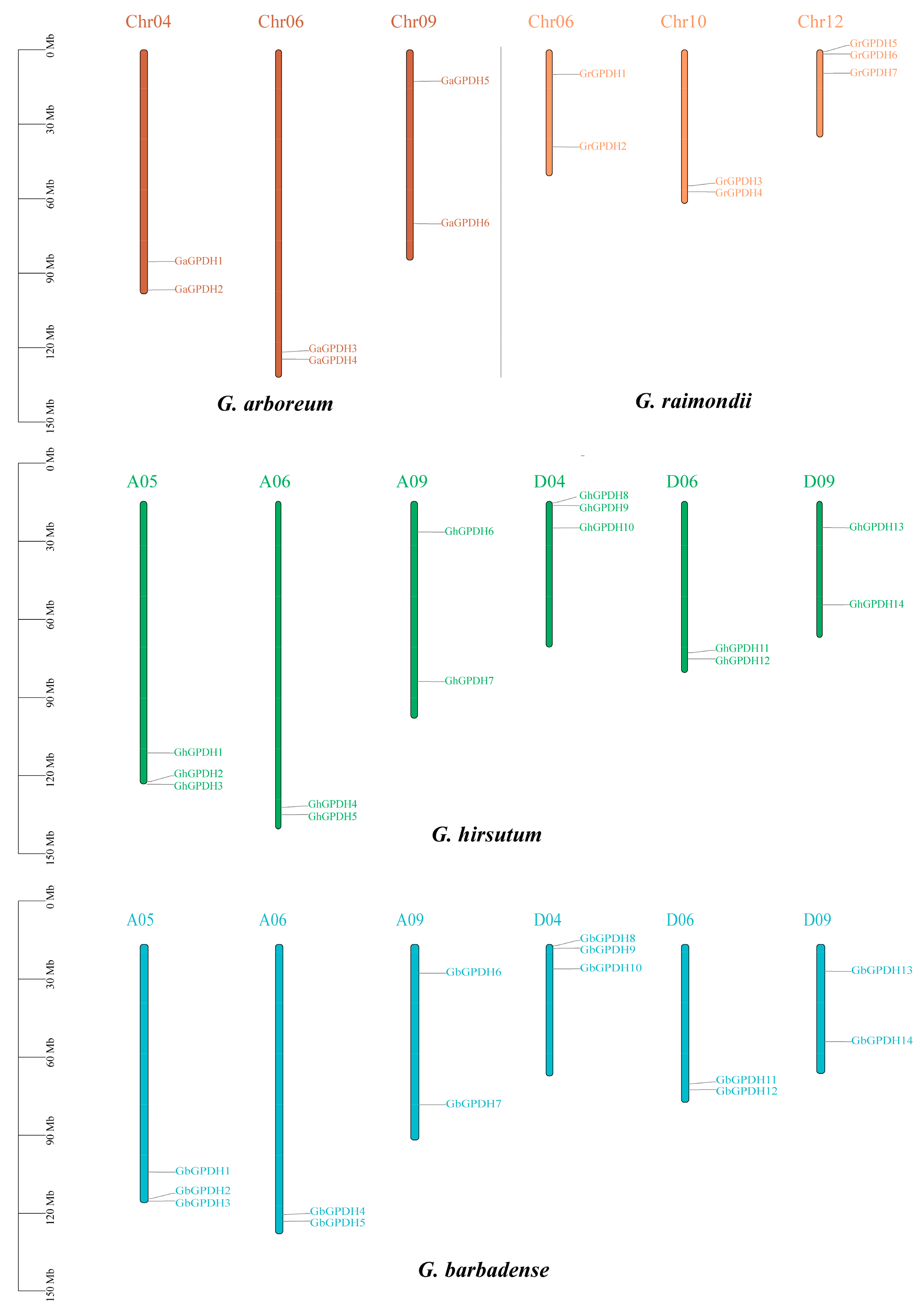
2.3. Chromosomal Localization of GPDH Genes in Four Cotton Species
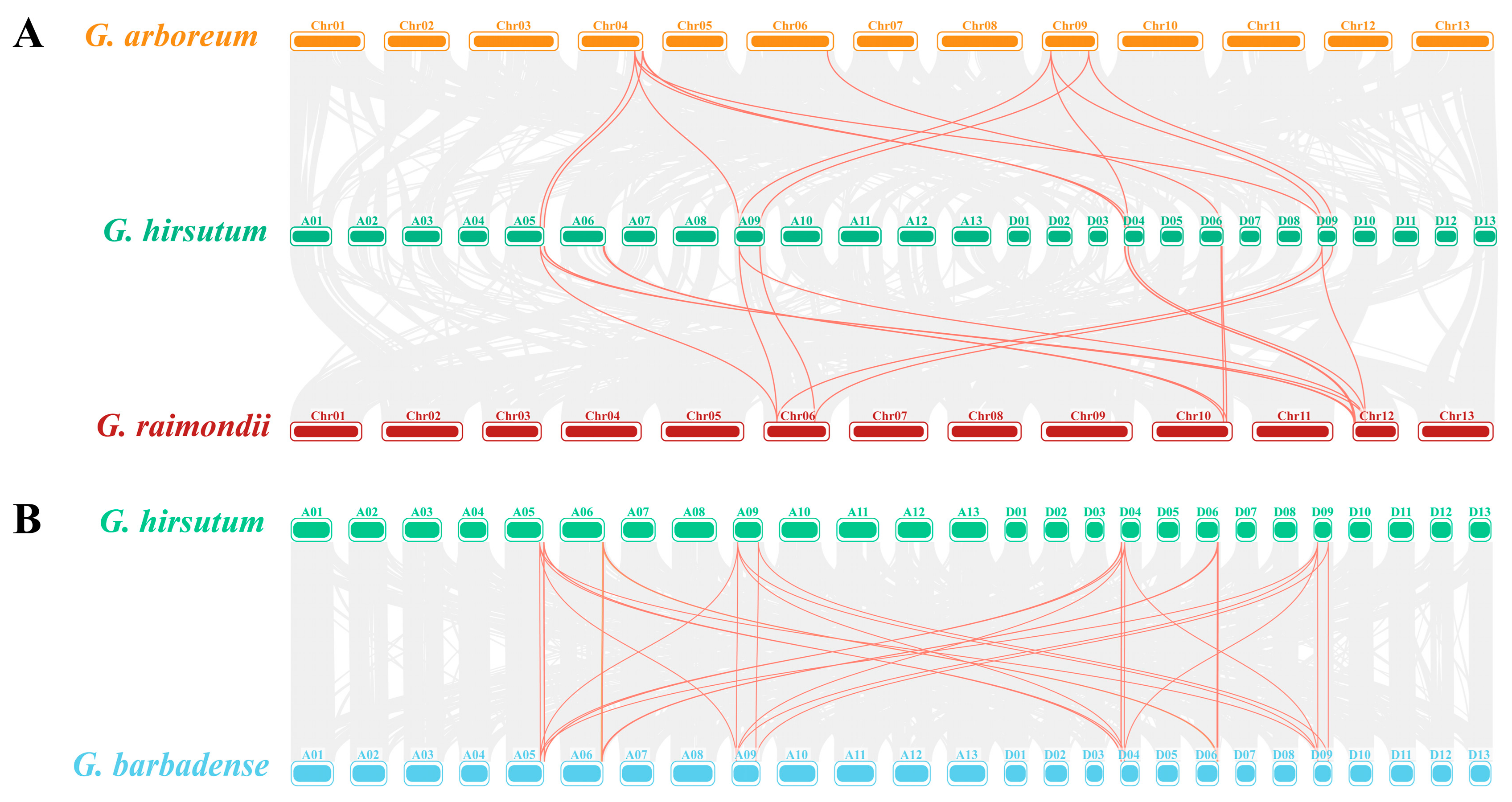
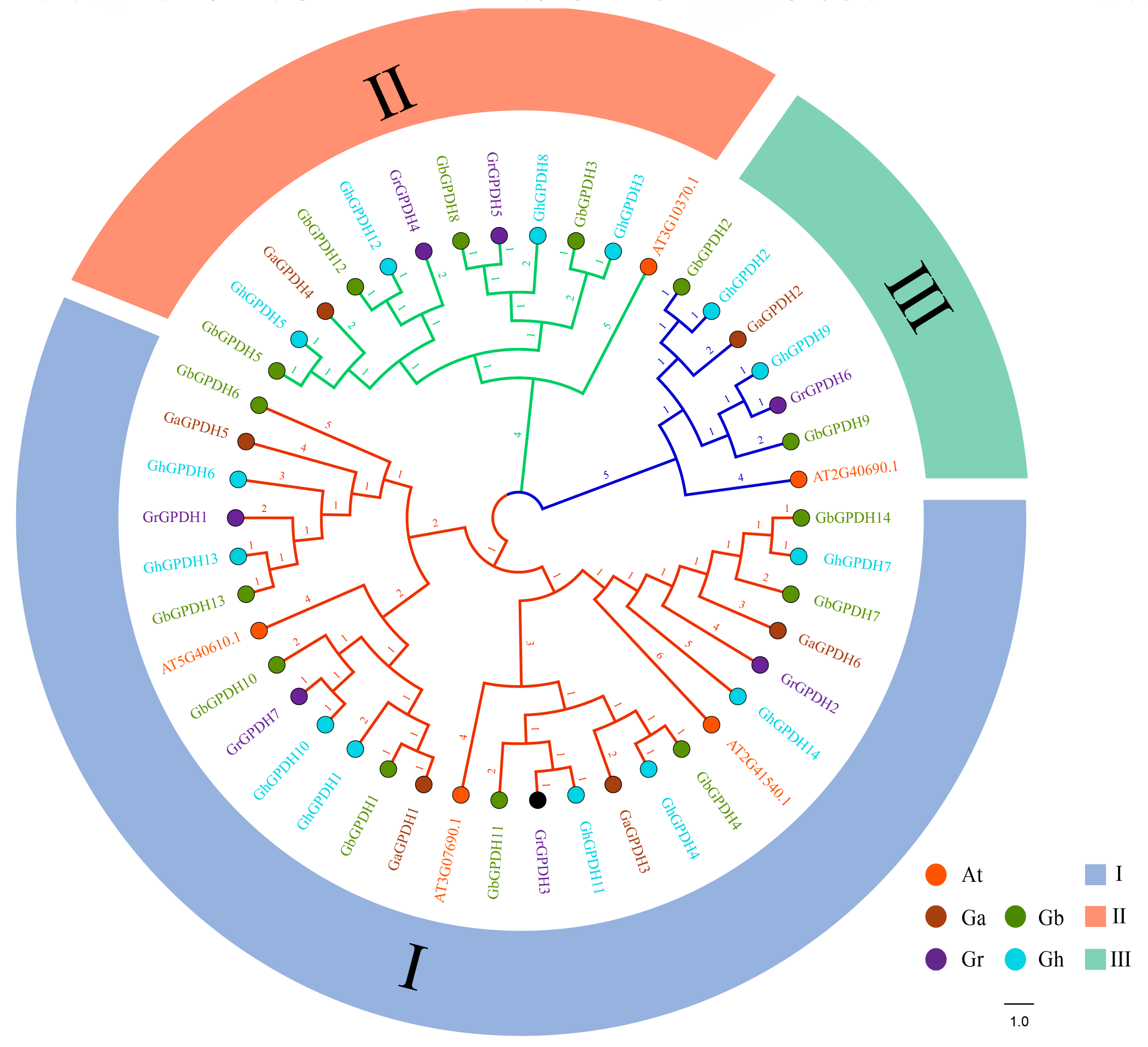
2.4. Replication Events and Phylogenetic Analysis
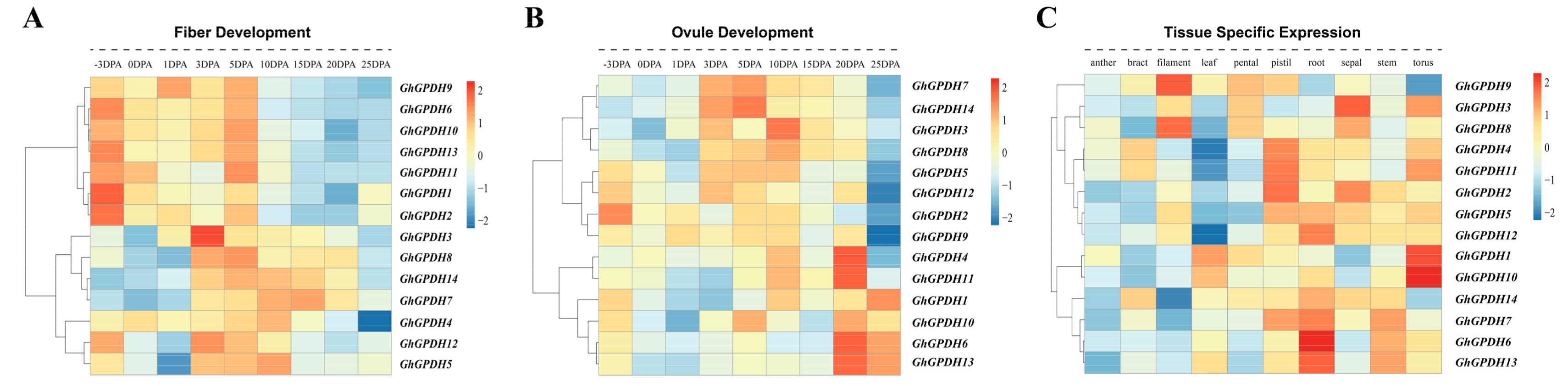
2.5. Gene Expression Profile Analysis of GPDH Family Members
2.6. Cis-Element Analysis of GPDH Promoter Region
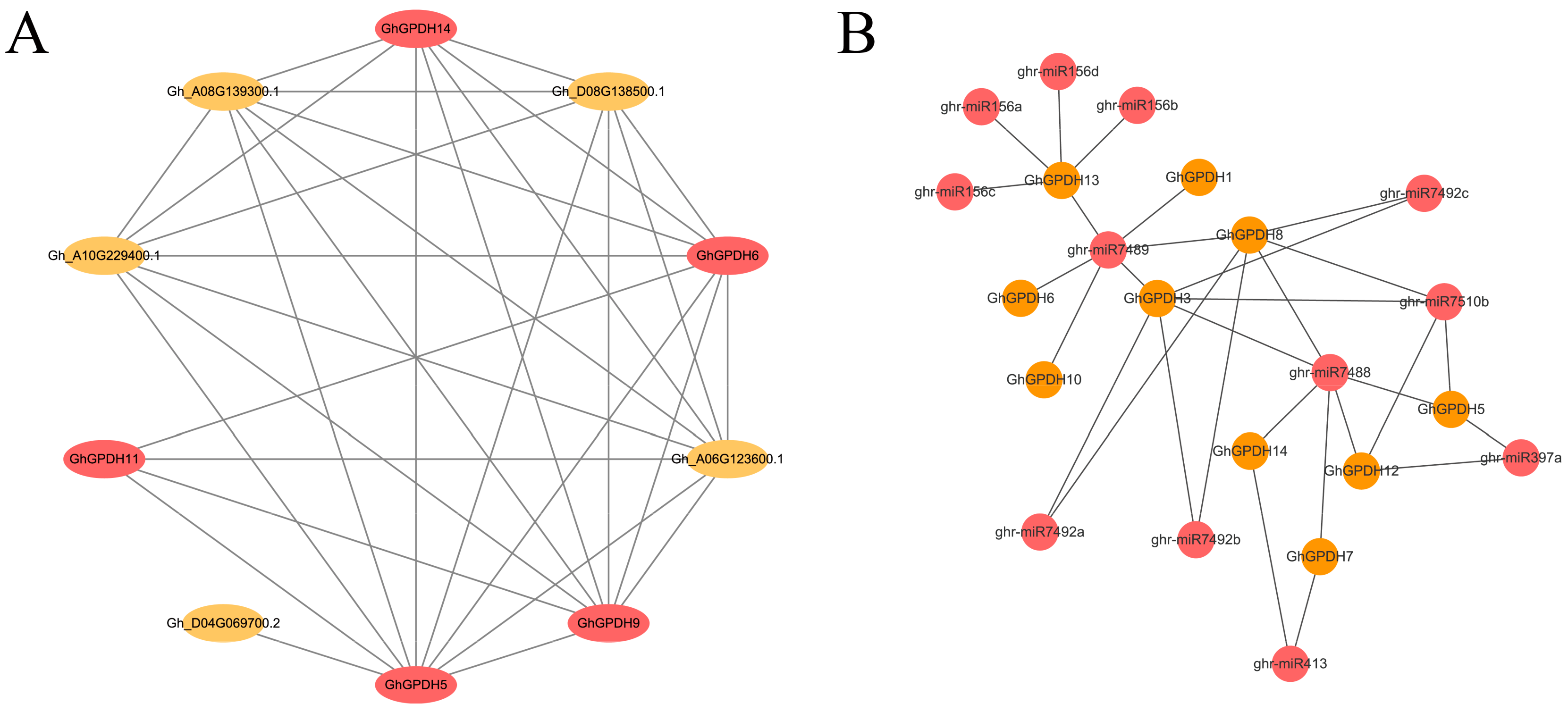
2.7. Protein Interaction Network Analysis of GPDH5 in G. hirsutum
2.8. Potential miRNAs That Target to GhGPDHs
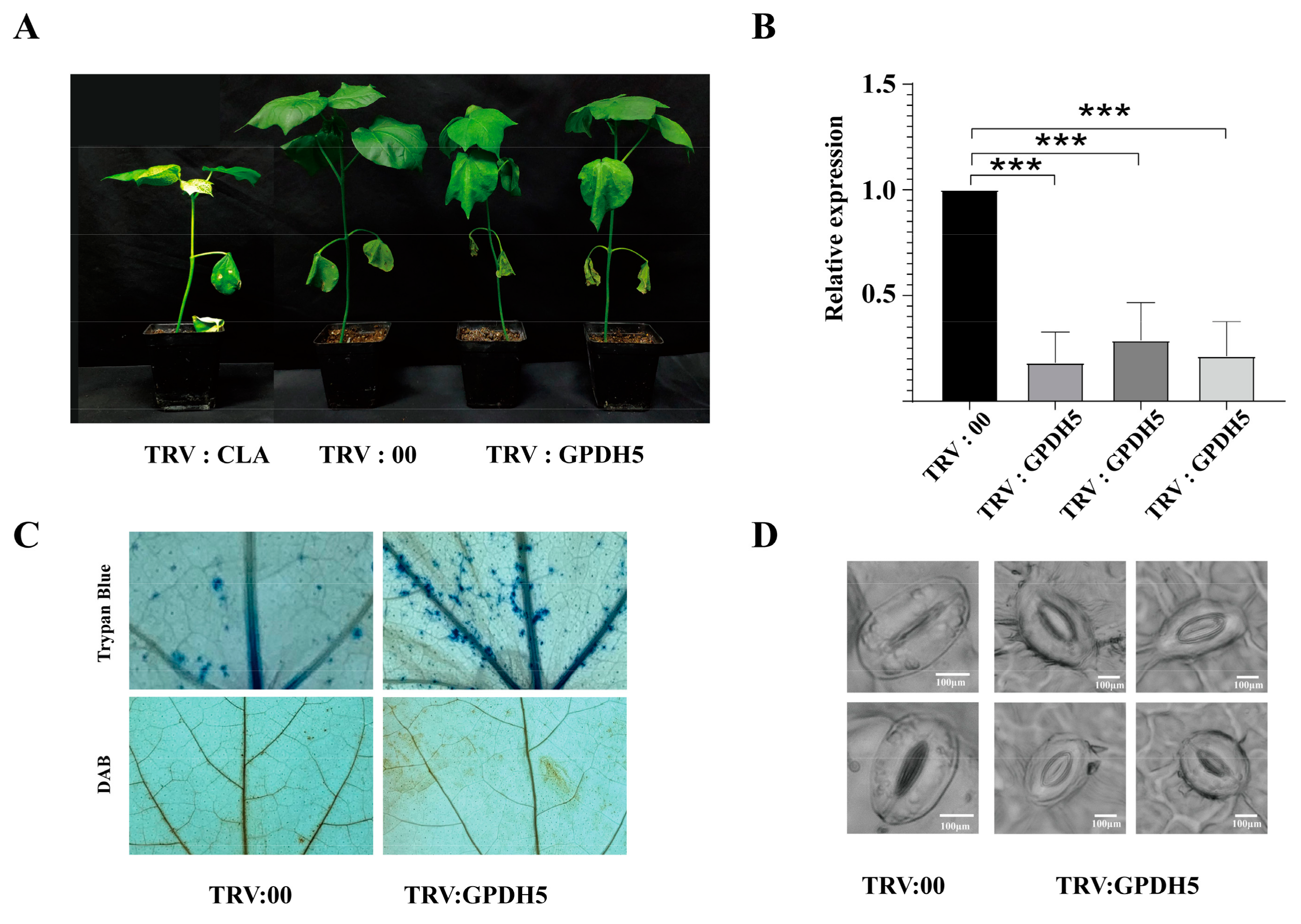
2.9. Preliminary Validation of GPDH5 Function in G. hirsutum
3. Discussion
4. Materials and Methods
4.1. Identification of GPDH Genes in Cotton
4.2. Gene Structures and Conserved Motifs Prediction
4.3. Phylogenetic Analysis
4.4. Chromosomal Distribution and Collinearity Analysis
4.5. Gene Expression Analysis of the GPDH Family in G. hirsutum
4.6. Analysis of Cis-Elements in the Promoter Region
4.7. MiRNA Prediction of GPDH Genes in G. hirsutum
4.8. Construction of the GhGPDH5 Protein-Protein Interaction Network
4.9. Gene Function Validation and Quantitative Real-Time PCR (qPCR)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TAIR | The Arabidopsis Information Resource |
| MUSCLE | Multiple Sequence Comparison by Log-Expectation |
| ML | Maximum Likelihood |
| BLAST | Basic Local Alignment Search Tool |
| Ka | The number of nonsynonymous substitutions per nonsynonymous site |
| Ks | The number of synonymous substitutions per synonymous site |
| DPA | Days Post Anthesis |
| CDS | Coding Sequence |
| Gh | G. hirsutum |
| Ga | G. arboreum |
| Gr | G. raimondii |
| Gb | G. barbadense |
| At | Arabidopsis |
| ABA | Abscisic acid |
| DAB | 3,3′-diaminobenzidine |
| TPB | Typan Bue |
| VIGS | Virus-Induced Gene Silencing |
| PI | Isoelectric point |
| MW | Molecular Weight |
| TRV | Tobacco Rattle Virus |
References
- Flowers, T.; Yeo, A. Breeding for salinity resistance in crop plants: Where next? Funct. Plant Biol. 1995, 22, 875–884. [Google Scholar] [CrossRef]
- Dos Reis, S.P.; Lima, A.M.; De Souza, C.R.B. Recent molecular advances on downstream plant responses to abiotic stress. Int. J. Mol. Sci. 2012, 13, 8628–8647. [Google Scholar] [CrossRef] [PubMed]
- FAO. Global Agriculture towards 2050, High-Level Expert Forum, How to Feed the World 2050; Food and Agriculture Organization of United Nations FAO: Rome, Italy, 2009. [Google Scholar]
- Abdelraheem, A.; Esmaeili, N.; O’Connell, M.; Zhang, J. Progress and perspective on drought and salt stress tolerance in cotton. Ind. Crops Prod. 2019, 130, 118–129. [Google Scholar] [CrossRef]
- Gerber, D.W.; Byerrum, R.U.; Gee, R.W.; Tolbert, N. Glycerol concentrations in crop plants. Plant Sci. 1988, 56, 31–38. [Google Scholar] [CrossRef]
- Eastmond, P.J. Glycerol-insensitive Arabidopsis mutants: Gli1 seedlings lack glycerol kinase, accumulate glycerol and are more resistant to abiotic stress. Plant J. 2004, 37, 617–625. [Google Scholar] [CrossRef]
- Bahieldin, A.; Sabir, J.S.M.; Ramadan, A.; Alzohairy, A.M.; Younis, R.A.; Shokry, A.M.; Gadalla, N.O.; Edris, S.; Hassan, S.M.; Al-Kordy, M.A.; et al. Control of glycerol biosynthesis under high salt stress in Arabidopsis. Funct. Plant Biol. 2013, 41, 87–95. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Avron, M. Isolation, Characterization, and Partial Purification of a Reduced Nicotinamide Adenine Dinucleotide Phosphate-dependent Dihydroxyacetone Reductase from the Halophilic Alga Dunaliella parva. Plant Physiol. 1974, 53, 628–631. [Google Scholar] [CrossRef][Green Version]
- Harding, J.W., Jr.; Pyeritz, E.A.; Copeland, E.S.; White, H.B., 3rd. Role of glycerol 3-phosphate dehydrogenase in glyceride metabolism. Effect of diet on enzyme activities in chicken liver. Biochem. J. 1975, 146, 223–229. [Google Scholar] [CrossRef]
- Vigeolas, H.; Waldeck, P.; Zank, T.; Geigenberger, P. Increasing seed oil content in oil-seed rape (Brassica napus L.) by over-expression of a yeast glycerol-3-phosphate dehydrogenase under the control of a seed-specific promoter. Plant Biotechnol. J. 2007, 5, 431–441. [Google Scholar] [CrossRef]
- Shen, W.; Wei, Y.; Dauk, M.; Tan, Y.; Taylor, D.C.; Selvaraj, G.; Zou, J. Involvement of a Glycerol-3-Phosphate Dehydrogenase in Modulating the NADH/NAD+ Ratio Provides Evidence of a Mitochondrial Glycerol-3-Phosphate Shuttle in Arabidopsis. Plant Cell 2006, 18, 422–441. [Google Scholar] [CrossRef]
- Wei, Y.; Periappuram, C.; Datla, R.; Selvaraj, G.; Zou, J. Molecular and biochemical characterizations of a plastidic glycerol-3-phosphate dehydrogenase from Arabidopsis § 1 This is National Research Council of Canada publication No. 43805. EMBL accession number for the Arabidopsis plastidic glycerol-3-phosphate dehydrogenase: AJ242602. Plant Physiol. Biochem. 2001, 39, 841–848. [Google Scholar]
- Shen, W.; Wei, Y.; Dauk, M.; Zheng, Z.; Zou, J. Identification of a mitochondrial glycerol-3-phosphate dehydrogenase fromArabidopsis thaliana: Evidence for a mitochondrial glycerol-3-phosphate shuttle in plants. FEBS Lett. 2003, 536, 92–96. [Google Scholar] [CrossRef]
- Chao, Z.; Sanxiong, F.; Rong, T.; Xiaoying, Z.; Huangsha; Lulu, W.; Kexing, Y.; Cunkou, Q. Genome-wide Identification of GPDH Gene Family In Brassica napus. Acta Agric. Boreali-Occident. Sin. 2020, 29, 363–373. [Google Scholar]
- Yuanshi, C.; Jiajia, G.; Hailin, L.; Qinghua, H.; Qinglian, Z. Cloing and Characterization a Cytoplasmic Glycerol 3-phosphate Dehydrogenase Gene from Dunaliella salina. Genom. Appl. Biol. 2020, 39, 4122–4129. [Google Scholar]
- Zhao, Y.; Li, X.; Wang, F.; Zhao, X.; Gao, Y.; Zhao, C.; He, L.; Li, Z.; Xu, J. Glycerol-3-phosphate dehydrogenase (GPDH) gene family in Zea mays L.: Identification, subcellular localization, and transcriptional responses to abiotic stresses. PLoS ONE 2018, 13, e0200357. [Google Scholar] [CrossRef]
- Guan, X.; Song, Q.; Chen, Z.J. Polyploidy and small RNA regulation of cotton fiber development. Trends Plant Sci. 2014, 19, 516–528. [Google Scholar] [CrossRef]
- Li, C.; Zhang, B. MicroRNAs in control of plant development. J. Cell. Physiol. 2016, 231, 303–313. [Google Scholar] [CrossRef]
- Gillani, S.M.N.; Iqbal, R.M.; Akram, M.; Ajmal, F.; Wasim, M.A.; Ijaz, M.; Pakistan, V.; Layyah, S.C.B. Performance of cotton (Gossypium hirsutum L) through foliar application of growth promoters. Pak. J. Life Soc. Sci. 2015, 13, 20–26. [Google Scholar]
- Chinnusamy, V.; Jagendorf, A.; Zhu, J.K. Understanding and improving salt tolerance in plants. Crop Sci. 2005, 45, 437–448. [Google Scholar] [CrossRef]
- Wu, Q.; Lan, Y.; Cao, X.; Yao, H.; Qiao, D.; Xu, H.; Cao, Y. Characterization and diverse evolution patterns of glycerol-3-phosphate dehydrogenase family genes in Dunaliella salina. Gene 2019, 710, 161–169. [Google Scholar] [CrossRef]
- Xu, F.-C.; Liu, H.-L.; Xu, Y.-Y.; Zhao, J.-R.; Guo, Y.-W.; Long, L.; Gao, W.; Song, C.-P. Heterogeneous expression of the cotton R2R3-MYB transcription factor GbMYB60 increases salt sensitivity in transgenic Arabidopsis. Plant Cell Tissue Organ Cult. (PCTOC) 2018, 133, 15–25. [Google Scholar] [CrossRef]
- Wendel, J.F. New World tetraploid cottons contain Old World cytoplasm. Proc. Natl. Acad. Sci. USA 1989, 86, 4132–4136. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.G.; Shan, J.X.; Shi, M.; Gao, J.P.; Lin, H.X. The miR156-SPL9-DFR pathway coordinates the relationship between development and abiotic stress tolerance in plants. Plant J. 2014, 80, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Stief, A.; Altmann, S.; Hoffmann, K.; Pant, B.D.; Scheible, W.R.; Baurle, I. Arabidopsis miR156 Regulates Tolerance to Recurring Environmental Stress through SPL Transcription Factors. Plant Cell 2014, 26, 1792–1807. [Google Scholar] [CrossRef] [PubMed]
- Schmalhausen, E.V.; Muronetz, V.I. An uncoupling of the processes of oxidation and phosphorylation in glycolysis. Biosci. Rep. 1997, 17, 521–527. [Google Scholar] [CrossRef][Green Version]
- Slaughter, M.R.; Thakkar, H.; O’Brien, P.J. Effect of diquat on the antioxidant system and cell growth in human neuroblastoma cells. Toxicol. Appl. Pharmacol. 2002, 178, 63–70. [Google Scholar] [CrossRef]
- Bange, G.G.J. On the quantitative explanation of stomatal transpiration. Acta Bot. Neerl. 1953, 2, 255–297. [Google Scholar] [CrossRef]
- Cowan, I.; Troughton, J. The relative role of stomata in transpiration and assimilation. Planta 1971, 97, 325–336. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Albertyn, J.; Hohmann, S.; Prior, B.A. Characterization of the osmotic-stress response in Saccharomyces cerevisiae: Osmotic stress and glucose repression regulate glycerol-3-phosphate dehydrogenase independently. Curr. Genet. 1994, 25, 12–18. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, M.; He, L.; Li, X.; Wang, F.; Yan, B.; Wei, J.; Zhao, C.; Li, Z.; Xu, J. A cytosolic NAD(+)-dependent GPDH from maize (ZmGPDH1) is involved in conferring salt and osmotic stress tolerance. BMC Plant Biol. 2019, 19, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, X.; Zhang, Z.; Pan, W.; Li, S.; Xing, Y.; Xin, W.; Zhang, Z.; Hu, Z.; Liu, C.; et al. GmGPDH12, a mitochondrial FAD-GPDH from soybean, increases salt and osmotic stress resistance by modulating redox state and respiration. Crop J. 2021, 9, 79–94. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, Q.; Wang, W.; Yang, J.; Zhang, X.; Yu, N.; Zhou, Y.; Wang, E. Medicago AP2-domain transcription factor WRI5a is a master regulator of lipid biosynthesis and transfer during mycorrhizal symbiosis. Mol. Plant 2018, 11, 1344–1359. [Google Scholar] [CrossRef]
- Ahmad, H.M.; Rahman, M.-u.; Ahmar, S.; Fiaz, S.; Azeem, F.; Shaheen, T.; Ijaz, M.; Bukhari, S.A.; Khan, S.A.; Mora-Poblete, F. Comparative genomic analysis of MYB transcription factors for cuticular wax biosynthesis and drought stress tolerance in Helianthus annuus L. Saudi J. Biol. Sci. 2021, 28, 5693–5703. [Google Scholar] [CrossRef]
- Chen, K.; Song, M.; Guo, Y.; Liu, L.; Xue, H.; Dai, H.; Zhang, Z. Md MYB 46 could enhance salt and osmotic stress tolerance in apple by directly activating stress-responsive signals. Plant Biotechnol. J. 2019, 17, 2341–2355. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Ho, T.D.; Liu, L.; Lee, D.H.; Lee, C.H.; Chen, Y.R.; Lin, S.Y.; Lu, C.A.; Yu, S.M. Sugar starvation-regulated MYBS2 and 14-3-3 protein interactions enhance plant growth, stress tolerance, and grain weight in rice. Proc. Natl. Acad. Sci. USA 2019, 116, 21925–21935. [Google Scholar] [CrossRef]
- Huang, J.; Guo, Y.; Sun, Q.; Zeng, W.; Li, J.; Li, X.; Xu, W. Genome-Wide Identification of R2R3-MYB Transcription Factors Regulating Secondary Cell Wall Thickening in Cotton Fiber Development. Plant Cell Physiol. 2019, 60, 687–701. [Google Scholar] [CrossRef]
- Inukai, S.; Kock, K.H.; Bulyk, M.L. Transcription factor-DNA binding: Beyond binding site motifs. Curr. Opin. Genet. Dev. 2017, 43, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, J.; Schemske, D.W. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu. Rev. Ecol. Syst. 1998, 29, 467–501. [Google Scholar] [CrossRef]
- Lynch, M.; Conery, J.S. The evolutionary fate and consequences of duplicate genes. Science 2000, 290, 1151–1155. [Google Scholar] [CrossRef]
- Paterson, A.H.; Wendel, J.F.; Gundlach, H.; Guo, H.; Jenkins, J.; Jin, D.; Llewellyn, D.; Showmaker, K.C.; Shu, S.; Udall, J. Repeated polyploidization of Gossypium genomes and the evolution of spinnable cotton fibres. Nature 2012, 492, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Shao-Fang, L.; Man, X.; Hui-Hui, W.; Ke-Bin, W.; Yun, G.C.; Gang, X.; Xing-Hua, X. Molecular cloning and expression profle of BnaGPDH gene in Brassica napus. Plant Physiol. J. 2017, 53, 1772–1780. [Google Scholar]
- Ansell, R.; Granath, K.; Hohmann, S.; Thevelein, J.M.; Adler, L. The two isoenzymes for yeast NAD+-dependent glycerol 3-phosphate dehydrogenase encoded by GPD1 and GPD2 have distinct roles in osmoadaptation and redox regulation. EMBO J. 1997, 16, 2179–2187. [Google Scholar] [CrossRef] [PubMed]
- Albertyn, J.; Hohmann, S.; Thevelein, J.M.; Prior, B.A. GPD1, which encodes glycerol-3-phosphate dehydrogenase, is essential for growth under osmotic stress in Saccharomyces cerevisiae, and its expression is regulated by the high-osmolarity glycerol response pathway. Mol. Cell. Biol. 1994, 14, 4135–4144. [Google Scholar] [PubMed]
- McAdam, S.A.; Duckett, J.G.; Sussmilch, F.C.; Pressel, S.; Renzaglia, K.S.; Hedrich, R.; Brodribb, T.J.; Merced, A. Stomata: The holey grail of plant evolution. Am. J. Bot. 2021, 108, 366. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, X.; Mao, Q.; Wan, H.; Zhou, G.; Cheng, Y. The SlWRKY81 transcription factor inhibits stomatal closure by attenuating nitric oxide accumulation in the guard cells of tomato under drought. Physiol. Plant. 2021, 172, 885–895. [Google Scholar] [CrossRef]
- Xu, B.; Long, Y.; Feng, X.; Zhu, X.; Sai, N.; Chirkova, L.; Betts, A.; Herrmann, J.; Edwards, E.J.; Okamoto, M.; et al. GABA signalling modulates stomatal opening to enhance plant water use efficiency and drought resilience. Nat. Commun. 2021, 12, 1952. [Google Scholar] [CrossRef]
- Pitaloka, M.K.; Harrison, E.L.; Hepworth, C.; Wanchana, S.; Toojinda, T.; Phetluan, W.; Brench, R.A.; Narawatthana, S.; Vanavichit, A.; Gray, J.E. Rice Stomatal Mega-Papillae Restrict Water Loss and Pathogen Entry. Front. Plant Sci. 2021, 12, 677839. [Google Scholar] [CrossRef]
- Joshi-Saha, A.; Valon, C.; Leung, J. A brand new START: Abscisic acid perception and transduction in the guard cell. Sci. Signal. 2011, 4, re4. [Google Scholar] [CrossRef]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nose, T.; Jikumaru, Y.; Kamiya, Y. ABA inhibits entry into stomatal-lineage development in A rabidopsis leaves. Plant J. 2013, 74, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Feitosa-Araujo, E.; da Fonseca-Pereira, P.; Pena, M.M.; Medeiros, D.B.; Perez de Souza, L.; Yoshida, T.; Weber, A.P.M.; Araujo, W.L.; Fernie, A.R.; Schwarzlander, M.; et al. Changes in intracellular NAD status affect stomatal development in an abscisic acid-dependent manner. Plant J. 2020, 104, 1149–1168. [Google Scholar] [CrossRef]
- Feitosa-Araujo, E.; da Fonseca-Pereira, P.; Knorr, L.S.; Schwarzländer, M.; Nunes-Nesi, A. NAD meets ABA: Connecting cellular metabolism and hormone signaling. Trends Plant Sci. 2021, 27, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Liang, C.; Meng, Z.; Sun, G.; Meng, Z.; Guo, S.; Zhang, R. CottonFGD: An integrated functional genomics database for cotton. BMC Plant Biol. 2017, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- Swarbreck, D.; Wilks, C.; Lamesch, P.; Berardini, T.Z.; Garcia-Hernandez, M.; Foerster, H.; Li, D.; Meyer, T.; Muller, R.; Ploetz, L. The Arabidopsis Information Resource (TAIR): Gene structure and function annotation. Nucleic Acids Res. 2007, 36 (Suppl. 1), D1009–D1014. [Google Scholar] [CrossRef]
- Eddy, S.R. Accelerated profile HMM searches. PLoS Comput. Biol. 2011, 7, e1002195. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the ExPASy server. In The Proteomics Protocols Handbook; Springer: New York, NY, USA, 2005; pp. 571–607. [Google Scholar]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef]
- Yu, C.S.; Chen, Y.C.; Lu, C.H.; Hwang, J.K. Prediction of protein subcellular localization. Proteins Struct. Funct. Bioinform. 2006, 64, 643–651. [Google Scholar] [CrossRef]
- Yu, C.S.; Lin, C.J.; Hwang, J.K. Predicting subcellular localization of proteins for Gram-negative bacteria by support vector machines based on n-peptide compositions. Protein Sci. 2004, 13, 1402–1406. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Terwilliger, T.C. Maximum-likelihood density modification. Acta Crystallogr. Sect. D Biol. Crystallogr. 2000, 56, 965–972. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Allaire, J. RStudio: Integrated Development Environment for R; RStudio: Boston, MA, USA, 2012; Volume 770, pp. 165–171. [Google Scholar]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Kumar, V.; Khare, T.; Shriram, V.; Wani, S.H. Plant small RNAs: The essential epigenetic regulators of gene expression for salt-stress responses and tolerance. Plant Cell Rep. 2018, 37, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhuang, Z.; Zhao, P.X. psRNATarget: A plant small RNA target analysis server (2017 release). Nucleic Acids Res. 2018, 46, W49–W54. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Lee, T.; Yang, S.; Kim, E.; Ko, Y.; Hwang, S.; Shin, J.; Shim, J.E.; Shim, H.; Kim, H.; Kim, C. AraNet v2: An improved database of co-functional gene networks for the study of Arabidopsis thaliana and 27 other nonmodel plant species. Nucleic Acids Res. 2015, 43, D996–D1002. [Google Scholar] [CrossRef]
- Wu, C.; Jia, L.; Goggin, F. The reliability of virus-induced gene silencing experiments using tobacco rattle virus in tomato is influenced by the size of the vector control. Mol. Plant Pathol. 2011, 12, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Schiff, M.; Marathe, R.; Dinesh-Kumar, S. Tobacco Rar1, EDS1 and NPR1/NIM1 like genes are required for N-mediated resistance to tobacco mosaic virus. Plant J. 2002, 30, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Ratcliff, F.; Martin-Hernandez, A.M.; Baulcombe, D.C. Technical advance: Tobacco rattle virus as a vector for analysis of gene function by silencing. Plant J. 2001, 25, 237–245. [Google Scholar] [CrossRef]
- Gao, X.; Wheeler, T.; Li, Z.; Kenerley, C.M.; He, P.; Shan, L. Silencing GhNDR1 and GhMKK2 compromises cotton resistance to Verticillium wilt. Plant J. 2011, 66, 293–305. [Google Scholar] [CrossRef]
- Fernández-Bautista, N.; Domínguez-Núñez, J.A.; Moreno, M.M.C.; Berrocal-Lobo, M. Plant tissue trypan blue staining during phytopathogen infection. Bio-Protocol 2016, 6, e2078. [Google Scholar] [CrossRef]
- van Wees, S. Phenotypic analysis of Arabidopsis mutants: Trypan blue stain for fungi, oomycetes, and dead plant cells. Cold Spring Harb. Protoc. 2008, 2008, prot4982. [Google Scholar] [CrossRef] [PubMed]
- Keogh, R.; Deverall, B.; McLeod, S. Comparison of histological and physiological responses to Phakopsora pachyrhizi in resistant and susceptible soybean. Trans. Br. Mycol. Soc. 1980, 74, 329–333. [Google Scholar] [CrossRef]
- Kumar, D.; Yusuf, M.A.; Singh, P.; Sardar, M.; Sarin, N.B. Modulation of antioxidant machinery in α-tocopherol-enriched transgenic Brassica juncea plants tolerant to abiotic stress conditions. Protoplasma 2013, 250, 1079–1089. [Google Scholar] [CrossRef]
- Kumar, D.; Yusuf, M.A.; Singh, P.; Sardar, M.; Sarin, N.B. Histochemical detection of superoxide and H2O2 accumulation in Brassica juncea seedlings. Bio-Protocol 2014, 4, e1108. [Google Scholar] [CrossRef]



| Type | ID | Name | MW | PI | Subcellular Location | Amino Acid Numbers | Region |
|---|---|---|---|---|---|---|---|
| I | Ga04G1449 | GaGPDH1 | 36,512.62 | 5.47 | Plasma Membrane | 336 | Chr04: 85365308–85367513 (+) |
| Gh_A05G359000 | GhGPDH1 | 26,610.66 | 5.15 | Cytoplasmic | 244 | A05: 96649619–96651585 (+) | |
| Gbar_A05G035870 | GbGPDH1 | 36,572.71 | 5.46 | Plasma Membrane | 336 | A05: 90403265–90406088 (+) | |
| Gorai.012G066600 | GrGPDH7 | 44,225.84 | 5.79 | Cytoplasmic | 402 | Chr12: 9501046–9503893 (-) | |
| Gbar_D04G006270 | GbGPDH10 | 41,212.08 | 5.26 | Cytoplasmic | 376 | D04: 9780994–9783914 (-) | |
| Gh_D04G066600 | GhGPDH10 | 41,154.04 | 5.35 | Cytoplasmic | 376 | D04: 10236191–10238979 (-) | |
| Gbar_A09G003620 | GbGPDH6 | 48,156.2 | 5.7 | Chloroplast | 439 | A09: 11430821–11435386 (-) | |
| Gh_A09G038400 | GhGPDH6 | 48,126.17 | 5.7 | Chloroplast | 439 | A09: 11794439–11796911 (-) | |
| Ga09G0401 | GaGPDH5 | 47,514.61 | 6.24 | Chloroplast | 434 | Chr09: 12758934–12762193 (-) | |
| Gorai.006G036200 | GrGPDH1 | 48,098.07 | 5.44 | Chloroplast | 438 | Chr06: 9950055–9952964 (-) | |
| Gbar_D09G003380 | GbGPDH13 | 48,128.19 | 5.6 | Chloroplast | 438 | D09: 10699107–10703615 (-) | |
| Gh_D09G035900 | GhGPDH13 | 48,186.23 | 5.51 | Chloroplast | 438 | D09: 10082117–10084586 (-) | |
| II | Ga04G1991 | GaGPDH2 | 47,231.15 | 9.47 | Mitochondrial | 437 | Chr04: 96767830–96770413 (-) |
| Gh_A05G409500 | GhGPDH2 | 47,186 | 9.47 | Mitochondrial | 437 | A05: 107687182–107689805 (-) | |
| Gbar_A05G040550 | GbGPDH2 | 47,233.12 | 9.47 | Mitochondrial | 437 | A05: 100948496–100952479 (-) | |
| Gh_D04G012900 | GhGPDH9 | 47,220.1 | 9.32 | Chloroplast | 437 | D04: 1567590-1570243 (+) | |
| Gbar_D04G001360 | GbGPDH9 | 47,161.08 | 9.25 | Chloroplast | 437 | D04: 1580690-1584522 (+) | |
| Gorai.012G015200 | GrGPDH6 | 47,186.08 | 9.32 | Chloroplast | 437 | Chr12: 1696170-1699874 (+) | |
| III | Gbar_A06G017080 | GbGPDH4 | 52,174.53 | 6.5 | Cytoplasmic | 466 | A06: 107255401-107259323 (-) |
| Gh_A06G186000 | GhGPDH4 | 52,174.53 | 6.5 | Cytoplasmic | 466 | A06: 117541104-117544857 (-) | |
| Ga06G1950 | GaGPDH3 | 52,075.37 | 6.31 | Cytoplasmic | 466 | Chr06: 121758944-121761694 (+) | |
| Gorai.010G191500 | GrGPDH3 | 52,161.46 | 6.36 | Cytoplasmic | 466 | Chr10: 54807050-54810899 (-) | |
| Gbar_D06G017760 | GbGPDH11 | 52,161.46 | 6.36 | Cytoplasmic | 466 | D06: 55366601-55370448 (-) | |
| Gh_D06G188200 | GhGPDH11 | 52,147.43 | 6.36 | Cytoplasmic | 466 | D06: 58158384-58162126 (-) | |
| Ga09G1387 | GaGPDH6 | 51,608.48 | 6.4 | Cytoplasmic | 464 | Chr09: 70045246-70047459 (+) | |
| Gbar_A09G013050 | GbGPDH7 | 51,622.51 | 6.4 | Mitochondrial | 464 | A09: 63713685-63716969 (+) | |
| Gbar_D09G012780 | GbGPDH14 | 51,564.44 | 6.4 | Cytoplasmic | 464 | D09: 38612288-38615590 (+) | |
| Gh_A09G139800 | GhGPDH7 | 51,622.51 | 6.4 | Mitochondrial | 464 | A09: 69189679-69191891 (+) | |
| Gh_D09G131000 | GhGPDH14 | 51,564.44 | 6.4 | Cytoplasmic | 464 | D09: 39773884-39776685 (+) | |
| IV | Gorai.006G135700 | GrGPDH2 | 51,564.44 | 6.4 | Cytoplasmic | 464 | Chr06: 39159929-39163138 (+) |
| Gbar_A06G018390 | GbGPDH5 | 69,021.82 | 8.71 | Mitochondrial | 634 | A06: 109949842-109955764 (+) | |
| Gh_A06G199100 | GhGPDH5 | 69,022.76 | 8.54 | Chloroplast | 634 | A06: 120372686-120377617 (+) | |
| Ga06G2073 | GaGPDH4 | 69,003.71 | 8.43 | Mitochondrial | 634 | Chr06: 124625099-124629571 (-) | |
| Gbar_D06G019050 | GbGPDH12 | 68,997.76 | 8.12 | Chloroplast | 634 | D06: 57729486-57734502 (+) | |
| Gh_D06G201500 | GhGPDH12 | 68,999.69 | 7.89 | Chloroplast | 634 | D06: 60515219-60519674 (+) | |
| Gorai.010G205900 | GrGPDH4 | 68,929.65 | 8.3 | Chloroplast | 633 | Chr10: 57195504-57200512 (+) | |
| Gbar_A05G041420 | GbGPDH3 | 59,564.11 | 7.55 | Chloroplast | 555 | A05: 101934946-101940926 (-) | |
| Gh_A05G417800 | GhGPDH3 | 68,627.3 | 8.14 | Mitochondrial | 631 | A05: 108672565-108677912 (-) | |
| Gh_D04G004200 | GhGPDH8 | 68,605.27 | 8.12 | Mitochondrial | 631 | D04: 562838-568622 (+) | |
| Gbar_D04G000480 | GbGPDH8 | 68,669.4 | 8.43 | Mitochondrial | 631 | D04: 553663-559666 (+) | |
| Gorai.012G005900 | GrGPDH5 | 68,618.3 | 8.29 | Mitochondrial | 631 | Chr12: 696866-702921 (+) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Cui, H.; Wu, B.; Wang, W.; Yang, Q.; Zhang, Y.; Yang, S.; Zhao, Y.; Xu, D.; Liu, G.; et al. Genome-Wide Identification of Cotton (Gossypium spp.) Glycerol-3-Phosphate Dehydrogenase (GPDH) Family Members and the Role of GhGPDH5 in Response to Drought Stress. Plants 2022, 11, 592. https://doi.org/10.3390/plants11050592
Sun J, Cui H, Wu B, Wang W, Yang Q, Zhang Y, Yang S, Zhao Y, Xu D, Liu G, et al. Genome-Wide Identification of Cotton (Gossypium spp.) Glycerol-3-Phosphate Dehydrogenase (GPDH) Family Members and the Role of GhGPDH5 in Response to Drought Stress. Plants. 2022; 11(5):592. https://doi.org/10.3390/plants11050592
Chicago/Turabian StyleSun, Jialiang, Hua Cui, Bingjie Wu, Weipeng Wang, Qiuyue Yang, Yaxin Zhang, Song Yang, Yuping Zhao, Dongbei Xu, Guoxiang Liu, and et al. 2022. "Genome-Wide Identification of Cotton (Gossypium spp.) Glycerol-3-Phosphate Dehydrogenase (GPDH) Family Members and the Role of GhGPDH5 in Response to Drought Stress" Plants 11, no. 5: 592. https://doi.org/10.3390/plants11050592
APA StyleSun, J., Cui, H., Wu, B., Wang, W., Yang, Q., Zhang, Y., Yang, S., Zhao, Y., Xu, D., Liu, G., & Qin, T. (2022). Genome-Wide Identification of Cotton (Gossypium spp.) Glycerol-3-Phosphate Dehydrogenase (GPDH) Family Members and the Role of GhGPDH5 in Response to Drought Stress. Plants, 11(5), 592. https://doi.org/10.3390/plants11050592






