Physiological Adaptation of Three Wild Halophytic Suaeda Species: Salt Tolerance Strategies and Metal Accumulation Capacity
Abstract
:1. Introduction
2. Results
2.1. Soil Physicochemical Properties
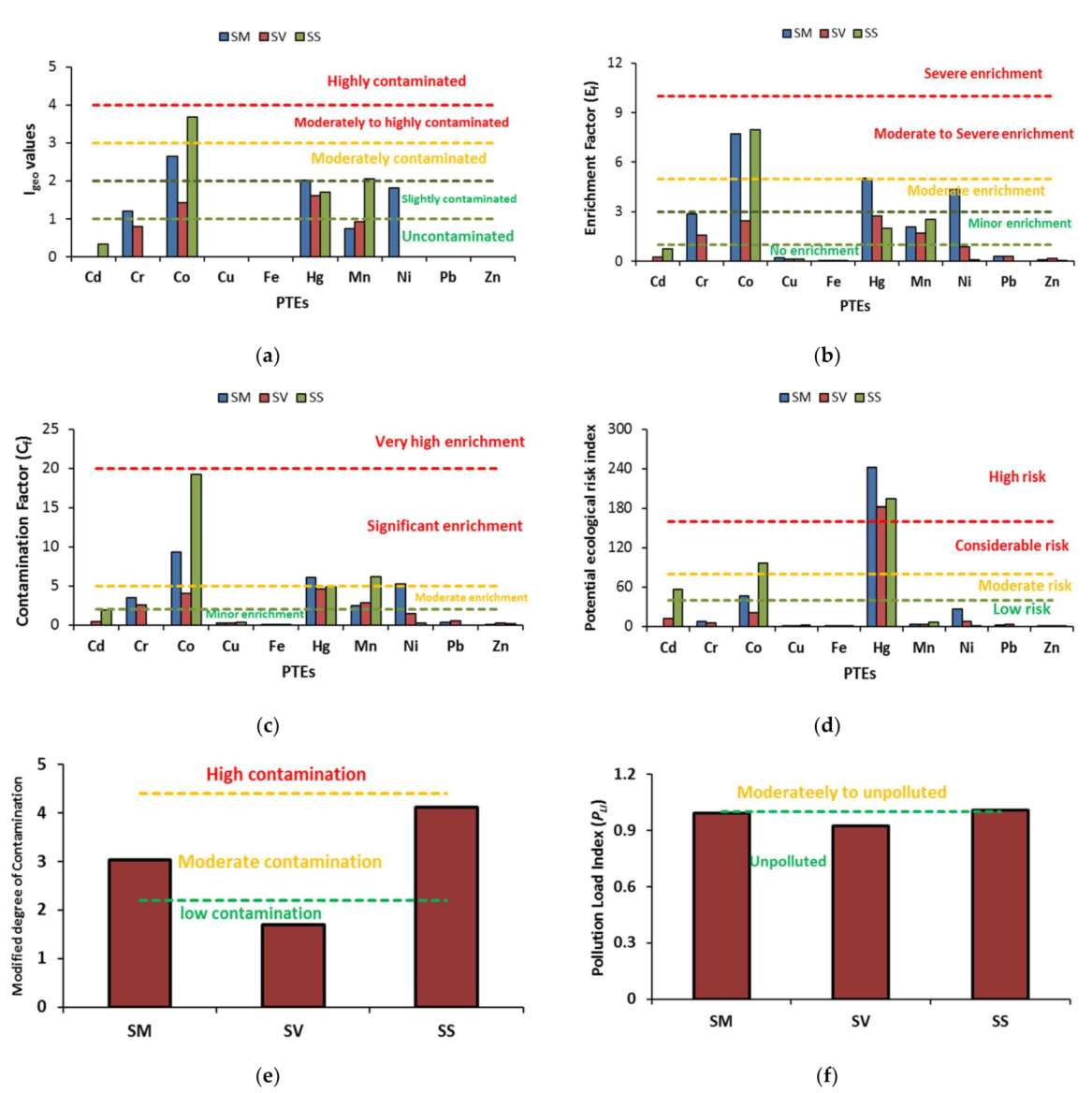
2.2. PTEs Concentration in the Tested Soils and Their Ecological Risk Assessment
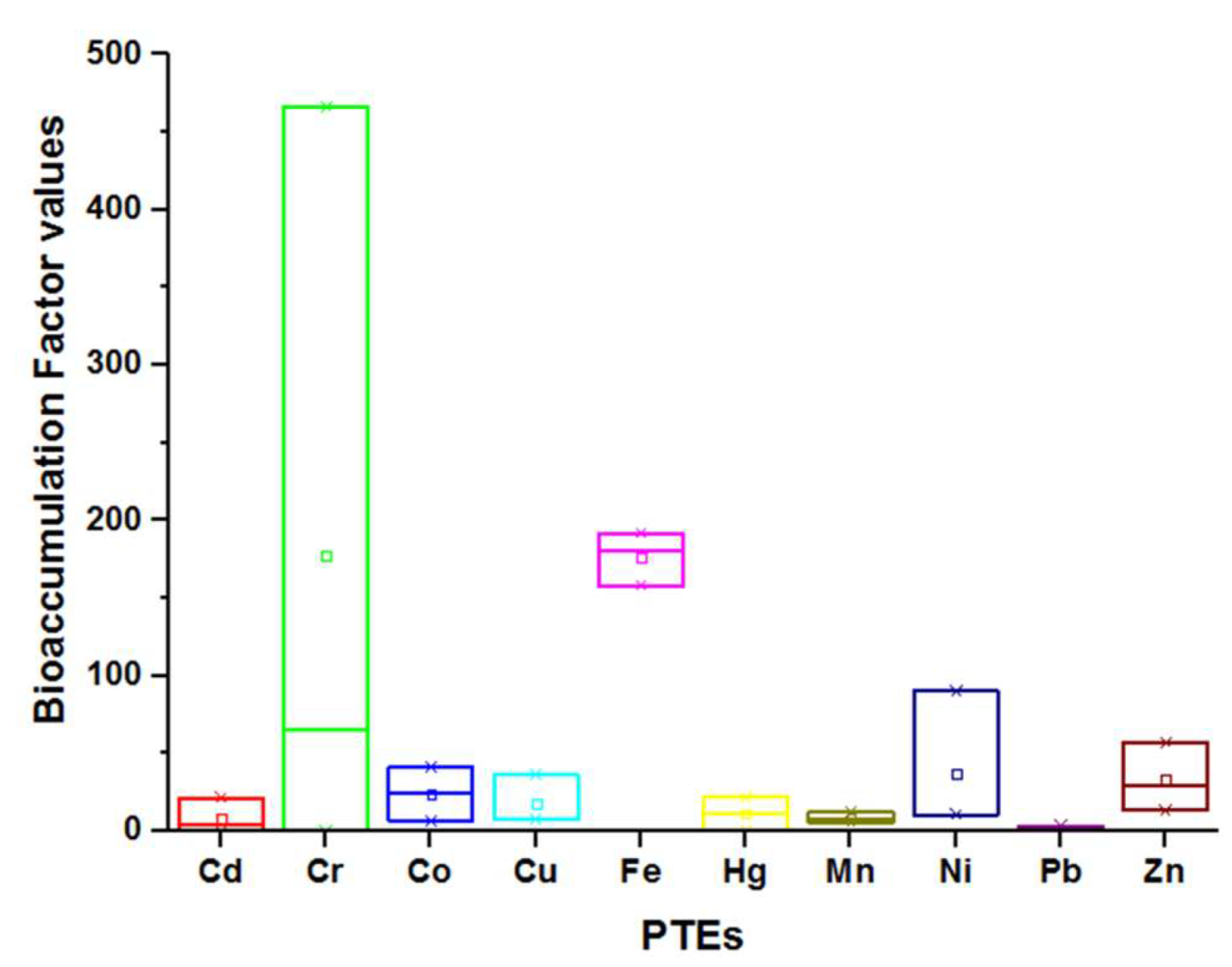
2.3. PTEs Concentration in the Tested Suaeda Species
2.4. Levels of Inorganic and Organic Nutrients in Leaves of the Investigated Suaeda Species
2.5. Variation in Photosynthetic Pigments and Carbohydrate Synthesis
2.6. Changes in Total Soluble Proteins and Amino Acid Profiles
2.7. Alterations in Oxidative Stress and Antioxidants Secondary Metabolites
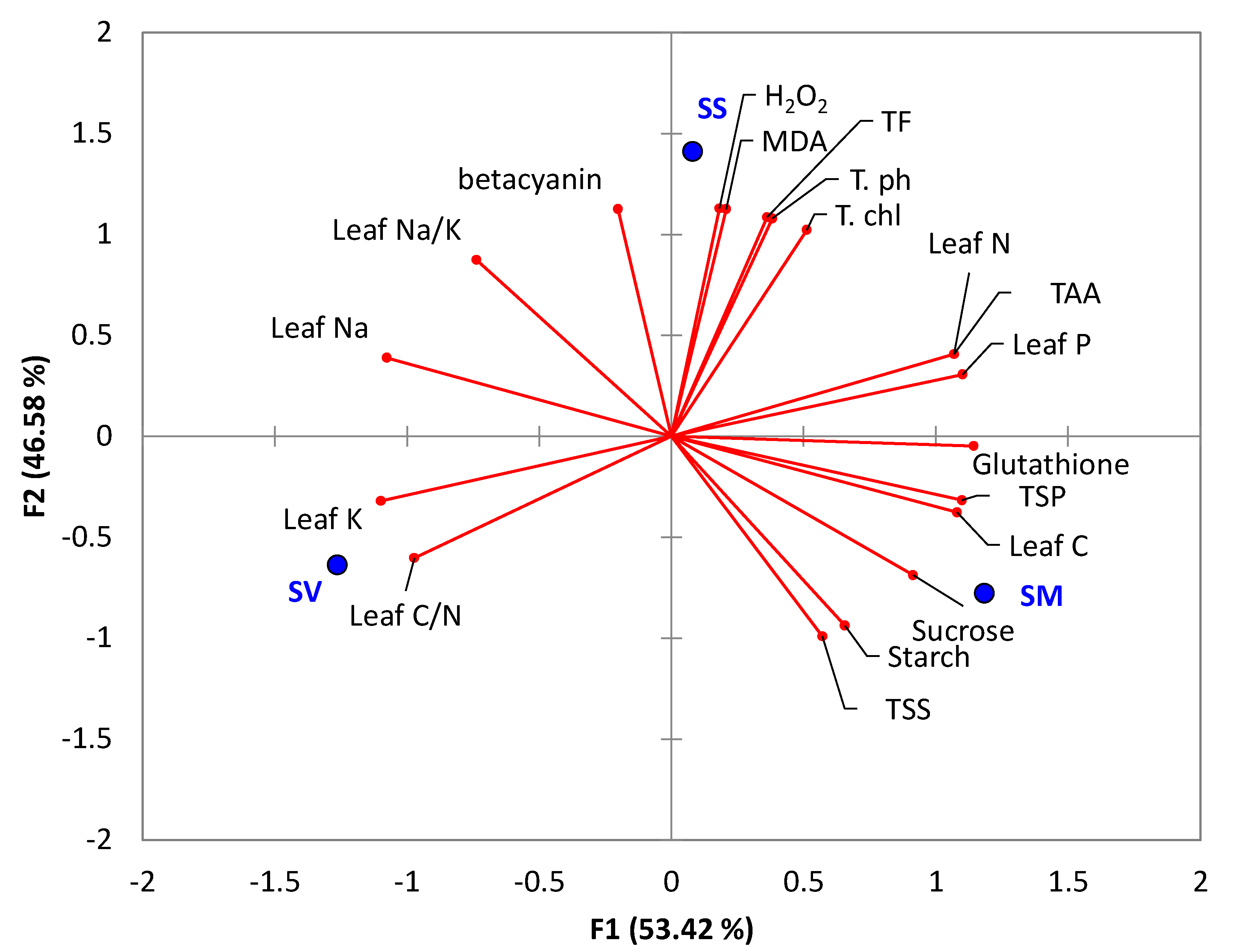
2.8. Correlation among the Tested Physiological Responses
3. Discussion
3.1. Soil Physicochemical Properties and the Relative Magnitudes of Salt Stress Imposed on the Investigated Suaeda Species
3.2. Utilization of Inorganic Ions as “Cheap” Osmotoica in the Tested Suaeda Species
3.3. Relative Physiological Responses of the Tested Suaeda Species in Response to Their Soil Microenvironment
3.4. Relative Oxidative Stress and Antioxidants Synthesis in the Tested Suaeda Species
4. Materials and Methods
4.1. Study Site and the Selected Suaeda Species
4.2. Plants and Soil Sampling
4.3. Soil Physicochemical and Plant Elemental Analyses
4.3.1. Soil PHYSICOCHEMICAL ANALYSES
4.3.2. Plant Analyses
4.4. Quality Control
4.5. Statistical Analysis
5. Conclusions
- -
- Suaeda species are exposed to varying levels of salinity stress along with nutrient stress either as deficiency of essential nutrients such as N, K, P, or as elevated levels of PTEs.
- -
- Suaeda species employ different and efficient adaptive strategies to maintain cellular homeostasis against increased levels of salinity in their rhizospheric soils.
- -
- The high accumulation potential of PTEs, based on the bioaccumulation index of the tested Suaeda species, highlights their potentiality as efficient phytoextractors of soil pollutants.
- -
- The obtained differences among the tested Suaeda species in the current study are driven mainly by species-specific tolerance strategies, and such specificity is shaped by the level of salinity and the genetic constitution of halophytic species.
- -
- In essence, the obtained results of this investigation fulfill the proposed specific objectives and support the set hypotheses of the current study.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szymansky, C.-M.; Muscolo, A.; Yeo, M.; Colville, L.; Clatworthy, I.; Salge, T.; Seal, C.E. Elemental localisation and a reduced glutathione redox state protect seeds of the halophyte Suaeda maritima from salinity during over-wintering and germination. Environ. Exp. Bot. 2021, 190, 104569. [Google Scholar] [CrossRef]
- Mahboob, S.; Ahmed, Z.; Khan, M.F.; Virik, P.; Al-Mulhm, N.; Baabbad, A.A. Assessment of heavy metals pollution in seawater and sediments in the Arabian Gulf, near Dammam, Saudi Arabia. J. King Saud Univ. Sci. 2021, 34, 101677. [Google Scholar] [CrossRef]
- El-Sorogy, A.; Al-Kahtany, K.; Youssef, M.; Al-Kahtany, F.; Al-Malky, M. Distribution and metal contamination in the coastal sediments of Dammam Al-Jubail area, Arabian Gulf, Saudi Arabia. Mar. Pollut. Bull. 2018, 128, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Seal, C.E.; Flowers, T.J.; Esnaud, C.; Yeo, M.; Muscolo, A. Is chloride toxic to seed germination in mixed-salt environments? A case study with the coastal halophyte Suaeda maritima in the presence of seawater. Plant Stress 2021, 2, 100030. [Google Scholar] [CrossRef]
- Geissler, N.; Hussin, S.; El-Far, M.M.; Koyro, H.-W. Elevated atmospheric CO2 concentration leads to different salt resistance mechanisms in a C3 (Chenopodium quinoa) and a C4 (Atriplex nummularia) halophyte. Environ. Exp. Bot. 2015, 118, 67–77. [Google Scholar] [CrossRef]
- Ben Hamed, K.; Castagna, A.; Ranieri, A.; García-Caparrós, P.; Santin, M.; Hernandez, J.A.; Espin, G.B. Halophyte based Mediterranean agriculture in the contexts of food insecurity and global climate change. Environ. Exp. Bot. 2021, 191, 104601. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant Salinity Stress: Many Unanswered Questions Remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [Green Version]
- Benjamin, J.J.; Lucini, L.; Jothiramshekar, S.; Parida, A. Metabolomic insights into the mechanisms underlying tolerance to salinity in different halophytes. Plant Physiol. Biochem. 2019, 135, 528–545. [Google Scholar] [CrossRef]
- Tipirdamaz, R.; Gagneul, D.; Duhazé, C.; Aïnouche, A.; Monnier, C.; Özkum, D.; Larher, F. Clustering of halophytes from an inland salt marsh in Turkey according to their ability to accumulate sodium and nitrogenous osmolytes. Environ. Exp. Bot. 2006, 57, 139–153. [Google Scholar] [CrossRef]
- Ahmed, H.A.I.; Shabala, L.; Shabala, S. Tissue-specificity of ROS-induced K+ and Ca2+ fluxes in succulent stems of the perennial halophyte Sarcocornia quinqueflora in the context of salinity stress tolerance. Plant Physiol. Biochem. 2021, 166, 1022–1031. [Google Scholar] [CrossRef]
- Bueno, M.; Lendínez, M.L.; Calero, J.; del Pilar Cordovilla, M. Salinity responses of three halophytes from inland saltmarshes of Jaén (southern Spain). Flora-Morphol. Distrib. Funct. Ecol. Plants 2020, 266, 151589. [Google Scholar] [CrossRef]
- Matinzadeh, Z.; Akhani, H.; Abedi, M.; Palacio, S. The elemental composition of halophytes correlates with key morphological adaptations and taxonomic groups. Plant Physiol. Biochem. 2019, 141, 259–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, H.; Khurshid, U.; Sarfraz, M.; Tousif, M.I.; Alamri, A.; Anwar, S.; Alamri, A.; Ahmad, I.; Abdallah, H.H.; Mahomoodally, F.M.; et al. A comprehensive phytochemical, biological, toxicological and molecular docking evaluation of Suaeda fruticosa (L.) Forssk.: An edible halophyte medicinal plant. Food Chem. Toxicol. 2021, 154, 112348. [Google Scholar] [CrossRef] [PubMed]
- Ravi, S.; Young, T.; Macinnis-Ng, C.; Nyugen, T.V.; Duxbury, M.; Alfaro, A.C.; Leuzinger, S. Untargeted metabolomics in halophytes: The role of different metabolites in New Zealand mangroves under multi-factorial abiotic stress conditions. Environ. Exp. Bot. 2020, 173, 103993. [Google Scholar] [CrossRef]
- Zhang, J.; He, N.; Liu, C.; Xu, L.; Chen, Z.; Li, Y.; Wang, R.; Yu, G.; Sun, W.; Xiao, C.; et al. Variation and evolution of C:N ratio among different organs enable plants to adapt to N-limited environments. Glob. Chang. Biol. 2020, 26, 2534–2543. [Google Scholar] [CrossRef]
- Ding, Z.; Alharbi, S.; Almaroai, Y.A.; Eissa, M.A. Improving quality of metal-contaminated soils by some halophyte and non-halophyte forage plants. Sci. Total Environ. 2021, 764, 142885. [Google Scholar] [CrossRef]
- El-Banna, M.F.; Mosa, A.; Gao, B.; Yin, X.; Wang, H.; Ahmad, Z. Scavenging effect of oxidized biochar against the phytotoxicity of lead ions on hydroponically grown chicory: An anatomical and ultrastructural investigation. Ecotoxicol. Environ. Saf. 2019, 170, 363–374. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Liu, R.; Cui, B.; Lu, X.; Song, J. The positive effect of salinity on nitrate uptake in Suaeda salsa. Plant Physiol. Biochem. 2021, 166, 958–963. [Google Scholar] [CrossRef]
- Capó, X.; Tejada, S.; Ferriol, P.; Pinya, S.; Mateu-Vicens, G.; Montero-González, I.; Box, A.; Sureda, A. Hypersaline water from desalinization plants causes oxidative damage in Posidonia oceanica meadows. Sci. Total Environ. 2020, 736, 139601. [Google Scholar] [CrossRef]
- Mu, Y.; Tang, D.; Mao, L.; Zhang, D.; Zhou, P.; Zhi, Y.; Zhang, J. Phytoremediation of secondary saline soil by halophytes with the enhancement of γ-polyglutamic acid. Chemosphere 2021, 285, 131450. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Jiang, L.; Zhang, K.; Tanveer, M.; Tian, C.; Zhao, Z. Reclamation of saline soil by planting annual euhalophyte Suaeda salsa with drip irrigation: A three-year field experiment in arid northwestern China. Ecol. Eng. 2021, 159, 106090. [Google Scholar] [CrossRef]
- Samundeeswari, S.; Lakshmi, S. Heavy metals phytoremediation potentiality of Suaeda maritima (L.) Dumort. and Sesuvium portulacastrum L. from influence of tannery effluents. Int. J. Curr. Res. Life Sci. 2018, 7, 1934–1941. [Google Scholar]
- Caparrós, P.G.; Ozturk, M.; Gul, A.; Batool, T.S.; Pirasteh-Anosheh, H.; Unal, B.T.; Altay, V.; Toderich, K.N. Halophytes have potential as heavy metal phytoremediators: A comprehensive review. Environ. Exp. Bot. 2021, 193, 104666. [Google Scholar] [CrossRef]
- Guo, J.; Du, M.; Lu, C.; Wang, B. NaCl improves reproduction by enhancing starch accumulation in the ovules of the euhalophyte Suaeda salsa. BMC Plant Biol. 2020, 20, 262. [Google Scholar] [CrossRef]
- Wang, S.-M.; Zhang, J.; Flowers, T.J. Low-Affinity Na+ Uptake in the Halophyte Suaeda maritima. Plant Physiol. 2007, 145, 559–571. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Li, L.; Yang, R.; Yi, X.; Zhang, B. Contribution and distribution of inorganic ions and organic compounds to the osmotic adjustment in Halostachys caspica response to salt stress. Sci. Rep. 2015, 5, 13639. [Google Scholar] [CrossRef] [Green Version]
- Al-Shamsi, N.; Hussain, M.I.; El-Keblawy, A. Physiological responses of the xerohalophyte Suaeda vermiculata to salinity in its hyper-arid environment. Flora Morphol. Distrib. Funct. Ecol. Plants 2020, 273, 151705. [Google Scholar] [CrossRef]
- Behr, J.H.; Bouchereau, A.; Berardocco, S.; Seal, C.E.; Flowers, T.J.; Zörb, C. Metabolic and physiological adjustment of Suaeda maritima to combined salinity and hypoxia. Ann. Bot. 2017, 119, 965–976. [Google Scholar]
- Li, Q.; Song, J. Analysis of widely targeted metabolites of the euhalophyte Suaeda salsa under saline conditions provides new insights into salt tolerance and nutritional value in halophytic species. BMC Plant Biol. 2019, 19, 388. [Google Scholar] [CrossRef] [Green Version]
- Collenette, S. An Illustrated guide to the flowers of Saudi Arabia. Brittonia 1985, 38, 430–432. [Google Scholar]
- Guo, J.; Chen, Y.; Lu, P.; Liu, M.; Sun, P.; Zhang, Z. Roles of endophytic bacteria in Suaeda salsa grown in coastal wetlands: Plant growth characteristics and salt tolerance mechanisms. Environ. Pollut. 2021, 287, 117641. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Ungar, I.A.; Showalter, A.M. The effect of salinity on the growth, water status, and ion content of a leaf succulent perennial halophyte, Suaeda fruticosa (L.) Forssk. J. Arid. Environ. 2000, 45, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, K.; Agarie, S. Physiological Roles of Betacyanin in a Halophyte, Suaeda japonicaMakino. Plant Prod. Sci. 2010, 13, 351–359. [Google Scholar] [CrossRef]
- El-Arby, A.M.; Elbordiny, M.M. Impact of reused wastewater for irrigation on availability of heavy metals in sandy soils and their uptake by plants. J. Appl. Sci. Res. 2006, 2, 106–111. [Google Scholar]
- Shaheen, S.M.; Shams, M.S.; Khalifa, M.R.; El-Dali, M.A.; Rinklebe, J. Various soil amendments and environmental wastes affect the (im)mobilization and phytoavailability of potentially toxic elements in a sewage effluent irrigated sandy soil. Ecotoxicol. Environ. Saf. 2017, 142, 375–387. [Google Scholar] [CrossRef]
- Alnaimy, M.A.; Shahin, S.A.; Vranayova, Z.; Zelenakova, M.; Abdel-Hamed, E.M.W. Long-Term Impact of Wastewater Irrigation on Soil Pollution and Degradation: A Case Study from Egypt. Water 2021, 13, 2245. [Google Scholar] [CrossRef]
- Ibraheem, F.; Al-Hazmi, N.; El-Morsy, M.; Mosa, A. Ecological Risk Assessment of Potential Toxic Elements in Salt Marshes on the East Coast of the Red Sea: Differential Physiological Responses and Adaptation Capacities of Dominant Halophytes. Sustainability 2021, 13, 11282. [Google Scholar] [CrossRef]
- Mosa, A.; Taha, A.A.; Elsaeid, M. In-situ and ex-situ remediation of potentially toxic elements by humic acid extracted from different feedstocks: Experimental observations on a contaminated soil subjected to long-term irrigation with sewage effluents. Environ. Technol. Innov. 2021, 23, 101599. [Google Scholar] [CrossRef]
- Hussain, M.; Al-Shaibani, A.; Al-Ramadan, K.; Wood, W.W. Geochemistry and isotopic analysis of brines in the coastal sabkhas, Eastern region, Kingdom of Saudi Arabia. J. Arid Environ. 2020, 178, 104142. [Google Scholar] [CrossRef]
- Wang, F.; Xu, Y.-G.; Wang, S.; Shi, W.; Liu, R.; Feng, G.; Song, J. Salinity affects production and salt tolerance of dimorphic seeds of Suaeda salsa. Plant Physiol. Biochem. 2015, 95, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.L.; Bidari, B.I.; Hebbara, M.; Katti, J.; Dilvaranaik, S.; Vishwanatha, S.; Geetanjali, H.M.; Dasog, G.S. Identification of soil fertility constraints by GIS in Bedwatti sub watershed under Northern dry zone of Karnataka for site specific recommendations. J. Farm Sci. 2017, 30, 206–211. [Google Scholar]
- El-Naggar, A.; Ahmed, N.; Mosa, A.; Niazi, N.K.; Yousaf, B.; Sharma, A.; Sarkar, B.; Cai, Y.; Chang, S.X. Nickel in soil and water: Sources, biogeochemistry, and remediation using biochar. J. Hazard. Mater. 2021, 419, 126421. [Google Scholar] [CrossRef] [PubMed]
- Moghanm, F.S.; El-Banna, A.; El-Esawi, M.A.; Abdel-Daim, M.M.; Mosa, A.; Abdelaal, K.A. Genotoxic and Anatomical Deteriorations Associated with Potentially Toxic Elements Accumulation in Water Hyacinth Grown in Drainage Water Resources. Sustainability 2020, 12, 2147. [Google Scholar] [CrossRef] [Green Version]
- Al Hassan, M.; Estrelles, E.; Soriano, P.; López-Gresa, M.P.; Bellés, J.M.; Boscaiu, M.; Vicente, O. Unraveling Salt Tolerance Mechanisms in Halophytes: A Comparative Study on Four Mediterranean Limonium Species with Different Geographic Distribution Patterns. Front. Plant Sci. 2017, 8, 1438. [Google Scholar] [CrossRef] [Green Version]
- González-Orenga, S.; Ferrer-Gallego, P.P.; Laguna, E.; López-Gresa, M.P.; Donat-Torres, M.P.; Verdeguer, M.; Vicente, O.; Boscaiu, M. Insights on Salt Tolerance of Two Endemic Limonium Species from Spain. Metabolites 2019, 9, 294. [Google Scholar] [CrossRef] [Green Version]
- Gil, R.; Bautista, I.; Boscaiu, M.; Lidón, A.L.; Wankhade, S.; Sánchez, H.; Llinares, J.; Vicente, O. Responses of five Mediterranean halophytes to seasonal changes in environmental conditions. AoB Plants 2014, 6, plu049. [Google Scholar] [CrossRef]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef]
- Flowers, T.J.; Dalmond, D. Protein synthesis in halophytes: The influence of potassium, sodium and magnesium in vitro. Genet. Asp. Plant Miner. Nutr. 1993, 146, 195–203. [Google Scholar]
- Cocozza, C.; Brilli, F.; Pignattelli, S.; Pollastri, S.; Brunetti, C.; Gonnelli, C.; Tognetti, R.; Centritto, M.; Loreto, F. The excess of phosphorus in soil reduces physiological performances over time but enhances prompt recovery of salt-stressed Arundo donax plants. Plant Physiol. Biochem. 2020, 151, 556–565. [Google Scholar] [CrossRef]
- Mahajan, S.; Pandey, G.K.; Tuteja, N. Calcium- and salt-stress signaling in plants: Shedding light on SOS pathway. Arch. Biochem. Biophys. 2008, 471, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Zaier, M.M.; Ciudad-Mulero, M.; Cámara, M.; Pereira, C.; Ferreira, I.C.; Achour, L.; Kacem, A.; Morales, P. Revalorization of Tunisian wild Amaranthaceae halophytes: Nutritional composition variation at two different phenotypes stages. J. Food Compos. Anal. 2020, 89, 103463. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, G.; Lilong, W.; Zhang, M.; Zhang, L.; Zhang, X.; Guanxiang, Z.; Xu, S. C:N:P Stoichiometry and Leaf Traits of Halophytes in an Arid Saline Environment, Northwest China. PLoS ONE 2015, 10, e0119935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alshaal, T.; El-Ramady, H.; Al-Saeedi, A.H.; Shalaby, T.; Elsakhawy, T.; Omara, A.E.D.; Gad, A.; Hamad, E.; El-Ghamry, A.; Mosa, A. The rhizosphere and plant nutrition under climate change. In Essential Plant Nutrients; Springer: Berlin, Germany, 2017; pp. 275–308. [Google Scholar]
- Bagheri, R.; Ahmad, J.; Bashir, H.; Iqbal, M.; Qureshi, M.I. Changes in rubisco, cysteine-rich proteins and antioxidant system of spinach (Spinacia oleracea L.) due to sulphur deficiency, cadmium stress and their combination. Protoplasma 2017, 254, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Mosa, A.; El-Banna, M.F.; Gao, B. Biochar filters reduced the toxic effects of nickel on tomato (Lycopersicon esculentum L.) grown in nutrient film technique hydroponic system. Chemosphere 2016, 149, 254–262. [Google Scholar] [CrossRef]
- El-Banna, M.F.; Mosa, A.; Gao, B.; Yin, X.; Ahmad, Z.; Wang, H. Sorption of lead ions onto oxidized bagasse-biochar mitigates Pb-induced oxidative stress on hydroponically grown chicory: Experimental observations and mechanisms. Chemosphere 2018, 208, 887–898. [Google Scholar] [CrossRef]
- Flowers, T.; Yeo, A. Ion Relations of Plants Under Drought and Salinity. Funct. Plant Biol. 1986, 13, 75–91. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant, Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Plant salt tolerance: Adaptations in halophytes. Ann. Bot. 2015, 115, 327–331. [Google Scholar] [CrossRef] [Green Version]
- Thalmann, M.; Santelia, D. Starch as a determinant of plant fitness under abiotic stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Sardans, J.; Zhang, J.; Peñuelas, J. Variations in foliar carbon:nitrogen and nitrogen:phosphorus ratios under global change: A meta-analysis of experimental field studies. Sci. Rep. 2020, 10, 12156. [Google Scholar] [CrossRef]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How Does Proline Treatment Promote Salt Stress Tolerance During Crop Plant Development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef]
- Bhaduri, A.M.; Fulekar, M.H. Antioxidant enzyme responses of plants to heavy metal stress. Rev. Environ. Sci. Bio/Technol. 2012, 11, 55–69. [Google Scholar] [CrossRef]
- Nxele, X.; Klein, A.; Ndimba, B. Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. South Afr. J. Bot. 2017, 108, 261–266. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of Reactive Oxygen Species and Antioxidant Defense in Plants under Salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef]
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 2014, 65, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.S.; Huang, S.Q.; Guo, K.; Mehta, S.K.; Zhang, P.C.; Yang, Z.M. Metabolic adaptations to mercury-induced oxidative stress in roots of Medicago sativa L. J. Inorg. Biochem. 2007, 101, 1–9. [Google Scholar] [CrossRef]
- Tang, B.; Chen, J.; Wang, Z.; Qin, P.; Zhang, X. Mercury accumulation response of rice plant (Oryza sativa L.) to elevated atmospheric mercury and carbon dioxide. Ecotoxicol. Environ. Saf. 2021, 224, 112628. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, X.; Zhao, J.; Yu, J. Regulation of Metabolites, Gene Expression, and Antioxidant Enzymes to Environmentally Relevant Lead and Zinc in the Halophyte Suaeda salsa. J. Plant Growth Regul. 2013, 32, 353–361. [Google Scholar] [CrossRef]
- Wang, C.-Q.; Chen, M.; Wang, B.-S. Betacyanin accumulation in the leaves of C3 halophyte Suaeda salsa L. is induced by watering roots with H2O2. Plant Sci. 2007, 172, 1–7. [Google Scholar] [CrossRef]
- Li, Y.; Cui, L.; Yao, X.; Ding, X.; Pan, X.; Zhang, M.; Li, W.; Kang, X. Trade-off between leaf chlorophyll and betacyanins in Suaeda salsa in the Liaohe estuary wetland in northeast China. J. Plant Ecol. 2018, 11, 569–575. [Google Scholar] [CrossRef]
- Mane, A.V.; Karadge, B.A.; Samant, J.S. Salinity induced changes in photosynthetic pigments and polyphenols of Cymbopogon nardus (L.) Rendle. J. Chem. Pharm. Res 2010, 2, 338–347. [Google Scholar]
- Gee, G.W.; Or, D. 2.4 Particle-size analysis. Methods Soil Analysis. Part 2002, 4, 255–293. [Google Scholar]
- Dewis, J.; Freitas, F. Physical and chemical methods of soil and water analysis. FAO Soils Bull. 1970, 10, 275. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis: Advanced Course; UW-Madison Libraries Parallel Press: Madison, WI, USA, 2005. [Google Scholar]
- Ullah, S.; Skidmore, A.K.; Groen, T.A.; Schlerf, M. Evaluation of three proposed indices for the retrieval of leaf water content from the mid-wave infrared (2–6 μm) spectra. Agric. For. Meteorol. 2013, 171–172, 65–71. [Google Scholar] [CrossRef]
- Dehan, K.; Tal, M. Salt tolerance in the wild relatives of the cultivated tomato: Responses of Solanum pennellii to high salinity. Irrig. Sci. 1978, 1, 71–76. [Google Scholar] [CrossRef]
- Vernon, L.P. Spectrophotometric determination of chlorophylls and pheophytins in plant extracts. Anal. Chem. 1960, 32, 1144–1150. [Google Scholar] [CrossRef]
- Skalicky, M.; Kubes, J.; Shokoofeh, H.; Tahjib-Ul-Arif, M.; Vachova, P.; Hejnak, V. Betacyanins and Betaxanthins in Cultivated Varieties of Beta vulgaris L. Compared to Weed Beets. Molecules 2020, 25, 5395. [Google Scholar] [CrossRef]
- Hansen, J.; Møller, I. Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone. Anal. Biochem. 1975, 68, 87–94. [Google Scholar] [CrossRef]
- Sadasivam, S.; Manickam, A. Determination of total carbohydrate by anthrone method. In Bochemical Methods; New age International (P) Limited: New Delhi, India, 1996; pp. 8–9. [Google Scholar]
- Yadav, S.; Srivastava, A.; Biswas, S.; Chaurasia, N.; Singh, S.K.; Kumar, S.; Srivastava, V.; Mishra, Y. Comparison and optimization of protein extraction and two-dimensional gel electrophoresis protocols for liverworts. BMC Res. Notes 2020, 13, 60. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ouyang, S.-Q.; Liu, Y.-F.; Liu, P.; Lei, G.; He, S.-J.; Ma, B.; Zhang, W.-K.; Zhang, J.-S.; Chen, S.-Y. Receptor-like kinase OsSIK1 improves drought and salt stress tolerance in rice (Oryza sativa) plants. Plant J. 2010, 62, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Alonsoborbalan, A.; Zorro, L.; Guillen, D.; Barroso, C.G. Study of the polyphenol content of red and white grape varieties by liquid chromatography–mass spectrometry and its relationship to antioxidant power. J. Chromatogr. A 2003, 1012, 31–38. [Google Scholar] [CrossRef]



| Physicochemical Parameters | S. monoica | S. vermiculata | S. schimperi | ||
|---|---|---|---|---|---|
| Soil physical properties | Particle size distribution (%) | Sand | 92.6 | 76.0 | 95.8 |
| Silt and clay | 6.9 | 23.3 | 3.7 | ||
| Texture | Sandy | Sandy clay loam | Sandy | ||
| Water holding capacity (%) | 31.98 ± 3.05 | 38.15 ± 3.52 | 35.02 ± 3.21 | ||
| Porosity (%) | 39.98 ± 3.53 | 49.93 ± 4.57 | 43.89 ± 3.93 | ||
| Soil chemical properties | EC (dS m−1) | 5.04 ± 0.55 | 18.37 ± 1.91 | 16.25 ± 1.04 | |
| pH | 8.10 ± 0.44 | 8.65 ± 0.38 | 7.78 ± 0.24 | ||
| CaCO3 (%) | 0.55 ± 060 | 0.79 ± 0.66 | 0.79 ± 0.30 | ||
| Water soluble anions (Cmol/100 g) | HCO3− | 5.57 ± 0.60 | 6.35 ± 0.66 | 5.33 ± 0.30 | |
| Cl− | 6.46 ± 0.67 | 13.76 ± 3.28 | 14.29 ± 2.29 | ||
| Water soluble cations (Cmol/100 g) | Na+ | 2.51 ± 0.15 | 5.49 ± 0.23 | 4.89 ± 0.30 | |
| K+ | 0.46 ± 0.09 | 1.10 ± 0.12 | 0.98 ± 0.10 | ||
| Ca2+ | 7.20 ± 0.39 | 6.15 ± 0.41 | 7.60 ± 0.44 | ||
| Mg2+ | 1.79 ± 0.08 | 5.66 ± 0.69 | 10.42 ± 0.89 | ||
| Elemental Concentrations | S. monoica | S. vermiculata | S. schimperi | |
|---|---|---|---|---|
| Available nutrients (mg kg−1) | P | 8.17 a ± 0.38 | 9.40 a ± 0.44 | 6.53 b ± 31 |
| K | 178.8 b ± 6.2 | 427.8 a ± 15.4 | 383.8 a ± 19.0 | |
| Ca | 1441.0 a ± 127.0 | 1229.9 b ± 114.9 | 1519.1 a ± 145.9 | |
| Mg | 215.3 c ± 6.3 | 679.4 b ± 3.7 | 1250.2 a ± 13.3 | |
| Available Na+ (mg kg−1) | 578.3 c ± 45 | 1262.1 a ± 124.3 | 1103.5 b ± 108.2 | |
| Total organic elements (%) | C | N.D. | 0.23 b ± 0.07 | 1.95 a ± 0.19 |
| N | N.D. | N.D. | 0.18 ± 0.13 | |
| H | 0.23 b ± 0.03 | 0.51 a ± 0.04 | 0.593 a ± 0.04 | |
| S | N.D. | N.D. | 0.47 ± 0.03 | |
| Toxic Elements | S. monoica | S. vermiculata | S. schimperi | |||
|---|---|---|---|---|---|---|
| Concentration (mg kg−1) | ||||||
| Soil | Plant | Soil | Plant | Soil | Plant | |
| Cd | ND | ND | 0.024 b ± 0.002 | 0.496 a ± 0.055 | 0.112 a ± 0.011 | 0.499 a ± 0.058 |
| Cr | 0.380 a ± 0.038 | 177.108 a ± 15.591 | 0.286 b ± 0.029 | 18.668 c ± 2.272 | ND | 50.166 b ± 5.262 |
| Co | 0.279 b ± 0.028 | 6.734 a ± 0.662 | 0.121 c ± 0.012 | 5.032 b ± 0.526 | 0.577 a ± 0.057 | 3.516 c ± 0.377 |
| Cu | 2.070 b ± 0.391 | 75.125 a ± 7.873 | 2.160 b ± 0.743 | 17.040 b ± 1.635 | 2.566 a ± 0.056 | 19.488 b ± 2.002 |
| Fe | 5.019 c ± 0.441 | 902.942 c ± 85.291 | 8.147 b ± 0.762 | 1286.0 b ± 113.4 | 10.643 a ± 1.082 | 2038.4 a ± 188.6 |
| Hg | 1.813 a ± 0.155 | ND | 1.367 b ± 0.123 | 29.229 a ± 2.545 | 1.456 b ± 0.147 | 16.871 b ± 1.132 |
| Mn | 11.884 b ± 0.295 | 146.304 b ± 15.319 | 13.511 b ± 0.576 | 102.29 c ± 11.52 | 29.237 a ± 1.2211 | 166.053 a ± 14.973 |
| Ni | 1.623 a ± 0.056 | 16.852 a ± 1.981 | 0.441 b ± 0.020 | 4.518 b ± 0.503 | 0.066 c ± 0.013 | 5.964 b ± 0.654 |
| Pb | 3.812 b ± 0.147 | ND | 5.617 a ± 0.216 | 18.651 a ± 2.342 | ND | 9.918 b ± 1.119 |
| Zn | 1.992 c ± 0.069 | 113.735 a ± 11.109 | 5.633 a ± 0.071 | 74.080 c ± 8.148 | 3.233 b ± 0.063 | 93.904 b ± 10.077 |
| Elements Concentration (mg g−1 DWT) | S. monoica | S. vermiculata | S. schimperi |
|---|---|---|---|
| P | 1.485 a ± 0.156 | 0.452 c ± 0.126 | 1.275 b ± 0.130 |
| Ca | 10.38 b ± 1.15 | 10.82 ab ± 1.22 | 12.63 a ± 1.27 |
| Mg | 4.99 b ± 0.51 | 6.51 a ± 0.71 | 6.74 a ± 0.63 |
| K | 10.04 b ± 0.87 | 12.69 a ± 1.33 | 10.55 b ± 1.19 |
| Na | 11.01 b ± 1.15 | 17.84 a ± 1.85 | 16.19 a ± 1.75 |
| Na/K ratio | 1.097 ± 0.045 | 1.407 ± 0.002 | 1.536 ± 0.009 |
| C | 322.56 a ± 33.22 | 245.76 b ± 24.58 | 265.16 b ± 24.25 |
| N | 32.04 a ± 3.65 | 11.96 c ± 1.60 | 29.79 b ± 4.03 |
| S | 0.377 a ± 0.031 | 0.357 a ± 0.025 | N.D. |
| C/N ratio | 10.07 | 20.55 | 9.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibraheem, F.; Al-Zahrani, A.; Mosa, A. Physiological Adaptation of Three Wild Halophytic Suaeda Species: Salt Tolerance Strategies and Metal Accumulation Capacity. Plants 2022, 11, 537. https://doi.org/10.3390/plants11040537
Ibraheem F, Al-Zahrani A, Mosa A. Physiological Adaptation of Three Wild Halophytic Suaeda Species: Salt Tolerance Strategies and Metal Accumulation Capacity. Plants. 2022; 11(4):537. https://doi.org/10.3390/plants11040537
Chicago/Turabian StyleIbraheem, Farag, Ateeq Al-Zahrani, and Ahmed Mosa. 2022. "Physiological Adaptation of Three Wild Halophytic Suaeda Species: Salt Tolerance Strategies and Metal Accumulation Capacity" Plants 11, no. 4: 537. https://doi.org/10.3390/plants11040537
APA StyleIbraheem, F., Al-Zahrani, A., & Mosa, A. (2022). Physiological Adaptation of Three Wild Halophytic Suaeda Species: Salt Tolerance Strategies and Metal Accumulation Capacity. Plants, 11(4), 537. https://doi.org/10.3390/plants11040537







