UPLC-PDA-MS/MS Profiling and Healing Activity of Polyphenol-Rich Fraction of Alhagi maurorum against Oral Ulcer in Rats
Abstract
:1. Introduction
2. Results
2.1. Extraction and Fractionation of A. maurorum
2.2. Determination of Total Polyphenolic and Flavonoid Content of A. maurorum
2.3. Identification of the Major Polyphenol Compound of EFAM
2.4. Standardization of EFAM Using Ultra Performance Liquid Chromatography (UPLC) Analysis
2.5. Characterization of EFAM Polyphenols by LC-MS/MS
2.6. In Vitro Assay
2.6.1. DPPH Assay
2.6.2. FRAP Assay
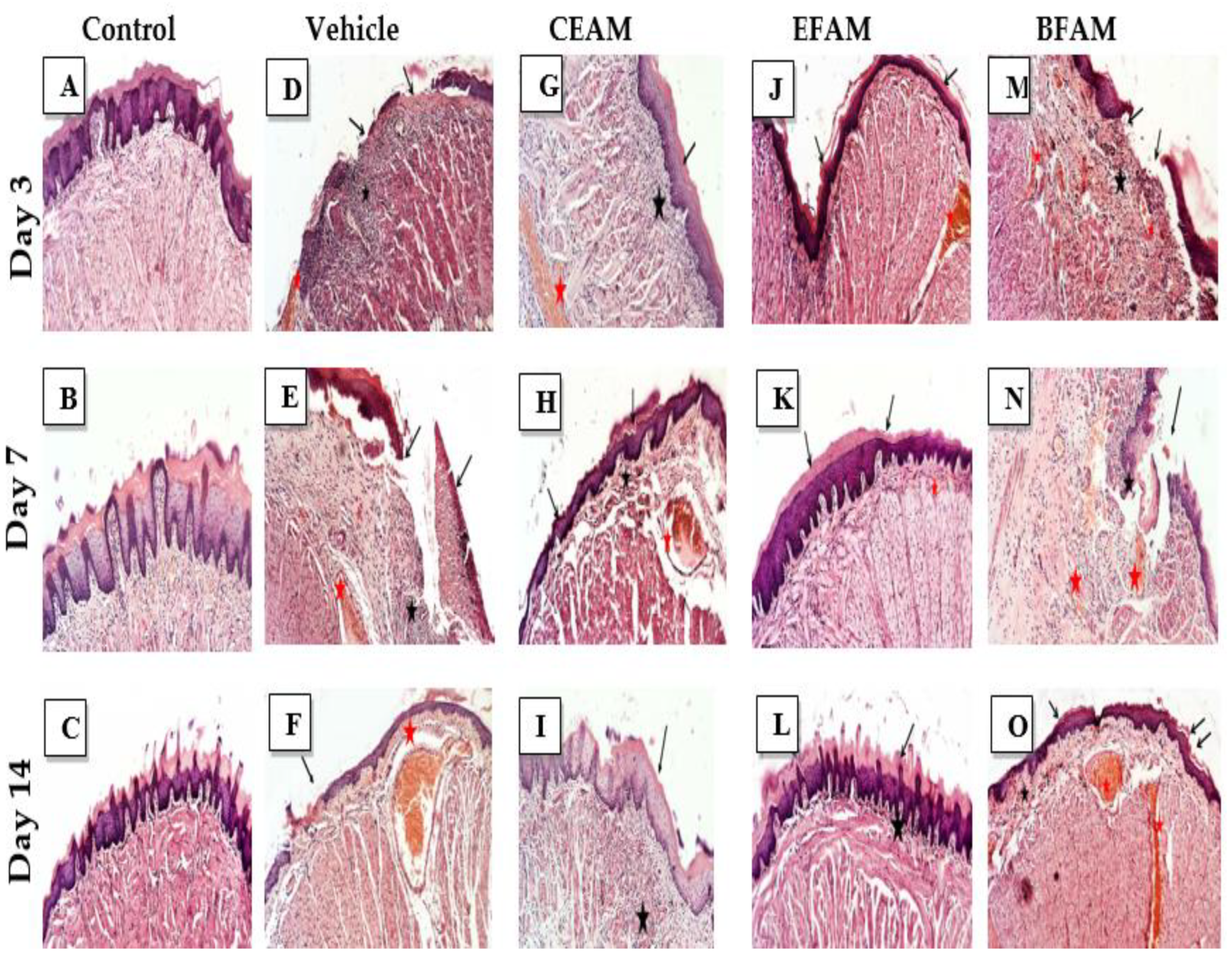
2.6.3. Evaluation of the Cytotoxic Activity of the Different Fractions of A. maurorum (MTT Assay)
2.7. In Vivo Assay
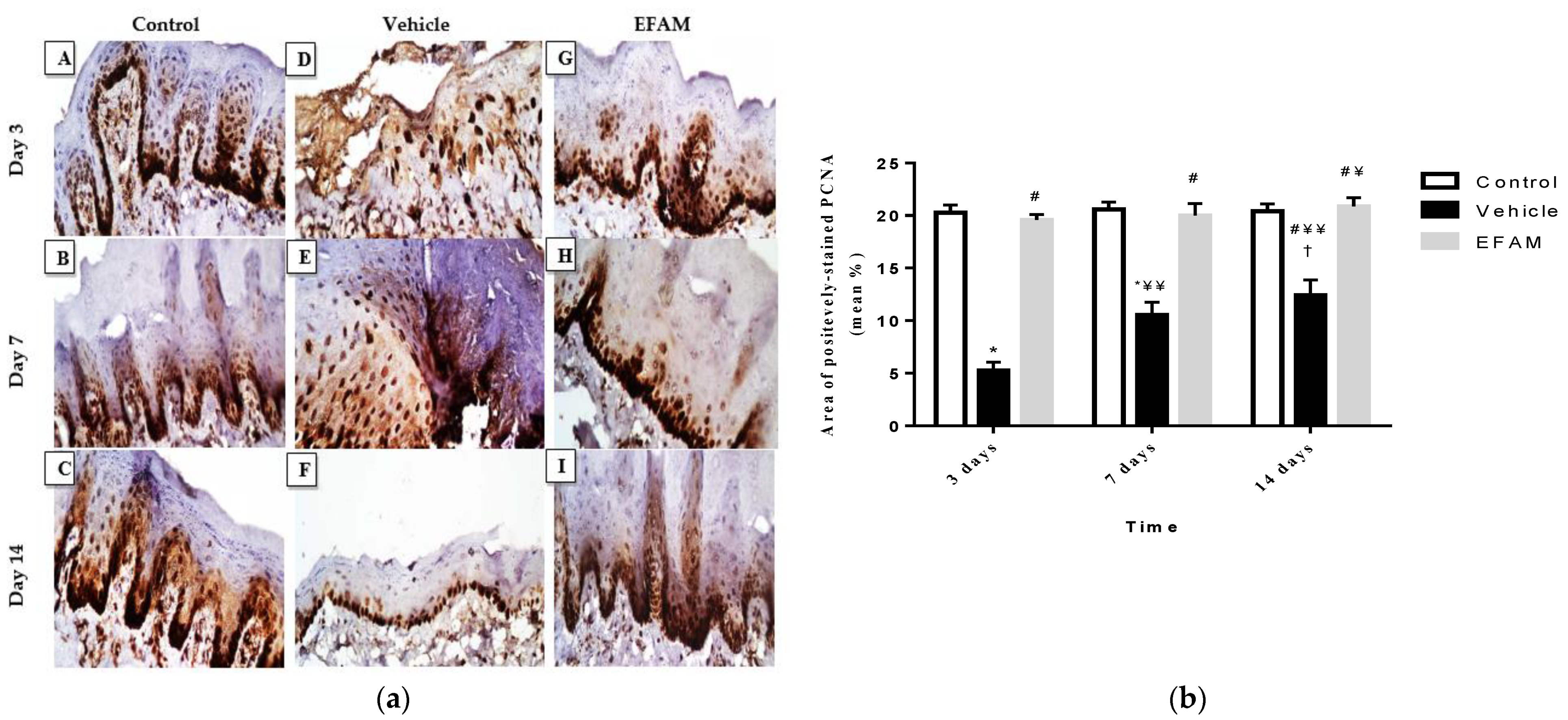
2.7.1. Histopathological Examination
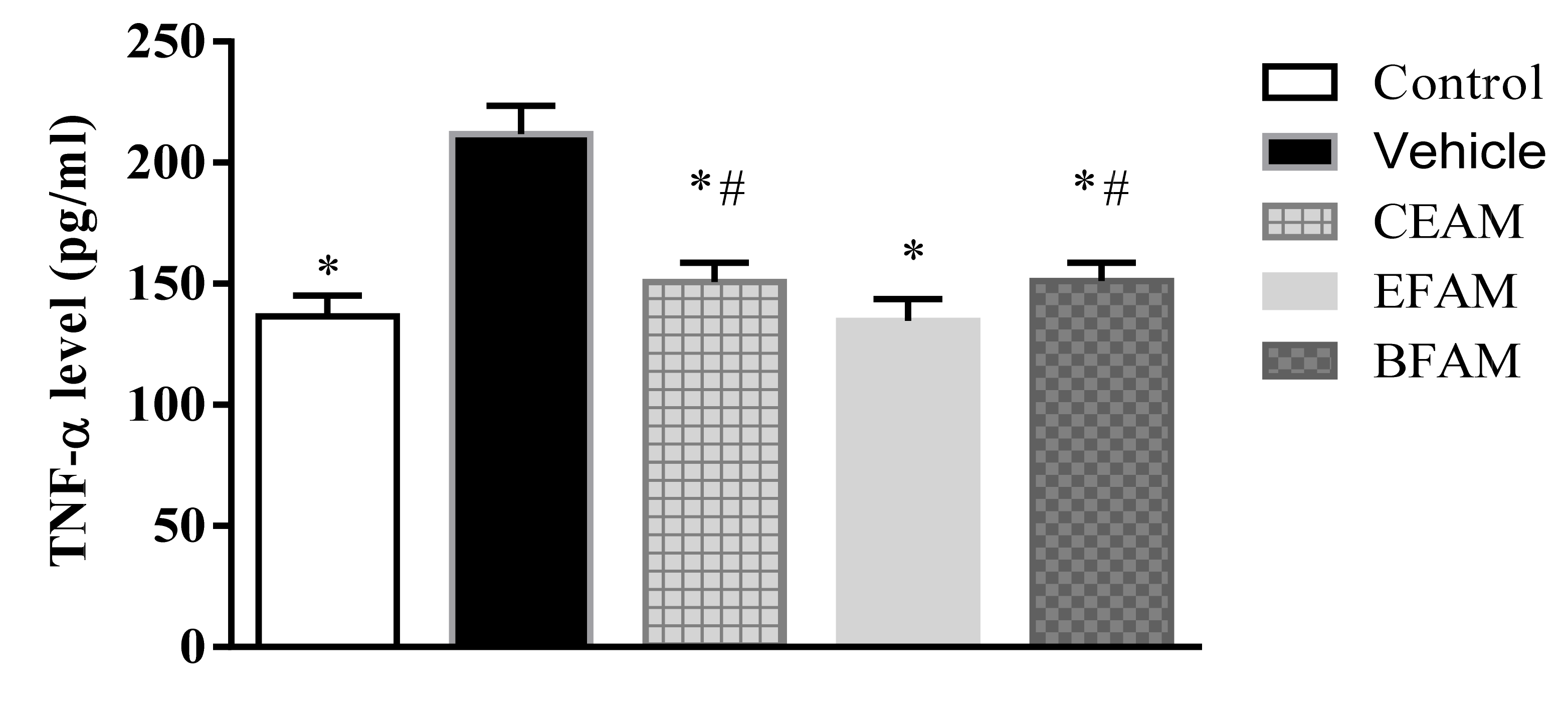
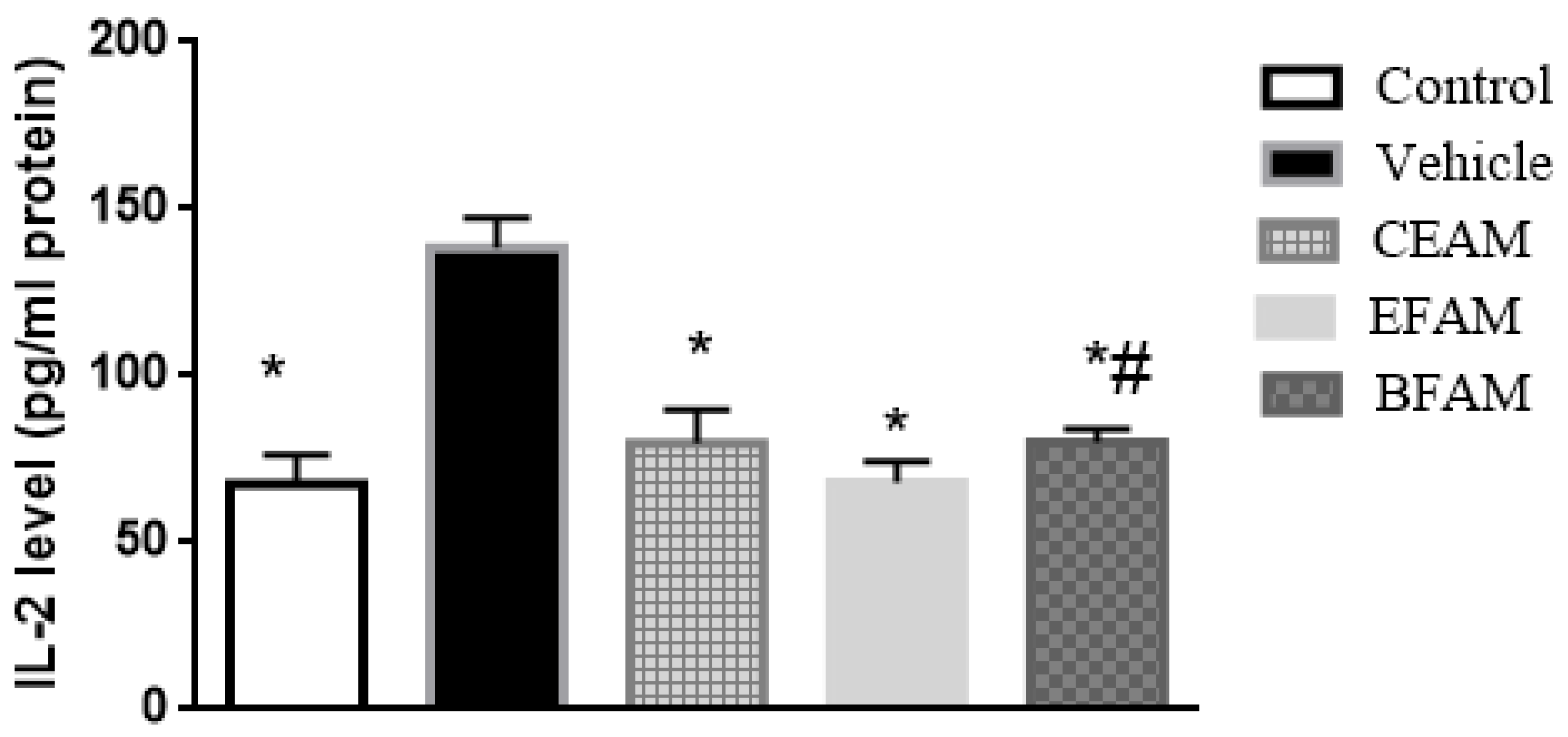
2.7.2. Inflammatory Markers
2.7.3. Immunohistochemical Staining of Proliferating Cell Nuclear Antigen (PCNA)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Plant Extraction and Fractionation of A. maurorum
4.3. Determination of Total Polyphenolic and Flavonoid Content of A. maurorum
4.4. Isolation and Identification of the Major Polyphenolic Compound of EFAM
4.5. Standardization of EFAM Using Ultra Performance Liquid Chromatography (UPLC) Analysis
4.6. UPLC/PDA/ESI/MS/MS Analysis of Polyphenol-Rich Fraction (EFAM)
4.7. In Vitro Studies
4.7.1. DPPH Assay
4.7.2. FRAP Assay
4.7.3. Cytotoxic Activity (MTT Assay)
4.8. In Vivo Traumatic Ulcer Healing Study
4.8.1. Animals
4.8.2. Induction of Tongue Ulcers
4.8.3. Treatment Preparation
4.8.4. Experimental Design
4.8.5. Biochemical Assessment of Inflammatory Markers
4.8.6. Tongue Tissue Preparation for Staining
4.8.7. Histomorphometric Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muñoz-Corcuera, M.; Esparza-Gómez, G.; González-Moles, M.A.; Bascones-Martínez, A. Oral ulcers: Clinical aspects. A tool for dermatologists. Part I. Acute ulcers. Clin. Exp. Dermatol. 2009, 34, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Minhas, S.; Sajjad, A.; Kashif, M.; Taj, F.; Alwadaani, H.; Khurshid, Z. Oral Ulcers Presentation in Systemic Diseases: An Update. Open Access Maced. J. Med Sci. 2019, 7, 3341–3347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çimen, M.Y.B.; Kaya, T.I.; Eskandari, G.; Tursen, U.; Ikizoglu, G.; Atik, U. Oxidant/antioxidant status in patients with recurrent aphthous stomatitis. Clin. Exp. Dermatol. 2003, 28, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Kim, G.-E.; Song, Y.-B.; Paudel, U.; Lee, N.-H.; Yun, B.-S.; Yu, M.-K.; Yi, H.-K. Davallialactone Reduces Inflammation and Repairs Dentinogenesis on Glucose Oxidase–induced Stress in Dental Pulp Cells. J. Endod. 2013, 39, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farzaei, M.H.; Abdollahi, M.; Rahimi, R. Role of dietary polyphenols in the management of peptic ulcer. World J. Gastroenterol. 2015, 21, 6499–6517. [Google Scholar] [CrossRef]
- Cuba, L.D.F.; Filho, A.B.; Cherubini, K.; Salum, F.G.; De Figueiredo, M.A.Z. Topical application of Aloe vera and vitamin E on induced ulcers on the tongue of rats subjected to radiation: Clinical and histological evaluation. Support. Care Cancer 2015, 24, 2557–2564. [Google Scholar] [CrossRef]
- Tsai, H.-C.; Li, Y.-C.; Young, T.-H.; Chen, M.-H. Citrus polyphenol for oral wound healing in oral ulcers and periodontal diseases. J. Formos. Med Assoc. 2015, 115, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, A.C.D.O.; Andrade, Z.D.A.; Costa, T.F.; Medrado, A.R.A.P. Wound Healing—A Literature Review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Gieringer, M.; Gosepath, J.; Naim, R. Radiotherapy and wound healing: Principles, management and prospects (Review). Oncol. Rep. 2011, 26, 299–307. [Google Scholar] [CrossRef]
- Pourahmad, M.; Rahiminejad, M.; Fadaei, S.; Kashafi, H. Effects of camel thorn distillate on recurrent oral aphthous lesions. JDDG J. der Dtsch. Dermatol. Ges. 2010, 8, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Belenguer-Guallar, I.; Jiménez-Soriano, Y.; Claramunt-Lozano, A. Treatment of recurrent aphthous stomatitis. A literature review. J. Clin. Exp. Dent. 2014, 6, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-L.; Huang, H.-L.; Hua, H.; Wang, W.-C. Efficacy and safety of topical herbal medicine treatment on recurrent aphthous stomatitis: A systemic review. Drug Des. Dev. Ther. 2015, 10, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Sannomiya, M.; Fonseca, V.B.; da Silva, M.; da Rocha, L.R.M.; Santos, L.; Hiruma-Lima, C.; Brito, A.S.; Vilegas, W. Flavonoids and antiulcerogenic activity from Byrsonima crassa leaves extracts. J. Ethnopharmacol. 2005, 97, 1–6. [Google Scholar] [CrossRef]
- Amani, A.A.; Maitland, D.; Soliman, G. Antiulcerogenic Activity ofAlhagi maurorum. Pharm. Biol. 2006, 44, 292–296. [Google Scholar] [CrossRef] [Green Version]
- Tavassoli, A.P.; Anushiravani, M.; Hoseini, S.M.; Nikakhtar, Z.; Baghdar, H.N.; Ramezani, M.; Ayati, Z.; Amiri, M.S.; Sa-hebkar, A.; Emami, S.A. Phytochemistry and therapeutic effects of Alhagi spp. and Tarangabin in traditional and modern medicine: A review. J. Herbmed Pharmacol. 2020, 9, 19. [Google Scholar] [CrossRef]
- Ahmad, N.; Bibi, Y.; Saboon; Raza, I.; Zahara, K.; Idrees, S.; Khalid, N.; Bashir, T.; Tabassum, S.; Mudrikah. Traditional uses and pharmacological properties of Alhagi maurorum: A review. Asian Pac. J. Trop. Dis. 2015, 5, 856–861. [Google Scholar] [CrossRef]
- Abdel-Mageed, W.M.; Al-Saleem, M.S.M.; Al-Wahaib, L.H.; Gouda, Y.G.; Sayed, H.M. Antioxidant flavonoids from Alhagi maurorum with hepatoprotective effect. Pharmacogn. Mag. 2019, 15, 592. [Google Scholar] [CrossRef]
- Laghari, A.H.; Memon, A.A.; Memon, S.; Nelofar, A.; Khan, K.M.; Yasmin, A. Determination of free phenolic acids and antioxidant capacity of methanolic extracts obtained from leaves and flowers of camel thorn (Alhagi maurorum). Nat. Prod. Res. 2011, 26, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-J.; Inbaraj, B.S.; Chen, B.-H. Determination of Phenolic Acids and Flavonoids in Taraxacum formosanum Kitam by Liquid Chromatography-Tandem Mass Spectrometry Coupled with a Post-Column Derivatization Technique. Int. J. Mol. Sci. 2011, 13, 260–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, H.; Chen, B.; Zhang, Y.; Ou, H.; Li, Y.; Li, S.; Shi, P.; Lin, X. Analysis of the Total Biflavonoids Extract from Selaginella doederleinii by HPLC-QTOF-MS and Its In Vitro and In Vivo Anticancer Effects. Molecules 2017, 22, 325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.-H.; Guo, H.; Xu, W.-B.; Ge, J.; Li, X.; Alimu, M.; He, D. Rapid Identification of Flavonoid Constituents Directly from PTP1B Inhibitive Extract of Raspberry (Rubus idaeus L.) Leaves by HPLC–ESI–QTOF–MS-MS. J. Chromatogr. Sci. 2016, 54, 805–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, S.; Riaz, N.; Saleem, M.; Jabbar, A.; Rehman, N.U.; Ashraf, M. Antioxidant flavonoids from Alhagi maurorum. J. Asian Nat. Prod. Res. 2010, 12, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Said, A.; Abo-Elfotouh, M.; Alshabrawy, A.; Hawas, O.; Louizzo, M.; Omar, A. Antitumor Evaluation of Alhagi Maurorum Extracts and Flavonoids. Middle East J. Appl. Sci. 2014, 4, 471–476. [Google Scholar]
- Pacifico, S.; Di Maro, A.; Petriccione, M.; Galasso, S.; Piccolella, S.; Di Giuseppe, A.M.; Scortichini, M.; Monaco, P. Chemical composition, nutritional value and antioxidant properties of autochthonous Prunus avium cultivars from Campania Region. Food Res. Int. 2014, 64, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Xue, W.J.; Zou, G.A.; Aisa, H.A. Chemical Composition of Alhagi sparsifolia Flowers. Chem. Nat. Compd. 2016, 52, 1095–1097. [Google Scholar] [CrossRef]
- Piwowarczyk, R.; Ochmian, I.; Lachowicz, S.; Kapusta, I.; Sotek, Z.; Błaszak, M. Phytochemical parasite-host relations and interactions: A Cistanche armena case study. Sci. Total Environ. 2020, 716, 137071. [Google Scholar] [CrossRef]
- Brito, A.; Ramirez, J.E.; Areche, C.; Sepúlveda, B.; Simirgiotis, M.J. HPLC-UV-MS Profiles of Phenolic Compounds and Antioxidant Activity of Fruits from Three Citrus Species Consumed in Northern Chile. Molecules 2014, 19, 17400–17421. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, H.; Wu, H.; Pan, Y.; Wang, K.; Jin, Y.; Zhang, C. Characterization and Quantification by LC-MS/MS of the Chemical Components of the Heating Products of the Flavonoids Extract in Pollen Typhae for Transformation Rule Exploration. Molecules 2015, 20, 18352–18366. [Google Scholar] [CrossRef] [Green Version]
- Benayad, Z.; Gómez-Cordovés, C.; Es-Safi, N.E. Characterization of Flavonoid Glycosides from Fenugreek (Trigonella foenum-graecum) Crude Seeds by HPLC–DAD–ESI/MS Analysis. Int. J. Mol. Sci. 2014, 15, 20668–20685. [Google Scholar] [CrossRef] [Green Version]
- Muratova, M.S.; Zou, G.A.; Jenis, J.; Aisa, H.A. Chemical Constituents of Alhagi sparsifolia. Chem. Nat. Compd. 2019, 55, 932–933. [Google Scholar] [CrossRef]
- Ye, M.; Yang, W.-Z.; Liu, K.-D.; Qiao, X.; Li, B.-J.; Cheng, J.; Feng, J.; Guo, D.-A.; Zhao, Y.-Y. Characterization of flavonoids in Millettia nitida var. hirsutissima by HPLC/DAD/ESI-MS n. J. Pharm. Anal. 2011, 2, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felipe, D.F.; Brambilla, L.Z.S.; Porto, C.; Pilau, E.J.; Cortez, D.A.G. Phytochemical Analysis of Pfaffia glomerata Inflorescences by LC-ESI-MS/MS. Molecules 2014, 19, 15720–15734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Wang, W.; Sun, J.; Zhao, Y.; Liu, Y.; Liang, H.; Guo, D.-A. Characterization of phenolic compounds in the crude extract ofHedysarum multijugum by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 3833–3841. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Jia, Q.; Huang, X.; Yao, G.; Ma, W.; Chang, Y.; Ouyang, H.; He, J. Study on Pharmacokinetic and Bioavailability of Tamarixetin after Intravenous and Oral Administration to Rats. Evidence-Based Complement. Altern. Med. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Srivastava, B.; Sharma, H.; Dey, Y.N.; Wanjari, M.M.; Jahdav, A.D. Alhagi pseudalhagi: A review of its phyto-chemistry, phar-macology, folklore claims and Ayurvedic studies. Int. J. Herb. Med. 2014, 2, 47–51. [Google Scholar]
- Moussa, A.; Mostafa, N.M.; Singab, A.N.B. Pulchranin A: First report of isolation from an endophytic fungus and its inhibitory activity on cyclin dependent kinases. Nat. Prod. Res. 2019, 34, 2715–2722. [Google Scholar] [CrossRef]
- Edmond, M.P.; Mostafa, N.M.; El-Shazly, M.; Singab, A.N.B. Two clerodane diterpenes isolated from Polyalthia longifolia leaves: Comparative structural features, anti-histaminic and anti-Helicobacter pylori activities. Nat. Prod. Res. 2020, 35, 5282–5286. [Google Scholar] [CrossRef]
- Ayoub, N.; Singab, A.N.; Mostafa, N.; Schultze, W. Volatile Constituents of Leaves ofFicus caricaLinn. Grown in Egypt. J. Essent. Oil Bear. Plants 2010, 13, 316–321. [Google Scholar] [CrossRef]
- Mostafa, N.M.; Mostafa, A.M.; Ashour, M.L.; Elhady, S.S. Neuroprotective Effects of Black Pepper Cold-Pressed Oil on Scopolamine-Induced Oxidative Stress and Memory Impairment in Rats. Antioxidants 2021, 10, 1993. [Google Scholar] [CrossRef]
- Abdallah, S.H.; Mostafa, N.M.; Mohamed, M.A.E.H.; Nada, A.S.; Singab, A.N.B. UPLC-ESI-MS/MS profiling and hepatoprotective activities of Stevia leaves extract, butanol fraction and stevioside against radiation-induced toxicity in rats. Nat. Prod. Res. 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, E.; Maher, A.; Mostafa, D.; Gad, S.; Nawwar, M.; Swilam, N. A Unique Acylated Flavonol Glycoside from Prunus persica (L.) var. Florida Prince: A New Solid Lipid Nanoparticle Cosmeceutical Formulation for Skincare. Antioxidants 2021, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, N.M.; Edmond, M.P.; El-Shazly, M.; Fahmy, H.A.; Sherif, N.H.; Singab, A.N.B. Phytoconstituents and renoprotective effect of Polyalthia longifolia leaves extract on radiation-induced nephritis in rats via TGF-β/smad pathway. Nat. Prod. Res. 2021, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, N.; Badr, N.; Al-Ghamdi, S.S.; Alsanosi, S.; Alzahrani, A.R.; Abdel-Naim, A.B.; Nematallah, K.A.; Swilam, N. HPLC/MSn Profiling and Healing Activity of a Muco-Adhesive Formula of Salvadora persica against Acetic Acid-Induced Oral Ulcer in Rats. Nutrients 2021, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, N.; Badr, N.; Al-Ghamdi, S.S.; Alzahrani, A.; Alsulaimani, R.; Nassar, A.; Qadi, R.; Afifi, I.K.; Swilam, N. GC/MS Profiling and Ex Vivo Antibacterial Activity of Salvadora persica (Siwak) against Enterococcus faecalis as Intracanal Medicament. Evidence-Based Complement. Altern. Med. 2021, 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, E.S.; Nawwar, M.A.; Mostafa, D.A.; Ragab, M.F.; Swilam, N. Karafsin, a unique mono-acylated flavonoid apiofurnoside from the leaves of Apium graveolens var. secalinum Alef: In vitro and in vivo anti-inflammatory assessment. Ind. Crop. Prod. 2020, 158, 112901. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; Mostafa, N.M.; Eldahshan, O.A.; Singab, A.N.B. A new antidiabetic and anti-inflammatory biflavonoid from Schinus polygama (Cav.) Cabrera leaves. Nat. Prod. Res. 2020, 1–9. [Google Scholar] [CrossRef]
- Al-Madhagy, S.A.; Mostafa, N.M.; Youssef, F.S.; Awad, G.E.A.; Eldahshan, O.A.; Singab, A.N.B. Metabolic profiling of a polyphenolic-rich fraction of Coccinia grandis leaves using LC-ESI-MS/MS and in vivo validation of its antimicrobial and wound healing activities. Food Funct. 2019, 10, 6267–6275. [Google Scholar] [CrossRef]
- Mostafa, N.M.; Ashour, M.L.; Eldahshan, O.A.; Singab, A.N.B. Cytotoxic activity and molecular docking of a novel biflavonoid isolated from Jacaranda acutifolia (Bignoniaceae). Nat. Prod. Res. 2015, 30, 2093–2100. [Google Scholar] [CrossRef]
- Avci, E.; Akarslan, Z.; Erten, H.; Cevher, C. Oxidative stress and cellular immunity in patients with recurrent aphthous ulcers. Braz. J. Med Biol. Res. 2014, 47, 355–360. [Google Scholar] [CrossRef] [Green Version]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in Wound Repair: Molecular and Cellular Mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunte, K.; Hensel, A.; Beikler, T. Polyphenols in the prevention and treatment of periodontal disease: A systematic review of in vivo, ex vivo and in vitro studies. Fitoterapia 2018, 132, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; LiHua, Y.; Qian, Y.; Yan, L. Effect of Lentinus edodes polysaccharide on oxidative stress, immunity activity and oral ulceration of rats stimulated by phenol. Carbohydr. Polym. 2009, 75, 115–118. [Google Scholar] [CrossRef]
- Miao, M.; Peng, M.; Xing, Z.; Liu, D. Effect of Shuangjinlian mixture on oral ulcer model in rat. Saudi J. Biol. Sci. 2019, 26, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Pourali, P.; Yahyaei, B. Wound healing property of a gel prepared by the combination ofPseudomonas aeruginosaalginate andAlhagi maurorumaqueous extract in rats. Dermatol. Ther. 2018, 32, e12779. [Google Scholar] [CrossRef] [Green Version]
- Vasilev, H.; Ross, S.; Šmejkal, K.; Maršík, P.; Jankovská, D.; Havlík, J.; Veselý, O. Flavonoid Glycosides from Endemic Bulgarian Astragalus aitosensis (Ivanisch.). Molecules 2019, 24, 1419. [Google Scholar] [CrossRef] [Green Version]
- Comalada, M.; Ballester, I.; Bailón, E.; Sierra, S.; Xaus, J.; Galvez, J.; de Medina, F.S.; Zarzuelo, A. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: Analysis of the structure–activity relationship. Biochem. Pharmacol. 2006, 72, 1010–1021. [Google Scholar] [CrossRef]
- Zaki, A.A.; Xu, X.; Wang, Y.; Shie, P.-H.; Qiu, L. A new anti-inflammatory flavonoid glycoside from tetraena aegyptia. Nat. Prod. Res. 2019, 35, 1985–1990. [Google Scholar] [CrossRef]
- Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A.; Martínez-Vitela, C.; Serna-Saldívar, S.O. Topical Anti-Inflammatory Effects of Isorhamnetin Glycosides Isolated from Opuntia ficus-indica. BioMed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Velagapudi, R.; Aderogba, M.; Olajide, O.A. Tiliroside, a dietary glycosidic flavonoid, inhibits TRAF-6/NF-κB/p38-mediated neuroinflammation in activated BV2 microglia. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 3311–3319. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutierrez-Grijalva, E.P.; Ambriz-Perez, D.L.; Heredia, J.B. Flavonoids as Cytokine Modulators: A Possible Therapy for Inflammation-Related Diseases. Int. J. Mol. Sci. 2016, 17, 921. [Google Scholar] [CrossRef] [PubMed]
- Recio, M.D.C.; Giner, R.M.; Máñez, S.; Talens, A.; Cubells, L.; Gueho, J.; Julien, H.R.; Hostettmann, K.; Rios, J.L. Anti-Inflammatory Activity of Flavonol Glycosides fromErythrospermum monticolumDepending on Single or Repeated Local TPA Administration. Planta Medica 1995, 61, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Salaverry, L.S.; Parrado, A.C.; Mangone, F.M.; Dobrecky, C.B.; Flor, S.A.; Lombardo, T.; Sotelo, A.D.; Saccodossi, N.; Rugna, A.Z.; Blanco, G.; et al. In vitro anti-inflammatory properties of Smilax campestris aqueous extract in human macrophages, and characterization of its flavonoid profile. J. Ethnopharmacol. 2019, 247, 112282. [Google Scholar] [CrossRef] [PubMed]
- Attard, E. A rapid microtitre plate Folin-Ciocalteu method for the assessment of polyphenols. Open Life Sci. 2013, 8, 48–53. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef]
- Swilam, N.; Nematallah, K.A. Polyphenols profile of pomegranate leaves and their role in green synthesis of silver nanoparticles. Sci. Rep. 2020, 10, 14851. [Google Scholar] [CrossRef]
- Alfaifi, M.; Alsayari, A.; Gurusamy, N.; Louis, J.; Elbehairi, S.E.; Venkatesan, K.; Annadurai, S.; Asiri, Y.I.; Shati, A.; Saleh, K.; et al. Analgesic, Anti-Inflammatory, Cytotoxic Activity Screening and UPLC-PDA-ESI-MS Metabolites Determination of Bioactive Fractions of Kleinia pendula. Molecules 2020, 25, 418. [Google Scholar] [CrossRef] [Green Version]
- Boly, R.; Lamkami, T.; Lompo, M.; Dubois, J.; Guissou, I.P. DPPH free radical scavenging activity of two extracts from Age-lanthus dodoneifolius (Loranthaceae) Leaves. Int. J Toxicol. Pharmacol. Res. 2016, 8, 29–34. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Alfano, B.; Barretta, L.; Del Giudice, A.; De Vito, S.; Di Francia, G.; Esposito, E.; Formisano, F.; Massera, E.; Miglietta, M.L.; Polichetti, T. A Review of Low-Cost Particulate Matter Sensors from the Depvelopers’ Perspectives. Sensors 2020, 20, 6819. [Google Scholar] [CrossRef]
- Lopes, G.; Sousa, C.; Silva, L.; Pinto, E.; Andrade, P.; Bernardo, J.; Mouga, T.; Valentão, P. Can Phlorotannins Purified Extracts Constitute a Novel Pharmacological Alternative for Microbial Infections with Associated Inflammatory Conditions? PLoS ONE 2012, 7, e31145. [Google Scholar] [CrossRef] [PubMed]
- Hitomi, S.; Ono, K.; Yamaguchi, K.; Terawaki, K.; Imai, R.; Kubota, K.; Omiya, Y.; Hattori, T.; Kase, Y.; Inenaga, K. The traditional Japanese medicine hangeshashinto alleviates oral ulcer-induced pain in a rat model. Arch. Oral Biol. 2016, 66, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Sapan, C.V.; Lundblad, R.L. Review of methods for determination of total protein and peptide concentration in biological samples. Proteom. Clin. Appl. 2015, 9, 268–276. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Buchwalow, I.B.; Böcker, W. Immunohistochemistry: Basics and Methods. In Immunohistochemistry: Basics and Methods; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2010; pp. 109–127. [Google Scholar]
- Fedchenko, N.; Reifenrath, J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue—A review. Diagn. Pathol. 2014, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
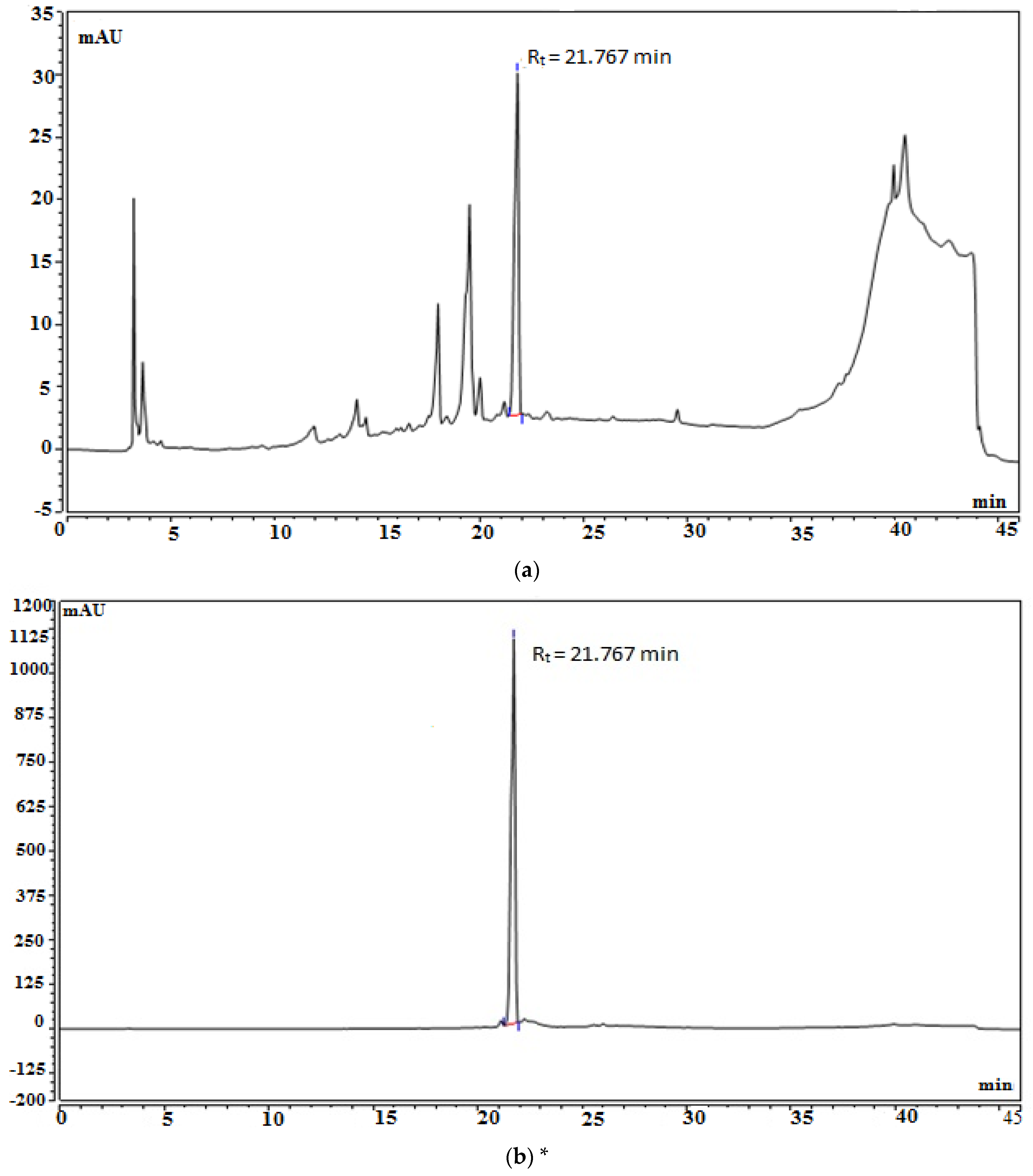
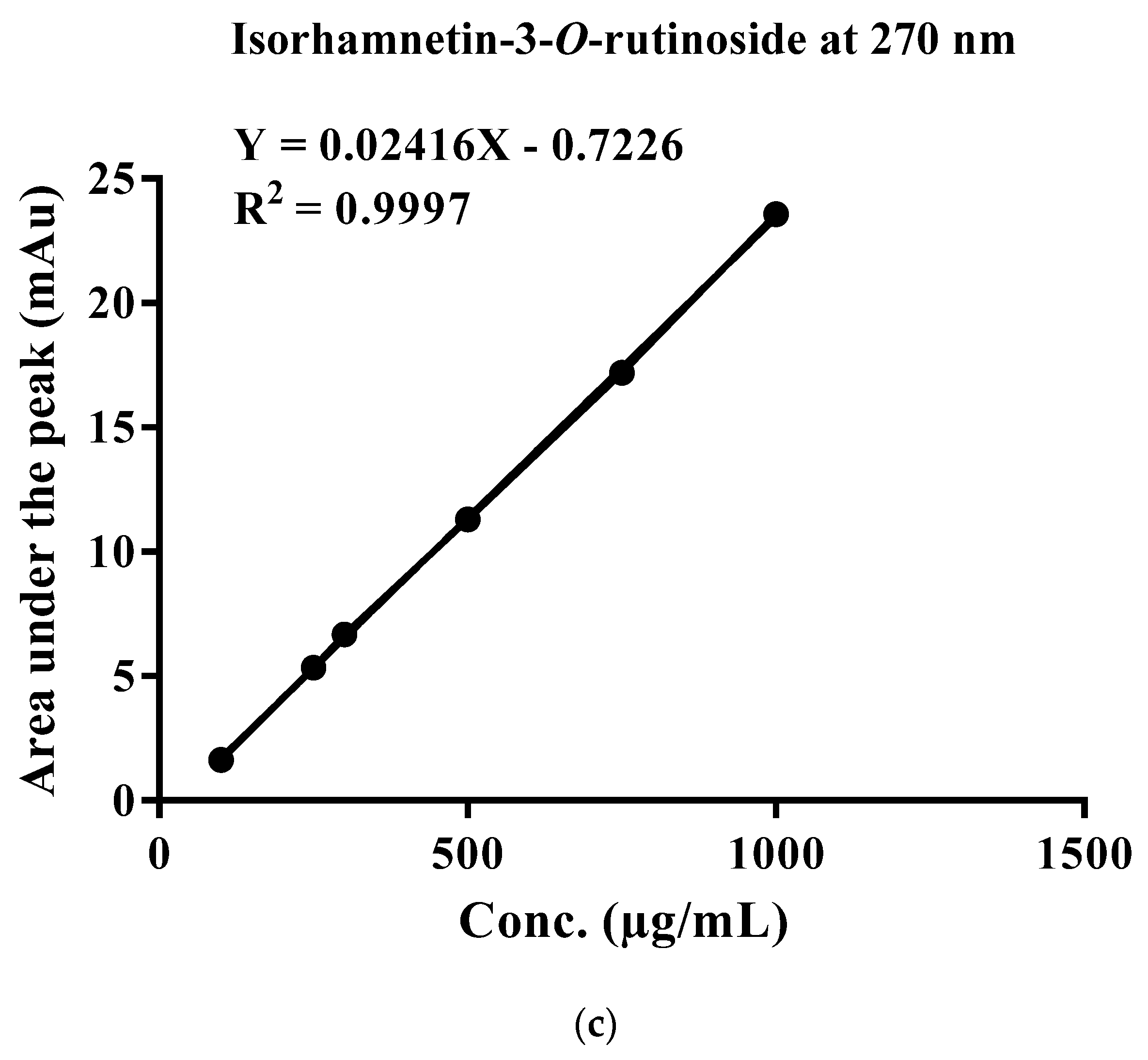
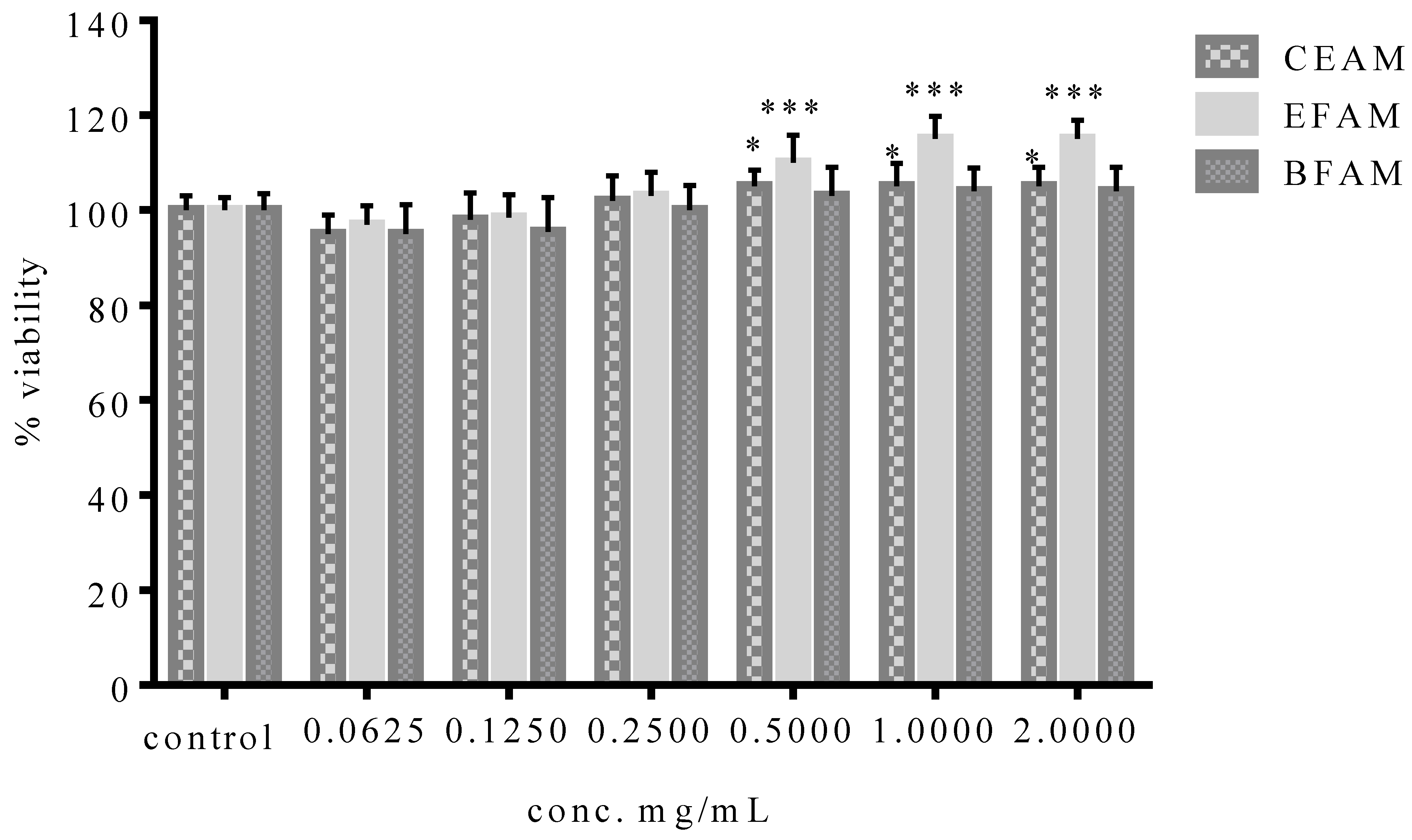
| Total Phenolic Content (mg GAE/g Dry Extract) | Total Flavonoid Content (mg RE/g Dry Extract) | |
|---|---|---|
| CEAM | 36.50 ± 2.2 | 22.90 ± 1.07 |
| EFAM | 53.19 ± 4.2 | 47.96 ± 3.1 |
| BFAM | 11.81 ± 0.36 | 4.21 ± 0.4 |
| Peak # | Rt (Min) | Identified Compound | UV-Vis (λmax) | [M − H]− (m/z) | Fragment Ions (m/z) | Percentage (%) | Occurrence | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.5 | Caffeic acid | --- | 179 | 135 | 0.2 | A. maurorum | [19] |
| 2 | 3.5 | Sinapic acid hexoside | 228 | 385.0 | 223, 179 | 0.65 | A. maurorum | [19] |
| 3 | 3.97 | Caffeoyl-hexose-deoxyhexoside | 318 | 487.0 | 308,179 | 6.40 | - | [20] |
| 4 | 4.5 | Chrysoeriolpentoside (chrysoeriol-7-O-xylosoid) | 220, 335 | 431.1 | 299 | 0.21 | A. maurorum | [15] |
| 5 | 7.85 | Dihydroxybiflavone derivative | 220, 332 | 521.01 | 375, 331 | 0.15 | - | [21] |
| 6 | 17.5 | Caffeoyl-hexose-deoxyhexoside | 320 | 487.0 | 308, 179 | 0.70 | - | [20] |
| 7 | 20.25 | Quercetin hexoside (Quercetin3-O-β-D-glucoside) | 221, 330 | 463 | 301, 179 | 1.56 | A. maurorum A. maurorum | [22,23,24] |
| 8 | 21.9 | Kampferol hexoside (Kampferol-3-O-β-D-glucoside) | 220, 330 | 447.02 | 285, 284 | 2.15 | A. maurorum A. sparsifolia | [24,25,26] |
| 9 | 22.12 | Quercetin diglucoside (Quercetin-3-O-di-glucoside) | 257, 335 | 625.3 | 271, 301,179 | 1.75 | A. maurorum | [27] |
| 10 | 22.77 | Quercetin rhamnoside (quercetin-3-O-β- rhamnoside) | 225, 332 | 447.23 | 301,179 | 3.85 | A. maurorum A. persarum | [22,23,24] |
| 11 | 23.7 | (Iso)rhamnetin rhamnoside | 235, 335 | 461 | 315 | 0.99 | - | [28] |
| 12 | 24.58 | Isorhamnetin-3-O-rhamnopyranosyl hexoside (Isorhamnetin-3-O-β-rutinoside) | 250, 342 | 623.12 | 315, 301, 179, 151 | 5.35 | A. maurorum A. maurorum | [18,24,28] |
| 13 | 26.5 | (Iso)rhamnetin-dirhamnopyranosyl- hexoside Isorhamnetin-3-O-rhamnosyl-rutinoside (Typhaneoside) | 230, 330 | 769.18 | 454, 315 | 0.63 | A. maurorum | [18,29] |
| 14 | 29.2 | (Iso)rhamnetin 3-O-hexosyl-neo-hesperidoside (Isorhamnetin 3-O-glucosylneo-hesperidoside) | 250, 350 | 803.21 | 623, 477, 315 | 0.75 | A. maurorum | [15] |
| 15 | 32.5 | Isorhamnetin-3-O-rhamnopyranosyl hexoside (Isorhamnetin-3-O-β-rutinoside) | 268, 342 | 623.21 | 315, 301, 179, 151 | 0.88 |
A. maurorum A. maurorum | [18,24,28] |
| 16 | 34.47 | Apigenin-rhamnosyl-hexoside (Apigenin-8-C-rhamnosyl-6-C-glucoside) | 272, 373 | 577.3 | 503, 473, 383, 353 | 2.98 | - | [30] |
| 17 | 35.0 | Kaempferol-3-O-rutinoside | 262, 362 | 593.17 | 285, 284 | 0.42 | A. maurorum | [28,31] |
| 18 | 35.5 | (Iso)rhamnetinhexoside (Isorhamnetin-3- O-β-D-glucopyranoside) | 268, 342 | 477.2 | 315 | 0.48 | A. saparsifolia | [26] |
| 19 | 36.12 | 3′-O-methylorobol | 283,357 | 299.12 | 284, 271 | 0.58 | A. maurorum | [23,32] |
| 20 | 36.8 | Quercetin | 254, 374 | 301.08 | 179, 151 | 0.83 |
A. maurorum A. saparsifolia | [18,26,28] |
| 21 | 37.0 | Kaempferol | 222, 335 | 285.9 | 239, 187 | 0.85 | A. maurorum A. saparsifolia | [18,26,33] |
| 22 | 37.80 | Quercetin hexoside (Quercetin-3-O-β-D-glucopyranoside) | 221, 330 | 463 | 301, 179 | 2.35 | A. maurorum A. maurorum | [22,23,24] |
| 23 | 38.98 | Dihydroxy methoxy diprenyl isoflavone (5,7-dihydroxy-4′-methoxy-6, 8-dipenylisoflavone) | 225, 335 | 419.08 | 404, 361 | 4.50 | - | [34] |
| 24 | 39.33 | Isorhamnetin | 224, 338 | 315.2 | 301, 272 | 2.75 | A. maurorum | [18,29] |
| 25 | 40.5 | Tamarixetin | 220, 331 | 315 | 300, 151 | 3.12 | A. maurorum | [35,36] |
| DPPH IC50 (μg/mL) | FRAP (μM TE/mg Extract) | |
|---|---|---|
| CEAM | 45.22 ± 2.11 | 139.90 ± 4.01 |
| EFAM | 27.73 ± 1.85 | 176.60 ± 5.21 |
| BFAM | 52.48 ± 35.85 | 78.04 ± 7.92 |
| Trolox | 22.35 ± 0.87 | - |
| Group | TNF-α (pg/mL Protein) | IL-2 (pg/mL Protein) |
|---|---|---|
| Control | 136.64 * ± 8.37 | 67.63 * ± 8.64 |
| Vehicle | 211.79 ± 11.69 | 138.33 ± 8.87 |
| CEAM | 150.65 *,# ± 8.08 | 79.87 * ± 9.84 |
| EFAM | 134.62 * ± 9.08 | 68.07 * ± 6.22 |
| BFAM | 151.16 *,# ± 7.47 | 80.02 *,# ± 4.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Zahar, H.; Menze, E.T.; Handoussa, H.; Osman, A.K.; El-Shazly, M.; Mostafa, N.M.; Swilam, N. UPLC-PDA-MS/MS Profiling and Healing Activity of Polyphenol-Rich Fraction of Alhagi maurorum against Oral Ulcer in Rats. Plants 2022, 11, 455. https://doi.org/10.3390/plants11030455
El-Zahar H, Menze ET, Handoussa H, Osman AK, El-Shazly M, Mostafa NM, Swilam N. UPLC-PDA-MS/MS Profiling and Healing Activity of Polyphenol-Rich Fraction of Alhagi maurorum against Oral Ulcer in Rats. Plants. 2022; 11(3):455. https://doi.org/10.3390/plants11030455
Chicago/Turabian StyleEl-Zahar, Hala, Esther T. Menze, Heba Handoussa, Ahmed K. Osman, Mohamed El-Shazly, Nada M. Mostafa, and Noha Swilam. 2022. "UPLC-PDA-MS/MS Profiling and Healing Activity of Polyphenol-Rich Fraction of Alhagi maurorum against Oral Ulcer in Rats" Plants 11, no. 3: 455. https://doi.org/10.3390/plants11030455
APA StyleEl-Zahar, H., Menze, E. T., Handoussa, H., Osman, A. K., El-Shazly, M., Mostafa, N. M., & Swilam, N. (2022). UPLC-PDA-MS/MS Profiling and Healing Activity of Polyphenol-Rich Fraction of Alhagi maurorum against Oral Ulcer in Rats. Plants, 11(3), 455. https://doi.org/10.3390/plants11030455








