Mechanistic Aspects and Effects of Selected Tank-Mix Partners on Herbicidal Activity of a Novel Fatty Acid Ester
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Species and Biological Test Conditions
2.2. Experimental Design of the Phytotron Trials
2.2.1. Herbicidal Compound and Tank-Mix Partners Tested
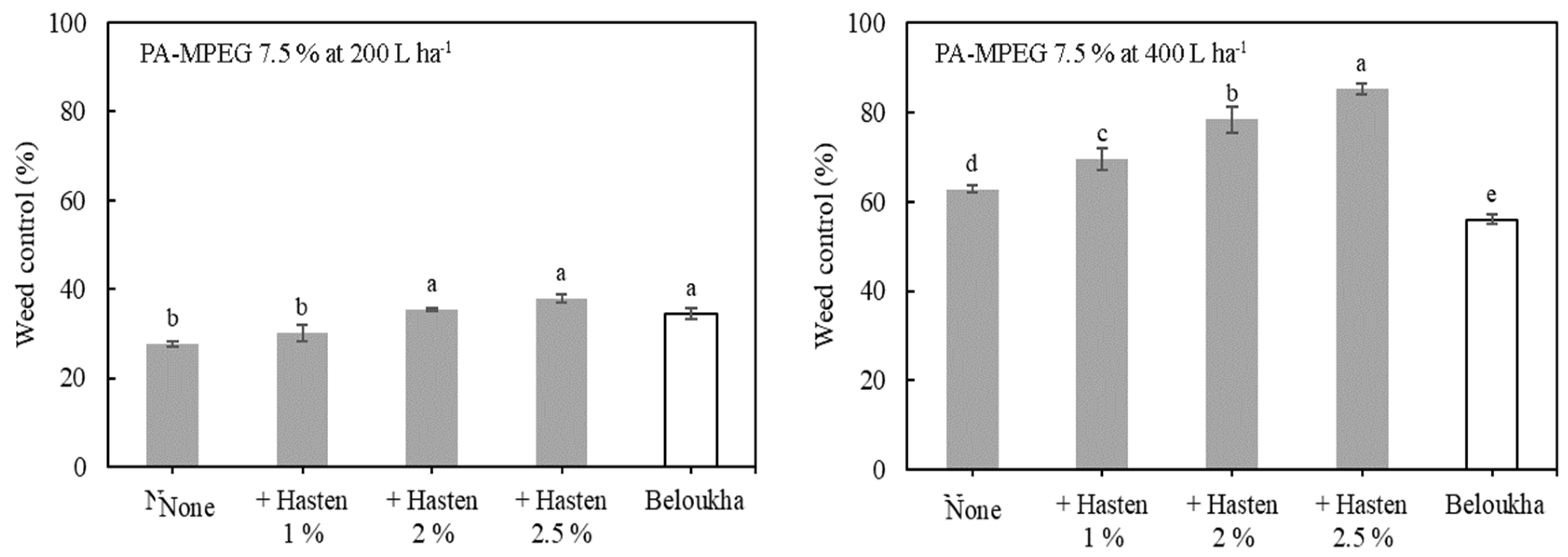
2.2.2. Interaction of the Hasten Concentration and the PA-MPEG Rate and Spray Volume
2.2.3. Phytotoxicity of Spray Tank Partners after Spraying and Single Droplet Application
2.3. Laboratory Experiments
2.3.1. Cuticular Penetration
2.3.2. Characterization of Spray Deposits on Glass Slides
2.3.3. Scanning Electron Microscope (SEM)
2.3.4. Cuticular Transpiration
2.3.5. Stomatal Conductance
2.4. Statistical Analysis
3. Results and Discussion
3.1. PA-MPEG Herbicidal Activity Affected by the Test Compounds Added to the Spray Tank
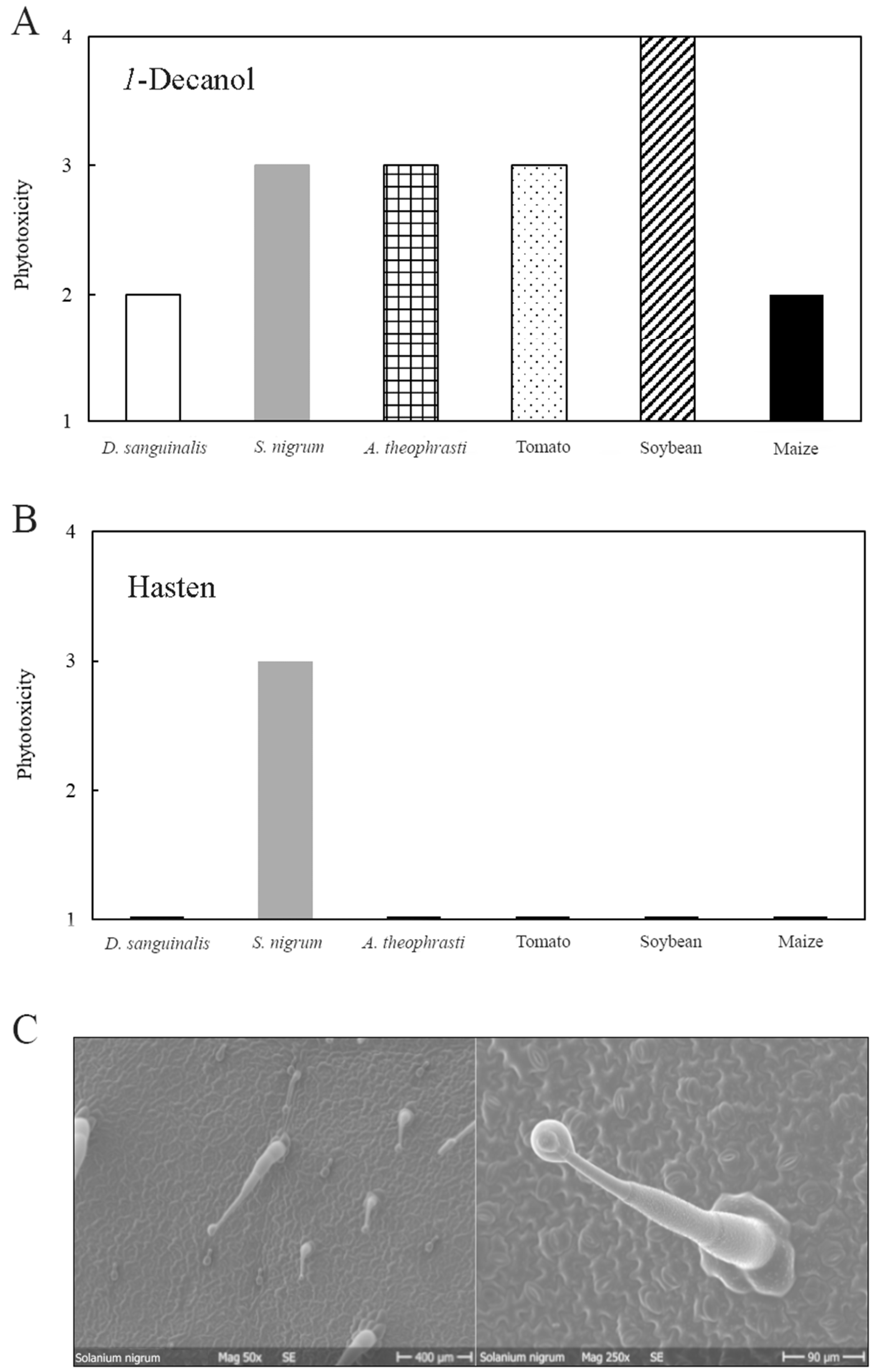
3.2. Phytotoxicity of 1-Decanol and Hasten
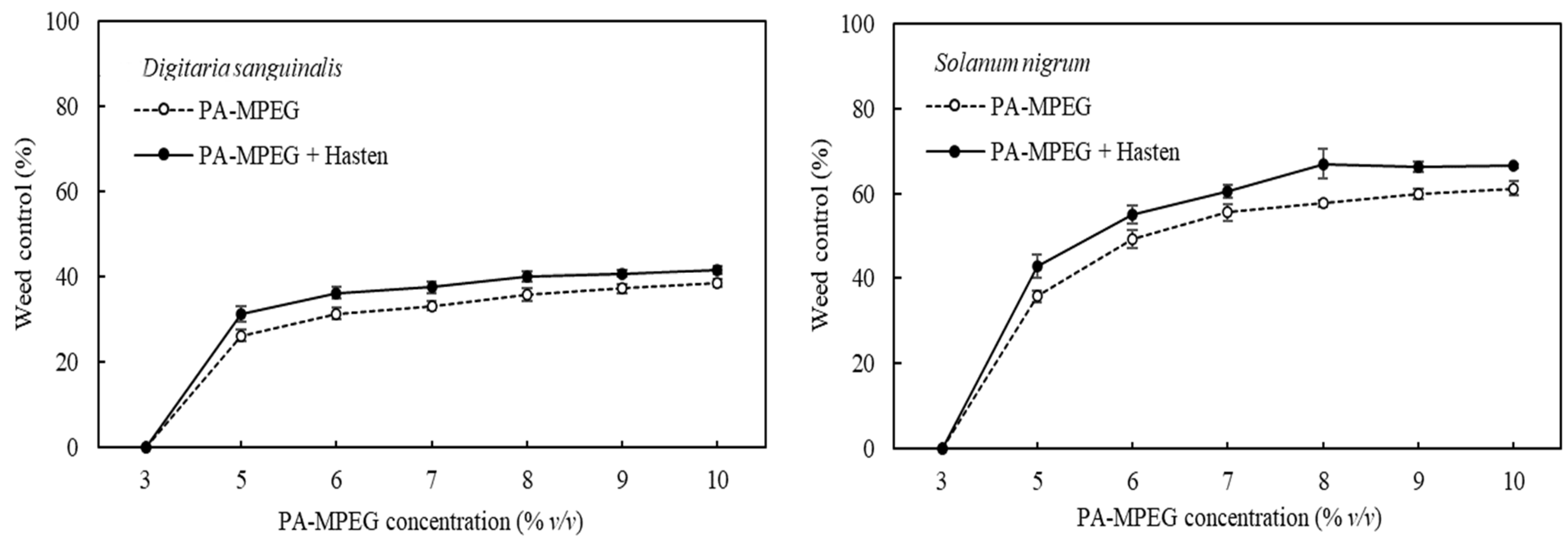
3.3. Concentration Dependence of the Adjuvant Effect on PA-MPEG Herbicidal Activity
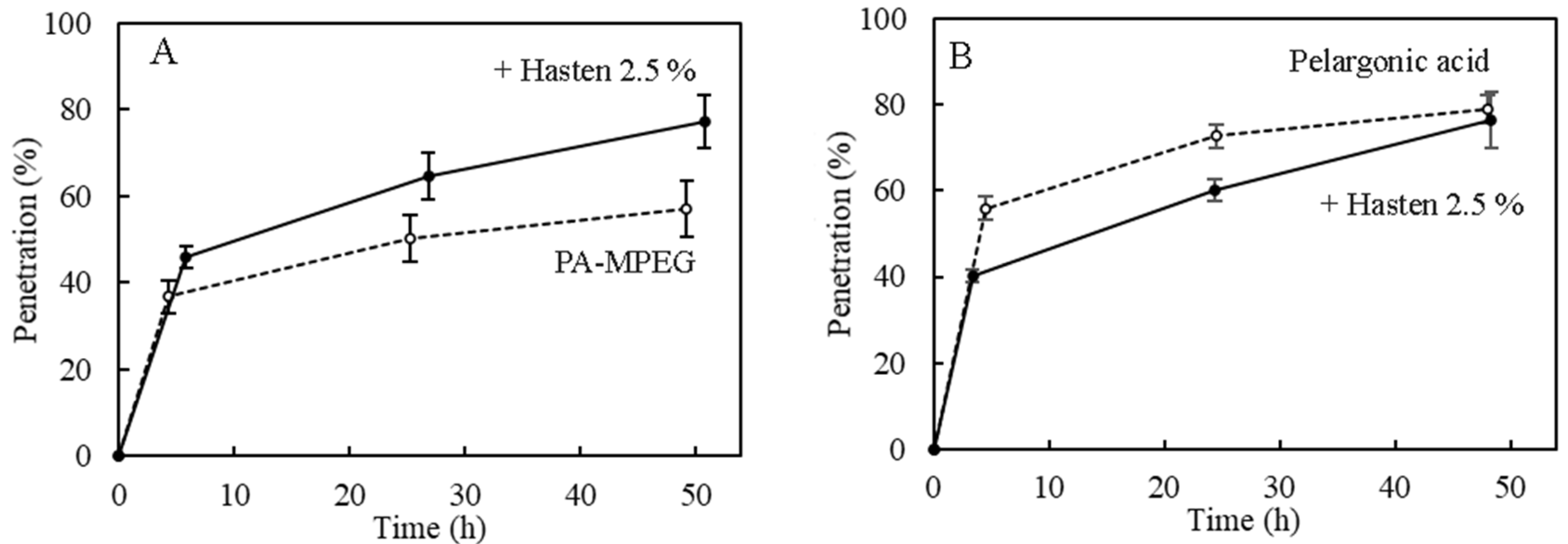
3.4. Pelargonic Acid and PA-MPEG Cuticular Penetration
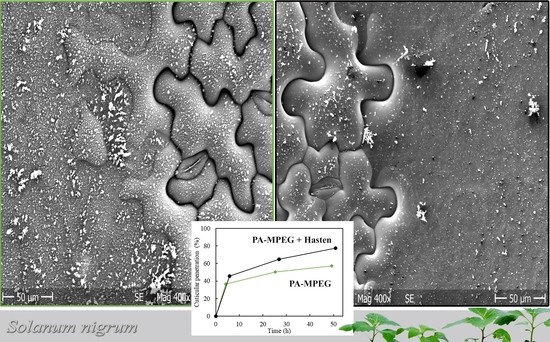
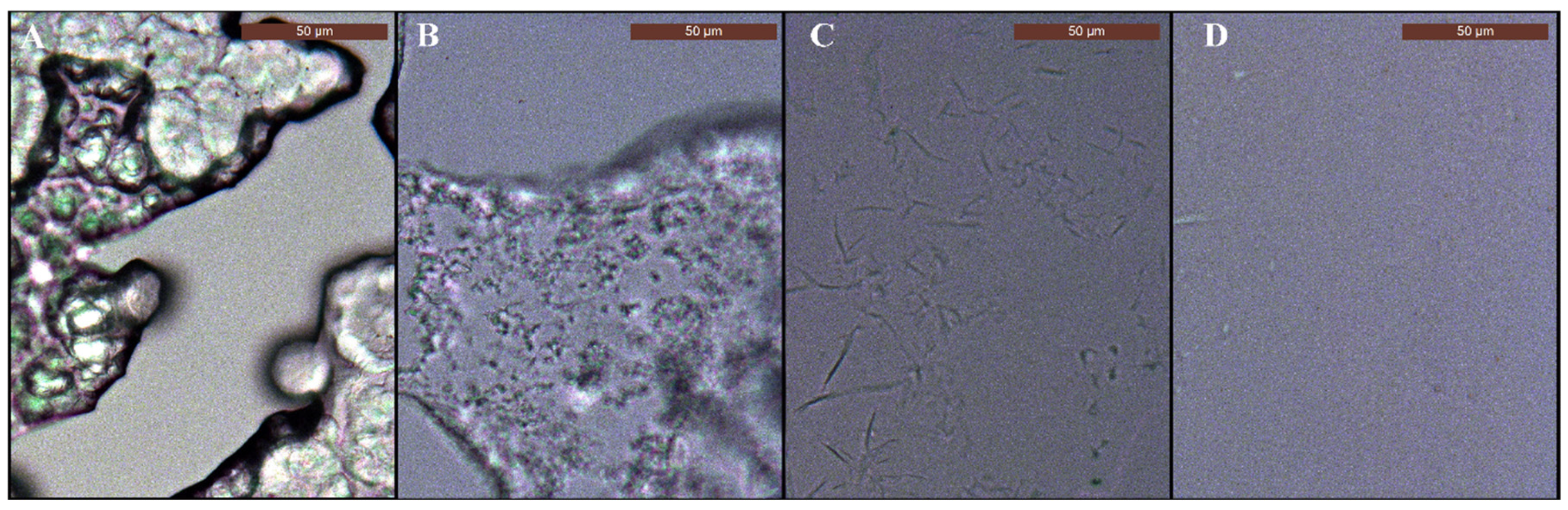
3.5. Characterization of Spray Deposits on Glass Slides
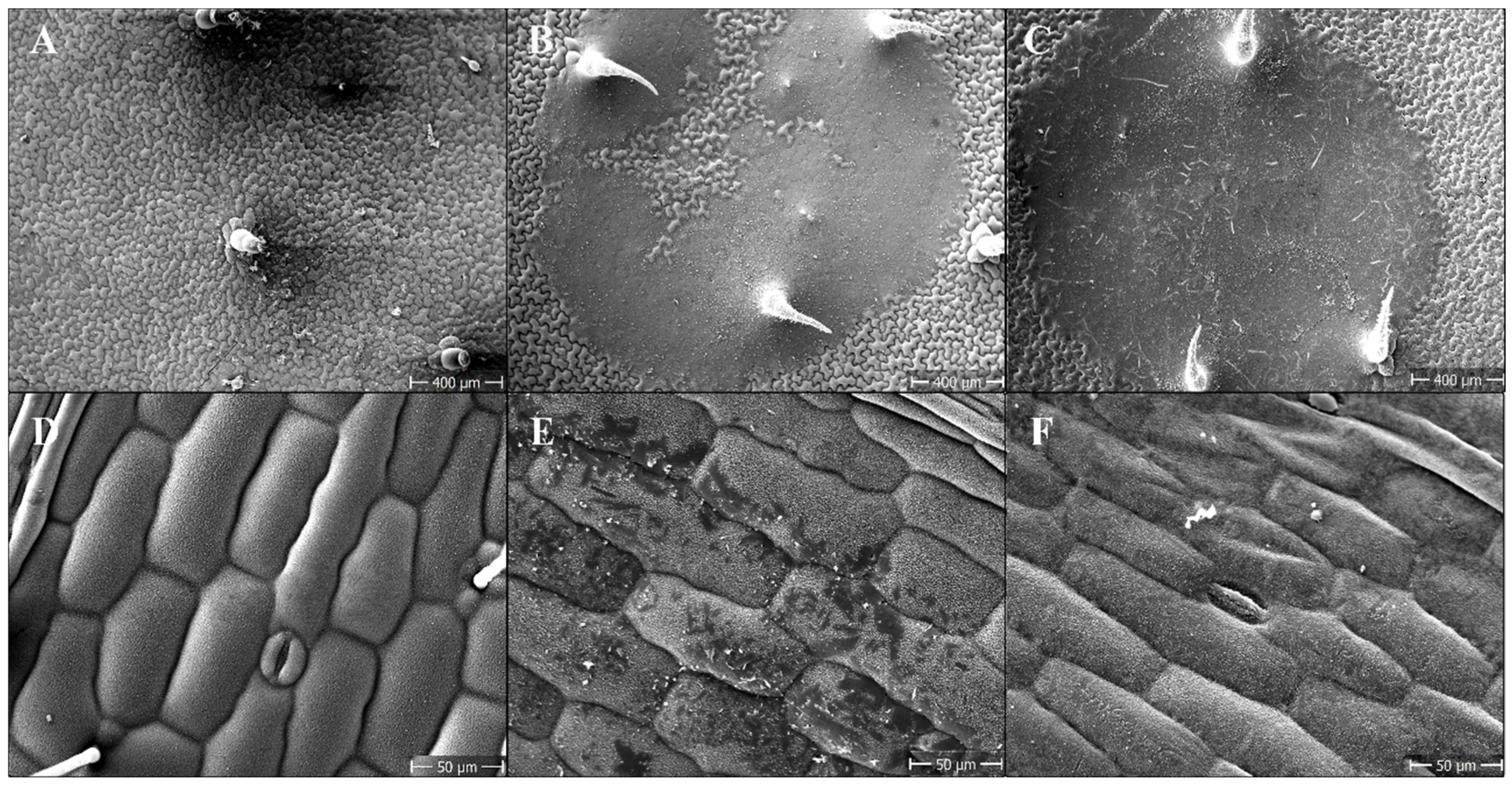
3.6. Scanning Electron Microscope (SEM)
3.7. The Mechanistic Aspects and High Use Rates of PA-MPEG and PA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hatcher, P.E.; Froud-Williams, R.J. Weed Research: Expanding Horizons, 1st ed.; John Wiley & Sons Ltd.: West Sussex, UK, 2017; p. 456. [Google Scholar]
- Jeschke, P.; Witschel, M.; Kräamer, W.; Schirmer, U. Modern Crop Protection Compounds, 3rd ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2019; p. 1784. [Google Scholar]
- Baur, P.; Aponte, J. Co-penetration of actives and adjuvants and its significance for the matched pair liaison. In Retention, Uptake, and Translocation of Agrochemicals in Plants; Myung, K., Satchivi, N.M., Kingston, C., Eds.; American Chemical Society: Washington, DC, USA, 2014; pp. 23–39. [Google Scholar]
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a-century herbicide. Pest. Manag. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef]
- Gatzweiler, E.; Krähmer, H.; Hacker, E.; Hills, M.; Trabold, K.; Bonfig-Picard, G. Weed Spectrum and Selectivity of Tembotrione under Varying Environmental Conditions. 25. Deutsche Arbeitsbesprechung über Fragen der Unkrautbiologie und -bekämpfung, Braunschweig, Germany; Nordmeyer, H., Ulber, L., Eds.; Julius-Kühn-Archiv: Braunschweig, Germany, 2012; pp. 385–391. [Google Scholar]
- Zimdahl, R. Fundamentals of Weed Science, 5th ed.; Academic Press: London, UK, 2018; p. 758. [Google Scholar]
- Eddleston, M. Paraquat and Diquat. In Critical Care Toxicology, 2nd ed.; Brent, J., Burkhart, K., Dargan, P., Hatten, B., Bruno Megarbane, B., Palmer, R., White, J., Eds.; Springer: Cham, Switzerland, 2017; pp. 1855–1874. [Google Scholar]
- The International Survey of Herbicide Resistant Weeds. Available online: https://weedscience.org (accessed on 1 October 2021).
- World Health Organization (WHO). Environmental Health Criteria 39, Paraquat and Diquat; WHO: Geneva, Switzerland, 1984. [Google Scholar]
- Dinham, B. Why Paraquat should be banned. Outlooks Pest Manag. 2004, 15, 268–271. [Google Scholar] [CrossRef]
- Fukuda, M.; Fujimori, T.; Tsujino, Y.; Wakabayashi, K.; Böger, P. Phytotoxic activity of middle-chain fatty acids I: Effects on cell constituents. Pesti. Biochem. Physiol. 2004, 80, 143–150. [Google Scholar] [CrossRef]
- Lederer, B.; Fujimori, T.; Tsujino, Y.; Wakabayashi, K.; Böger, P. Phytotoxic activity of middle-chain fatty acids II: Peroxidation and membrane effects. Pesti. Biochem. Physiol. 2004, 80, 151–156. [Google Scholar] [CrossRef]
- Coleman, R.; Penner, D. Desiccant Activity of Short Chain Fatty Acids. Weed Technol. 2006, 20, 410–415. [Google Scholar] [CrossRef]
- Belchim Crop Protection. Beloukha®. Available online: https://belchim-agro.de (accessed on 21 October 2018).
- Westbridge Agricultural Products. SUPPRESS® HERBICIDE EC. Available online: https://westbridge.com (accessed on 20 June 2020).
- Certis Europe. Finalsan. Available online: https://www.certiseurope.com (accessed on 20 June 2020).
- Campos, J.; Mansour, P.; Verdeguer, M.; Baur, P. Rate, Spray Volume, and Application Parameters of a Novel Contact Herbicide. 2021; submitted. [Google Scholar]
- Ciriminna, R.; Fidalgo, A.; Ilharco, L.; Pagliaro, M. Herbicides based on pelargonic acid: Herbicides of the bioeconomy. Biofuels Bioprod. Biorefin. 2019, 13, 1476–1482. [Google Scholar] [CrossRef]
- Chiotti, D.; Leonard, R.; Schlenk, D.; Wilen, C.; Schiff, K. Alternatives to Glyphosate for Vegetation Management in Los Angeles County; Technical Committee Report. Southern California Coastal Water Research Project; Southern California Coastal Water Research: Costa Mesa, CA, USA, 2020. [Google Scholar]
- BPDB: Bio-Pesticides DataBase—Pelargonic Acid. Available online: http://sitem.herts.ac.uk (accessed on 1 October 2021).
- Baur, P.; Bauer, M.; Bodelon, L.; Campos-Cuevas, J.; Giessler, S.; Hövelmann, F. Fatty Acid Derivatives for Use as Herbicides. International Patent WO 2019/162484 A1, 25 February 2019. [Google Scholar]
- Merritt, J.; Webber, C.; Shrefler, J. The Economic Value of Pelargonic Acid as a Natural Herbicide in Sweet Bell Peppers. In Proceedings of the Southern Agricultural Economics Association’s 2016 Annual Meeting, San Antonio, TX, USA, 6–9 February 2016. [Google Scholar]
- Campos, J.; Verdeguer, M.; Baur, P. Capped polyethylene glycol esters of fatty acids as novel active principles for weed control. Pest Manag. Sci. 2021, 77, 4648–4657. [Google Scholar] [CrossRef] [PubMed]
- Crmaric, I.; Keller, M.; Krauss, J.; Delabays, N. Efficacy of Natural Fatty Acid Based Herbicides on Mixed Weed Stands. 28. Deutsche Arbeitsbesprechung über Fragen der Unkrautbiologie und -bekämpfung, Braunschweig, Germany; Nordmeyer, H., Ulber, L., Eds.; Julius-Kühn-Archiv: Braunschweig, Germany, 2018; pp. 328–333. [Google Scholar]
- Wehtje, G.; Altland, J.; Gilliam, C. Interaction of glyphosate and pelargonic acid in ready-to-use weed control products. Weed Technol. 2009, 23, 544–549. [Google Scholar] [CrossRef]
- Little, K.; Nadel, R. Testing pelargonic acid and pyraflufen-ethyl with glyphosate as alternatives to paraquat dichloride for the preparation of fire-break tracer lines at Underberg, South Africa. South. For. J. For. Sci. 2014, 74, 67–73. [Google Scholar] [CrossRef]
- Baur, P.; Bauer, M.; Bodelon, L.; Campos-Cuevas, J.; Giessler, S.; Hövelmann, F. Fatty Acid Derivatives for Improving the Effect of Agrochemical Actives. International Patent WO 2020/173717 A1, 14 February 2020. [Google Scholar]
- Hazen, J.L. Adjuvants—Terminology, Classification and Chemistry. Weed Technol. 2000, 14, 773–784. [Google Scholar] [CrossRef]
- Pacanoski, Z. Herbicides and Adjuvants. In Herbicides, Physiology of Action, and Safety; Price, A., Kelton, J., Sarunaite, L., Eds.; IntechOpen: London, UK, 2015; pp. 125–147. [Google Scholar]
- Clariant. Brochure Crop Solutions. Available online: https://clariant.com (accessed on 13 October 2021).
- Victoria Chemical Company. HASTEN Spray Adjuvant. Available online: https://vicchem.com/ (accessed on 20 June 2020).
- Baur, P.; Bodelon, L.; Lowe, B. Novel Adjuvant Blend for In-Can and Tank-Mix Application Potential Combining Sustainability and Performance as Versatile Penetration Enhancer. In Pesticide Formulation and Delivery Systems: 33rd Volume, “Sustainability: Contributions from Formulation Technology”; Sesa, C., Ed.; ASTM International: West Conshohocken, PA, USA, 2014; pp. 87–106. [Google Scholar]
- Cronfeld, P.; Lader, K.; Baur, P. Classification of Adjuvants and Adjuvant Blends by Effects on Cuticular Penetration. In Pesticide Formulations and Application Systems; Viets, A.K., Tann, R.S., Mueninghoff, J.C., Eds.; ASTM International: West Conshohocken, PA, USA, 2001; Volume 20, pp. 80–94. [Google Scholar]
- Pathan, A.; Bond, J.; Gaskin, R. Sample preparation for SEM of plant surfaces. Mater. Today 2010, 12, 32–43. [Google Scholar] [CrossRef]
- Geyer, U.; Schönherr, J. In Vitro Test for Effects of Surfactants and Formulations on Permeability of Plant Cuticles. In Pesticides Formulation. Innovations and Developments; Comstock, J., Ed.; American Chemical Society: Washington, DC, USA, 1988; pp. 22–33. [Google Scholar] [CrossRef]
- Zhang, S.; Ji, M.; Gu, Z.; Yang, Q. The hydrolytic and photolytic properties of 2,4-D isooctyl ester. Chin. J. Pestic. Sci. 2019, 21, 125–130. [Google Scholar] [CrossRef]
- Coleman, R.; Penner, D. Organic Acid Enhancement of Pelargonic Acid. Weed Technol. 2008, 22, 38–41. [Google Scholar] [CrossRef]
- Webber, C.; Shrefler, J.W.; Taylor, M.J. Adjuvants Affect Duckweed (Lemna minor) Control with Pelargonic Acid. J. Agric. Sci. 2014, 12, 1–8. [Google Scholar] [CrossRef]
- Campos, J.; (Global Innovation & Technology, Clariant, Frankfurt am Main, Germany). Unpublished Work. 2019.
- Yu, Y.; Zhu, H.; Frantz, J.M.; Redubf, M.E.; Chan, K.C.; Ozkan, H.E. Evaporation and coverage area of pesticide droplets on hairy and waxy leaves. Biosyst. Eng. 2009, 104, 324–334. [Google Scholar] [CrossRef]
- Baur, P.; Grayson, B.T.; Schönherr, J. Polydisperse ethoxylated fatty alcohol surfactants as accelerators of cuticular penetration. 1. Effects of ethoxy chain length and the size of the penetrants. Pestic. Sci. 1997, 51, 131–152. [Google Scholar] [CrossRef]
- Baur, P.; Schönherr, J.; Grayson, B.T. Polydisperse ethoxylated fatty alcohol surfactants as accelerators of cuticular penetration. 2: Separation of effects on driving force and mobility and reversibility of surfactant action. Pestic. Sci. 1999, 55, 831–842. [Google Scholar] [CrossRef]
- Baur, P. Surfactant effects on Cuticular penetration of neutral polar compounds: Dependence on humidity and temperature. J. Agric. Food Chem. 1999, 47, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Baur, P. Mechanistic aspects of foliar penetration of agrochemicals and the effect of adjuvants. Recent Res. Develop. Agric. Food Chem. 1998, 2, 809–837. [Google Scholar]
- Baur, P.; Buchholz, A.; Schönherr, J. Diffusion in plant cuticles as affected by temperature and size of organic solutes: Similarity and diversity among species. Plant Cell Environ. 1997, 20, 982–994. [Google Scholar] [CrossRef]
- American Society of Agricultural and Biological Engineers (ASABE). Spray Nozzle Classification by Droplet Spectra, ANSI/ASAE S572.2. Available online: https://www.asabe.org/ (accessed on 10 October 2021).
- Smith, D.; Askew, S.; Morris, W.; Shaw, D.; Boyette, M. Droplet size and leaf morphology effects on pesticide spray deposition. Trans. ASAE 2000, 43, 255–259. [Google Scholar] [CrossRef]
- Janardhan, K.V.; Janakiraman, N.; Nataraju, S.P.; Setty, M.V.N. Decanol—An effective suckericide for flue-cured tobacco production in Karnataka. Tob. Res. 1990, 16, 57–60. [Google Scholar]
- Grains Research & Development Corporation. Adjuvants—Oils, Surfactants and Other Additives for Farm Chemicals—Revised 2012 Edition. Available online: https://grdc.com.au/ (accessed on 20 June 2021).
- Lewis, K.A.; Tzilivakis, J.; Warner, D.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- Baur, P.; Schönherr, J. Penetration of an ethoxylated fattyalcohol surfactant across lead cuticles as affected by concentration, additives, and humidity. J. Plant Dis. Prot. 1997, 104, 380–393. [Google Scholar]
- Dubovik, V.; Dalinova, A.; Berestetskiy, A. Effect of Adjuvants on Herbicidal Activity and Selectivity of Three Phytotoxins Produced by the Fungus, Stagonospora cirsii. Plants 2020, 9, 1621. [Google Scholar] [CrossRef] [PubMed]
- Wernecke, A.; Eckert, J.H.; Forster, R.; Kurlemann, N.; Odemer, R. Inert agricultural spray adjuvants may increase the adverse effects of selected insecticides on honeybees (Apis mellifera L.) under laboratory conditions. J. Plant Dis. Prot. 2021. [Google Scholar] [CrossRef]
- Henriques, J.; Lima, M.; Rosa, S.; Días, A.; Días, L. Allelopathic Plants. XVIII. Solanum nigrum L. Allelopath. J. 2006, 17, 1–16. [Google Scholar]
- Baur, P.; Pontzen, R. Basic features of plant surface wettability and deposit formation and the impact of adjuvants. In Proceedings of the 8th International Symposium on Adjuvants for Agrochemicals (ISAA); International Society for Agrochecmicals Adjuvants: Columbus, OH, USA, 2007. [Google Scholar]
- Schreiber, L.; Schönherr, J. Water and Solute Permeability of Plant Cuticles: Measurement and Data Analysis; Springer: Berlin/Heidelberg, Germany, 2009; p. 299. [Google Scholar]
- Cook, R.; Lupette, J.; Benning, C. The Role of Chloroplast Membrane Lipid Metabolism in Plant Environmental Responses. Cells. 2021, 10, 706. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.; Cejudo, F.J. Chloroplast Lipids Metabolism and Function. A Redox Perspective. Front. Plant Sci. 2021, 12, 1636. [Google Scholar] [CrossRef] [PubMed]
- Briggs, G.; Bromilow, R. Influence of Physicochemical Properties on Uptake and Loss of Pesticides and Adjuvants from the Leaf Surface. In Interactions between Adjuvants, Agrochemicals and Target Organisms; Holloway, P.J., Rees, R.T., Stock, D., Eds.; Springer: Berlin/Heidelberg, Germany, 1994; Volume 12, pp. 1–26. [Google Scholar] [CrossRef]
- Schenk, J.; Espino, S.; Romo, D.; Nima, N.; Do, A.; Michaud, J.; Papahadjopoulos-Sternberg, B.; Yang, J.; Zuo, Y.; Steppe, K.; et al. Xylem Surfactants Introduce a New Element to the Cohesion-Tension Theory. Plant Physiol. 2017, 173, 1177–1196. [Google Scholar] [CrossRef] [PubMed]
- Sperry, J.; Tyree, M. Mechanism of Water Stress-Induced Xylem Embolism. Plant Physiol. 1988, 88, 581–587. [Google Scholar] [CrossRef] [PubMed]
| Test Compounds | Description | Use Rate (% v/v) 1 | pH Spray Mixture 2 |
|---|---|---|---|
| Phosphoric acid | Solution–85 wt. % in H2O. | 0.60 | 1.9 |
| D-glucose | 97.5% purity. | 1.00 | 5.8 |
| Potassium carbonate | 99.0% purity. | 1.00 | 10.3 |
| 1-decanol | 99.0% purity. | 1.00 | 6.1 |
| Synergen® TS 7 3 | Blend of docusate sodium and ethoxylated fatty alcohol (sum 100%). | 0.15 | 5.8 |
| Polyglykol 400 3 | Polyethylene glycol (PEG) with a molar weight of 400. | 1.50 | 5.9 |
| Genapol® C 050 3 | Coconut fatty alcohol polyglycol ether with 5 EO. | 1.00 | 5.8 |
| HastenTM4 | Emulsifiable concentrate of esterified vegetable oil and non-ionic surfactants. | 2.50 | 6.1 |
| Rate | Description |
|---|---|
| 1 | No damage |
| 2 | Slight symptoms (discoloration of tissue) |
| 3 | Slight necrotic spots |
| 4 | Strong symptoms (Complete necrosis) |
| Test Compound | Concentration (%) 1 | Weed Control (%) | |
|---|---|---|---|
| D. sanguinalis | S. nigrum | ||
| None | 29 d * | 50 bc * | |
| 1-Decanol | 1.00 | 43 a | 74 a |
| Phosphoric acid | 0.63 | 33 cd | 59 b |
| D-Glucose | 1.00 | 29 d | 47 c |
| Potassium Carbonate | 1.00 | 30 cd | 48 c |
| Genapol C 050 | 1.00 | 31 cd | 52 bc |
| Polyglycol 400 | 1.50 | 31 cd | 53 bc |
| Synergen TS 7 | 0.15 | 36 bc | 58 b |
| Hasten | 2.50 | 39 ab | 68 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, J.; Bodelon, L.; Verdeguer, M.; Baur, P. Mechanistic Aspects and Effects of Selected Tank-Mix Partners on Herbicidal Activity of a Novel Fatty Acid Ester. Plants 2022, 11, 279. https://doi.org/10.3390/plants11030279
Campos J, Bodelon L, Verdeguer M, Baur P. Mechanistic Aspects and Effects of Selected Tank-Mix Partners on Herbicidal Activity of a Novel Fatty Acid Ester. Plants. 2022; 11(3):279. https://doi.org/10.3390/plants11030279
Chicago/Turabian StyleCampos, Javier, Luciana Bodelon, Mercedes Verdeguer, and Peter Baur. 2022. "Mechanistic Aspects and Effects of Selected Tank-Mix Partners on Herbicidal Activity of a Novel Fatty Acid Ester" Plants 11, no. 3: 279. https://doi.org/10.3390/plants11030279
APA StyleCampos, J., Bodelon, L., Verdeguer, M., & Baur, P. (2022). Mechanistic Aspects and Effects of Selected Tank-Mix Partners on Herbicidal Activity of a Novel Fatty Acid Ester. Plants, 11(3), 279. https://doi.org/10.3390/plants11030279