Possibilities of Using Seed Meals in Control of Herbicide-Susceptible and -Resistant Biotypes of Rye Brome (Bromus secalinus L.) in Winter Wheat
Abstract
1. Introduction
2. Results
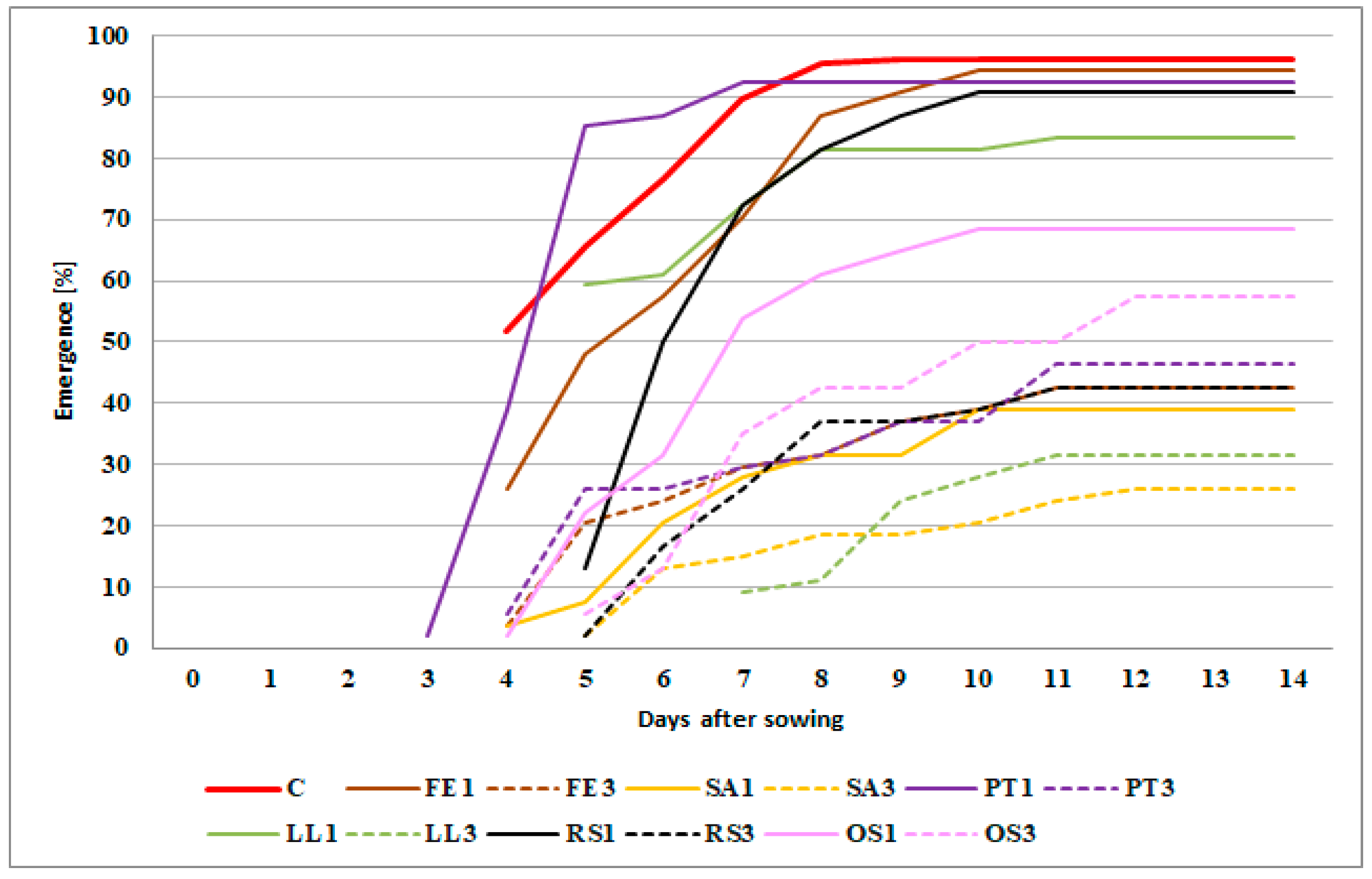
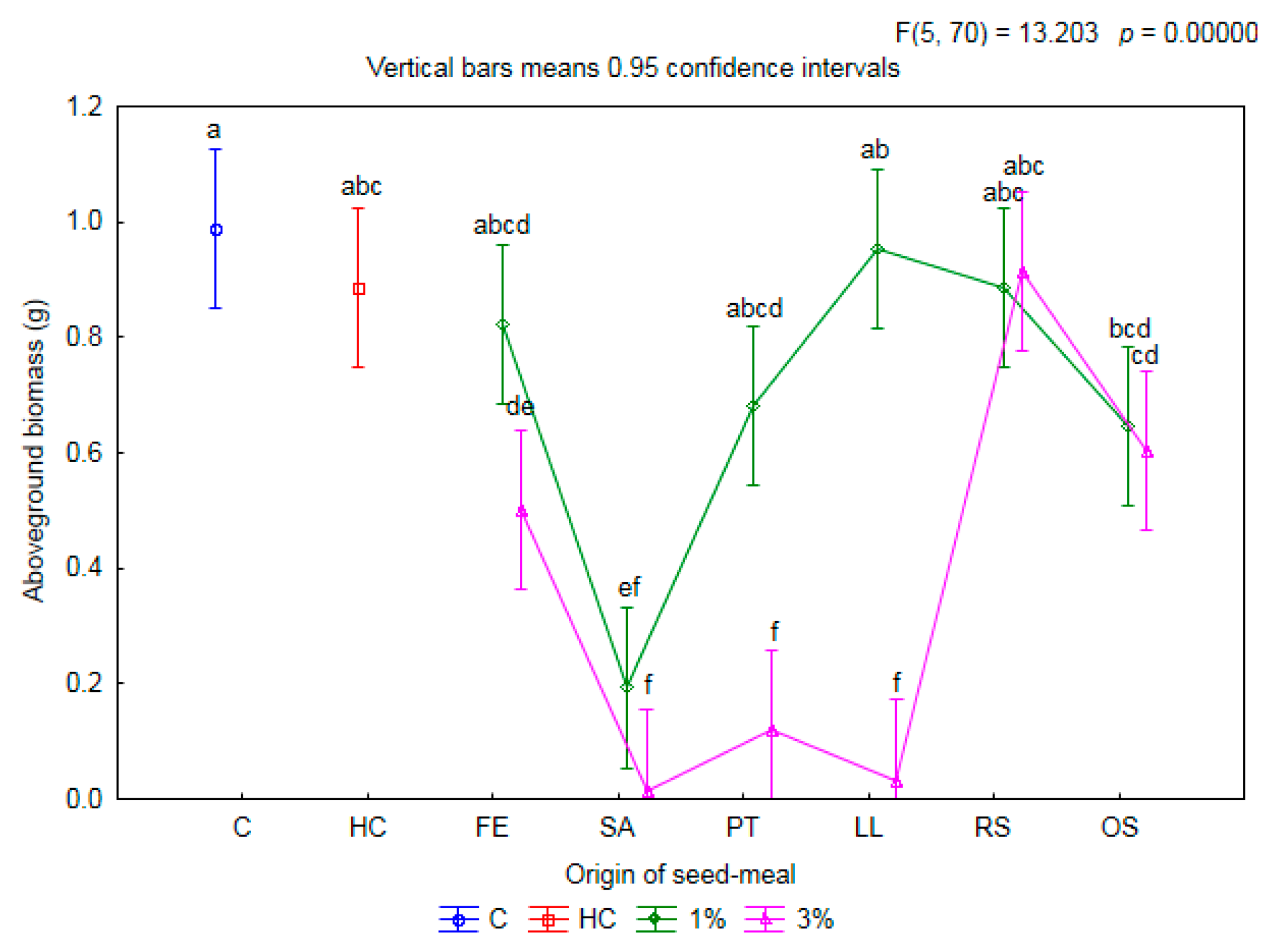
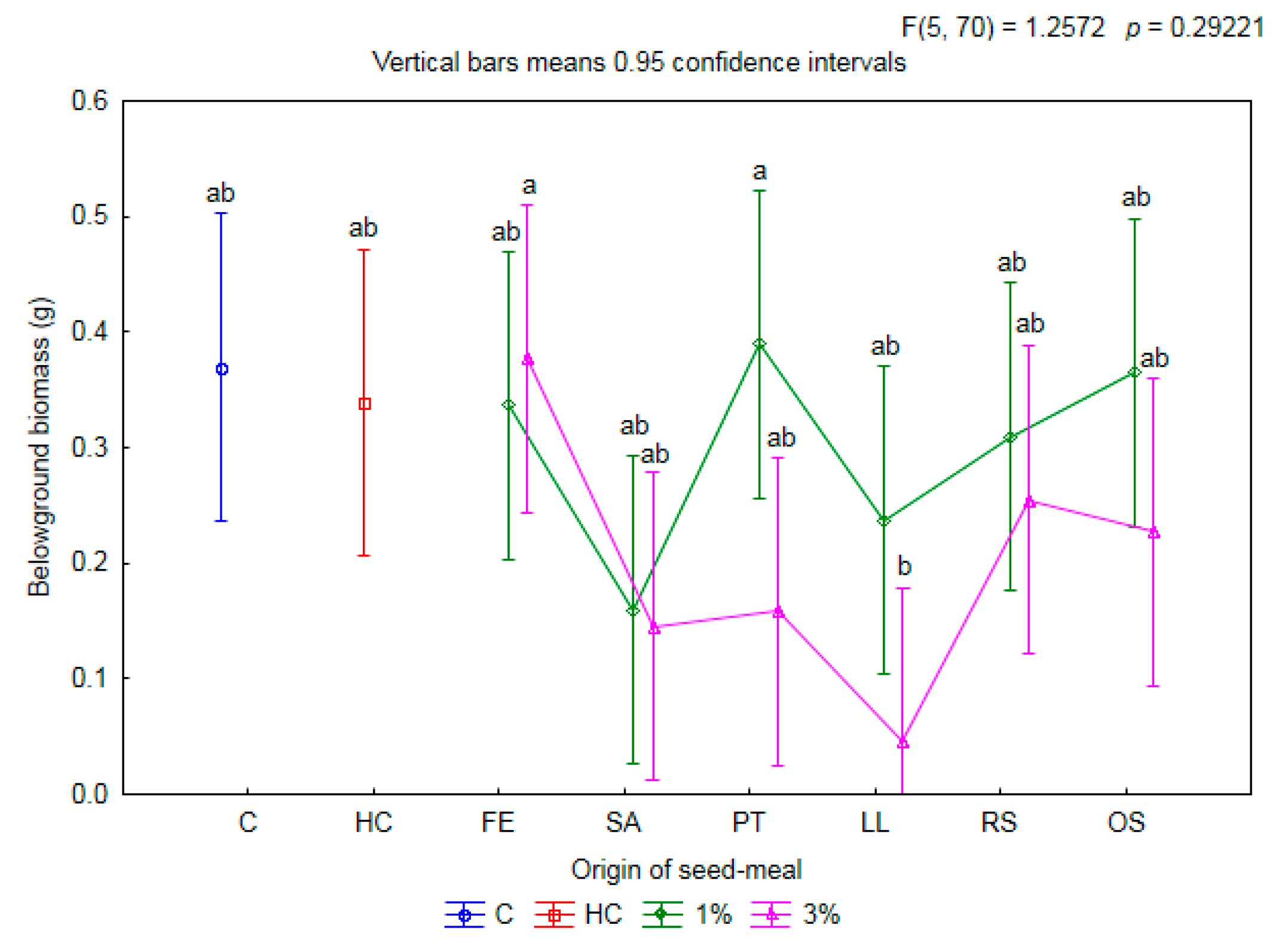
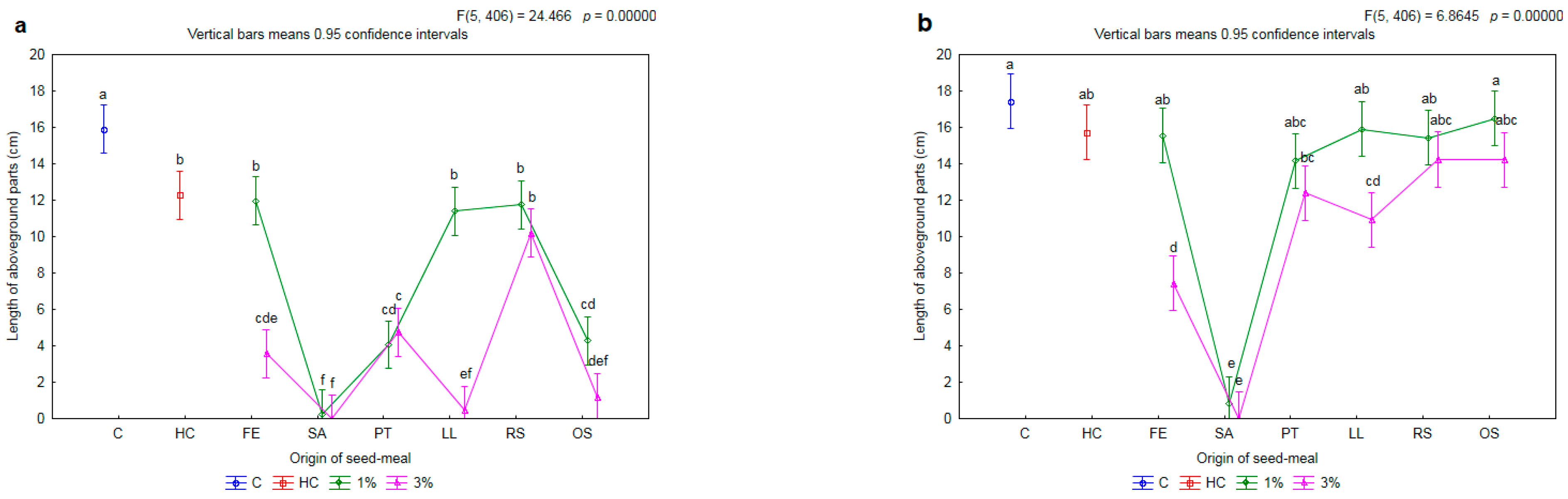
2.1. Influence of Seed Meals on Winter Wheat
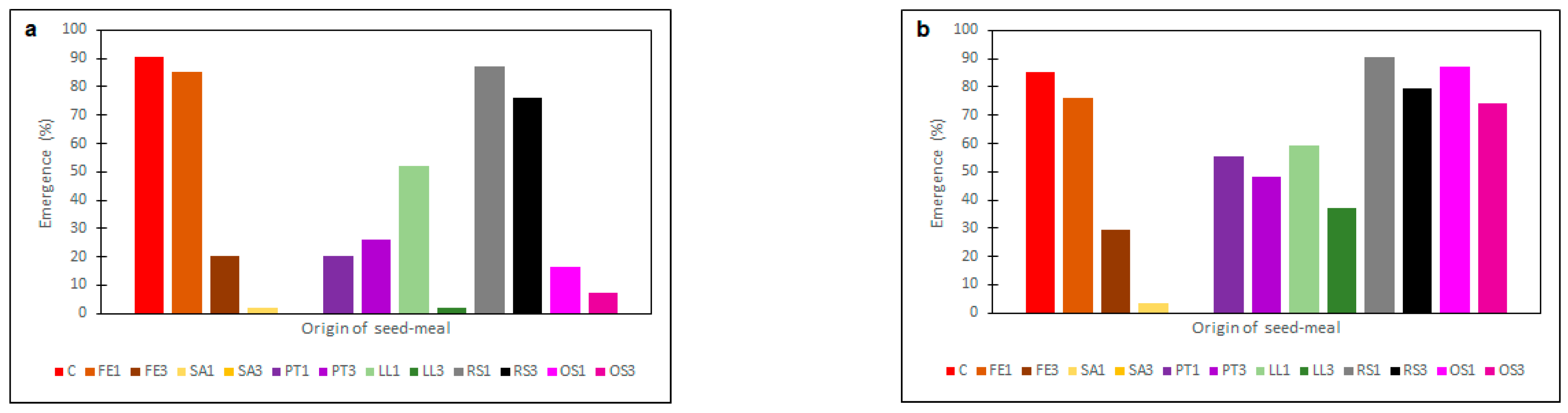
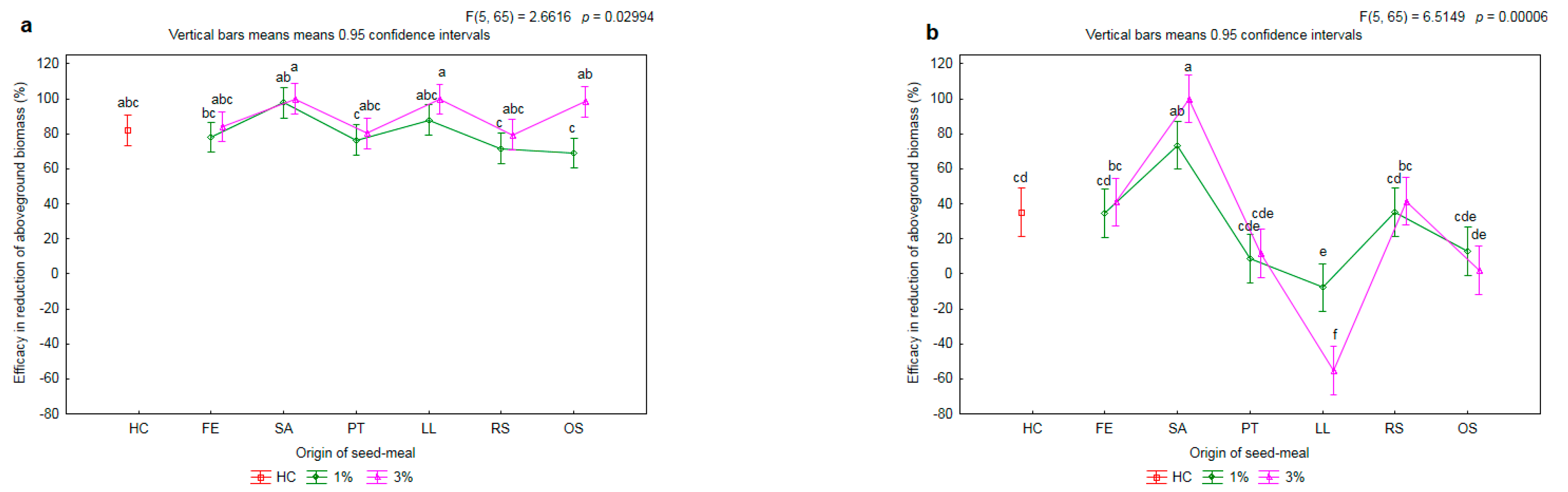
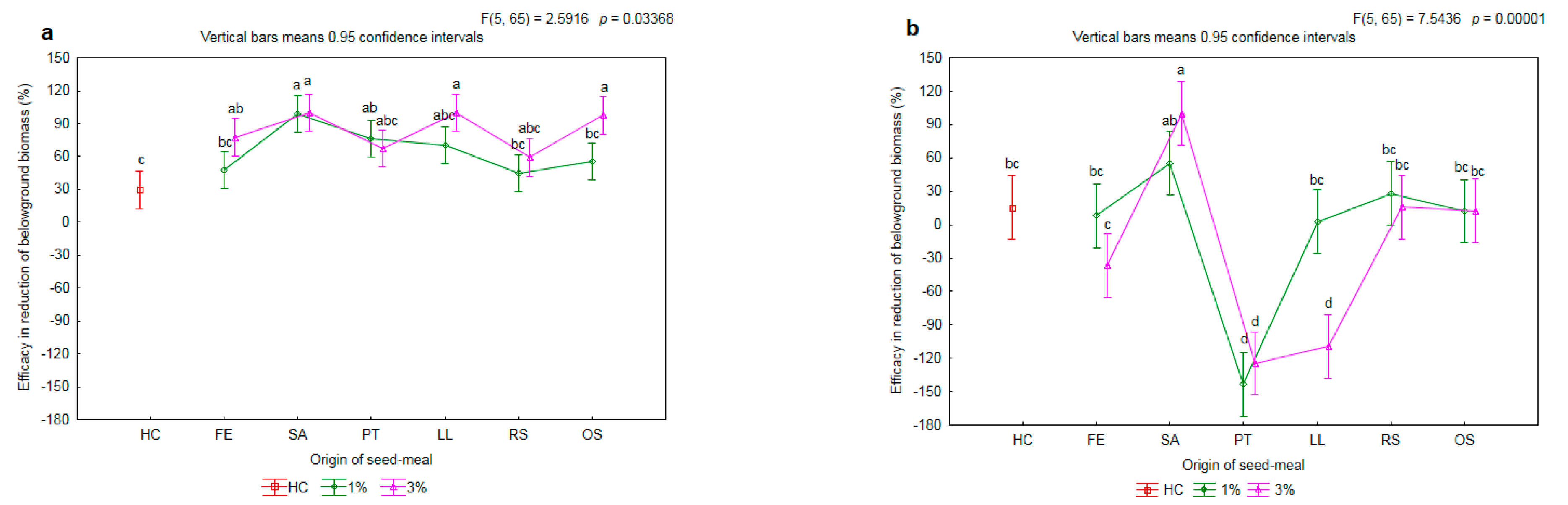
2.2. Effectiveness of Seed Meals in Reduction of Rye Brome Growth
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Seed Meals and Their Preparation
4.3. Herbicide Characteristics
4.4. Soil Characteristics
4.5. Set-up and Management of Pot Experiments
4.6. Measurement Range
4.6.1. Winter Wheat
4.6.2. Rye Brome
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spalton, L.M. An analysis of the characters of Bromus racemosus L., B. commutatus Schrad. and B.secalinus L. (Poaceae). Watsonia 2002, 24, 193–202. [Google Scholar]
- Zech-Matterne, V.; Derreumaux, M.; Pradat, B.; Luccioni, P.; Ruas, M.-P.; Toulemonde, F. Should Bromus secalinus (rye brome) be considered a crop? Analysis of Bromus rich assemblages from protohistoric and historic sites in northern France and textual references. Veget. Hist. Archaeobot. 2021, 30, 773–787. [Google Scholar] [CrossRef]
- Kapeluszny, J.; Haliniarz, M. Selected elements of germination biology of flixweed (Descurainia sophia Webb. ex Prantl.) and rye brome (Bromus secalinus L.). Ann. Univ. Mariae Curie-Skłodowska. Sect. E Agric. 2007, 62, 226–233. (In Polish) [Google Scholar]
- World Checklist of Selected Plant Families. Available online: http://wcsp.science.kew.org/ (accessed on 13 October 2021).
- Jun, M.-J.; Liao, G.-I.; Kuo, C.-S. Notes on alien Bromus grasses in Taiwan. Taiwania 2006, 51, 131–138. [Google Scholar]
- Dastgheib, F.; Rolston, M.P.; Archie, W.J. Chemical control of Brome grasses (Bromus spp.) in cereals. N. Z. Plant Prot. 2003, 56, 227–232. [Google Scholar] [CrossRef]
- Davies, L.R.; Onkokesung, N.; Brazier-Hicks, M.; Edwards, R.; Moss, S. Detection and characterization of resistance to acetolactate synthase inhibiting herbicides in Anisantha and Bromus species in the United Kingdom. Pest Manag. Sci. 2020, 76, 2473–2482. [Google Scholar] [CrossRef]
- Koch, M.A.; Meyer, N.; Engelhardt, M.; Thiv, M.; Bernhard, K.-G.; Michling, F. Morphological and genetic variation of highly endangered Bromus species and the status of these Neolithic weeds in Central Europe. Plant Syst. Evol. 2016, 302, 515–525. [Google Scholar] [CrossRef]
- Georgescu, M.I.; Ionescu, N.; Penescu, A.; Vrabie, F.C.; Săvulescu, E.; Luchian, V.; Popa, V.I. Some observations on the morphology and germination of Bromus secalinus L. caryopsis. Sci. Papers Ser. A Agron. 2019, 52, 513–518. [Google Scholar]
- Rzymowska, Z.; Skrzyczyńska, J.; Affek-Straczewska, A. Occurrence and some morphological features of Bromus secalinus L. in agrocenoses of the Podlaski Przełom Bugu Mesoregion. Fragm. Agron. 2010, 27, 102–110. (In Polish) [Google Scholar]
- Kącki, Z.; Szczęśniak, E.; Czarniecka, M. Bromus secalinus (Poaceae) in Lower Silesia–occurrence and threats. Acta Bot. Sil. Suppl. 2011, 1, 66–68. (In Polish) [Google Scholar]
- Skrajna, T.; Kubicka, H.; Rzymowska, Z. Phenotypic variation in relation to seed storage protein polymorphism in Bromus secalinus L. (Gramineae) populations from North-Eastern Poland. Pol. J. Ecol. 2012, 60, 41–65. [Google Scholar]
- Stokłosa, A.; Puła, J. Selected morphological traits of cheat (Bromus secalinus L.) and cereals grown in mixtures. Mod. Phytomorphol. 2012, 2, 17–18. [Google Scholar]
- Kubicka, H.; Gozdowski, D.; Skrajna, T. Multivariate evaluation of variability of Bromus genotypes from north-eastern Poland. Appl. Ecol. Environ. Res. 2014, 13, 809–818. [Google Scholar] [CrossRef]
- Adamczewski, K.; Kaczmarek, S.; Kierzek, R.; Urban, M. Germination biology and weed thresholds of rye brome (Bromus secalinus L.) in wheat (Trtiticum aestivum L.). Pak. J. Agri. Sci. 2015, 52, 989–995. [Google Scholar]
- Stone, A.E.; Peeper, T.F.; Solie, J.B. Cheat (Bromus secalinus) control with herbicides applied to mature seeds. Weed Technol. 2001, 15, 382–386. [Google Scholar] [CrossRef]
- Stone, J.C.; Peeper, T.F.; Stone, A.E. Rotational cropping systems to reduce cheat (Bromus secalinus) densities. Weed Technol. 2006, 20, 445–452. [Google Scholar] [CrossRef]
- Nourozi, M.; Sheidai, M.; Noori, A.; Assadi, M. Bromus secalinus L. (Poaceae), a new record for flora of Iran. Iran. J. Bot. 2005, 11, 71–73. [Google Scholar]
- Malara, J.; Gancarczyk-Gola, M. Characteristic of species Bromus secalinus L. Pam. Puł. 2009, 150, 337–343. (In Polish) [Google Scholar]
- Korniak, T.; Dynowski, P. Bromus secalinus (Poaceae)—A vanishing or a widespread weed species of cereal crops in North-Eastern Poland? Fragm. Florist. Geobot. Pol. 2011, 18, 341–348. (In Polish) [Google Scholar]
- Mirek, Z.; Zarzycki, K.; Wojewoda, W.; Szeląg, Z. Red List of Plants and Fungi in Poland; Inst. Bot. PAN: Kraków, Poland, 2006; p. 99. (In Polish) [Google Scholar]
- Chmara, R.; Rekowska, E. New locality of Bromus secalinus (Poaceae) in central Poland. Fragm. Florist. Geobot. Polon. 2020, 27, 731–733. (In Polish) [Google Scholar] [CrossRef]
- Korbas, M.; Strażyński, P.; Horoszkiewicz-Janka, J.; Jajor, E.; Miklaszewska, K.; Danielewicz, J.; Kalinowska, A. Recommendations for the Protection of Plants for 2020/21. Winter Cereals; IOR-PIB: Poznań, Poland, 2020; p. 547. (In Polish) [Google Scholar]
- Moss, S.; Ulber, L.; den Hoed, I. A herbicide resistance risk matrix. Crop Prot. 2019, 115, 13–19. [Google Scholar] [CrossRef]
- Heap, I. The International Herbicide-Resistant Weed Database. Available online: https://www.weedscience.org (accessed on 5 October 2021).
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 5 October 2021).
- Herbicide Resistance Action Committee. Available online: https://hracglobal.com (accessed on 19 October 2021).
- Das, T.K.; Tuti, M.D.; Sharma, R.; Paul, T.; Mirja, P.R. Weed management research in India: An overview. Indian J. Agron. 2012, 57, 148–156. [Google Scholar]
- Shahzad, M.; Hussain, M.; Jabran, K.; Farooq, M.; Farooq, S.; Gašparovič, K.; Barboricova, M.; Aljuaid, B.S.; El-Shehawi, A.M.; Zuan, A.T.K. The impact of different crop rotations by weed management strategies’ interactions on weed infestation and productivity of wheat (Triticum aestivum L.). Agronomy 2021, 11, 2088. [Google Scholar] [CrossRef]
- Głąb, L.; Sowiński, J.; Bough, R.; Dayan, F.E. Allelopathic potential of sorghum (Sorghum bicolor (L.) Moench) in weed control: A comprehensive review. Adv. Agron. 2017, 145, 43–95. [Google Scholar] [CrossRef]
- Sturm, D.J.; Kunz, C.; Gerhards, R. Inhibitory effects of cover crop mulch on germination and growth of Stellaria media (L.) Vill., Chenopodium album L. and Matricaria chamomilla L. Crop Prot. 2016, 90, 125–131. [Google Scholar] [CrossRef]
- Pużyńska, K.; Jop, B.; Gala-Czekaj, D.; Synowiec, A.; Bocianowski, J. Effect of allelopathic seed meals on the weed infestation and yielding of maize. Acta Physiol. Plant. 2019, 41, 193. [Google Scholar] [CrossRef]
- Rice, A.R.; Johnson-Maynard, J.L.; Thill, D.C.; Morra, M.J. Vegetable crop emergence and weed control following amendment with different Brassicaceae seed meals. Renew. Agric. Food Syst. 2007, 22, 204–212. [Google Scholar] [CrossRef]
- Webber, C.L., III; White, P.M., Jr.; Boydston, R.; Shrefer, J. Impact of mustard seed meal applications on direct-seeded cucurbits and weed control. J. Agric. Sci. 2017, 9, 81–90. [Google Scholar] [CrossRef][Green Version]
- Mehmood, Z.; Ashiq, M.; Noorka, I.R.; Ali, A.; Tabasum, S.; Iqbal, M.S. Chemical control of monocot weeds in wheat (Triticum aestivum L.). Am. J. Plant Sci. 2014, 5, 1272–1276. [Google Scholar] [CrossRef][Green Version]
- Synowiec, A.; Jop, B.; Domaradzki, K.; Podsiadło, C.; Gawęda, D.; Wacławowicz, R.; Wenda-Piesik, A.; Nowakowski, M.M.; Bocianowski, J.; Marcinkowska, K.; et al. Environmental factors effects on winter wheat competition with herbicide-resistant or susceptible silky bentgrass (Apera spica-venti L.) in Poland. Agronomy 2021, 11, 871. [Google Scholar] [CrossRef]
- Kelley, P.; Peeper, T.F. MON 37500 application timing affects cheat (Bromus secalinus) control and winter wheat. Weed Sci. 2003, 51, 231–236. [Google Scholar] [CrossRef]
- Vázquez-García, J.G.; Castro, P.; Cruz-Hipólito, H.E.; Millan, T.; Palma-Bautista, C.; De Prado, R. Glyphosate resistance confirmation and field management of red brome (Bromus rubens L.) in perennial crops grown in southern Spain. Agronomy 2021, 11, 535. [Google Scholar] [CrossRef]
- Davies, L.R.; Hull, R.; Moss, S.; Neve, P. The first cases of evolving glyphosate resistance in UK poverty brome (Bromus sterilis) populations. Weed Sci. 2019, 67, 41–47. [Google Scholar] [CrossRef]
- Adamczewski, K.; Matysiak, K.; Kierzek, R.; Kaczmarek, S. Significant increase of weed resistance to herbicides in Poland. J. Plant. Prot. Res. 2019, 59, 139–150. [Google Scholar] [CrossRef]
- Stankiewicz-Kosyl, M.; Haliniarz, M.; Wrochna, M.; Synowiec, A.; Wenda-Piesik, A.; Tendziagolska, E.; Sobolewska, M.; Domaradzki, K.; Skrzypczak, G.; Łykowski, W.; et al. Herbicide resistance of Centaurea cyanus L. in Poland in the context of its management. Agronomy 2021, 11, 1954. [Google Scholar] [CrossRef]
- Stankiewicz-Kosyl, M.; Synowiec, A.; Haliniarz, M.; Wenda-Piesik, A.; Domaradzki, K.; Parylak, D.; Wrochna, M.; Pytlarz, E.; Gala-Czekaj, D.; Marczewska-Kolasa, K.; et al. Herbicide resistance and management options of Papaver rhoeas L. and Centaurea cyanus L. in Europe: A review. Agronomy 2020, 10, 874. [Google Scholar] [CrossRef]
- Boutin, C.; Strandberg, B.; Carpenter, D.; Mathiassen, S.K.; Thomas, P.J. Herbicide impact on non-target plant reproduction: What are the toxicological and ecological implications? Environ. Pollut. 2014, 185, 295–306. [Google Scholar] [CrossRef]
- Mejri, D.; Gamalero, E.; Tombolini, R.; Massa, N.; Berta, G.; Souissi, T. Biological control of great brome (Bromus diandrus) in durum wheat (Triticum durum): Specificity, physiological traits and impact on plant growth and root architecture of the fluorescent pseudomonad strain X33d. BioControl 2010, 55, 561–572. [Google Scholar] [CrossRef]
- Kennedy, A.C.; Johnson, B.N.; Stubbs, T.L. Host range of a deleterious rhizobacterium for biological control of downy brome. Weed Sci. 2001, 49, 792–797. [Google Scholar] [CrossRef]
- Reinhart, K.O.; Carlson, C.H.; Feris, K.P.; Germino, M.J.; Jandreau, C.J.; Lazarus, B.E.; Mangold, J.; Pellatz, D.W.; Ramsey, P.; Rinella, M.J.; et al. Weeds suppressive bacteria fail to control Bromus tectorum under field conditions. Rangel. Ecol. Manag. 2020, 73, 760–765. [Google Scholar] [CrossRef]
- Synowiec, A.; Kalemba, D. Composition and herbicidal effect of Heracleum sosnowskyi essential oil. Open Life Sci. 2015, 10, 425–432. [Google Scholar] [CrossRef]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop. Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Duke, S.O.; Dayan, F.E.; Rimando, A.M.; Schrader, K.K.; Aliotta, G.; Oliva, A. Chemicals from nature for weed management. Weed Sci. 2002, 50, 138–151. [Google Scholar] [CrossRef]
- Gala-Czekaj, D.; Jop, B.; Synowiec, A. The influence of meal from seeds and perianth of Sosnovskyi hogweed (Heracleum Sosnowskyi Manden.) on initial growth of maize and two weeds species. Fragm. Agron. 2018, 35, 29–39. (In Polish) [Google Scholar] [CrossRef]
- Boydston, R.A.; Anderson, T. Mustard (Sinapis alba) seed meal suppresses weeds in container-grown ornamentals. Hortic. Sci. 2008, 43, 800–803. [Google Scholar] [CrossRef]
- Dhima, K.V.; Vasilakoglou, I.; Gatsis, T.D.; Panou-Philotheou, E.; Eleftherohorinos, I. Effects of aromatic plants incorporated as green manure on weed and maize development. Field Crops Res. 2009, 110, 235–241. [Google Scholar] [CrossRef]
- Saini, R.; Singh, S. Use of natural products for weed management in high-value Crops: An overview. Preprints 2018, 2018100737. [Google Scholar] [CrossRef]
- Schappert, A.; Schumacher, M.; Gerhards, R. Weed control ability of single sown cover crops compared to species mixtures. Agronomy 2019, 9, 294. [Google Scholar] [CrossRef]
- Tursun, N.; Işık, D.; Demir, Z.; Jabran, K. Use of living, mowed, and soil-incorporated cover crops for weed control in apricot orchards. Agronomy 2018, 8, 150. [Google Scholar] [CrossRef]
- Dhima, K.; Vasilakoglou, I.; Garane, V.; Ritzoulis, C.; Lianopoulou, V.; Panou-Philotheou, E. Competitiveness and essential oil phytotoxicity of seven annual aromatic plants. Weed Sci. 2010, 58, 457–465. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Masi, M.; Cimmino, A.; Vilariño, S.; Evidente, A. Allelopathic effect of quercetin, a flavonoid from Fagopyrum esculentum roots in the radicle growth of phelipanche ramosa: Quercetin natural and semisynthetic analogues were used for a structure-activity relationship investigation. Plants 2021, 10, 543. [Google Scholar] [CrossRef] [PubMed]
- Szwed, M.; Wiczkowski, W.; Szawara-Nowak, D.; Obendorf, R.L.; Horbowicz, M. Allelopathic influence of common buckwheat root residues on selected weed species. Acta Physiol. Plant. 2019, 41, 92. [Google Scholar] [CrossRef]
- Kalinova, J.; Vrchotova, N.; Triska, J. Exudation of allelopathic substances in buckwheat (Fagopyrum esculentum Moench). J. Agric. Food Chem. 2007, 55, 6453–6459. [Google Scholar] [CrossRef] [PubMed]
- Szwed, M.; Mitrus, J.; Wiczkowski, W.; Dębski, H.; Horbowicz, M. If phenolic compounds in the soil with buckwheat residues affect the emergence and growth of weed seedlings? Acta Physiol. Plant. 2020, 42, 154. [Google Scholar] [CrossRef]
- Mioduszewska, H.; Klocek, J.; Horbowicz, M.; Wolska, K. Effect of water extracts from tissues of common buckwheat on seed germination and seedling growth of winter wheat and lettuce. Acta Sci. Pol. Agric. 2013, 12, 45–54. [Google Scholar]


| Biotype | ED50 (g ha−1) | Site (Coordinate) |
|---|---|---|
| S | 16.26 | Wrocław (51.132663 N 17.117230 E) |
| R | 48.86 | Wielowieś (51.339435 N 16.373906 E) |
| Name | Cultivar | Abbreviation | |
|---|---|---|---|
| English | Latin | ||
| Common buckwheat | Fagopyrum esculentum Moench. | Panda | FE |
| White mustard | Sinapis alba L. | Bardena | SA |
| Lacy phacelia | Phacelia tanacetifolia Benth. | Anabela | PT |
| Yellow lupin | Lupinus luteus L. | Mister | LL |
| Fodder radish | Raphanus sativus L. var. oleiformis Pers. | Adagio | RS |
| Common birdsfoot | Ornithopus sativus Brot. | Bydgoska 1 | OS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pytlarz, E.; Gala-Czekaj, D. Possibilities of Using Seed Meals in Control of Herbicide-Susceptible and -Resistant Biotypes of Rye Brome (Bromus secalinus L.) in Winter Wheat. Plants 2022, 11, 331. https://doi.org/10.3390/plants11030331
Pytlarz E, Gala-Czekaj D. Possibilities of Using Seed Meals in Control of Herbicide-Susceptible and -Resistant Biotypes of Rye Brome (Bromus secalinus L.) in Winter Wheat. Plants. 2022; 11(3):331. https://doi.org/10.3390/plants11030331
Chicago/Turabian StylePytlarz, Elżbieta, and Dorota Gala-Czekaj. 2022. "Possibilities of Using Seed Meals in Control of Herbicide-Susceptible and -Resistant Biotypes of Rye Brome (Bromus secalinus L.) in Winter Wheat" Plants 11, no. 3: 331. https://doi.org/10.3390/plants11030331
APA StylePytlarz, E., & Gala-Czekaj, D. (2022). Possibilities of Using Seed Meals in Control of Herbicide-Susceptible and -Resistant Biotypes of Rye Brome (Bromus secalinus L.) in Winter Wheat. Plants, 11(3), 331. https://doi.org/10.3390/plants11030331






