Effect of Inoculation with Arbuscular Mycorrhizal Fungi and Fungicide Application on the Secondary Metabolism of Solanum tuberosum Leaves
Abstract
:1. Introduction
2. Results
2.1. Identification and Quantification of Phenolic Compounds
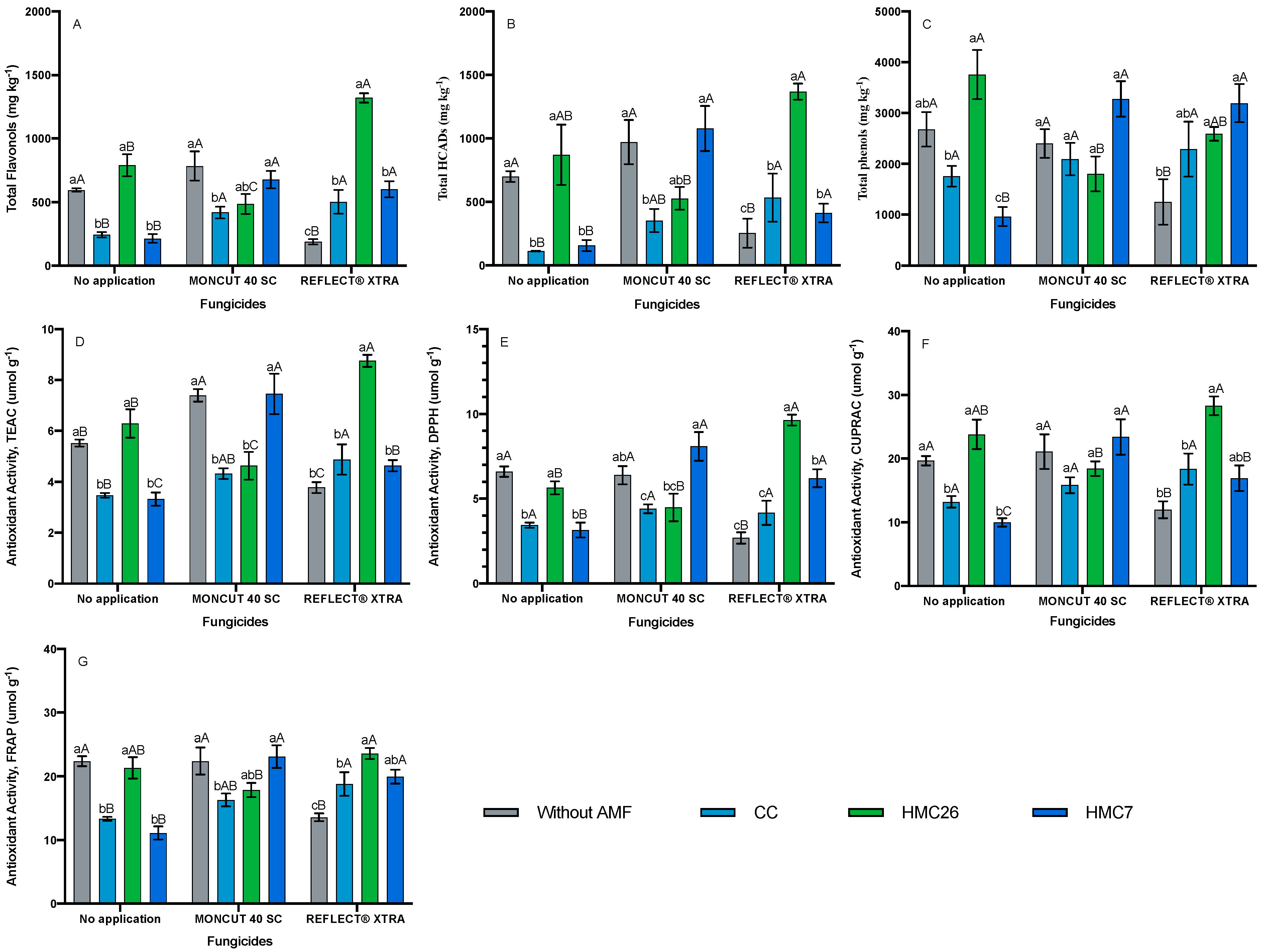
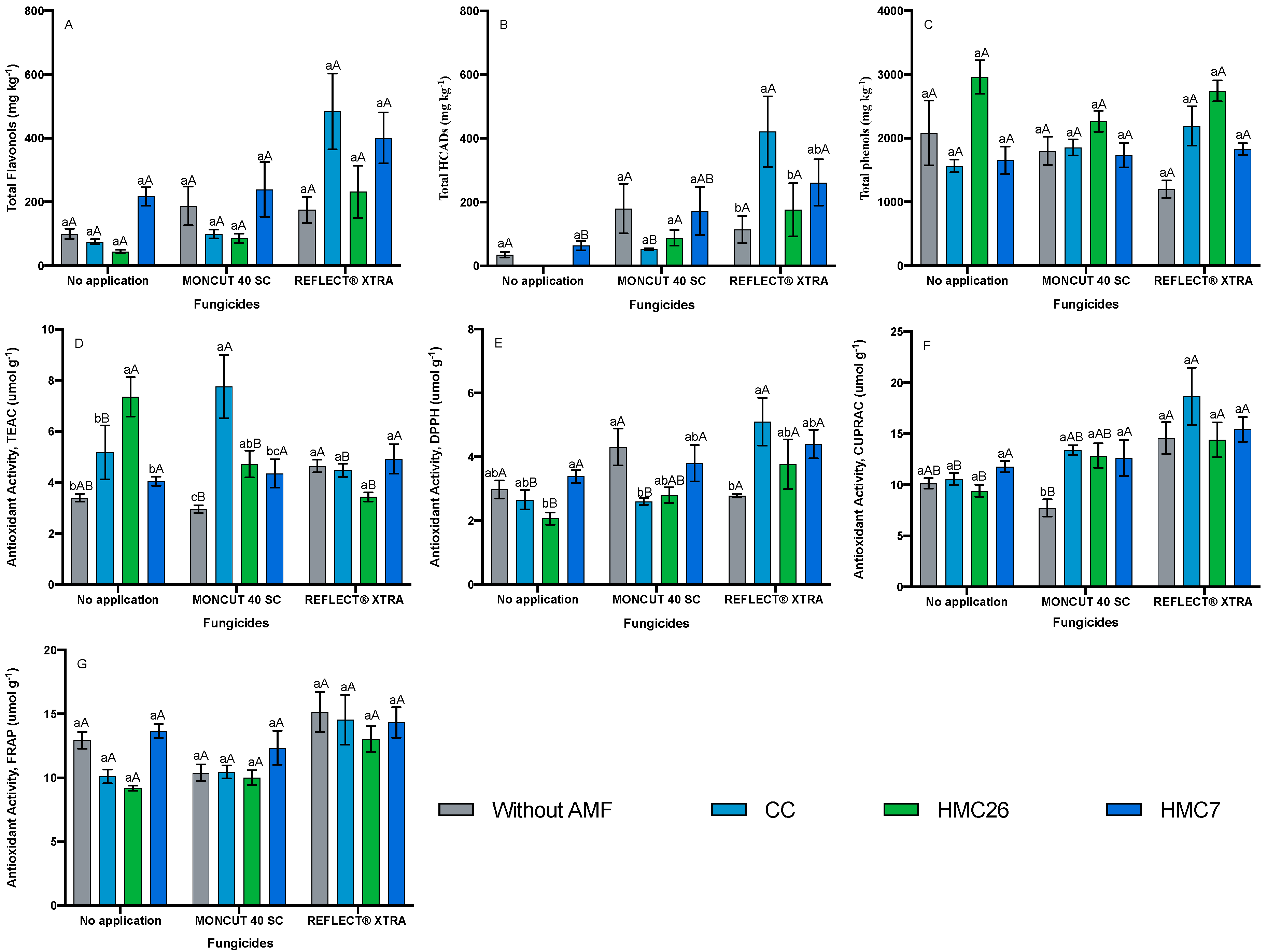
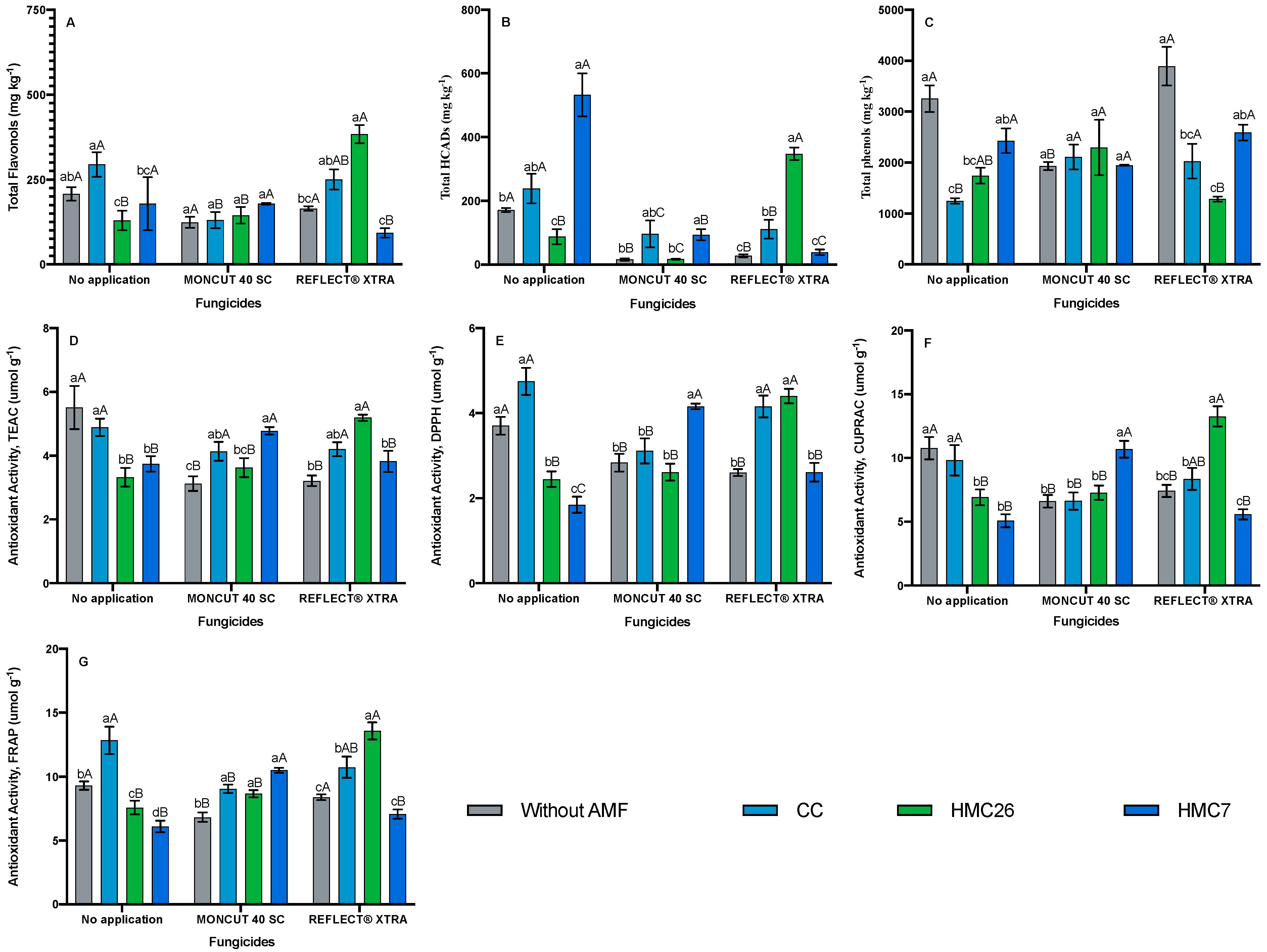
2.2. Total Phenols Concentrations
2.3. Antioxidant Activity
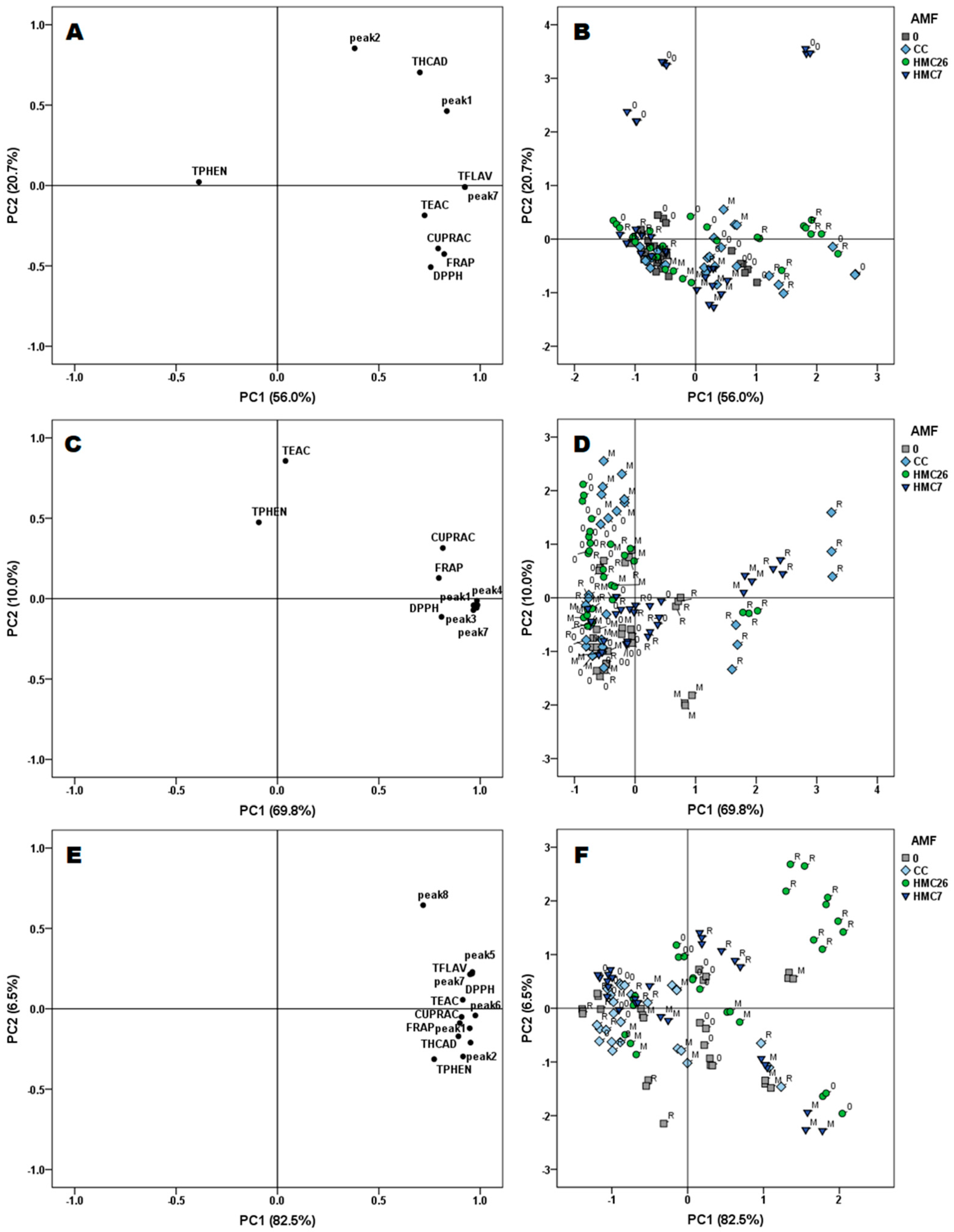
2.4. Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Samples
4.2. Identification and Quantification of Phenolic Compounds
4.3. Determination of Total Phenols Using the Folin-Ciocalteu Method
4.4. Antioxidant Activity Determinations
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Organization of the United Nations [Online]. 2019. Available online: http://www.fao.org/faostat/es/#data (accessed on 25 October 2021).
- Zarzecka, K.; Gugała, M.; Sikorska, A.; Mystkowska, I.; Baranowska, A.; Niewęgłowski, M.; Dołęga, H. The effect of herbicides and biostimulants on polyphenol content of potato (Solanum tuberosum L) tubers and leaves. J. Saudi Soc. Agric. Sci. 2019, 18, 102–106. [Google Scholar] [CrossRef]
- Koch, M.; Naumann, M.; Pawelzik, E.; Gransee, A.; Thiel, H. The importance of nutrient management for potato production Part I: Plant nutrition and yield. Potato Res. 2020, 63, 97–119. [Google Scholar] [CrossRef] [Green Version]
- Akyol, H.; Riciputi, Y.; Capanoglu, E.; Caboni, M.F.; Verardo, V. Phenolic compounds in the potato and its byproducts: An overview. Int. J. Mol. Sci. 2016, 17, 835. [Google Scholar] [CrossRef] [PubMed]
- Agu, K.C.; Okolie, P.N. Proximate composition, phytochemical analysis, and in vitro antioxidant potentials of extracts of Annona muricata (Soursop). Food Sci. Nutr. 2017, 5, 1029–1036. [Google Scholar] [CrossRef] [Green Version]
- Murniece, I.; Kruma, Z.; Skrabule, I.; Vaivode, A. Carotenoids and phenols of organically and conventionally cultivated potato varieties. Int. J. Chem. Eng. Appl. 2013, 4, 342–348. [Google Scholar] [CrossRef]
- Ruiz, A.; Aguilera, A.; Ercoli, S.; Parada, J.; Winterhalter, P.; Contreras, B.; Cornejo, P. Effect of the frying process on the composition of hydroxycinnamic acid derivatives and antioxidant activity in flesh colored potatoes. Food Chem. 2018, 268, 577–584. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazur, J.; Roy, K.; Shigdar, S.; Kanwar, J.R. Efficacy of promising flavonoids from Festuca, Lonicera, and Acacia genera against glioblastoma multiforme; potential for the Dandenong Ranges. In Advances and Avenues in the Development of Novel Carriers for Bioactives and Biological Agents; Elsevier: Amsterdam, The Netherlands, 2020; pp. 383–422. [Google Scholar] [CrossRef]
- Baur, S.; Frank, S.; Hauslader, H.; Huckelhoven, R.; Hofmann, T.; Eisenreich, W.; Dawid, C. Biosynthesis of α-solanine and α-chaconine in potato leaves (Solanum tuberosum L.)—A 13CO2 study. Food Chem. 2021, 365, 130461. [Google Scholar] [CrossRef] [PubMed]
- Nafady, N.A.; Hashem, M.; Hassan, E.A.; Ahmed, H.A.; Alamri, S.A. The combined effect of arbuscular mycorrhizae and plant-growth-promoting yeast improves sunflower defense against Macrophomina phaseolina diseases. Biol. Control. 2019, 138, 104049. [Google Scholar] [CrossRef]
- Fu, S.F.; Sun, P.F.; Lu, H.Y.; Wei, J.Y.; Xiao, H.S.; Fang, W.T.; Cheng, B.Y.; Chou, J.Y. Plant growth-promoting traits of yeasts isolated from the phyllosphere and rhizosphere of Drosera spatulata. Fungal Biol. 2016, 120, 433–448. [Google Scholar] [CrossRef]
- Shrivastava, G.; Ownley, B.H.; Auge, R.M.; Toler, H.; Dee, M.; Vu, A.; Kollner, T.G.; Chen, F. Colonization by arbuscular mycorrhizal and endophytic fungi enhanced terpene production in tomato plants and their defense against an herbivorous insect. Symbiosis 2015, 65, 65–74. [Google Scholar] [CrossRef]
- Sharma, I.P.; Sharma, A.K. Physiological and biochemical changes in tomato cultivar PT-3 with dual inoculation of mycorrhiza and PGPR against root-knot nematode. Symbiosis 2017, 71, 175–183. [Google Scholar] [CrossRef]
- Chen, S.; Jin, W.; Liu, A.; Zhang, S.; Liu, D.; Wang, F.; He, C. Arbuscular mycorrhizal fungi (AMF) increase growth and secondary metabolism in cucumber subjected to low temperature stress. Sci. Hortic. 2013, 160, 222–229. [Google Scholar] [CrossRef]
- Li, F.; Christensen, M.J.; Gao, P.; Li, Y.; Duan, T. An arbuscular mycorrhizal fungus and Epichloë festucae var. lolii reduce Bipolaris sorokiniana disease incidence and improve perennial ryegrass growth. Mycorrhiza 2018, 28, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.S.; Cheon, K.S.; Hong, S.Y.; Cho, J.H.; Im, J.S.; Mekapogu, M.; Park, T.H. Complete chloroplast genome sequences of Solanum commersonii and its application to chloroplast genotype in somatic hybrids with Solanum tuberosum. Plant Cell Rep. 2016, 35, 2113–2123. [Google Scholar] [CrossRef] [PubMed]
- Tarwackaa, J.; Polkowska-Kowalczyka, L.; Kolano, B.; Sliwka, J.; Wielgat, B. Interspecifics matic hybrids Solanum villosum (+) S. tuberosum, resistant to Phytophthora infestans. J. Plant Physiol. 2013, 170, 1541–1548. [Google Scholar] [CrossRef]
- Alarcon, S.; Tereucan, G.; Cornejo, P.; Contreras, B.; Ruiz, A. Metabolic and antioxidant effects of inoculation with arbuscular mycorrhizal fungi in crops of flesh-coloured Solanum tuberosum treated with fungicides. J. Sci. Food Agric. 2021. [Google Scholar] [CrossRef] [PubMed]
- Zocco, D.; Fontaine, J.; Lozanova, E.; Renard, L.; Bivort, C.; Durand, R.; Grandmougin-Ferjani, A.; Declerck, S. Effects of two sterol biosynthesis inhibitor fungicides (fenpropimorph and fenhexamid) on the development of an arbuscular mycorrhizal fungus. Mycol. Res. 2008, 112, 592–601. [Google Scholar] [CrossRef]
- Hage-Ahmed, K.; Rosner, K.; Steinkellner, S. Arbuscular mycorrhizal fungi and their response to pesticides. Pest Manag. Sci. 2019, 75, 583–590. [Google Scholar] [CrossRef]
- Santander, C.; Ruiz, A.; García, S.; Aroca, R.; Cumming, J.; Cornejo, P. Efficiency of two arbuscular mycorrhizal fungal inocula to improve saline stress tolerance in lettuce plants by changes of antioxidant defense mechanisms. J. Sci. Food Agric. 2020, 100, 1577–1587. [Google Scholar] [CrossRef]
- Rivero, J.; Álvarez, D.; Flors, V.; Azcón-Aguilar, C.; Pozo, M.J. Root metabolic plasticity underlies functional diversity in mycorrhiza-enhanced stress tolerance in tomato. New Phytol. 2018, 220, 1322–1336. [Google Scholar] [CrossRef] [Green Version]
- Parada, J.; Valenzuela, T.; Gómez, F.; Tereucán, G.; García, S.; Cornejo, P.; Winterhalter, P.; Ruiz, A. Effect of fertilization and arbuscular mycorrhizal fungal inoculation on antioxidant profiles and activities in Fragaria ananassa fruit. J. Sci. Food Agric. 2019, 99, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Avio, L.; Sbrana, C.; Giovannetti, M.; Frassinetti, S. Arbuscular mycorrhizal fungi affect total phenolics content and antioxidant activity in leaves of oak leaf lettuce varieties. Sci. Hortic. 2017, 224, 265–271. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Antonino, L.M.; Ruta, C.; Mauromicale, G. In vitro micropropagation and mycorrhizal treatment influences the polyphenols content profile of globe artichoke under field conditions. Int. Food Res. J. 2017, 99, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, H.; Zhang, X.; Tang, M. Arbuscular mycorrhizal symbiosis alleviates salt stress in black locust through improved photosynthesis, water status, and K+/Na+ homeostasis. Front. Plant Sci. 2017, 8, 1739. [Google Scholar] [CrossRef] [PubMed]
- Sbrana, C.; Avio, L.; Giovannetti, M. Beneficial mycorrhizal symbionts affecting the production of health-promoting phytochemicals. Electrophoresis 2014, 35, 1535–1546. [Google Scholar] [CrossRef]
- Santander, C.; Aroca, R.; Cartes, P.; Vidal, G.; Cornejo, P. Aquaporins and cation transporters are differentially regulated by two arbuscular mycorrhizal fungi strains in lettuce cultivars growing under salinity conditions. Plant Physiol. Biochem. 2021, 158, 396–409. [Google Scholar] [CrossRef]
- Río, J.A.; Gómez, P.; Báidez, A.; Fuster, M.D.; Ortuño, A.; Frias, V. Phenolic Compounds Have a Role in the Defence Mechanism Protecting Grapevine against the Fungi Involved in Petri Disease. Phytopathol. Mediterr. 2004, 43, 87–94. [Google Scholar] [CrossRef]
- Clough, J.M. The strobilurins, oudemansins, and myxothiazols, fungicidal derivatives of beta-methoxyacrylic acid. Nat. Prod. Rep. 1993, 10, 565–574. [Google Scholar] [CrossRef]
- Bartlett, D.W.; Clough, J.M.; Godwin, J.R.; Hall, A.A.; Hamer, M.; Parr-Dobrzanski, B. The strobilurin fungicides. Pest Manag. Sci. 2002, 58, 649–662. [Google Scholar] [CrossRef]
- Schiavon, M.; Petelewicz, P.; Orlinski, P.M.; Baird, J.H. Effects of fungicides on creeping bentgrass health and rooting characteristics under abiotic stress. Int. Turfgrass Soc. Res. J. 2021, 1–9. [Google Scholar]
- Nwankno, A.J.; Gordon, S.L.; Verrall, S.R.; Brennan, R.M.; Hancock, R.D. Treatment with fungicides influences phytochemical quality of blackcurrant juice. Ann. Appl. Biol. 2012, 160, 86–96. [Google Scholar] [CrossRef]
- Rivero, J.; Gamir, J.; Aroca, R.; Pozo, M.J.; Flors, V. Metabolic transition in mycorrhizal tomato roots. Front. Microbiol. 2015, 6, 598. [Google Scholar] [CrossRef] [PubMed]
- López-Ráez, J.A.; Verhage, A.; Fernández, I.; García, J.M.; Azcón-Aguilar, C.; Flors, V.; Pozo, M.J. Hormonal and transcriptional profiles highlight common and differential host responses to arbuscular mycorrhizal fungi and the regulation of the oxylipin pathway. J. Exp. Bot. 2010, 61, 2589–2601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweiger, R.; Baier, M.C.; Persicke, M.; Müller, C. High specificity in plant leaf metabolic responses to arbuscular mycorrhiza. Nat. Commun. 2014, 5, 3886. [Google Scholar] [CrossRef] [Green Version]
- Aroca, R.; Ruiz-Lozano, J.M.; Zamarreño, A.M.; Paz, J.A.; García-Mina, J.M.; Pozo, M.J.; López-Ráez, J. M; Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J. Integr. Plant Biol. 2013, 170, 47–55. [Google Scholar] [CrossRef]
- Santander, C.; Sanhueza, M.; Olave, J.; Borie, F.; Valentine, A.; Cornejo, P. Arbuscular mycorrhizal colonization promotes the tolerance to salt stress in lettuce plants through an efficient modification of ionic balance. J. Soil Sci. Plant Nutr. 2019, 19, 321–331. [Google Scholar] [CrossRef]
- Avio, L.; Maggini, R.; Ujvári, G.; Incrocci, L.; Giovannetti, M.; Turrini, A. The phenol content and antioxidant activity in the leaves of two artichoke cultivars are differentially affected by six mycorrhizal symbionts. Sci. Hortic. 2020, 264, 109153. [Google Scholar] [CrossRef]
- Ruiz, A.; Sanhueza, M.; Gómez, F.; Tereucán, G.; Valenzuela, T.; García, S.; Hermosín-Gutiérrez, I. Changes in the content of anthocyanins, flavonols, and antioxidant activity in Fragaria ananassa var. Camarosa fruits under traditional and organic fertilization. J. Sci. Food Agric. 2019, 99, 2404–2410. [Google Scholar] [PubMed]
- Ajigboye, O.O.; Murchie, E.; Ray, R.V. Foliar application of isopyrazam and epoxiconazole improves photosystem II efficiency, biomass and yield in winter wheat. Pestic Biochem. Physiol. 2014, 114, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, A.; Tereucán, G.; Ercoli, S.; Cornejo, P.; Gomez, M.R.; Uhlmann, L.; Ruiz, A. Influence of Organic and Chemical Fertilisation on Antioxidant Compounds Profiles and Activities in Fruits of Fragaria ananassa var. Camarosa. J. Soil Sci. Plant Nutr. 2020, 1–10. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.P.; Magalhaes, L.M.; Reis, S.; Lima, J.L.; Segundo, M.A. High-throughput total cupric ion reducing antioxidant capacity of biological samples determined using flow injection analysis and microplate-based methods. Anal. Sci. 2011, 27, 483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maldonado, P.D.; Rivero-Cruz, I.; Mata, R.; Pedraza-Chaverrí, J. Antioxidant activity of A-type proanthocyanidins from Geranium niveum (Geraniaceae). J. Agric. Food Chem. 2005, 53, 1996–2001. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Aspee, F.; Quispe, C.; Soriano, M.; del Pilar, M.; Fuentes Gonzalez, J.; Hüneke, E.; Theoduloz, C.; Schmeda-Hirschmann, G. Antioxidant activity and characterization of constituents in copao fruits (Eulychnia acida Phil., Cactaceae) by HPLC–DAD–MS/MSn. Int. Food Res. J. 2014, 62, 286–298. [Google Scholar] [CrossRef]

| Peak | tR (min) | Identifications | Λmax (nm) | [M-H]- | Product Ions |
|---|---|---|---|---|---|
| 1 | 6.4 | 5-caffeoylquinic acid | 324 | 353.0 | 190.9 |
| 2 | 13.0 | caffeoylquinic acid | 327 | 353.9 | 191.0; 179.9; 173.0; 135.0 |
| 3 | 13.3 | quercetin-3-glucosylrutinoside | 352 | 771.8 | 300.1 |
| 4 | 13.9 | quercetin-dihexoside | 352 | 624.9 | 300.1 |
| 5 | 15.1 | quercetin-pentoside-rutinoside | 351 | 740.9 | 300.1 |
| 6 | 16.4 | no identified | 351 | ||
| 7 | 16.9 | quercetin-rutinoside | 353 | 609.0 | 300.1 |
| 8 | 19.6 | kaempferol-rutinoside | 345 | 592.9 | 284.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fritz, V.; Tereucán, G.; Santander, C.; Contreras, B.; Cornejo, P.; Ferreira, P.A.A.; Ruiz, A. Effect of Inoculation with Arbuscular Mycorrhizal Fungi and Fungicide Application on the Secondary Metabolism of Solanum tuberosum Leaves. Plants 2022, 11, 278. https://doi.org/10.3390/plants11030278
Fritz V, Tereucán G, Santander C, Contreras B, Cornejo P, Ferreira PAA, Ruiz A. Effect of Inoculation with Arbuscular Mycorrhizal Fungi and Fungicide Application on the Secondary Metabolism of Solanum tuberosum Leaves. Plants. 2022; 11(3):278. https://doi.org/10.3390/plants11030278
Chicago/Turabian StyleFritz, Valentina, Gonzalo Tereucán, Christian Santander, Boris Contreras, Pablo Cornejo, Paulo Ademar Avelar Ferreira, and Antonieta Ruiz. 2022. "Effect of Inoculation with Arbuscular Mycorrhizal Fungi and Fungicide Application on the Secondary Metabolism of Solanum tuberosum Leaves" Plants 11, no. 3: 278. https://doi.org/10.3390/plants11030278
APA StyleFritz, V., Tereucán, G., Santander, C., Contreras, B., Cornejo, P., Ferreira, P. A. A., & Ruiz, A. (2022). Effect of Inoculation with Arbuscular Mycorrhizal Fungi and Fungicide Application on the Secondary Metabolism of Solanum tuberosum Leaves. Plants, 11(3), 278. https://doi.org/10.3390/plants11030278







