Major Plant in Herbal Mixture Gan-Mai-Da-Zao for the Alleviation of Depression in Rat Models
Abstract
:1. Introduction
2. Results
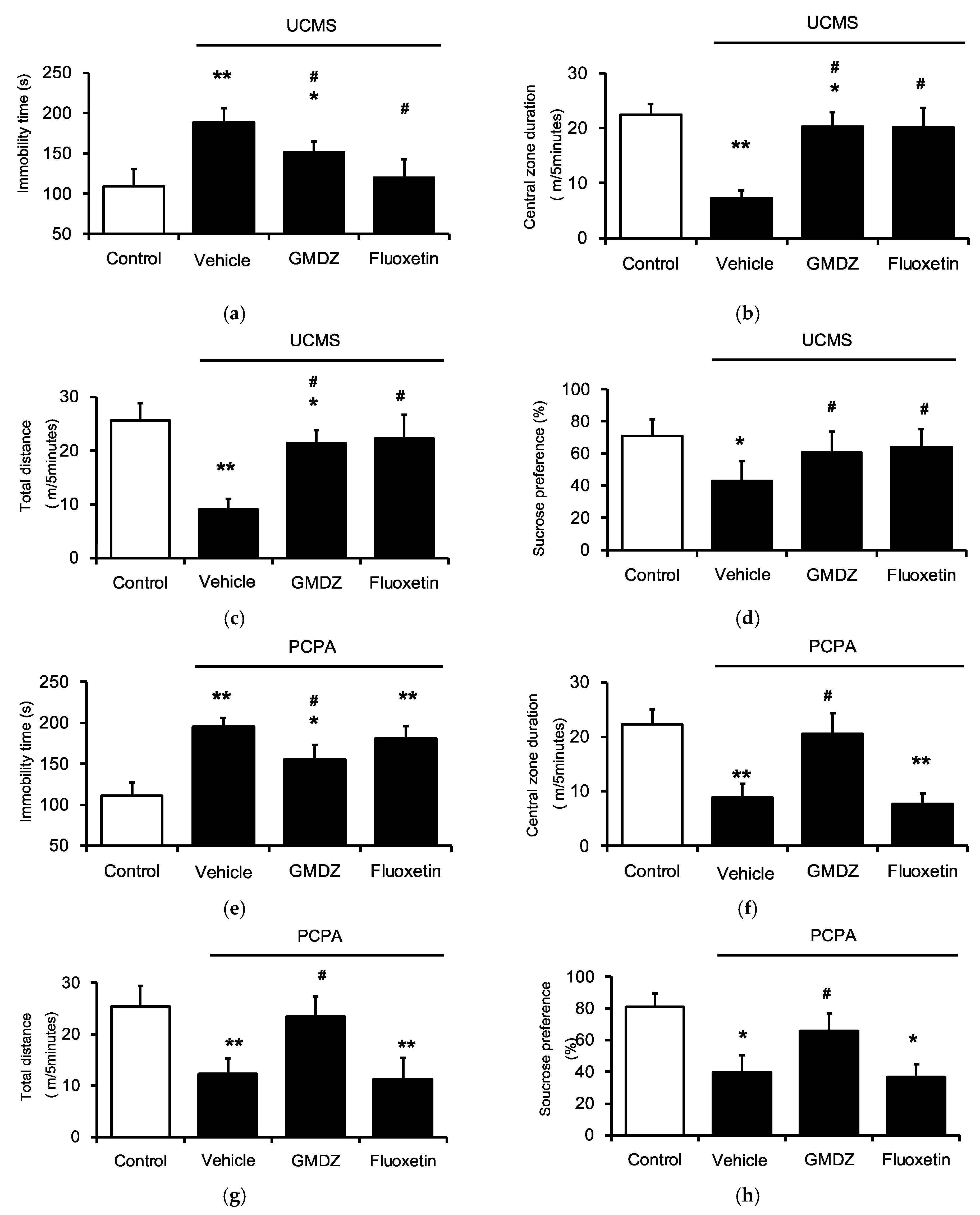
2.1. Chronic GMDZ Treatment Ameliorated Depression-Like Behaviors in UCMS Rats and PCPA Treated Rats
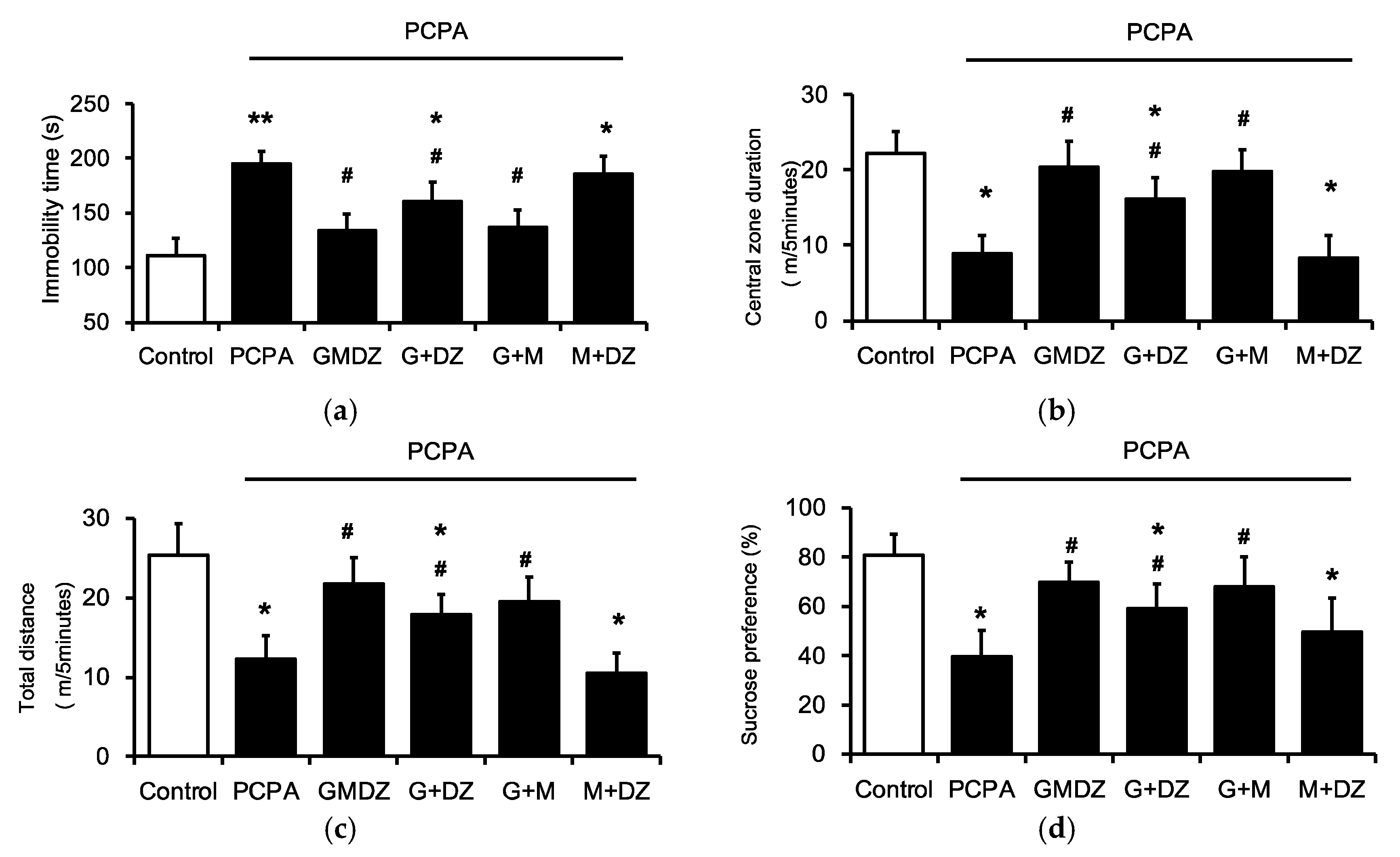
2.2. Effects of Two-Herb Mixture of GMDZ on Behavioral Tests in PCPA-Induced Rats
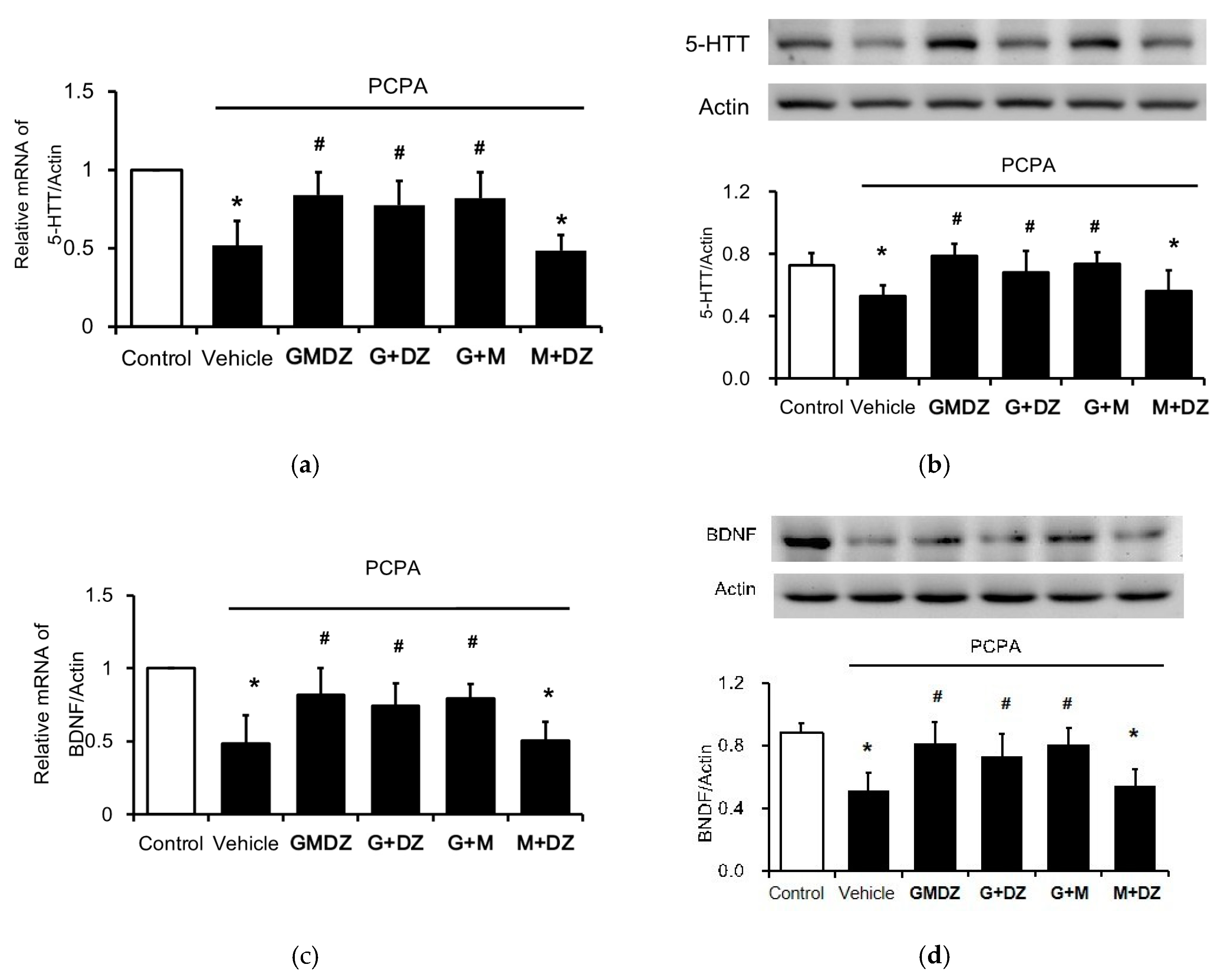
2.3. Chronic GMDZ Treatment Restores the 5-HTT and BDNF Levels in the Hippocampus of PCPA Treated Rats
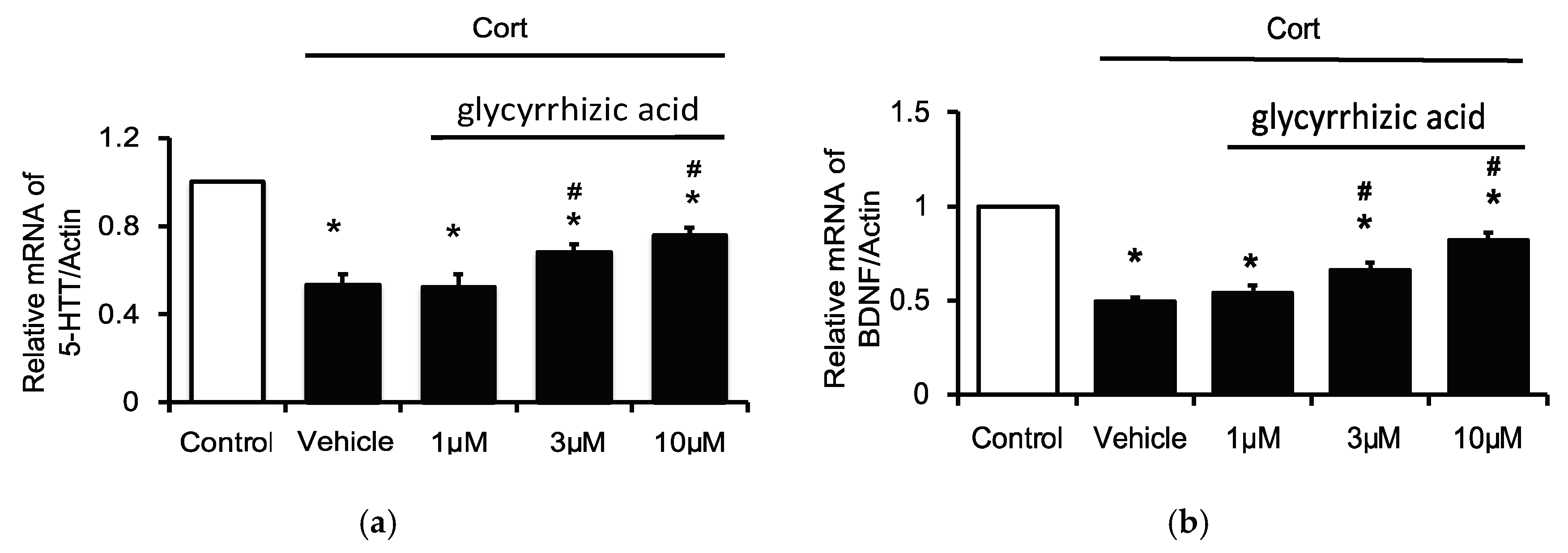
2.4. Effects of Glycyrrhizic Acid on 5-HTT and BDNF Expression in the Corticosterone-Treated H19-7 Cell Line
3. Discussion
4. Materials and Methods
4.1. Preparation of Extracts of the GMDZ Decoction
4.2. Experimental Animals
4.3. Experimental Design
4.4. UCMS Procedure
4.5. Behavioral Tests
4.6. Tissue Preparation
4.7. Cell Cultures
4.8. Western Blotting Analysis
4.9. Real-Time Reverse Transcription-Polymerase Chain Reaction
- BDNF F: 5′-GCAGTCAAGTGCCTTTGGAG-3′;
- BDNF R: 5′-CGGCATCCAGGTAATTTTTG -3′;
- 5-HTT F: 5′-CATCAGCCCTCTGTTTCTCC -3′;
- 5-HTT R: 5′-CGGACGACATCCCTATGC -3′;
- β-actin F: 5′-CTAAGGCCAACCGTGAAAAG-3′;
- β-actin R: 5′-GCCTGGATGGCTACGTACA-3′.
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lepine, J.P.; Briley, M. The increasing burden of depression. Neuropsychiatr. Dis. Treat. 2011, 7, 3–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansson, S.R.; Mezey, E.; Hoffman, B.J. Serotonin transporter messenger RNA in the developing rat brain: Early expression in serotonergic neurons and transient expression in non-serotonergic neurons. Neuroscience 1998, 83, 1185–1201. [Google Scholar] [CrossRef]
- Molteni, R.; Cattaneo, A.; Calabrese, F.; Macchi, F.; Olivier, J.D.; Racagni, G.; Ellenbroek, B.A.; Gennarelli, M.; Riva, M.A. Reduced function of the serotonin transporter is associated with decreased expression of BDNF in rodents as well as in humans. Neurobiol. Dis. 2010, 37, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Kastner, R.; Wetmore, C.; Olson, L. Comparative study of brain-derived neurotrophic factor messenger RNA and protein at the cellular level suggests multiple roles in hippocampus, striatum and cortex. Neuroscience 1996, 74, 161–183. [Google Scholar] [CrossRef]
- Lesch, K.P.; Bengel, D.; Heils, A.; Sabol, S.Z.; Greenberg, B.D.; Petri, S.; Benjamin, J.; Muller, C.R.; Hamer, D.H.; Murphy, D.L. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996, 274, 1527–1531. [Google Scholar] [CrossRef] [PubMed]
- Karege, F.; Perret, G.; Bondolfi, G.; Schwald, M.; Bertschy, G.; Aubry, J.M. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002, 109, 143–148. [Google Scholar] [CrossRef]
- Shirayama, Y.; Chen, A.C.; Nakagawa, S.; Russell, D.S.; Duman, R.S. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J. Neurosci. 2002, 22, 3251–3261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, F.; Wu, X.A.; Tang, Y.; Huang, Q.; Zhang, Z.J.; Yuan, J.H. Meta-analysis of randomized controlled trials to assess the effectiveness and safety of Free and Easy Wanderer Plus, a polyherbal preparation for depressive disorders. J. Psychiatr. Res. 2011, 45, 1518–1524. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, R.; Huang, X. Meta-analysis of the clinical effectiveness of traditional Chinese medicine formula Chaihu-Shugan-San in depression. J. Ethnopharmacol. 2012, 141, 571–577. [Google Scholar] [CrossRef]
- Yeung, W.F.; Chung, K.F.; Ng, K.Y.; Yu, Y.M.; Ziea, E.T.; Ng, B.F. A meta-analysis of the efficacy and safety of traditional Chinese medicine formula Ganmai Dazao decoction for depression. J. Ethnopharmacol. 2014, 153, 309–317. [Google Scholar] [CrossRef]
- Jun, J.H.; Lee, J.A.; Choi, T.Y.; Yun, K.J.; Lim, H.J.; Lee, M.S. Herbal medicine (Gan Mai Da Zao decoction) for depression: A systematic review protocol. BMJ Open 2014, 4, e003690. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Robins, E.; Endicott, J. Research Diagnostic Criteria (RDC) for a Selected Group of Functional Disorders; New York State Psychiatric Institute: New York, NY, USA, 1978. [Google Scholar]
- Yang, F.; Qiao, Q.; Gong, Y. Antidepressant effect of gan-maida-zao tang on 30 women with postpartum depression. Shanxi J TCM 2009, 30, 851–852. [Google Scholar]
- Wu, J. Antidepressant effect of gan-mai-da-zao tang on depression compared with fluoxetine. J. Chin. Physician 2002, 11, 18–19. [Google Scholar]
- Kurebayashi, L.F.; Turrini, R.N.; Kuba, G.; Shimizu, M.H.; Takiguch, R.S. Chinese phytotherapy to reduce stress, anxiety and improve quality of life: Randomized controlled trial. Rev. Esc. Enferm. USP 2016, 50, 853–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, J.S.; Li, C.Y.; Yang, X.C.; Fang, J.; Yang, Y.X.; Guo, J.Y. Protective effect of gan mai da zao decoction in unpredictable chronic mild stress-induced behavioral and biochemical alterations. Pharm. Biol. 2010, 48, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.D.; Manadas, B.J.; Melo, C.V.; Gomes, J.R.; Mendes, C.S.; Graos, M.M.; Carvalho, R.F.; Carvalho, A.P.; Duarte, C.B. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005, 12, 1329–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEwen, B.S. Stress and hippocampal plasticity. Annu. Rev. Neurosci. 1999, 22, 105–122. [Google Scholar] [CrossRef] [Green Version]
- Willner, P.; Towell, A.; Sampson, D.; Sophokleous, S.; Muscat, R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology 1987, 93, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Lei, J.; Sun, X.; Liu, G.; Zhao, S. Stress-induced anhedonia correlates with lower hippocampal serotonin transporter protein expression. Brain Res. 2013, 1513, 127–134. [Google Scholar] [CrossRef]
- McClung, C.A.; Nestler, E.J. Neuroplasticity mediated by altered gene expression. Neuropsychopharmacology 2008, 33, 3–17. [Google Scholar] [CrossRef]
- Ren-Patterson, R.F.; Cochran, L.W.; Holmes, A.; Sherrill, S.; Huang, S.J.; Tolliver, T.; Lesch, K.P.; Lu, B.; Murphy, D.L. Loss of brain-derived neurotrophic factor gene allele exacerbates brain monoamine deficiencies and increases stress abnormalities of serotonin transporter knockout mice. J. Neurosci. Res. 2005, 79, 756–771. [Google Scholar] [CrossRef]
- Mossner, R.; Daniel, S.; Albert, D.; Heils, A.; Okladnova, O.; Schmitt, A.; Lesch, K.P. Serotonin transporter function is modulated by brain-derived neurotrophic factor (BDNF) but not nerve growth factor (NGF). Neurochem. Int. 2000, 36, 197–202. [Google Scholar] [CrossRef]
- Zetterstrom, T.S.; Pei, Q.; Madhav, T.R.; Coppell, A.L.; Lewis, L.; Grahame-Smith, D.G. Manipulations of brain 5-HT levels affect gene expression for BDNF in rat brain. Neuropharmacology 1999, 38, 1063–1073. [Google Scholar] [CrossRef]
- Dhingra, D.; Sharma, A. Antidepressant-like activity of Glycyrrhiza glabra L. in mouse models of immobility tests. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2006, 30, 449–454. [Google Scholar] [CrossRef]
- Hatano, T.; Fukuda, T.; Miyase, T.; Noro, T.; Okuda, T. Phenolic constituents of licorice. III. Structures of glicoricone and licofuranone, and inhibitory effects of licorice constituents on monoamine oxidase. Chem. Pharm. Bull. 1991, 39, 1238–1243. [Google Scholar] [CrossRef] [Green Version]
- Dhingra, D.; Sharma, A. Evaluation of antidepressant-like activity of glycyrrhizin in mice. Indian J. Pharmacol. 2005, 37, 390–395. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.L.; Li, Y.X.; Niu, Y.T.; Zheng, J.; Wu, J.; Shi, G.J.; Ma, L.; Niu, Y.; Sun, T.; Yu, J.Q. Observing anti-inflammatory and anti-nociceptive activities of glycyrrhizin through regulating COX-2 and pro-inflammatory cytokines expressions in mice. Inflammation 2015, 38, 2269–2278. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cao, S.; Zhang, X. Modulation of gut microbiota-brain axis by probiotics, prebiotics, and diet. J. Agric. Food Chem. 2015, 63, 7885–7895. [Google Scholar] [CrossRef]
- Borah, M.; Sarma, P.; Das, S. A study of the protective effect of triticum aestivum L. in an experimental animal model of chronic fatigue syndrome. Pharmacogn. Res. 2014, 6, 285–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.M.; Park, J.G.; Lee, Y.H.; Lee, C.G.; Min, B.S.; Kim, J.H.; Lee, H.K. Anti-complementary activity of triterpenoides from fruits of Zizyphus jujuba. Biol. Pharm. Bull. 2004, 27, 1883–1886. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Sim, Y.B.; Kang, Y.J.; Kim, S.S.; Kim, C.H.; Kim, S.J.; Suh, H.W. Mechanisms involved in the antinociceptive effects of orally administered oleanolic acid in the mouse. Arch. Pharmacal Res. 2013, 36, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Oyebanji, B.O.; Saba, A.B.; Oridupa, O.A. Studies on the anti-inflammatory, analgesic and antipyrexic activities of betulinic acid derived from Tetracera potatoria. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 30–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.L.; Lim, S.L.; Lu, K.H.; Sheen, L.Y. Antidepressant-like effects of Gan-Mai-Dazao-Tang via monoamine regulatory pathways on forced swimming test in rats. J. Tradit. Complement. Med. 2018, 8, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, L.; Shiha, A.A.; Bascunana, P.; de Cristobal, J.; Fernandez de la Rosa, R.; Delgado, M.; Pozo, M.A. Serotonin depletion does not modify the short-term brain hypometabolism and hippocampal neurodegeneration induced by the lithium-pilocarpine model of status epilepticus in rats. Cell. Mol. Neurobiol. 2016, 36, 513–519. [Google Scholar] [CrossRef]
- Kornum, B.R.; Licht, C.L.; Weikop, P.; Knudsen, G.M.; Aznar, S. Central serotonin depletion affects rat brain areas differently: A qualitative and quantitative comparison between different treatment schemes. Neurosci. Lett. 2006, 392, 129–134. [Google Scholar] [CrossRef]
- Tianzhu, Z.; Shihai, Y.; Juan, D. Antidepressant-like effects of cordycepin in a mice model of chronic unpredictable mild stress. Evid.-Based Complement. Altern. Med. 2014, 2014, 438506. [Google Scholar] [CrossRef]
- Rowland, N.E. Food or fluid restriction in common laboratory animals: Balancing welfare considerations with scientific inquiry. Comp. Med. 2007, 57, 149–160. [Google Scholar] [PubMed]
- Bessa, J.M.; Ferreira, D.; Melo, I.; Marques, F.; Cerqueira, J.J.; Palha, J.A.; Almeida, O.F.; Sousa, N. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol. Psychiatry 2009, 14, 739, 764–773. [Google Scholar] [CrossRef]
- Nielsen, C.K.; Arnt, J.; Sanchez, C. Intracranial self-stimulation and sucrose intake differ as hedonic measures following chronic mild stress: Interstrain and interindividual differences. Behav. Brain Res. 2000, 107, 21–33. [Google Scholar] [CrossRef]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Dunn, A.J.; Swiergiel, A.H. Effects of interleukin-1 and endotoxin in the forced swim and tail suspension tests in mice. Pharmacol. Biochem. Behav. 2005, 81, 688–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.W.; Lai, M.C.; Cheng, J.T.; Tsai, J.J.; Huang, C.C.; Wu, S.N. Pregabalin attenuates excitotoxicity in diabetes. PLoS ONE 2013, 8, e65154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piron, M.; Villereal, M.L. Chronic exposure to stress hormones alters the subtype of store-operated channels expressed in H19-7 hippocampal neuronal cells. J. Cell. Physiol. 2013, 228, 1332–1343. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.H.; Hsu, C.T.; Niu, H.S.; Niu, C.S.; Cheng, J.T.; Chen, Z.C. Ginsenoside Rh2 improves cardiac fibrosis via PPARdelta-STAT3 signaling in type 1-Like diabetic rats. Int. J. Mol. Sci. 2017, 18, 1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-X.; Cheng, K.-C.; Hsu, C.-T.; Cheng, J.-T.; Yang, T.-T. Major Plant in Herbal Mixture Gan-Mai-Da-Zao for the Alleviation of Depression in Rat Models. Plants 2022, 11, 258. https://doi.org/10.3390/plants11030258
Li Y-X, Cheng K-C, Hsu C-T, Cheng J-T, Yang T-T. Major Plant in Herbal Mixture Gan-Mai-Da-Zao for the Alleviation of Depression in Rat Models. Plants. 2022; 11(3):258. https://doi.org/10.3390/plants11030258
Chicago/Turabian StyleLi, Ying-Xiao, Kai-Chun Cheng, Chao-Tien Hsu, Juei-Tang Cheng, and Ting-Ting Yang. 2022. "Major Plant in Herbal Mixture Gan-Mai-Da-Zao for the Alleviation of Depression in Rat Models" Plants 11, no. 3: 258. https://doi.org/10.3390/plants11030258
APA StyleLi, Y.-X., Cheng, K.-C., Hsu, C.-T., Cheng, J.-T., & Yang, T.-T. (2022). Major Plant in Herbal Mixture Gan-Mai-Da-Zao for the Alleviation of Depression in Rat Models. Plants, 11(3), 258. https://doi.org/10.3390/plants11030258





