1. Introduction
Rice (
Oryza sativa L.) is a very important edible starchy and staple cereal food crop, as more than one half of the world’s population (>3.5 billion) depends on rice for their consumption and livelihood [
1,
2]. The Food and Agriculture Organization (FAO) estimates that, by 2029, the world’s food production will have to increase by 50% [
3]. Thus, doubling rice production by 2050 is urgently needed to feed more than 9 billion people globally [
3,
4]. The productivity of released cultivars has plateaued due to narrow genetic distance in the parental lines applied in breeding programs [
5], and hybrid rice is an important strategy to overcome this plateau. Hybrid rice technology provides an advantage in yield up to 20% higher than the best regular inbred rice varieties, due to a higher harvest index at the maturity stage and the accumulation of more plant biomass before flowering [
6].
Large-scale cultivated hybrid rice is limited due to low amounts of hybrid seed and the high cost of seed production. To overcome this constraint, it is necessary to enhance the outcrossing ability of CMS lines for faster and wider hybrid rice adoption [
7,
8]. Applying anther indehiscence with exerted stigmas was hindered by trait fixation and propagation in hybrid rice breeding. The exerted stigma of rice seems to improve the outcrossing rate of the sterile lines [
9,
10,
11]. The outcrossing in CMS lines is significantly increased by improving the floral traits, such as a longer duration of the floret opening, a longer stigma, a wider angle of the floret opening, and an exertion of higher stigma [
8]. Consequently, in hybrid rice breeding programs, the floral traits of CMS lines are crucial and important. Moreover, yield traits are essential for ensuring high yield heterosis. In China, success in breeding and growing hybrid rice commercially has attracted the awareness of rice breeders worldwide [
12]. In Egypt, breeding a high-yield hybrid rice is considered one of the most promising potential strategies for increasing rice production to cope with population growth [
12]. Several factors influence multiplication cytoplasmic male sterile (CMS) and hybrid seed production, such as seeding time, field conditions, planting pattern, weather conditions at flowering, the synchronization of flowering between the parental lines, and supplementary pollination techniques. These practices can regulate panicle exertion from the flag leaf, enhance the rate of stigma exertion, adjust plant height, promote the duration of the floret opening, and enhance the length and productivity of late developing tillers [
13,
14].
Sustainable agricultural development needs novel tools for producing safe food with minimum environmental pollution. One of these is the use of plant growth promoting bacteria (PGPB), which are defined as free-living soil rhizosphere microbes. These bacteria have a good influence on both the rhizosphere and phyllosphere of the plants [
15].
Cyanobacteria (blue-green algae and a Gram-negative bacteria) are an ancient group of photosynthetic microorganisms and contribute to oxygen production in the Earth’s atmosphere [
16]. They are beneficial microbes found in various environments, including soil, oceans, bare rocks, and freshwater [
17]. Cyanobacteria have the ability to fix atmospheric N2, solubilize phosphate, produce plant growth regulators, suppress the growth of some pathogenic microbes in soil, catalyze the nutrient cycling, produce some bioactive compounds such as vitamins and hormones that contribute to plant growth, decompose organic wastes, and detoxify pesticides, heavy metals, and other xenobiotics [
18]. These microorganisms adapt to environmental changes and grow quickly and densely. However, their fast growth rate depends on biotic factors, a differentiation in nutrients levels, global warming, and climate change [
19,
20].
Plant growth-promoting bacteria such as rhizospheric bacteria [
21] and symbiotic rhizobia [
22] are considered PHPB (plant health-promoting bacteria) agents. Additionally, in recent years, cyanobacteria have gained a great importance due to the fact that they have a significant role in regulating plant productivity [
18]. In view of their benefits for soil fertility and crop production, this type of bacteria releases varied amounts of phytohormones (cytokinins, gibberellins, and auxins), amino acids, ammonia and polypeptides [
23], siderophores [
24], and polysaccharides [
25] during active cell growth for plant growth and development [
26]. These different types of substances represent important factors with stimulating effects on plant growth [
27]. Notably, cyanobacterial biomass represents an effective bio-fertilizer source that plays an important role in improving soil physico-chemical characteristics such as the mineral nutrient status of the degraded lands and water-holding capacity [
18]. Nowadays, cyanobacterial beneficial effects in the case of inoculation are reported in different crops such as cereals [
28,
29], pea plants [
30], and romaine lettuce [
31]. The application of cyanobacteria allows for the reduction of chemical fertilizer doses up to 50%, with a non-significant shortage in growth yield and plant biomass as a result for plant enrichment with auxin (IAA), cytokinins, gibberellic acids (Gibberellins), microelements (S, Zn, Fe, Mn, Cu, Mo, Co), amino acids, polyamines, macronutrients (N, P, K, Ca, Mg), and other secondary metabolites [
32,
33,
34,
35,
36,
37].
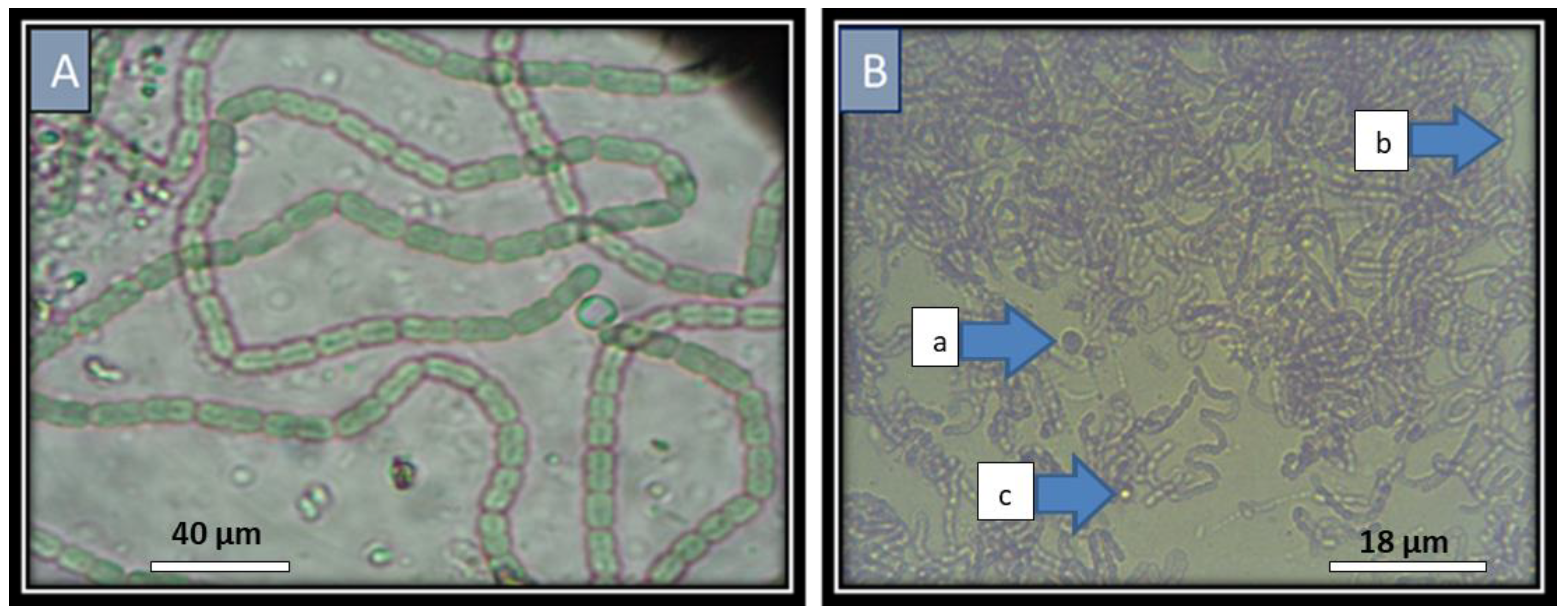
Traditionally, cyanobacterial species (i.e.,
Nostoc and
Anabaena) are differentiated on the basis of morphological and life cycle characteristics. Even the 16S rRNA sequence for taxonomy is wide-spread in bacteria, and this technique represents a low variability in this region among species and strains [
38].
Nostoc strains seem to form a monophyletic cluster that possibly contains more than one genus. The morphological features are stable and enough to separate different phylogenetic clusters (i.e., the length and width of akinetes are considered useful characteristics in classification of the
Anabaena) [
39].
In general, the application of cyanobacteria influences the spikelet opening angle, panicle exertion, and other floral traits, leading to a higher yield because of its ability to secrete Gibberellic acid (GA3). This can reflect on plant growth and thus on its productivity [
35,
40], growth, plant height, weight of 1000 grain, and grain yield of rice [
41,
42]. The present study aimed at: (1) isolating a new cyanobacterial isolate from the local area, (2) evaluating their production from IAA, and (3) exploring the influence of isolated cyanobacterial species such as
Anabaena and
Nostoc on enhancing floral traits and outcrossing rates in diverse cytoplasmic male sterile lines in rice and, subsequently, improving hybrid rice seed production.
3. Discussion
The CMS outcrossing rate was considered the main restricted reason in commercializing the hybrid rice. Therefore, enhancing the ability of outcrossing is necessary to increase hybrid seed production. Floral characters are very important and affect the efficacy of the outcrossing rate in CMS lines. Enhancing the CMS floral traits allows the opposition of foreign pollens, subsequently improving the process of cross-pollination and the production of the hybrid seed [
43,
44]. Cyanobacteria (blue-green algae) can fix atmospheric N in the wetland rice ecosystem and could significantly supply rice plants and soil with their need for nitrogen. The two genera
Nostoc and
Anabaena represent 80% of isolates in the rhizosphere. The two isolated cyanobacteria were identified based on their morphological characteristics. Makra et al [
38] tried to determine the taxonomic relationships among
Anabaena and
Nostoc strains by 16S rRNA and
rbcLX gene sequences. They found that some strains seemed to be identical when a comparison between them was performed by 16S rRNA or
rbcLX sequence alignment.
The identified isolates are efficient in enhancing seed germination and growth in cereal crops such as rice and wheat via high protein accumulation in addition to IAA production [
45,
46,
47]. The two isolated cyanobacterial strains produced up to 11.74 and 37.01 µg/100 mL of IAA and its intermediate compounds (i.e., indole-3-pyruvate (IPyA)) after 21 days of incubation. As reported, indole-3-pyruvate (IPyA) is the major intermediate and pathway for IAA biosynthesis in
Rhizobium tropici CIAT 899 [
48]. After adding tryptophan to the growth medium of
Azospirillum brasilense Sp7, indole-3-pyruvic acid was detected in the broth, indicating the function of an aminotransferase pathway in IAA synthesis [
49].
In the present study, the implementation of solely cyanobacteria (T4) remarkably improved all evaluated floral traits compared to the untreated control (T1). By applying T4 treatment (hybrid inoculum from
Anabaena Oryzae and
Nostoc muscorum), CMS lines had a longer duration of spikelet opening, a wider spikelet opening angle, a longer stigma, a wider stigma, and a higher stigma brush. Increasing the size of floret organs and the duration of the floret opening are favored features in out-crossing pollination. In this perspective, Zheng et al., Pathak et al., Majeed et al., and Kalavathi et al. [
50,
51,
52,
53] disclosed positive impacts of GA3, IAA, and NAA (Naphthalene acetic acid) on floral traits. Furthermore, the evaluated CMS lines exhibited highly significant variations in all evaluated floral traits. L4 and L1 displayed the uppermost evaluated floral traits, particularly under T4. Similar genotypic differences among CMS lines in floral traits were detected by El Sabagh et al. and Zheng et al. [
8,
43,
54].
The enhancement of plant vegetative growth considerably reinforced hybrid seed production. The cyanobacterial mixture significantly enhanced flag leaf angle, plant height, panicle length, and panicle exertion of all evaluated CMS lines. The growth regulators produced by cyanobacteria enhance vegetative growth by boosting cell division, cell elongation, cell differentiation and enlargement, protein synthesis, content of chlorophyll, and efficiency of photosynthesis [
55,
56]. In Egypt, a study carried out by Salama [
57] reported an increase in rice plant heights with values of 86.92 cm and 83.75 cm against the control (70.21 cm) after applying a treatment with
Nostoc sp.,
Anabaena sp., and
Calothrix sp. Additionally, findings indicated that individual or combinations of these cyanobacteria significantly increased the crop yield and 1000 grain weight of rice.
Poor panicle exertion of CMS lines considers a serious limitation in hybrid seed production. In this respect, the exogenous cyanobacterial mixture exhibited a significant increase in panicle length, panicle exertion, spikelet opening angle, duration of spikelet opening, total stigma length, stigma width, flag leaf angle, and leaf area index compared to untreated plants. Similar positive and significant impacts of growth regulators (cyanobacteria produce IAA) on panicle length, panicle exertion, and outcrossing rate were disclosed by Abo-Youssef et al., Hasan et al., and Pin et al. [
58,
59,
60]. Furthermore, Gadallah [
61]; Pal, et al. [
62]; Hussain et al. (2021) [
56] depicted that the application of growth regulators enhanced the plant height, panicle exertion, panicle length, and flag leaf angle of CMS lines. In the same manner, CMS lines exhibited highly significant differences in the genetic behavior for growth traits. L4 and L1 displayed the highest flag leaf angle, panicle length, plant height, and panicle exertion compared to the other evaluated CMS lines, particularly under the T4 treatment.
The total cyanobacterial count increased in all growth stages of nitrogen fertilization in rice plants [
49]. The applied mixed cyanobacteria enhanced plant growth, which was reflected positively in seed yield and its contributing traits. This treatment reflected the highest improvement in panicle weight, grain yield, number of fertile panicles per hill, seed set, and harvest index. In light of that, Tiwari et al. [
63]; Biswas et al. [
64]; Pal et al. [
62]; Hussain et al. [
56] manifested that growth regulators boosted grain yield and its contributing traits in CMS lines.
Both cyanobacteria are plasticity and their ability to fix nitrogen allows the reduction of inorganic atmospheric nitrogen (N
2) into organic nitrogen. The formed organic nitrogen is an ideal source of biofertilizer for plants. As a photosynthetic, cyanobacteria can also form symbiotic associations with other microorganisms and plants, subsequently using carbon dioxide (CO
2) and water to generate monosaccharides and oxygen, which the plants can use [
22]. Furthermore, cyanobacteria work well as PGPR (plant growth-promoting rhizobacteria) biofertilizers with PGP (plant growth promoting) traits, such as plant growth hormone production, nutrient solubilization, and siderophore production, which act as an antagonism to pathogenic fungi. Notably, they produce a source of bioactive molecules such as the diversity of secondary metabolites, vitamins, insecticides, immune suppressive activities, and herbicides [
35].
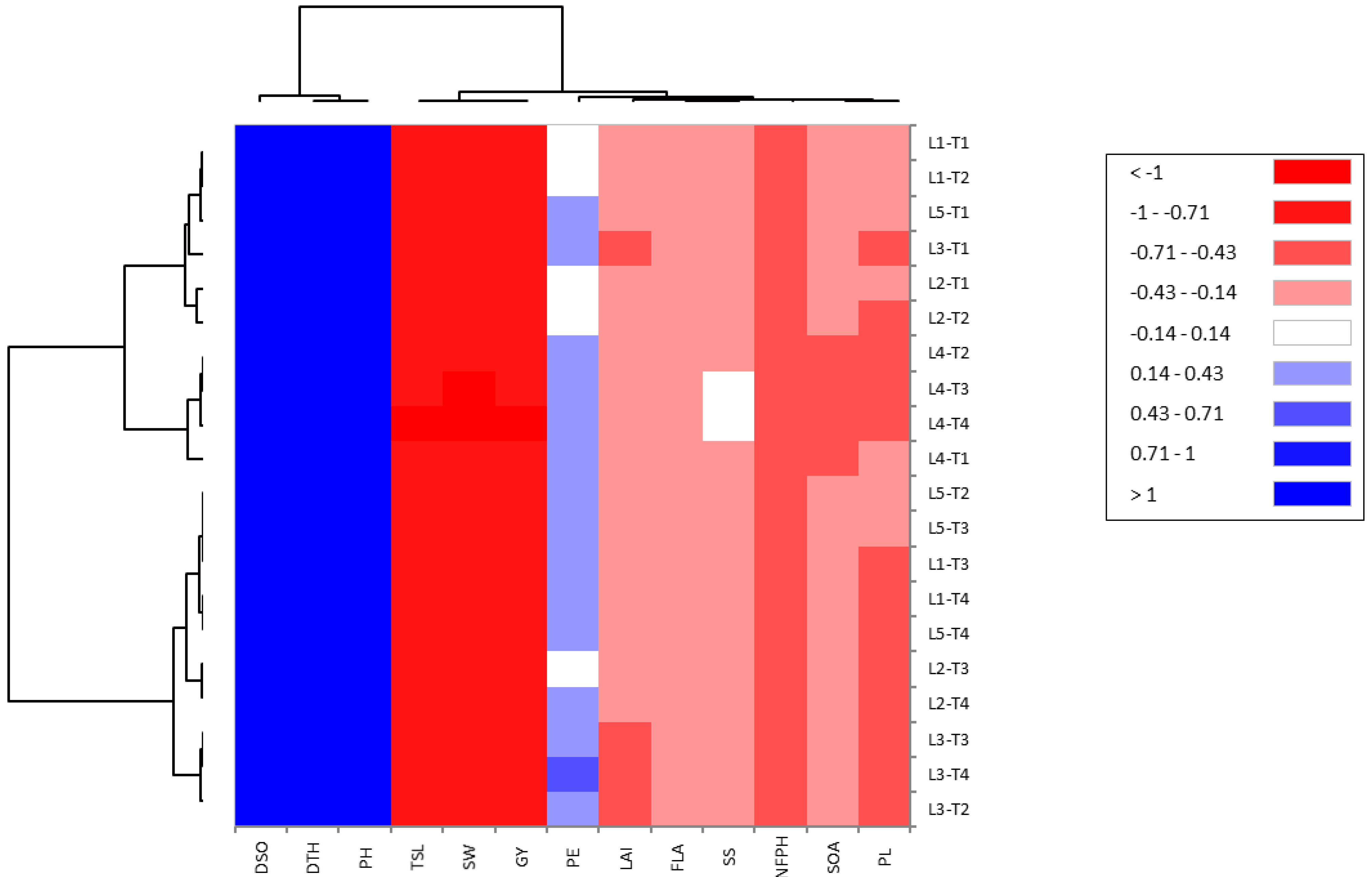
To estimate the association between studied traits, the PCA biplot is a suitable method for this purpose. Our results from this approach reinforced the abovementioned results. Robust positive associations were identified among all evaluated floral and growth traits with yield traits in CMS lines under cyanobacteria treatment. Our findings confirmed that the application of T4 treatment with promising CMS lines, such as L4 and L1, enhances floral traits, plant growth, and plant productivity and hence increases the outcrossing rate and hybrid seed production in CMS lines.