How Carbon Nanoparticles, Arbuscular Mycorrhiza, and Compost Mitigate Drought Stress in Maize Plant: A Growth and Biochemical Study
Abstract
1. Introduction
2. Material and Methods
2.1. Compost Formation
2.2. Carbon Nanoparticles (CNPs)
2.3. Experimental Set-Up
2.4. Environmental Growth Condition
2.5. Soil Structure and Composition
2.6. Determination of Photosynthesis Rate
2.7. Determination of Rubisco Activity
2.8. Determination of Sugars
2.9. Determination of Organic Acids
2.10. Determination of Amino Acid Levels and Metabolism
2.11. Determination of Fatty Acids
2.12. Polyamine Metabolism
2.13. Statistical Analysis
3. Results
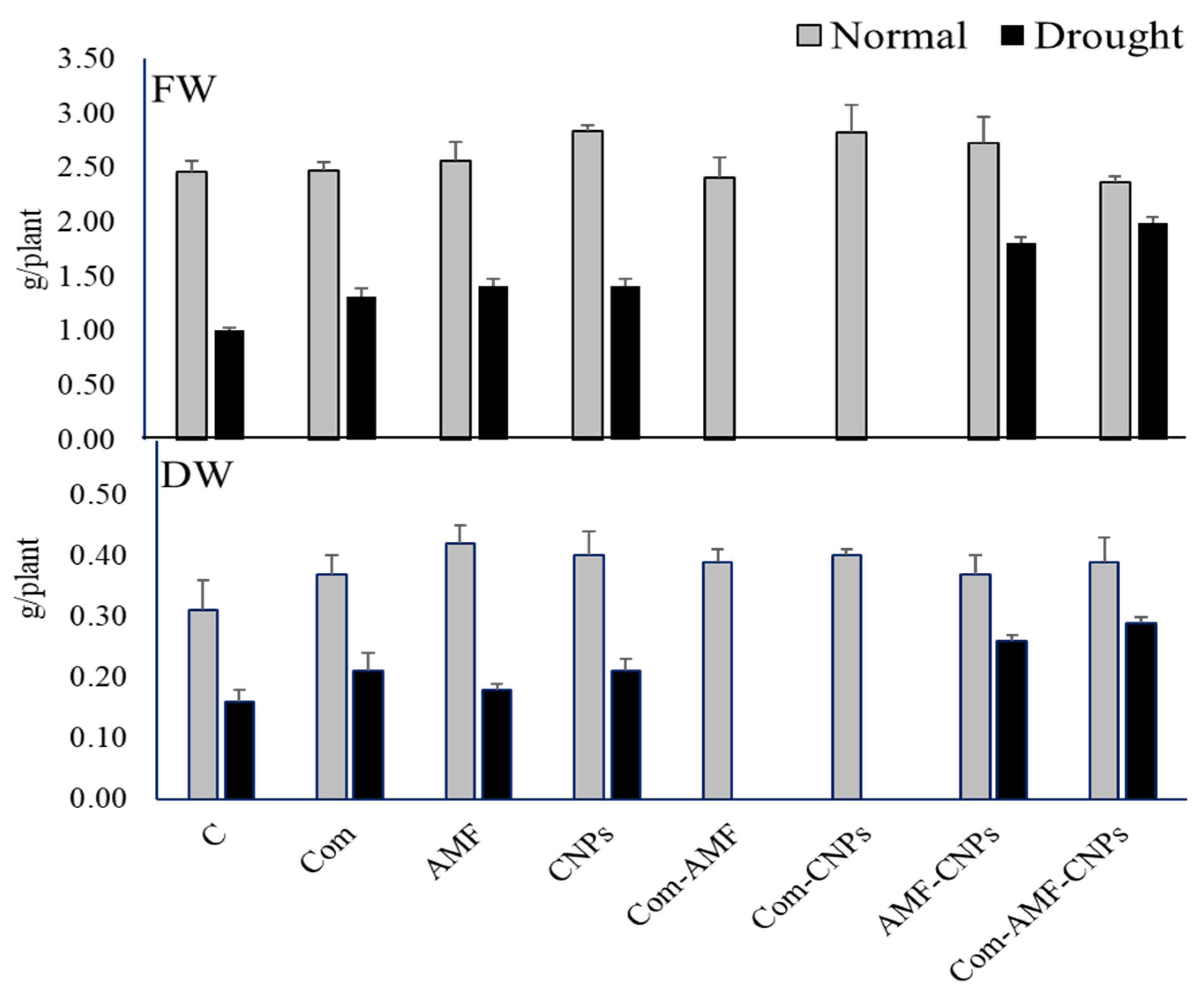
3.1. Effect of Different Treatments on Maize Growth
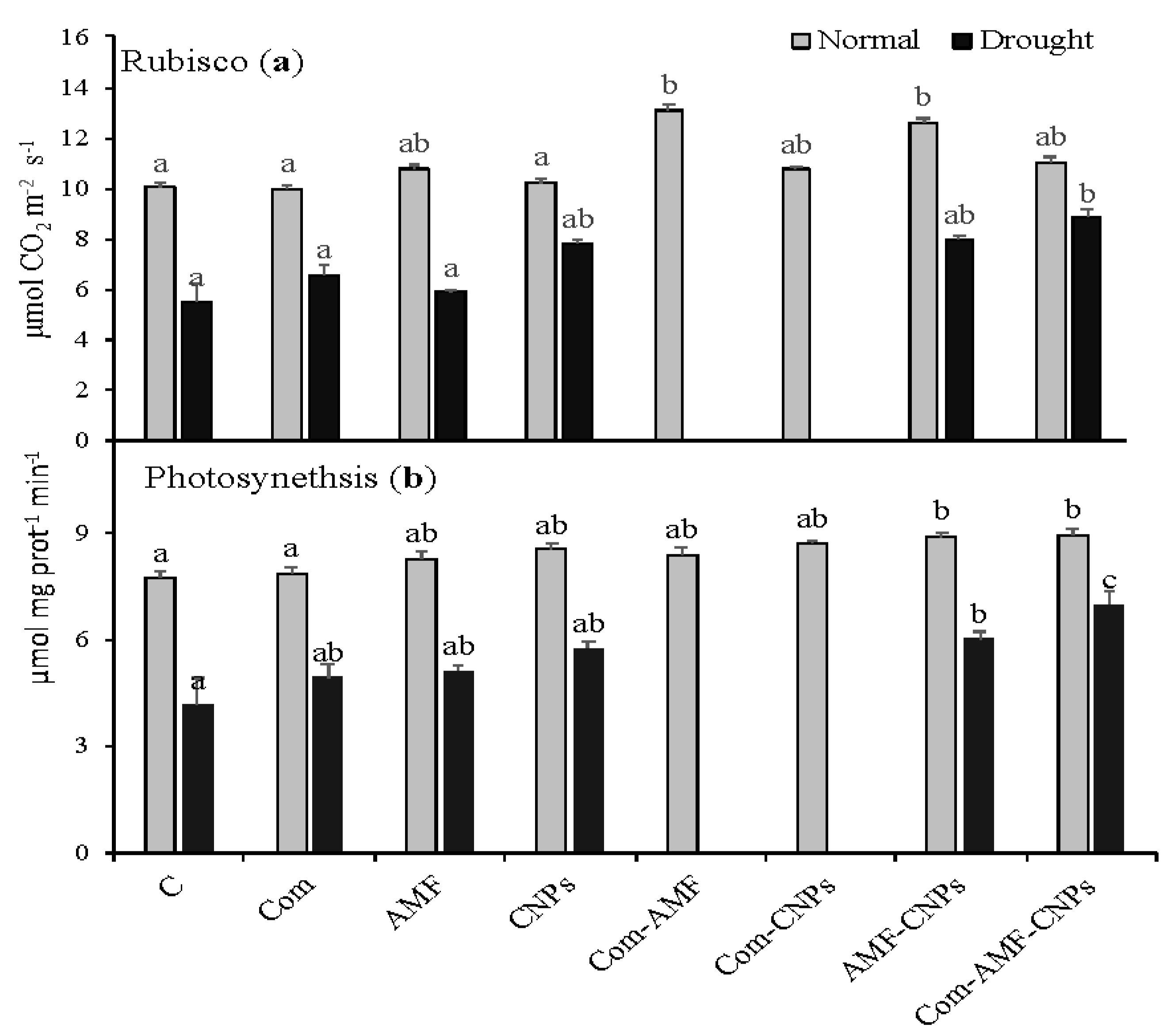
3.2. Photosynthetic Parameters
3.3. Sugar Metabolism
3.4. Organic Acid Level
3.5. Amino Acid Metabolism
3.6. Fatty Acid Level
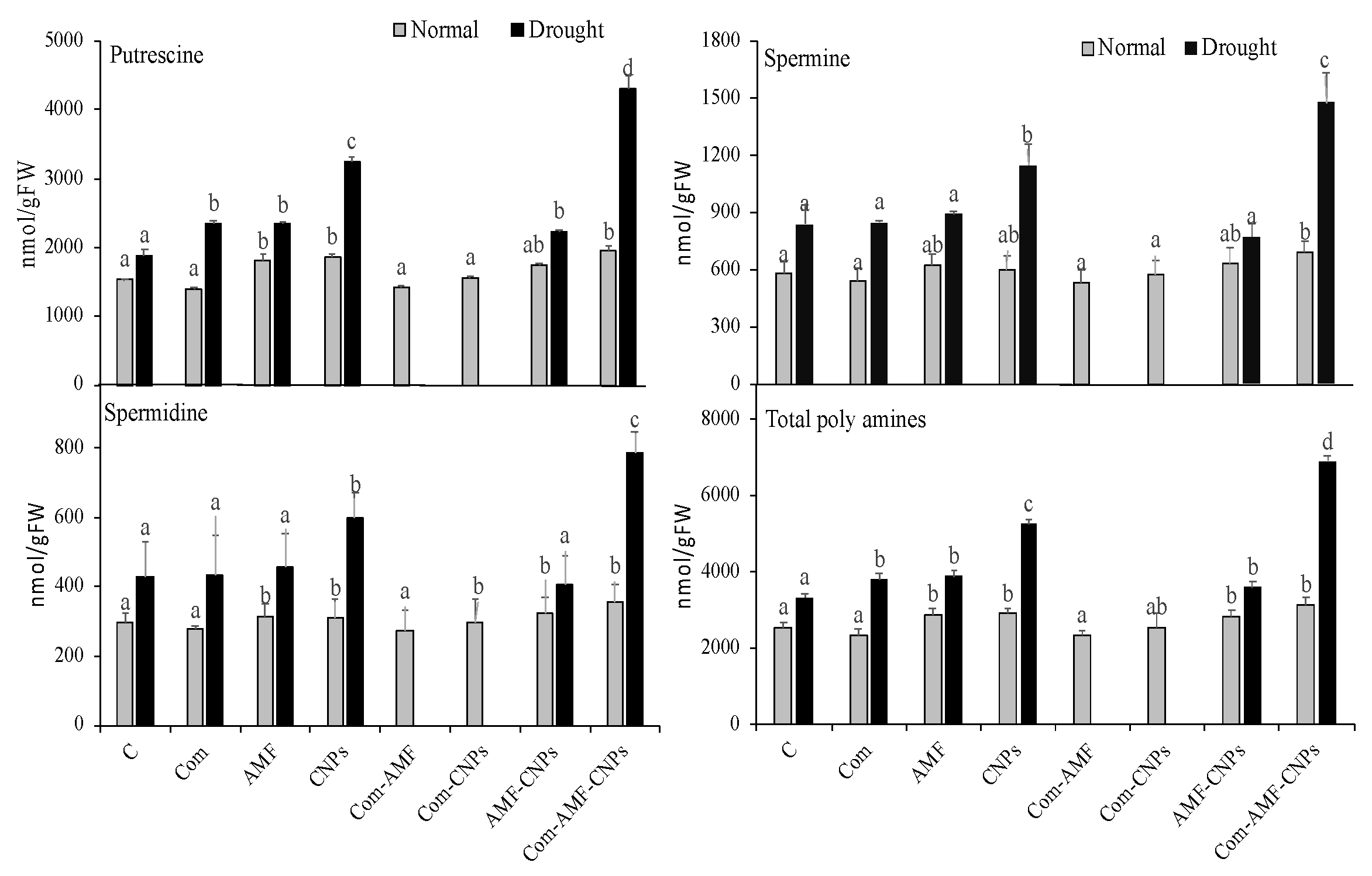
3.7. Polyamine Metabolism
4. Discussion
4.1. Drought Conditions Affect Growth and Metabolism
4.2. Com, AMF, and CNPs Mitigated Drought Stress Effect on Maize Plant Metabolism
4.2.1. Primary Metabolism
4.2.2. Secondary Metabolism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatt, R.M.; Rao, N.K.S. Influence of pod load on response of okra to water stress. Indian J. Plant Physiol. 2005, 10, 54–59. [Google Scholar]
- Cataldo, E.; Fucile, M.; Mattii, G.B. Leaf Eco-Physiological Profile and Berries Technological Traits on Potted Vitis vinifera L. cv Pinot Noir Subordinated to Zeolite Treatments under Drought Stress. Plants 2022, 11, 1735. [Google Scholar] [CrossRef] [PubMed]
- Razmjoo, K.; Heydarizadeh, P.; Sabzalian, M.R. Effect of salinity and drought stresses on growth parameters and essential oil content of Matricaria chamomile. Int. J. Agric. Biol. 2008, 10, 451–454. [Google Scholar]
- Al-Wabel, M.I.; Sallam, A.; Ahmad, M.; Elanazi, K.; Usman, A.R.A. Extent of Climate Change in Saudi Arabia and Its Impacts on Agriculture: A Case Study from Qassim Region. In Environment, Climate, Plant and Vegetation Growth; Fahad, S., Hasanuzzaman, M., Alam, M., Ullah, H., Saeed, M., Khan, I.A., Adnan, M., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S. Response of plants to water stress. Front Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef]
- Chen, T.H.H.; Murata, N. Glycinebetaine: An effective protectant against abiotic stress in plants. Trends Plant Sci. 2008, 13, 499–505. [Google Scholar] [CrossRef]
- Cataldo, E.C.; Salvi, L.S.; Paoli, F.P.; Fucile, M.F.; Masciandaro, G.M.; Manzi, D.M.; Masini, C.M.M.; Mattii, G.B.M. Effects of natural clinoptilolite on physiology, water stress, sugar, and anthocyanin content in Sanforte (Vitis vinifera L.) young vineyard. J. Agric. Sci. 2021, 159, 488–499. [Google Scholar] [CrossRef]
- Chaves, M.M.; Costa, J.M.; Saibo, N.J.M. Recent advances in photosynthesis under drought and salinity. Adv. Bot. Res. 2011, 57, 49–104. [Google Scholar]
- Noiraud, N.; Maurousset, L.; Lemoine, R. Transport of polyols in higher plants. Plant Physiol. Biochem. 2001, 39, 717–728. [Google Scholar] [CrossRef]
- Mekki, A.; Mdhaffar, S.; Sayadi, S. Advance in Mediterranean soil properties followingcompost amendment. Int. J. Agr. Pol. Res. 2014, 2, 373–379. [Google Scholar] [CrossRef]
- Bokobana, A.; Toundou, O.; Odah, K.; Dossou, K.S.; Tozo, K. Enhancement of proline content and antioxidant enzyme activities induced by drought stress in maize (Zea mays L.) by application of compost. Int. J. Biol. Chem. Sci. 2019, 13, 2978–2990. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Jeffries, P.; Gianinazzi, S.; Perotto, S.; Turnau, K.; Barea, J.M. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol. Fert. Soils 2003, 37, 1–16. [Google Scholar] [CrossRef]
- Jeffries, P.; Barea, J.M. 4 Arbuscular Mycorrhiza: A Key Component of Sustainable Plant–Soil Ecosystems. Fung. Assoc. 2012, 9, 51–75. [Google Scholar]
- Anli, M.; Baslam, M.; Tahiri, A.; Raklami, A.; Symanczik, S.; Boutasknit, A.; Ait-El-Mokhtar, M.; Ben-Laouane, R.; Toubali, S.; Ait Rahou, Y.; et al. Biofertilizers as strategies to improve photosynthetic apparatus, growth, and drought stress tolerance in the date palm. Front Plant Sci. 2020, 11, 516818. [Google Scholar] [CrossRef]
- Khodakovskaya, M.V.; Kim, B.; Kim, J.N.; Mohammad, M.A.; Dervishi, E.; Mustafa, T.; Carl, E.; Cernigla, C.E. Carbon nanotubes as plant growth regulators: Effects on tomato growth, reproductive system, and soil microbial community. Small 2013, 9, 115–123. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.C.; Zapata, L.; Chalbi, N.; Carvajal, M. Multiwalled carbon nanotubes enter broccoli cells enhancing growth and water uptake of plants exposed to salinity. J. Nanobiotechnol. 2016, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Majumdar, S.; Servin, A.D.; Pagano, L.; Dhankher, O.P.; White, J.C. Carbon nanomaterials in agriculture: A critical review. Front. Plant Sci. 2016, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Xin, X.; Cao, Y.; Su, D.; Ji, P.; Zhu, Z.; He, Z. Use of Carbon Nanoparticles to Improve Soil Fertility, Crop Growth and Nutrient Uptake by Corn (Zea mays L.). Nanomaterials 2021, 11, 2717. [Google Scholar] [CrossRef]
- Khan, I.; Zakari, A.; Zhang, J.; Dagar, V.; Singh, S.A. Study of trilemma energy balance, clean energy transitions, and economic expansion in the midst of environmental sustainability: New insights from three trilemma leadership. Energy 2022, 248, 123619. [Google Scholar] [CrossRef]
- Al nashwan, O.S.; Al rwis, K.N.; Ghanem, A.M.; Ahamed, S.B.; Aldawdahi, N.M. Maize production strategy in Saudi Arabia. J. Exp. Biol. Agricul. Sci. 2019, 7, 545–553. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Peshev, D.; Zinta, G.; Van den Ende, W.; Janssens, I.A.; Asard, H. Climate extreme effects on the chemical composition of temperate grassland species under ambient and elevated CO2: A comparison of fructan and non-fructan accumulators. PLoS ONE 2014, 9, e92044. [Google Scholar] [CrossRef] [PubMed]
- Vergauwen, R.; Van den Ende, W.; Van Laere, A. The role of fructans in flowering of Campanula rapunculoides. J. Exp. Bot. 2000, 51, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Madany, M.M.Y.; Khalil, R.R. Fenugreek seed extract enhanced the growth of vicia faba and zea mays seedlings. Egyp. J. Bot. 2017, 57, 363–377. [Google Scholar] [CrossRef]
- Almuhayawi, M.S.; Mohamed, M.S.M.; Abdel-Mawgoud, M.; Selim, S.; Al Jaouni, S.K.; AbdElgawad, H. Bioactive Potential of Several Actinobacteria Isolated from Microbiologically Barely Explored Desert Habitat, Saudi Arabia. Biology 2021, 10, 235. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, H.; Ali, A.; Kame, L.S.; Rahmann, C.; Abdel-Moamen, M.A. A modified manta ray foraging optimizer for planning inverter-based photovoltaic with battery energy storage system and wind turbine in distribution networks. IEEE Access 2021, 9, 91062–91079. [Google Scholar] [CrossRef]
- Al-Habeeb, A.; Altwaijri, Y.A.; Al-Subaie, A.S.; Bilal, L.; Almeharish, A.; Sampson, N.A.; Liu, H.; Kessler, R.C. Twelve-month treatment of mental disorders in the Saudi National Mental Health Survey. Int. J. Methods Psychiatr. Res. 2020, 29, e1832. [Google Scholar] [CrossRef]
- Bregoli, A.M.; Scaramagli, S.; Costa, G.; Sabatini, E.; Ziosi, V.; Biondi, S.; Torrigiani, P. Peach (Prunus persica) fruit ripening: Aminoethoxyvinylglycine (AVG) and exogenous polyamines affect ethylene emission and flesh firmness. Physiol. Plant. 2002, 114, 472–481. [Google Scholar] [CrossRef]
- Cason, A.L.; Ikeguchi, Y.; Skinner, C.; Wood, T.C.; Holden, K.R.; Lubs, H.A.; Martinez, F.; Simensen, R.J.; Stevenson, R.E.; Pegg, A.E.; et al. (X-linked spermine synthase gene (SMS) defect: The first polyamine deficiency syndrome. Eur. J. Hum. Genet 2003, 11, 937–944. [Google Scholar] [CrossRef]
- Selmar, I.; Kleinwächter, M. Influencing the product quality by deliberately applying drought stress during the cultivation of medicinal plants. Ind. Crops Prod. 2013, 42, 558–566. [Google Scholar] [CrossRef]
- Ballhorn, D.J.; Pietrowski, A.; Lieberei, R. Direct trade-off between cyanogenesis and resistance to a fungal pathogen in lima bean(PhaseoluslunatusL.). J. Ecol. 2010, 98, 226–236. [Google Scholar] [CrossRef]
- Sengupta, A.; Bamel, U.; Singh, P. Value proposition framework: Implications for employer branding. Decision 2015, 42, 307–323. [Google Scholar] [CrossRef]
- Podlešáková, K.; Ugena, L.; Spíchal, L.L.; Doležal, K.; De Diego, N. Phytohormones and polyamines regulate plant stress responses by altering GABA pathway. New Biotech. 2019, 48, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Sheen, J. Master Regulators in Plant Glucose Signaling Networks. J. Plant Biol. 2014, 57, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Keunen, E.; Peshev, D.; Vangronsveld, J.; Ende, W.; Cuypers, A. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef]
- Pandey, N.; Sharma, C.P. Effect of heavy metals Co2+, Ni2+ and Cd2+ on growth and metabolism of cabbage. Plant Sci. 2002, 163, 753–758. [Google Scholar] [CrossRef]
- Pathak, H.; Jain, N.; Bhatia, A.; Mohanty, S.; Gupta, N. Global warming mitigation potential of biogas plants in India. Environ. Monit. Assess 2009, 157, 407–418. [Google Scholar] [CrossRef]
- Spormann, S.; Soares, C.; Teixeira, J.; Fidalgo, F. Polyamines as key regulatory players in plants under metal stress—A way for an enhanced tolerance. Ann. Appl. Biol. 2021, 178, 209–226. [Google Scholar] [CrossRef]
- Liu, J.; Dietz, T.; Carpenter, S.R.; Alberti, M.; Folke, C.; Moran, E.; Pell, A.N.; Deadman, P.; Kratz, T.; Lubchenco, J.; et al. Complexity of Coupled Human and Natural Systems. Science 2007, 317, 5844. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Warman, P.R. Phytoavailability and fractionation of copper, manganese, and zinc in soil following application of two composts to four crops. Environ. Poll. 2004, 131, 187–195. [Google Scholar] [CrossRef]
- Haghighi, T.M.; Saharkhiz, M.J. Mycorrhizal colonization and silicon nutrition mitigates drought stress in Licorice (Glycyrrhiza glabra L.) with morphophysiological and biochemical perspectives. Ind. Crops Prod. 2022, 178, 114650. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Ukita, M.; Imai, T.; Higuchi, T. Recycling mineral nutrients to farmland via compost application. Water Sci Technol. 2006, 53, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Lakhdar, A.; Hafsi, C.; Rabhi, M.; Debez, A.; Montemurro, F.; Abdelly, C.; Jedidi, N.; Ouerghi, Z. Application of municipal solid waste compost reduces the negative effects of saline water in Hordeum maritimum L. Biores. Technol. 2008, 99, 7160–7167. [Google Scholar] [CrossRef] [PubMed]
- Ghani, M.I.; Ali, A.; Atif, M.J.; Ali, M.; Amin, B.; Cheng, Z. Arbuscular Mycorrhizal Fungi and Dry Raw Garlic Stalk Amendment Alleviate Continuous Monocropping Growth and Photosynthetic Declines in Eggplant by Bolstering its Antioxidant System and Accumulation of Osmolytes and Secondary Metabolites. Front. Plant Sci. 2022, 13, 849521. [Google Scholar] [CrossRef]
- Ming, X.; Xu, F.; Jiang, Y.; Zong, P.; Wang, B.; Li, J.; Qiao, Y.; Tian, Y. Thermal degradation of food waste by TG-FTIR and Py-GC/MS: Pyrolysis behaviors, products, kinetic and thermodynamic analysis. J. Clean. Prod. 2020, 244, 118713. [Google Scholar] [CrossRef]
- Materechera, S.A.; Mkhabela, T.S.; Antony, J. The effectiveness of lime, chicken manure and leaf litter ash in ameliorating acidity in a soil previously under black wattle (Acacia mearnsii) plantation. Bioresour. Technol. 2002, 85, 9–16. [Google Scholar] [CrossRef]
- Schulze, E.D.; Beck, E.; Buchmann, N.; Clemens, S.; Müller-Hohenstein, K.; Scherer-Lorenzen, M. Water deficiency (Drought). In Plant Ecology; Springer: Berlin, Germany, 2019; pp. 165–202. [Google Scholar]
- Gao, F.; Liu, C.; Qu, C.; Zheng, L.; Yang, F.; Su, M.; Hong, F. Was improvement of spinach growth by nano-TiO2 treatment related to the changes of rubisco activase? Biometals 2008, 21, 211–217. [Google Scholar] [CrossRef]
- El-Henawy, A.; El-Sheikh, I.; Hassan, A.; Madein, A.; El-Sheikh, A.; El-Yamany, A.; Radwan, A.; Mohamed, F.; Khamees, M.; Ramadan, M.; et al. Response of cultivated broccoli and red cabbage crops to mineral, organic and nano-fertilizers. Environ. Biodiv. Soil Secur. 2018, 2, 221–223. [Google Scholar] [CrossRef]
- Kiers, E.K.; Duhamel, M.; Beesetty, Y.; Mensah, J.A.; Franken, O.; Verbruggen, E.; Fellbaum, C.R.; Kowalchuk, G.A.; Hart, M.M.; Bago, A.; et al. Reciprocal Rewards Stabilize Cooperation in the Mycorrhizal Symbiosis. Science 2011, 333, 880–882. [Google Scholar] [CrossRef]
- Lahiani, M.H.M.; Dervishi, E.; Chen, J.; Nima, Z.; Gaume, A.; Biris, A.S.; Khodakovskaya, M.V. Impact of Carbon Nanotube Exposure to Seeds of Valuable Crops. ACS Appl. Mater. 2013, 5, 7965–7973. [Google Scholar] [CrossRef]
- Wang, B.; Lucy, K.A.; Schuman, J.S.; Sigal, I.A.; Bilonick, R.A.; Lu, C.; Liu, J.; Grulkowski, J.; Nadler, Z.; Ishikawa, H.; et al. Tortuous Pore Path Through the Glaucomatous Lamina Cribros. Sci. Rep. 2018, 8, 7281. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Nevins, J.E.; Nadler, Z.; Wollstein, G.; Ishikawa, H.; Bilonick, R.A.; Kagemann, L.; Sigal, I.A.; Grulkowski, I.; Liu, J.J.; et al. Reproducibility of in-vivo OCT measured three-dimensional human lamina cribrosa microarchitecture. PLoS ONE 2014, 189, e95526. [Google Scholar] [CrossRef] [PubMed]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of sugars under abiotic stress. Plant Phys. Bioch. 2016, 109, 54–61. [Google Scholar] [CrossRef]
- Lancien, M.; Gadal, P.; Hodges, M. Enzyme Redundancy and the Importance of 2-Oxoglutarate in Higher Plant Ammonium Assimilation. Plant Physiol. 2000, 123, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Malundo, T.M.M.; Shewfelt, R.L.; Ware, G.O.; Baldwin, E.A. Sugars and Acids Influence Flavor Properties of Mango (Mangifera indica). J. Amer. Soc. Hort. Sci. 2001, 126, 115–121. [Google Scholar] [CrossRef]
- Obaid, M.M.; Ibrahim, I.; Udin, N.M. Zakat and Tax Compliance Behaviour in Yemen: A Conceptual Study. J. Adv. Res. Bus. Manag. Stud. 2020, 19, 1–14. [Google Scholar] [CrossRef]
- Ruiz-Lozano, M.; Azcon, R.; Gomez, M. Effects of arbuscular-mycorrhizal glomus species on drought tolerance: Physiological and nutritional plant responses. Appl. Environ. Microbiol. 1995, 61, 456–460. [Google Scholar] [CrossRef]
- Azcón, R.; Gómez, M.; Tobar, R. Physiological and nutritional responses by Lactuca sativa L. to nitrogen sources and mycorrhizal fungi under drought conditions. Biol. Fertil. Soils 1996, 22, 156–161. [Google Scholar] [CrossRef]
- Yooyongwech, S.; Phaukinsang, N.; Cha-um, S. Supaibulwatana, K. Arbuscular mycorrhiza improved growth performance in Macadamia tetraphylla L. grown under water deficit stress involves soluble sugar and proline accumulation. Plant Growth Regul. 2013, 69, 285–293. [Google Scholar] [CrossRef]
- Aroca, R.; Vernieri, P.; Ruiz-Lozano, J.M. Mycorrhizal and non-mycorrhizal Lactuca sativa plants exhibit contrasting responses to exogenous ABA during drought stress and recovery. J. Exp. Bot. 2008, 59, 2029–2041. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abbaspour, H.; Saeid-Sar, S.; Afshari, H.; Abdel-Wahhab, M.A. Tolerance of mycorrhiza infected Pistachio (Pistacia vera L.) seedlings to drought stress under glasshouse conditions. J. Plant Physiol. 2012, 169, 704–709. [Google Scholar] [CrossRef]
- Asrar, G.R.; Ryabinin, V.; Detemmerman, V. Climate science and services: Providing climate information for adaptation, sustainable development and risk management. Curr. Opin. Environ. Sustain. 2012, 4, 88–100. [Google Scholar] [CrossRef]
- Doubková, P.; Vlasáková, E.; Sudová, R. Arbuscular mycorrhizal symbiosis alleviates drought stress imposed on Knautia arvensis plants in serpentine soil. Plant Soil 2013, 370, 149–161. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef]
- Galazzi, A.; Adamini, I.; Consonni, D.; Roselli, P.; Rancati, D.; Ghilardi, G.; Greco, G.; Salinaro, G.; Laquintana, D. Accidental removal of devices in intensive care unit: An eight-year observational study. Intensive Crit. Care Nurs. 2019, 54, 34–38. [Google Scholar] [CrossRef]
- Kaliamurthi, S.; Selvaraj, G.; Hou, L.; Li, Z.; Wei, Y.; Gu, K.; Wei, D. Synergism of essential oils with lipid based nanocarriers: Emerging trends in preservation of grains and related food products. Grain Oil Sci. Technol. 2019, 2, 21–26. [Google Scholar] [CrossRef]
- Al Jaouni, S.; Selim, S.; Hassan, S.H.; Mohamad, H.S.H.; Wadaan, M.A.M.; Hozzein, W.N.; Asard, H.; AbdElgawad, H. Vermicompost Supply Modifies Chemical Composition and Improves Nutritive and Medicinal Properties of Date Palm Fruits from Saudi Arabia. Front. Plant Sci. 2019, 10, 424. [Google Scholar] [CrossRef]
- Lotfabadi, E.; Weisany, W.; Tahir, A.N.; Torkashvand, M.A. Arbuscular mycorrhizal fungi species improve the fatty acids profile and nutrients status of soybean cultivars grown under drought stress. J. Appl. Microbiol. 2022, 132, 2177–2188. [Google Scholar] [CrossRef]
- Bais, P.H.; Ravishankar, G. Role of polyamines in the ontogeny of plants and their biotechnological applications. Plant Cell Tissue Organ Cult. 2022, 69, 1–34. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fujita, M.; Oku, H.; Islam, M.T. Plant Tolerance to Environmental Stress: Role of Phytoprotectants, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar] [CrossRef]
- Niemi, K.; Vuorinen, T.; Ernstsen, A.; Haggman, H. Ectomycorrhizal fungi and exogenous auxins influence root and mycorrhiza formation Of Scots pine hypocotyl cuttings in vitro. Tree Physiol. 2002, 22, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Sarjala, T. Effect of organic and inorganic nitrogen sources on endogenous polyamines and growth of ectomycorrhizal fungi in pure culture. Mycorrhiza 1999, 8, 277–281. [Google Scholar] [CrossRef]
| Glucose mg/g FW | Fructose mg/g FW | Sucrose ug/g FW | Total S Sugars | Sucrose P Synthase | Invertase | Sucrose Synthase | |
|---|---|---|---|---|---|---|---|
| C | 2.11 ± 0.03 a | 0.86 ± 0.1 a | 2.24 ± 0.13 ac | 4.18 ± 0.13 c | 0.27 ± 0 d | 0.6 ± 0.01 d | 6.19 ± 0.81 c |
| Com | 2.1 ± 0.01 a | 0.96 ± 0.11 a | 2.34 ± 0.16 c | 4.31 ± 0.09 c | 0.26 ± 0 d | 0.55 ± 0 d | 6.69 ± 0.88 c |
| AMF | 2.64 ± 0.13 a | 1.2 ± 0.12 a | 2.61 ± 0.22 c | 5.38 ± 0.17 b | 0.32 ± 0 c | 0.61 ± 0.03 d | 6.96 ± 0.71 c |
| CNPs | 2.59 ± 0.01 a | 1.09 ± 0.13 a | 2.29 ± 0.13 c | 5.12 ± 0.12 b | 0.25 ± 0 d | 0.65 ± 0 d | 4.78 ± 0.63 e |
| Com-AMF | 2.28 ± 0.02 a | 1.19 ± 0.14 a | 2.81 ± 0.23 b | 4.92 ± 0.09 c | 0.26 ± 0 d | 0.56 ± 0 d | 6.78 ± 0.89 d |
| Com-CNPs | 2.5 ± 0.03 a | 1.17 ± 0.14 a | 3.2 ± 0.06 b | 5.55 ± 0.23 b | 0.42 ± 0 c | 0.9 ± 0.01 c | 7.32 ± 0.98 d |
| AMF-CNPs | 2.21 ± 0.02 a | 1.03 ± 0.12 a | 2.6 ± 0.13 c | 4.67 ± 0.12 c | 0.31 ± 0 c | 0.68 ± 0 d | 7.73 ± 1.02 d |
| Com-AMF-CNPs | 2.1 ± 0.05 a | 0.98 ± 0.11 a | 2.93 ± 0.07 c | 4.95 ± 0.24 c | 0.34 ± 0 c | 1.12 ± 0.02 c | 6.26 ± 0.8 c |
| D | 1.9 ± 0.04 a | 0.86 ± 0.1 a | 3.06 ± 0.12 b | 4.75 ± 0.35 c | 0.5 ± 0 b | 1.41 ± 0.03 b | 6.21 ± 0.79 c |
| D-Com | 2.33 ± 0.08 a | 1.43 ± 0.21 b | 4.27 ± 0.28 a | 6.52 ± 0.63 a | 0.55 ± 0.02 b | 1.6 ± 0.06 a | 6.56 ± 0.84 c |
| D-AMF | 1.9 ± 0.02 a | 1.22 ± 0.15 a | 4.06 ± 0.15 b | 5.77 ± 0.43 b | 0.59 ± 0 b | 1.82 ± 0.02 a | 6.39 ± 0.84 c |
| D-CNPs | 2.56 ± 0.03 a | 1.14 ± 0.11 a | 3.89 ± 0.14 b | 6.13 ± 0.32 a | 0.6 ± 0.02 b | 1.4 ± 0.1 b | 8.59 ± 1.42 b |
| D-Com-AMF | 2.67 ± 0.05 a | 1.2 ± 0.13 a | 4.4 ± 0.2 a | 6.78 ± 0.53 a | 0.86 ± 0.01 a | 2.14 ± 0.05 a | 12.52 ± 1.61 a |
| D-Com-AMF-CNPs | 2.82 ± 0.01 a | 1.47 ± 0.18 b | 4.67 ± 0.07 a | 7.18 ± 0.42 a | 0.7 ± 0 b | 1.8 ± 0.01 a | 13.05 ± 1.72 a |
| Oxalic Acid | Malic Acid | Succinic Acid | Citric Acid | Isobutyric Acid | Fumaric Acid | |
|---|---|---|---|---|---|---|
| C | 2.36 ± 0.03 a | 9.41 ± 0.1 b | 2.33 ± 0.04 b | 1.25 ± 0.02 d | 3.92 ± 0.07 a | 0.31 ± 0 a |
| Com | 1.97 ± 0.02 a | 7.25 ± 0.08 c | 1.96 ± 0.02 b | 3.27 ± 0.03 b | 2.69 ± 0.03 b | 0.27 ± 0 a |
| AMF | 2.4 ± 0.02 a | 10.7 ± 0.13 b | 1.92 ± 0.02 b | 2.37 ± 0.04 c | 3.49 ± 0.04 a | 0.27 ± 0 a |
| CNPs | 1.11 ± 0.02 b | 13.24 ± 0.14 a | 2.04 ± 0.08 b | 4.41 ± 0.05 a | 2.64 ± 0.03 b | 0.26 ± 0 a |
| Com-AMF | 2.94 ± 0.03 a | 14.76 ± 0.16 a | 1.33 ± 0.28 c | 1.28 ± 0.02 d | 1.93 ± 0.02 b | 0.21 ± 0 a |
| Com-CNPs | 2.23 ± 0.02 a | 11.06 ± 0.12 b | 1.29 ± 0.02 c | 2.71 ± 0.03 c | 3.2 ± 0.03 a | 0.22 ± 0 a |
| AMF-CNPs | 2.25 ± 0.02 a | 10.59 ± 0.11 b | 2.04 ± 0.08 b | 1.3 ± 0.02 d | 3.45 ± 0.14 a | 0.27 ± 0 a |
| Com-AMF-CNPs | 2.35 ± 0.03 a | 10.38 ± 0.11 b | 2.12 ± 0.08 b | 1.35 ± 0.02 d | 3.6 ± 0.15 a | 0.29 ± 0 a |
| D | 1.19 ± 0.02 b | 14.1 ± 0.15 a | 1.74 ± 0.05 b | 2.47 ± 0.02 c | 2.81 ± 0.03 b | 0.28 ± 0 a |
| D-Com | 1.98 ± 0.02 a | 16.9 ± 0.18 a | 1.63 ± 0.06 b | 5.36 ± 0.06 a | 2.81 ± 0.03 | 0.28 ± 0 a |
| D-AMF | 2.46 ± 0.03 a | 12.37 ± 0.13 b | 1.7 ± 0.04 b | 2.6 ± 0.05 c | 2.73 ± 0.11 b | 0.26 ± 0 a |
| D-CNPs | 1.26 ± 0.05 b | 9.53 ± 0.18 b | 0.91 ± 0.04 c | 1.04 ± 0.01 d | 2.26 ± 0.11 b | 0.19 ± 0 a |
| D-Com-AMF | 2.68 ± 0.03 a | 11.59 ± 0.12 b | 1.44 ± 0.02 b | 2.79 ± 0.03 c | 3.27 ± 0.04 a | 0.22 ± 0 a |
| D-Com-AMF-CNPs | 2.22 ± 0.13 a | 12.3 ± 0.76 b | 3.34 ± 0.13 a | 2.14 ± 0.03 c | 2.38 ± 0.12 b | 0.28 ± 0.02 a |
| Glycine | Lysine | Histidine | Alanine | Arginie | Ornithine | Glutamine | Asparagine | Isoleucine | Leucine | Mean | Threonine | Valine | Serine | Phenylalanine | GlutamIic acid | Aspartate | Cystine | Mean | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | 59.02 ± 0.38 b | 3.61 ± 0.05 c | 1.39 ± 0.02 b | 4.43 ± 0.03 d | 0.55 ± 0.01 b | 0.28 ± 0.02 c | 0.83 ± 0.03 c | 0.66 ± 0 c | 0.1 ± 0 d | 0.03 ± 0 d | 0.05 ± 0 d | 0.23 ± 0.02 e | 0.38 ± 0 c | 0.41 ± 0.03 b | 0.59 ± 0 d | 0.11 ± 0 e | 0.06 ± 0.03 c | 0.16 ± 0.02 | 0.36 ± 0 c |
| Com | 39.08 ± 0.25 c | 2.81 ± 0.03 d | 1.59 ± 0.08 b | 11.86 ± 0.08 b | 1.44 ± 0.1 a | 0.26 ± 0.01 d | 0.36 ± 0.01 d | 1.23 ± 0.01 a | 0.17 ± 0 d | 0.03 ± 0 d | 0.08 ± 0.01 c | 0.48 ± 0.04 c | 0.81 ± 0.01 a | 0.29 ± 0.01 c | 0.47 ± 0.03 d | 0.61 ± 0 c | 0.09 ± 0 c | 0.22 ± 0.02 c | 0.38 ± 0 c |
| AMF | 43.11 ± 0.4 c | 4.6 ± 0.04 b | 1.72 ± 0.05 a | 18.69 ± 0.15 a | 1.47 ± 0.05 a | 0.45 ± 0.05 b | 1.55 ± 0.07 b | 0.79 ± 0.06 c | 0.48 ± 0 b | 0.07 ± 0 c | 0.07 ± 0.01 c | 0.46 ± 0.07 c | 0.75 ± 0.03 a | 0.51 ± 0.03 a | 0.71 ± 0.05 c | 0.8 ± 0.01 c | 0.14 ± 0.01 b | 0.37 ± 0.05 b | 0.61 ± 0.01 a |
| CNPs | 35.18 ± 0.23 c | 2.47 ± 0.05 d | 1.78 ± 0.05 a | 6.21 ± 0.05 c | 1.24 ± 0.01 a | 0.52 ± 0.04 b | 1.51 ± 0.09 b | 2.28 ± 0.03 a | 0.8 ± 0.01 a | 0.29 ± 0.01 a | 0.41 ± 0.02 a | 0.66 ± 0.02 b | 0.43 ± 0 b | 0.31 ± 0.02 c | 0.88 ± 0.05 c | 0.52 ± 0.04 d | 0.23 ± 0.01 b | 0.56 ± 0.04 a | 0.72 ± 0.01 a |
| Com-AMF | 43.9 ± 0.3 c | 6.5 ± 0.04 a | 1.59 ± 0.07 b | 9.66 ± 0.07 b | 1.48 ± 0.01 a | 0.29 ± 0.01 c | 1.22 ± 0.01 b | 0.73 ± 0.01 c | 0.18 ± 0 d | 0.04 ± 0 d | 0.04 ± 0 d | 0.17 ± 0.01 e | 0.52 ± 0.04 | 0.24 ± 0 c | 0.44 ± 0.04 d | 0.72 ± 0 c | 0.08 ± 0 c | 0.38 ± 0.06 b | 0.78 ± 0.01 a |
| Com-CNPs | 73.3 ± 0.47 a | 4.27 ± 0.05 c | 1.76 ± 0.05 a | 12.87 ± 0.08 b | 1.11 ± 0.01 a | 0.39 ± 0.01 c | 0.88 ± 0.02 c | 0.63 ± 0.01 c | 0.2 ± 0 d | 0.09 ± 0 c | 0.08 ± 0 d | 0.37 ± 0.03 d | 0.71 ± 0.01 a | 0.52 ± 0.03 a | 0.52 ± 0.04 d | 0.52 ± 0.04 d | 0.16 ± 0 b | 0.45 ± 0.06 b | 0.91 ± 0.01 a |
| AMF-CNPs | 48.44 ± 0.31 b | 3.4 ± 0.04 c | 1.24 ± 0.02 b | 7.57 ± 0.05 c | 1.55 ± 0.01 a | 0.33 ± 0.01 c | 1.36 ± 0 b | 0.5 ± 0.01 c | 0.28 ± 0.01 c | 0.37 ± 0.01 a | 0.22 ± 0 b | 0.34 ± 0.04 d | 0.75 ± 0.01 b | 0.32 ± 0.01 b | 0.71 ± 0.04 c | 0.88 ± 0 c | 0.11 ± 0 c | 0.32 ± 0.04 b | 0.62 ± 0 a |
| Com-AMF-CNPs | 54.62 ± 0.35 b | 3.72 ± 0.06 c | 1.94 ± 0.05 a | 5.97 ± 0.04 d | 1.11 ± 0.01 a | 0.43 ± 0.01 b | 2.78 ± 0.19 a | 0.71 ± 0 c | 0.29 ± 0.01 c | 0.37 ± 0.01 a | 0.51 ± 0.03 a | 0.24 ± 0.03 e | 0.52 ± 0.04 b | 0.29 ± 0.01 c | 0.66 ± 0.05 c | 0.95 ± 0.01 c | 0.11 ± 0 c | 0.29 ± 0.03 c | 0.54 ± 0 b |
| D | 68.44 ± 0.43 a | 5.45 ± 0.08 b | 2.25 ± 0.03 a | 16.78 ± 0.11 a | 0.76 ± 0.05 b | 0.61 ± 0.01 a | 0.76 ± 0.02 c | 0.57 ± 0.01 c | 0.24 ± 0 c | 0.12 ± 0 b | 0.11 ± 0.02 c | 0.66 ± 0.07 b | 0.52 ± 0.04 b | 0.62 ± 0.03 a | 1.39 ± 0.06 b | 1.4 ± 0.01 c | 0.2 ± 0 b | 0.43 ± 0.06 b | 0.88 ± 0.01 a |
| D-Com | 72.01 ± 0.48 a | 5.78 ± 0.04 b | 1.33 ± 0.02 b | 17.52 ± 0.12 a | 1.28 ± 0 | 0.58 ± 0.01 a | 0.76 ± 0.02 c | 0.57 ± 0.01 c | 0.24 ± 0 c | 0.12 ± 0 d | 0.11 ± 0 c | 0.66 ± 0.07 b | 1.28 ± 0.01 a | 0.62 ± 0.03 a | 1.7 ± 0.09 a | 2 ± 0.01 a | 0.33 ± 0.01 a | 0.43 ± 0.06 b | 0.88 ± 0.01 a |
| D-AMF | 62.67 ± 0.42 a | 3.26 ± 0.08 c | 2.79 ± 0.07 a | 10.93 ± 0.08 b | 0.81 ± 0.01 b | 0.82 ± 0.05 a | 0.76 ± 0.01 c | 0.92 ± 0.02 b | 0.42 ± 0 b | 0.37 ± 0.01 a | 0.53 ± 0.03 a | 0.95 ± 0.03 a | 0.85 ± 0.01 a | 0.51 ± 0.02 a | 1.09 ± 0.06 b | 1.32 ± 0.01 c | 0.25 ± 0.01 b | 0.52 ± 0.04 a | 0.69 ± 0 a |
| D-CNPs | 50.74 ± 0.1 b | 6.25 ± 0.12 a | 1.44 ± 0.03 b | 11.59 ± 0.1 b | 0.45 ± 0.02 b | 0.42 ± 0.01 b | 1.07 ± 0.04 b | 1.02 ± 0.09 b | 0.15 ± 0 d | 0.29 ± 0 a | 0.13 ± 0 c | 0.49 ± 0.04 c | 0.87 ± 0.01 a | 0.31 ± 0.03 c | 1.26 ± 0.12 b | 1.71 ± 0.05 a | 0.2 ± 0 b | 0.5 ± 0.05 a | 0.57 ± 0 b |
| D-Com-AMF | 54.94 ± 0.35 b | 5.74 ± 0.05 b | 1.31 ± 0.02 b | 12.37 ± 0.08 b | 0.58 ± 0.03 b | 0.52 ± 0.08 a | 1.37 ± 0.09 b | 1.81 ± 0.01 a | 0.14 ± 0 d | 0.12 ± 0 d | 0.14 ± 0.01 c | 0.57 ± 0.04 c | 0.89 ± 0.01 a | 0.63 ± 0.05 a | 1.73 ± 0.06 a | 1.5 ± 0.01 a | 0.24 ± 0 b | 0.39 ± 0.03 b | 0.62 ± 0 a |
| D-Com-AMF-CNPs | 62.24 ± 3.34 a | 6.93 ± 0.42 a | 2.18 ± 0.01 a | 18.91 ± 0.13 a | 0.66 ± 0.06 b | 0.67 ± 0.08 a | 1.19 ± 0.05 b | 1.33 ± 0.08 a | 0.11 ± 0 d | 0.24 ± 0 b | 0.11 ± 0 | 0.5 ± 0.05 c | 0.95 ± 0.05 a | 0.55 ± 0.06 a | 2.02 ± 0.07 a | 2.08 ± 0.11 a | 0.3 ± 0.02 a | 0.64 ± 0.08 a | 0.61 ± 0.04 a |
| Proline | P5CS | P5CR | OAT | PRODH | |
|---|---|---|---|---|---|
| C | 2.23 ± 0.06 c | 2.73 ± 0.04 b | 0.41 ± 0.01 b | 3.56 ± 0.06 d | 5.42 ± 0.09 c |
| Com | 2.43 ± 0.11 c | 2.73 ± 0.02 b | 0.41 ± 0.02 b | 3.54 ± 0.03 d | 5.47 ± 0.04 c |
| AMF | 2.92 ± 0.14 c | 2.88 ± 0.12 b | 0.43 ± 0.02 b | 3.77 ± 0.16 d | 5.74 ± 0.23 c |
| CNPs | 2.98 ± 0.12 c | 2.39 ± 0.01 b | 0.26 ± 0.01 c | 2.72 ± 0.01 d | 4.05 ± 0.02 d |
| Com-AMF | 2.25 ± 0.1 c | 2.25 ± 0.02 c | 0.39 ± 0.02 b | 3.58 ± 0.02 d | 5.52 ± 0.04 c |
| Com-CNPs | 2.26 ± 0.1 c | 2.57 ± 0.03 b | 0.43 ± 0.02 b | 4.13 ± 0.04 c | 5.99 ± 0.06 c |
| AMF-CNPs | 2.69 ± 0.12 c | 2.23 ± 0.02 c | 0.44 ± 0.02 b | 4.12 ± 0.03 c | 6.33 ± 0.05 c |
| Com-AMF-CNPs | 2.72 ± 0.16 c | 2.48 ± 0.05 b | 0.37 ± 0.02 b | 3.52 ± 0.08 d | 4.84 ± 0.1 c |
| D | 5.15 ± 0.12 a | 3.64 ± 0.07 b | 0.45 ± 0.01 b | 4.05 ± 0.08 c | 5.52 ± 0.1 c |
| D-Com | 5.15 ± 0.11 a | 3.37 ± 0.11 b | 0.48 ± 0.01 b | 4.47 ± 0.15 c | 6.25 ± 0.21 c |
| D-AMF | 6.01 ± 0.27 a | 3.86 ± 0.03 b | 0.44 ± 0.02 b | 4.13 ± 0.04 c | 5.28 ± 0.05 c |
| D-CNPs | 5.01 ± 0.04 b | 3.66 ± 0.15 b | 0.57 ± 0.01 b | 5.92 ± 0.44 c | 8.47 ± 0.64 b |
| D-Com-AMF | 6.74 ± 0.14 a | 4.72 ± 0.1 a | 0.81 ± 0.02 a | 9.27 ± 0.19 a | 13.3 ± 0.27 a |
| D-Com-AMF-CNPs | 7.04 ± 0.23 a | 5.77 ± 0.02 a | 0.85 ± 0.03 a | 7.5 ± 0.03 b | 10.86 ± 0.05 b |
| Myristic (C14:0) | Palmitic (C16:0) | Heptadecanoic (C17:0) | Stearic (C18:0) | Arachidic (C20:0) | Docosanoic (C22:0) | Tricosanoic (C23:0) | Pentacosanoic (C25:0) | Palmitoleic (C16:1) | Heptadecenoic (C17:1) | Oleic (C18:1) | Linoleic (C18:2) | Linolenic (C18:3 ω−3) | Eicosenoic (C20:1) | Eicosenoic (C20:1) | Tetracosenoic (C24:1) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | 0.34 ± 0 c | 19.95 ± 0.44 c | 0.03 ± 0 b | 1.19 ± 0.02 c | 1.16 ± 0.02 b | 0.52 ± 0.01 c | 0.022 ± 0 c | 0.01 ± 0 c | 0.08 ± 0 c | 0.15 ± 0.01 c | 37.03 ± 0.31 c | 0.014 ± 0 c | 0.11 ± 0.02 c | 0.91 ± 0.03 b | 0.06 ± 0 b | 0.01 ± 0 a |
| Com | 0.4 ± 0 b | 19.87 ± 0.1 c | 0.03 ± 0 b | 1.54 ± 0.03 b | 1.16 ± 0.01 b | 0.55 ± 0.01 c | 0.027 ± 0 c | 0.03 ± 0 c | 0.07 ± 0 c | 0.15 ± 0.01 c | 33.7 ± 0.54 c | 0.021 ± 0 b | 0.021 ± 0 b | 0.9 ± 0.01 b | 0.06 ± 0 b | 0.02 ± 0 |
| AMF | 0.46 ± 0.02 b | 19.12 ± 0.62 c | 0.04 ± 0 b | 1.47 ± 0.04 b | 1.46 ± 0.07 b | 0.68 ± 0.04 b | 0.034 ± 0 c | 0.02 ± 0 c | 0.07 ± 0 c | 0.16 ± 0.01 c | 43.88 ± 2.23 c | 0.02 ± 0 b | 0.02 ± 0 b | 0.97 ± 0.01 b | 0.06 ± 0 b | 0.016 ± 0 a |
| CNPs | 0.5 ± 0 b | 17.88 ± 0.28 c | 0.03 ± 0 b | 1.81 ± 0.04 b | 1.43 ± 0.01 b | 0.64 ± 0.01 b | 0.036 ± 0 c | 0.02 ± 0 c | 0.08 ± 0 c | 0.11 ± 0.01 c | 40.1 ± 0.4 c | 0.018 ± 0 b | 0.013 ± 0 c | 0.51 ± 0.03 c | 0.04 ± 0 c | 0.015 ± 0 a |
| Com-AMF | 0.36 ± 0 c | 17.06 ± 0.14 c | 0.03 ± 0 b | 1.5 ± 0.04 b | 1.26 ± 0.01 b | 0.69 ± 0.01 b | 0.027 ± 0 c | 0.01 ± 0 c | 0.07 ± 0 c | 0.15 ± 0.01 c | 34.4 ± 0.55 c | 0.022 ± 0 b | 0.022 ± 0 b | 0.98 ± 0.02 b | 0.06 ± 0 b | 0.016 ± 0 a |
| Com-CNPs | 0.35 ± 0 c | 18.52 ± 0.18 c | 0.07 ± 0 a | 1.37 ± 0.02 b | 1.38 ± 0.01 b | 0.62 ± 0.03 b | 0.05 ± 0 b | 0.01 ± 0 c | 0.1 ± 0 b | 0.17 ± 0.01 c | 37.36 ± 0.65 c | 0.016 ± 0 b | 0.02 ± 0 b | 1.13 ± 0.01 b | 0.07 ± 0 b | 0.017 ± 0 a |
| AMF-CNPs | 0.43 ± 0 b | 16.81 ± 0.21 c | 0.04 ± 0 b | 1.7 ± 0.04 b | 1.22 ± 0.01 b | 0.59 ± 0.01 c | 0.034 ± 0 b | 0.03 ± 0 c | 0.08 ± 0 c | 0.18 ± 0.01 b | 42.09 ± 0.67 c | 0.02 ± 0 b | 0.021 ± 0 a | 1.11 ± 0.05 b | 0.07 ± 0 b | 0.018 ± 0 a |
| Com-AMF-CNPs | 0.33 ± 0 c | 19.22 ± 0.16 c | 0.07 ± 0 a | 1.21 ± 0.03 c | 1.15 ± 0.02 c | 0.52 ± 0.01 c | 0.05 ± 0 b | 0.02 ± 0 c | 0.13 ± 0.01 b | 0.14 ± 0.01 c | 50.45 ± 3.64 b | 0.021 ± 0 b | 0.023 ± 0 b | 1.3 ± 0.06 b | 0.05 ± 0 b | 0.018 ± 0 a |
| D | 0.58 ± 0.01 b | 30.15 ± 0.88 b | 0.08 ± 0 a | 2.11 ± 0.03 a | 1.03 ± 0.02 c | 0.52 ± 0.01 c | 0.06 ± 0 b | 0.012 ± 0 b | 0.18 ± 0 a | 0.15 ± 0 c | 52.4 ± 2.41 b | 0.02 ± 0 b | 0.015 ± 0 b | 0.99 ± 0.03 b | 0.06 ± 0 b | 0.017 ± 0 a |
| D-Com | 1.02 ± 0.01 a | 30.64 ± 1.05 b | 0.11 ± 0 a | 2.5 ± 0.04 a | 1.04 ± 0.01 c | 0.7 ± 0.01 b | 0.07 ± 0.01 a | 0.017 ± 0 b | 0.22 ± 0.01 a | 0.14 ± 0.01 c | 60.3 ± 0.61 a | 0.012 ± 0 c | 0.017 ± 0 b | 1.02 ± 0.04 b | 0.06 ± 0 b | 0.017 ± 0 a |
| D-AMF | 1.15 ± 0.04 a | 33.77 ± 1.83 b | 0.11 ± 0.01 a | 2.98 ± 0.07 a | 1.21 ± 0.03 b | 0.88 ± 0.03 b | 0.08 ± 0.01 a | 0.014 ± 0 b | 0.17 ± 0 a | 0.19 ± 0.01 b | 64.18 ± 3.78 a | 0.019 ± 0 b | 0.02 ± 0 b | 1.23 ± 0.03 b | 0.08 ± 0 b | 0.02 ± 0 a |
| D-CNPs | 0.91 ± 0.04 a | 33.58 ± 1.62 b | 0.12 ± 0.01 a | 2.35 ± 0.1 a | 2 ± 0.1 a | 0.99 ± 0.05 a | 0.07 ± 0 a | 0.021 ± 0 a | 0.2 ± 0.01 a | 0.25 ± 0 a | 85.29 ± 5.56 a | 0.035 ± 0 a | 0.03 ± 0 a | 1.57 ± 0.04 b | 0.12 ± 0 a | 0.02 ± 0 a |
| D-Com-AMF | 0.95 ± 0.01 a | 36.2 ± 0.4 b | 0.13 ± 0 a | 3.01 ± 0.04 a | 1.43 ± 0.03 b | 0.99 ± 0.08 a | 0.09 ± 0 a | 0.021 ± 0 a | 0.21 ± 0.01 a | 0.3 ± 0.01 a | 51.05 ± 8.84 b | 0.046 ± 0 a | 0.032 ± 0 a | 2.31 ± 0.08 a | 0.14 ± 0 a | 0.03 ± 0 a |
| D-Com-AMF-CNPs | 1.11 ± 0.04 a | 42.73 ± 0.78 a | 0.1 ± 0 a | 3.1 ± 0.1 a | 2.13 ± 0.01 a | 1.36 ± 0.08 a | 0.059 ± 0 b | 0.033 ± 0 a | 0.17 ± 0 a | 0.28 ± 0.01 a | 55.24 ± 1.95 b | 0.037 ± 0 a | 0.037 ± 0 a | 1.81 ± 0.08 a | 0.11 ± 0 a | 0.03 ± 0 a |
| Orinthnine Decarboxylase (µ mol/mg Protein min) | S-adenosyl-L-Methionine Decarboxylase (µ mol/mg Protein min) | Spermidine (Spd) Synthase (µ mol/mg Protein min) | Spd Synthase (µ mol/mg Protein min) | |
|---|---|---|---|---|
| C | 0.12 ± 0 c | 13.28 ± 0.28 d | 38.64 ± 0.29 c | 17.99 ± 0.06 c |
| Com | 0.1 ± 0 c | 18.73 ± 0.14 c | 35.23 ± 0.55 c | 16.54 ± 0.13 c |
| AMF | 0.12 ± 0 c | 18.12 ± 0.62 c | 41.92 ± 0.61 b | 19.06 ± 0.1 c |
| CNPs | 0.13 ± 0 c | 17.13 ± 0.25 c | 41.5 ± 0.41 b | 18.54 ± 0.08 c |
| Com-AMF | 0.11 ± 0 b | 16.21 ± 0.12 c | 36.1 ± 0.56 c | 16.34 ± 0.11 c |
| Com-CNPs | 0.17 ± 0 a | 17.56 ± 0.18 c | 39.23 ± 0.64 c | 17.75 ± 0.15 c |
| AMF-CNPs | 0.13 ± 0 b | 17.78 ± 0.46 c | 43.9 ± 0.7 b | 19.38 ± 0.18 c |
| Com-AMF-CNPs | 0.21 ± 0.01 a | 18.36 ± 0.13 c | 48.83 ± 1.7 b | 21.74 ± 0.36 c |
| D | 0.28 ± 0 a | 27.68 ± 0.47 b | 55.82 ± 0.49 b | 26 ± 0.08 c |
| D-Com | 0.35 ± 0.01 a | 24.03 ± 0.44 c | 58.93 ± 1.25 b | 26.03 ± 0.29 c |
| D-AMF | 0.22 ± 0.01 | 27.98 ± 1.41 b | 58.9 ± 0.54 b | 27.66 ± 0.05 c |
| D-CNPs | 0.33 ± 0.01 a | 32.33 ± 1.55 b | 81.57 ± 1.9 a | 36.27 ± 1.0 b |
| D-Com-AMF | 0.34 ± 0.01 a | 35.44 ± 0.33 b | 38.95 ± 1.04 c | 24.63 ± 0.3 c |
| D-Com-AMF-CNPs | 0.37 ± 0.02 a | 49.42 ± 2.6 a | 81.06 ± 2.97 a | 47.6 ± 2.2 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsherif, E.A.; Almaghrabi, O.; Elazzazy, A.M.; Abdel-Mawgoud, M.; Beemster, G.T.S.; Sobrinho, R.L.; AbdElgawad, H. How Carbon Nanoparticles, Arbuscular Mycorrhiza, and Compost Mitigate Drought Stress in Maize Plant: A Growth and Biochemical Study. Plants 2022, 11, 3324. https://doi.org/10.3390/plants11233324
Alsherif EA, Almaghrabi O, Elazzazy AM, Abdel-Mawgoud M, Beemster GTS, Sobrinho RL, AbdElgawad H. How Carbon Nanoparticles, Arbuscular Mycorrhiza, and Compost Mitigate Drought Stress in Maize Plant: A Growth and Biochemical Study. Plants. 2022; 11(23):3324. https://doi.org/10.3390/plants11233324
Chicago/Turabian StyleAlsherif, Emad A., Omar Almaghrabi, Ahmed M. Elazzazy, Mohamed Abdel-Mawgoud, Gerrit T. S. Beemster, Renato Lustosa Sobrinho, and Hamada AbdElgawad. 2022. "How Carbon Nanoparticles, Arbuscular Mycorrhiza, and Compost Mitigate Drought Stress in Maize Plant: A Growth and Biochemical Study" Plants 11, no. 23: 3324. https://doi.org/10.3390/plants11233324
APA StyleAlsherif, E. A., Almaghrabi, O., Elazzazy, A. M., Abdel-Mawgoud, M., Beemster, G. T. S., Sobrinho, R. L., & AbdElgawad, H. (2022). How Carbon Nanoparticles, Arbuscular Mycorrhiza, and Compost Mitigate Drought Stress in Maize Plant: A Growth and Biochemical Study. Plants, 11(23), 3324. https://doi.org/10.3390/plants11233324









