Seed Priming with Carbon Nanomaterials Improves the Bioactive Compounds of Tomato Plants under Saline Stress
Abstract
:1. Introduction
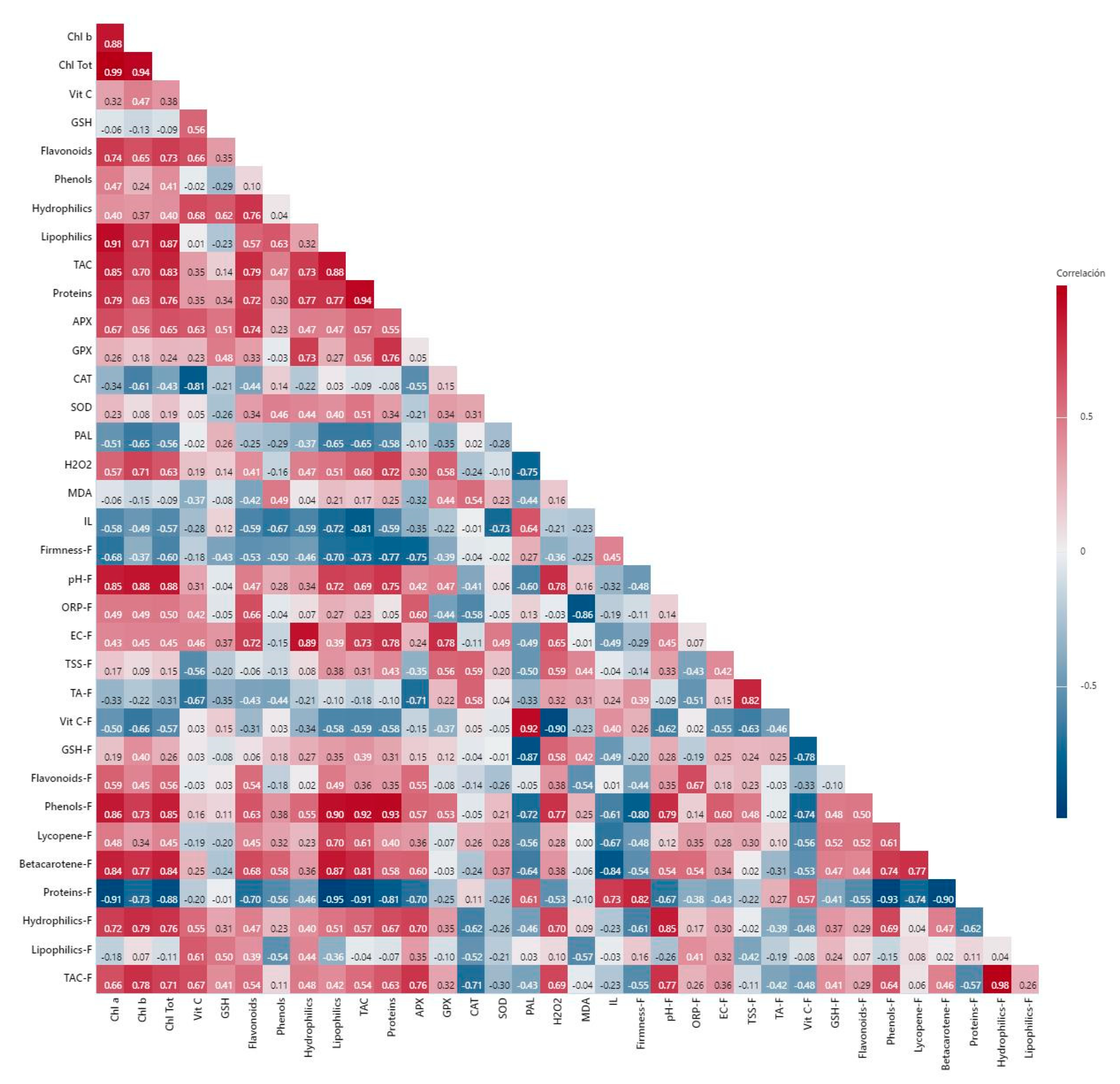
2. Results
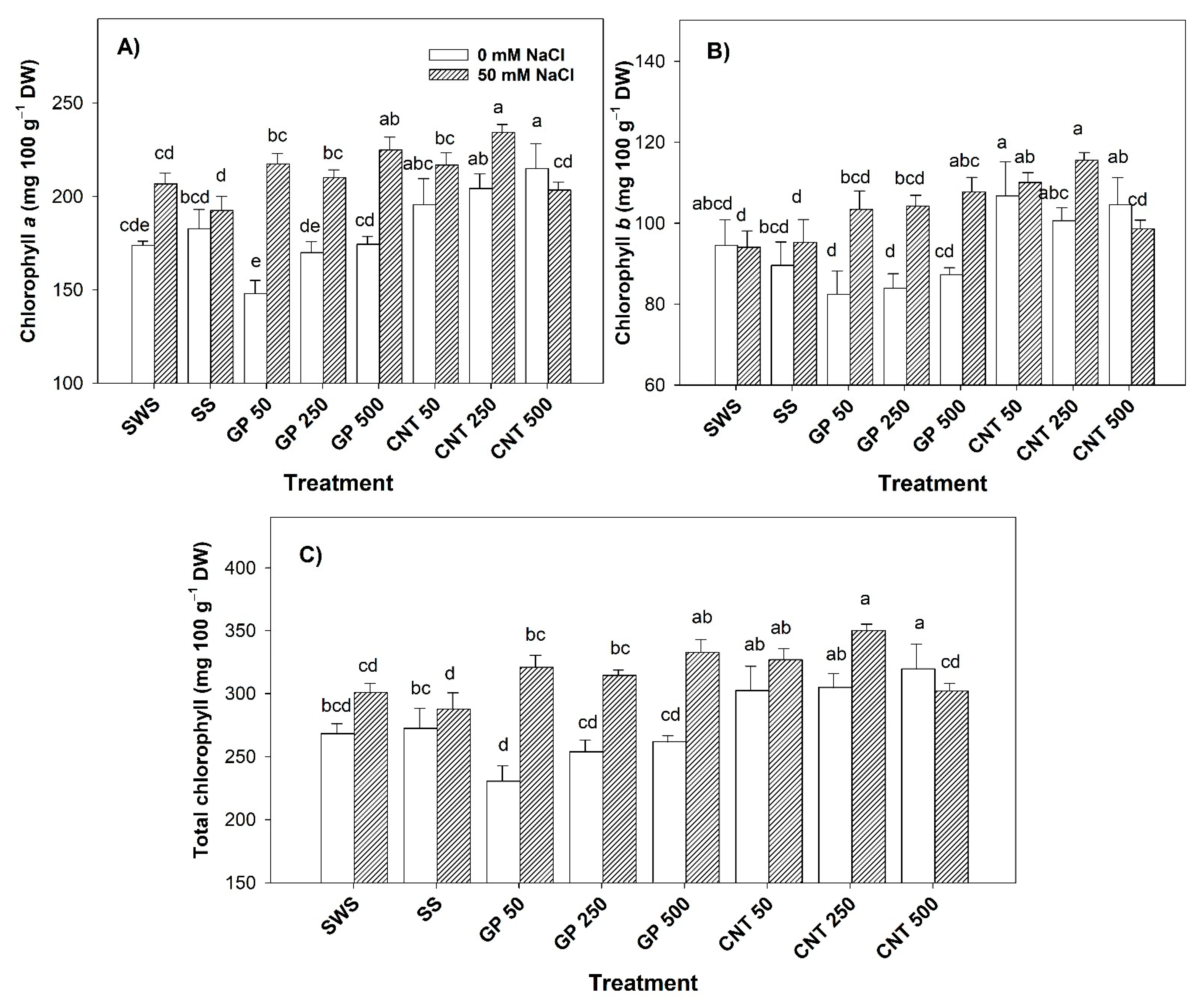
2.1. Content of Photosynthetic Pigments in Leaves
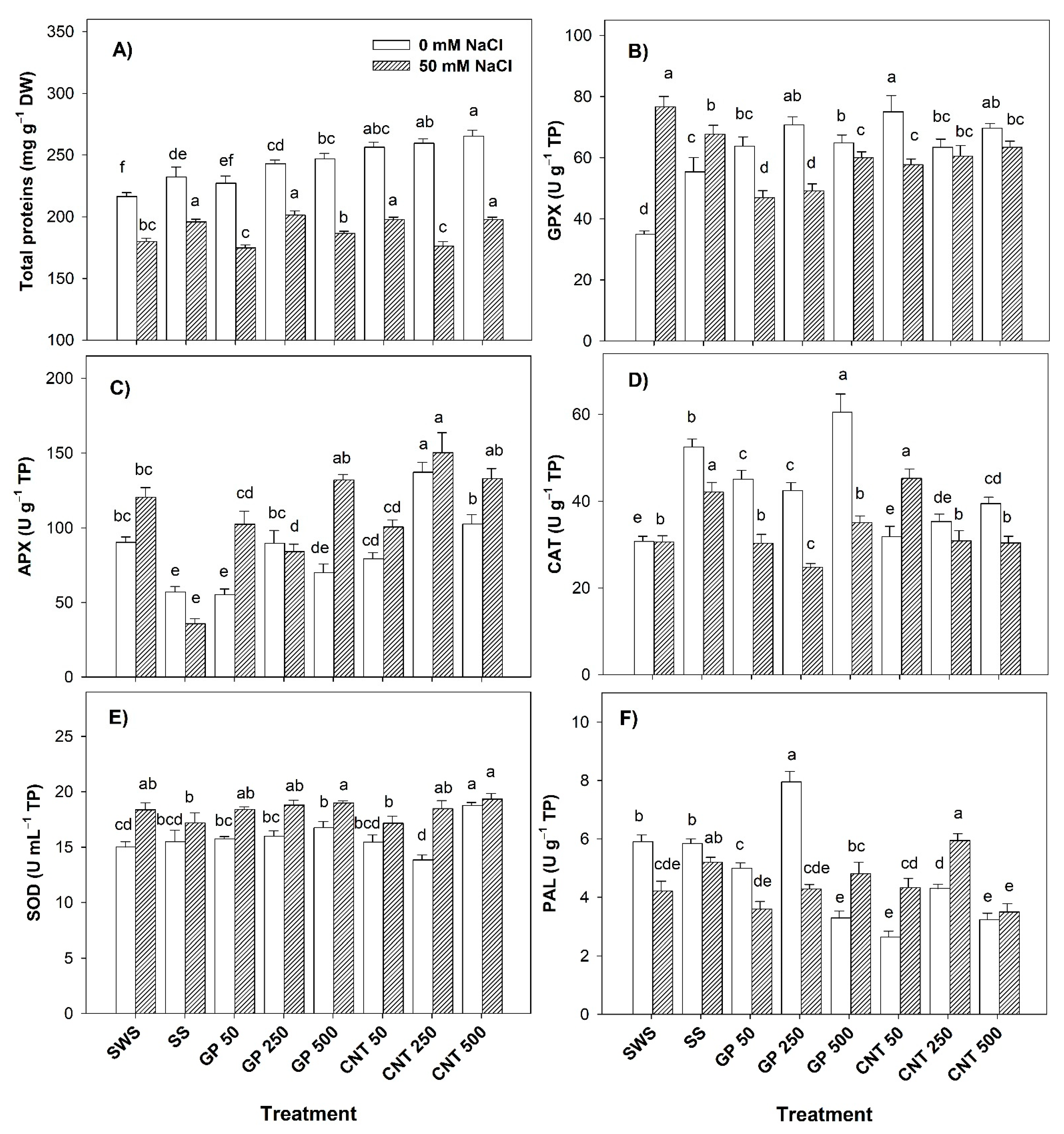
2.2. Stress Biomarkers in Tomato Leaves
2.3. Proteins and Enzymatic Activity in Leaves
2.4. Non-Enzymatic Antioxidant Compounds in Leaves
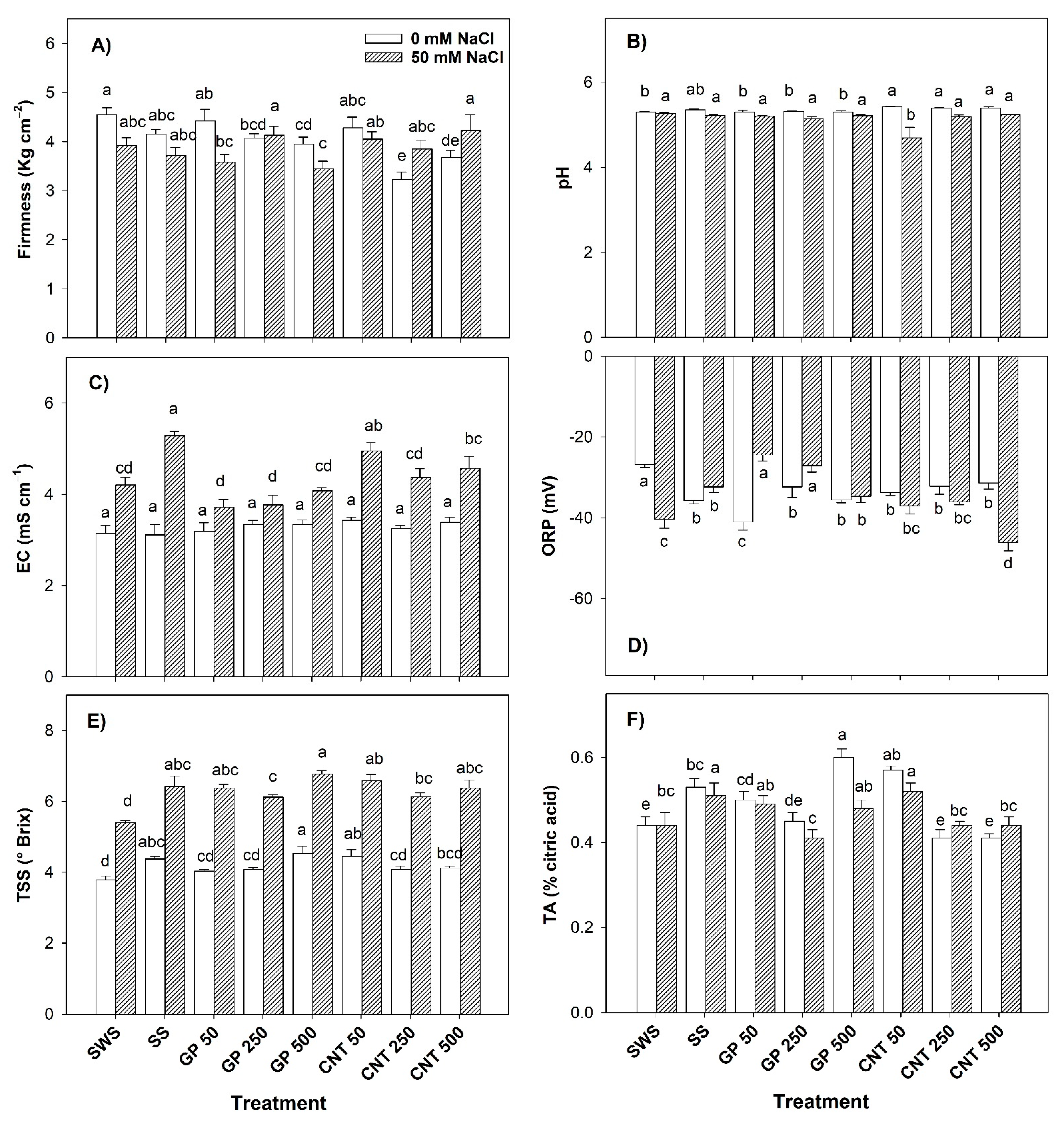
2.5. Physico-Chemical Characteristics of the Fruits
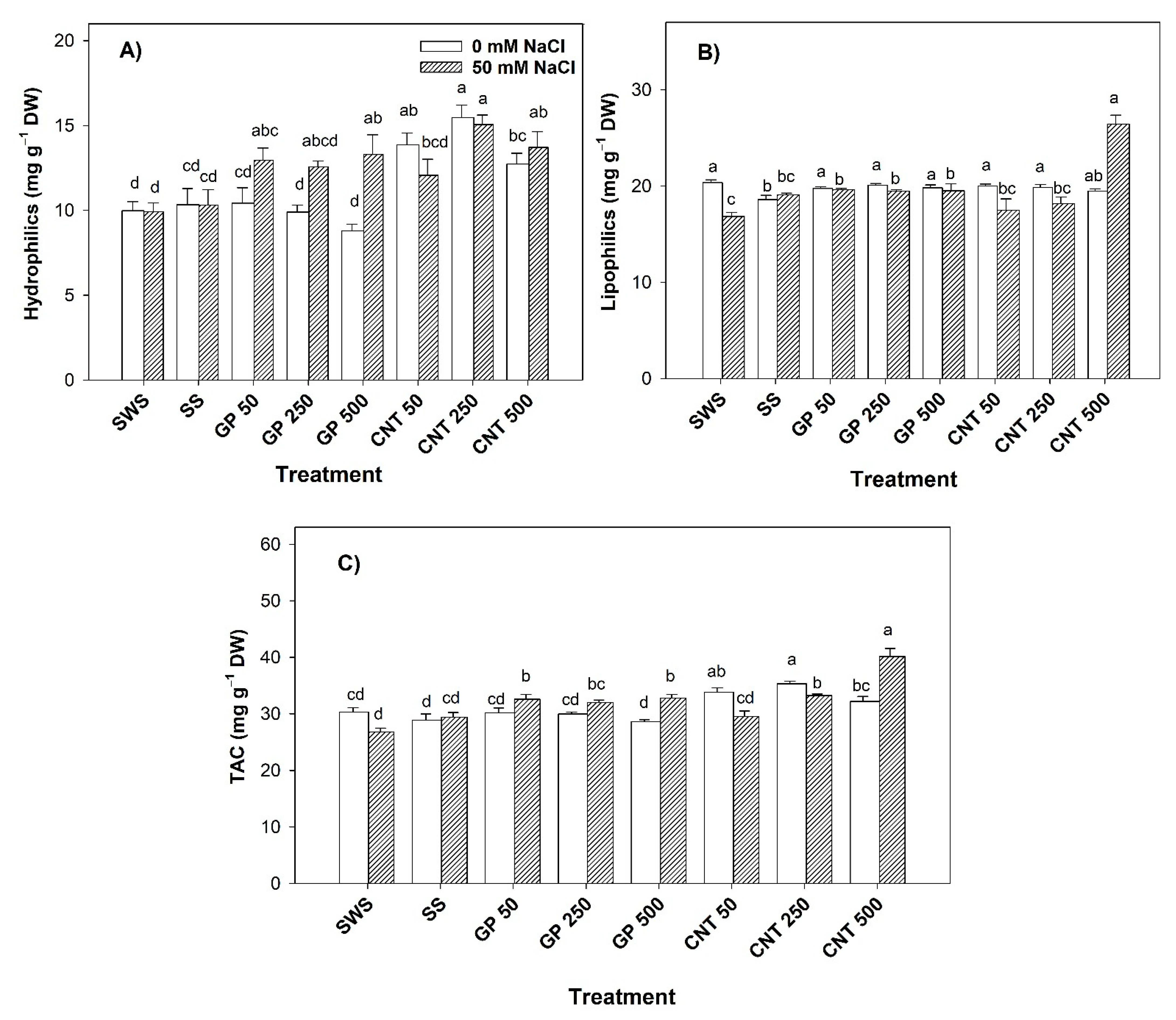
2.6. Bioactive Compounds in Fruits
2.7. Antioxidant Capacity in Fruits
3. Discussion
4. Materials and Methods
4.1. Plant Material and Carbon Nanomaterials
4.2. Seed Treatment
4.3. Crop Development
4.4. Physico-Chemical Analysis of the Fruits
4.5. Antioxidant Compounds and Proteins
4.6. Stress Biomarkers
4.7. Enzymatic Activity
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 8263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Khatun, M.; Baskin, C.C.; Murata, Y.; Hasanuzzaman, M. Seed Priming with Phytohormones: An Effective Approach for the Mitigation of Abiotic Stress. Plants 2020, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, F.; Iqbal, M.; Ali, M.; Ashraf, M.A. Fullerenol regulates oxidative stress and tissue ionic homeostasis in spring wheat to improve net-primary productivity under salt-stress. Ecotoxicol. Environ. Saf. 2021, 211, 111901. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Mor, V.S.; Tokas, J.; Punia, H.; Malik, S.; Malik, K.; Sangwan, S.; Tomar, S.; Singh, P.; Singh, N.; et al. Biostimulant-Treated Seedlings under Sustainable Agriculture: A Global Perspective Facing Climate Change. Agronomy 2020, 11, 14. [Google Scholar] [CrossRef]
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; AlMutairi, K.A.; Siddiqui, Z.H. Role of nanomaterials in plants under challenging environments. Plant Physiol. Biochem. 2017, 110, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Kranjc, E.; Drobne, D. Nanomaterials in plants: A review of hazard and applications in the agri-food sector. Nanomaterials 2019, 9, 1094. [Google Scholar] [CrossRef] [Green Version]
- Hatami, M.; Naghdi Badi, H.; Ghorbanpour, M. Nano-elicitation of secondary pharmaceutical metabolites in plant cells: A review. J. Med. Plants 2019, 18, 6–36. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Ashraf, M. Nanoparticles potentially mediate salt stress tolerance in plants. Plant Physiol. Biochem. 2021, 160, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Zhang, P.; Guo, Z.; Chetwynd, A.J.; Zhang, M.; Adeel, M.; Li, M.; Guo, K.; Gao, R.; Li, J.; et al. Different physiological responses of C3 and C4 plants to nanomaterials. Environ. Sci. Pollut. Res. 2021, 28, 25542–25551. [Google Scholar] [CrossRef]
- Carniel, F.C.; Fortuna, L.; Nepi, M.; Cai, G.; Del Casino, C.; Adami, G.; Bramini, M.; Bosi, S.; Flahaut, E.; Martín, C.; et al. Beyond graphene oxide acidity: Novel insights into graphene related materials effects on the sexual reproduction of seed plants. J. Hazard. Mater. 2020, 393, 122380. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Keller, A.A. Omics to address the opportunities and challenges of nanotechnology in agriculture. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2595–2636. [Google Scholar] [CrossRef]
- Samadi, S.; Asgari Lajayer, B.; Moghiseh, E.; Rodríguez-Couto, S. Effect of carbon nanomaterials on cell toxicity, biomass production, nutritional and active compound accumulation in plants. Environ. Technol. Innov. 2021, 21, 101323. [Google Scholar] [CrossRef]
- Gilbertson, L.M.; Pourzahedi, L.; Laughton, S.; Gao, X.; Zimmerman, J.B.; Theis, T.L.; Westerhoff, P.; Lowry, G.V. Guiding the design space for nanotechnology to advance sustainable crop production. Nat. Nanotechnol. 2020, 15, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Tommonaro, G.; Abbamondi, G.R.; Nicolaus, B.; Poli, A.; D’Angelo, C.; Iodice, C.; De Prisco, R. Productivity and Nutritional Trait Improvements of Different Tomatoes Cultivated with Effective Microorganisms Technology. Agriculture 2021, 11, 112. [Google Scholar] [CrossRef]
- Flores, I.R.; Vásquez-Murrieta, M.S.; Franco-Hernández, M.O.; Márquez-Herrera, C.E.; Ponce-Mendoza, A.; del Socorro López-Cortéz, M. Bioactive compounds in tomato (Solanum lycopersicum) variety saladette and their relationship with soil mineral content. Food Chem. 2021, 344, 128608. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Sina, A.A.I.; Khandker, S.S.; Neesa, L.; Tanvir, E.M.; Kabir, A.; Khalil, M.I.; Gan, S.H. Nutritional Composition and Bioactive Compounds in Tomatoes and Their Impact on Human Health and Disease: A Review. Foods 2020, 10, 45. [Google Scholar] [CrossRef]
- Godoy, F.; Olivos-Hernández, K.; Stange, C.; Handford, M. Abiotic Stress in Crop Species: Improving Tolerance by Applying Plant Metabolites. Plants 2021, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Vaishnav, A.; Singh, J.; Singh, P.; Rajput, R.S.; Singh, H.B.; Sarma, B.K. Sphingobacterium sp. BHU-AV3 induces salt tolerance in tomato by enhancing antioxidant activities and energy metabolism. Front. Microbiol. 2020, 11, 443. [Google Scholar] [CrossRef] [PubMed]
- Amjad, M.; Ameen, N.; Murtaza, B.; Imran, M. Comparative physiological and biochemical evaluation of salt and nickel tolerance mechanisms in two contrasting tomato genotypes. Physiol. Plant. 2019, 168, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Soltabayeva, A.; Ongaltay, A.; Omondi, J.O.; Srivastava, S. Morphological, Physiological and Molecular Markers for Salt-Stressed Plants. Plants 2021, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, S.P.; Kumari, N.; Mishra, P.; Prasad Moharana, D.; Aamir, M.; Singh, B.; Prasanna, H.C. Transcriptional regulation-mediating ROS homeostasis and physio-biochemical changes in wild tomato (Solanum chilense L.) and cultivated tomato (Solanum lycopersicum L.) under high salinity. Saudi J. Biol. Sci. 2020, 27, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Abdel Latef, A.; Kordrostami, M.; Zakir, A.; Zaki, H.; Saleh, O. Eustress with H2O2 Facilitates Plant Growth by Improving Tolerance to Salt Stress in Two Wheat Cultivars. Plants 2019, 8, 303. [Google Scholar] [CrossRef] [Green Version]
- Joshi, A.; Sharma, L.; Kaur, S.; Dharamvir, K.; Nayyar, H.; Verma, G. Effect of Multiwalled Carbon Nanotubes on Growth, Anatomy, Yield and Grain Composition of Rice. Bionanoscience 2020, 10, 430–445. [Google Scholar] [CrossRef]
- Miralles, P.; Johnson, E.; Church, T.L.; Harris, A.T. Multiwalled carbon nanotubes in alfalfa and wheat: Toxicology and uptake. J. R. Soc. Interface 2012, 9, 3514–3527. [Google Scholar] [CrossRef] [PubMed]
- Hatami, M.; Hadian, J.; Ghorbanpour, M. Mechanisms underlying toxicity and stimulatory role of single-walled carbon nanotubes in Hyoscyamus niger during drought stress simulated by polyethylene glycol. J. Hazard. Mater. 2017, 324, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Bárzana, G.; Garcia-Gomez, P.; Carvajal, M. Nanomaterials in plant systems: Smart advances related to water uptake and transport involving aquaporins. Plant Nano Biol. 2022, 1, 100005. [Google Scholar] [CrossRef]
- Martinez-Ballesta, M.C.; Chelbi, N.; Lopez-Zaplana, A.; Carvajal, M. Discerning the mechanism of the multiwalled carbon nanotubes effect on root cell water and nutrient transport. Plant Physiol. Biochem. 2020, 146, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, H.; Jamil, M.; Haq, A.; Ali, S.; Ahmad, R.; Malik, Z.; Parveen, D. Salt stress manifestation on plants, mechanism of salt tolerance and potassium role in alleviating it: A review. Zemdirbyste-Agriculture 2016, 103, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Pérez-Labrada, F.; López-Vargas, E.R.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Responses of Tomato Plants under Saline Stress to Foliar Application of Copper Nanoparticles. Plants 2019, 8, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhury, S.; Panda, P.; Sahoo, L.; Kumar, P.S. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal. Behav. 2013, 8, e23681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younes, N.A.; Dawood, M.F.A.; Wardany, A.A. Biosafety assessment of graphene nanosheets on leaf ultrastructure, physiological and yield traits of Capsicum annuum L. and Solanum melongena L. Chemosphere 2019, 228, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Khodakovskaya, M.V.; De Silva, K.; Nedosekin, D.A.; Dervishi, E.; Biris, A.S. Complex genetic, photothermal, and photoacoustic analysis of nanoparticle-plant interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 1028–1033. [Google Scholar] [CrossRef] [Green Version]
- Rifna, E.J.; Ramanan, K.R.; Mahendran, R. Emerging technology applications for improving seed germination. Trends Food Sci. Technol. 2019, 86, 95–108. [Google Scholar] [CrossRef]
- Ratnikova, T.A.; Podila, R.; Rao, A.M.; Taylor, A.G. Tomato Seed Coat Permeability to Selected Carbon Nanomaterials and Enhancement of Germination and Seedling Growth. Sci. World J. 2015, 2015, 419215. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, S.; Zhang, Y.; Zhu, X.; Yang, X.; Zhang, C.; Li, H.; Feng, X. Generation of male-sterile soybean lines with the CRISPR/Cas9 system. Crop J. 2021, 9, 1270–1277. [Google Scholar] [CrossRef]
- Lahiani, M.H.; Nima, Z.A.; Villagarcia, H.; Biris, A.S.; Khodakovskaya, M.V. Assessment of Effects of the Long-Term Exposure of Agricultural Crops to Carbon Nanotubes. J. Agric. Food Chem. 2018, 66, 6654–6662. [Google Scholar] [CrossRef]
- Zhang, D.; He, S.; Fu, Y.; Yu, R.; Gao, X.; Wang, Z.; Liu, Z.; Guo, Y.; Chen, M. Transcriptome analysis reveals key genes in response to salinity stress during seed germination in Setaria italica. Environ. Exp. Bot. 2021, 191, 104604. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Abu Alhmad, M.F.; Abdelfattah, K.E. The Possible Roles of Priming with ZnO Nanoparticles in Mitigation of Salinity Stress in Lupine (Lupinus termis) Plants. J. Plant Growth Regul. 2017, 36, 60–70. [Google Scholar] [CrossRef]
- Fan, X.; Xu, J.; Lavoie, M.; Peijnenburg, W.J.G.M.; Zhu, Y.; Lu, T.; Fu, Z.; Zhu, T.; Qian, H. Multiwall carbon nanotubes modulate paraquat toxicity in Arabidopsis thaliana. Environ. Pollut. 2018, 233, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, J.P.; Landry, M.P.; Faltermeier, S.M.; McNicholas, T.P.; Iverson, N.M.; Boghossian, A.A.; Reuel, N.F.; Hilmer, A.J.; Sen, F.; Brew, J.A.; et al. Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat. Mater. 2014, 13, 400–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baz, H.; Creech, M.; Chen, J.; Gong, H.; Bradford, K.; Huo, H. Water-Soluble Carbon Nanoparticles Improve Seed Germination and Post-Germination Growth of Lettuce under Salinity Stress. Agronomy 2020, 10, 1192. [Google Scholar] [CrossRef]
- Joshi, A.; Kaur, S.; Singh, P.; Dharamvir, K.; Nayyar, H.; Verma, G. Tracking multi-walled carbon nanotubes inside oat (Avena sativa L.) plants and assessing their effect on growth, yield, and mammalian (human) cell viability. Appl. Nanosci. 2018, 8, 1399–1414. [Google Scholar] [CrossRef]
- Gohari, G.; Safai, F.; Panahirad, S.; Akbari, A.; Rasouli, F.; Dadpour, M.R.; Fotopoulos, V. Modified multiwall carbon nanotubes display either phytotoxic or growth promoting and stress protecting activity in Ocimum basilicum L. in a concentration-dependent manner. Chemosphere 2020, 249, 126171. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Q.; Zhao, Y.; Rui, Q.; Wang, D. Toxicity and translocation of graphene oxide in Arabidopsis thaliana. Environ. Toxicol. Pharmacol. 2015, 39, 145–156. [Google Scholar] [CrossRef]
- Park, S.; Ahn, Y.J. Multi-walled carbon nanotubes and silver nanoparticles differentially affect seed germination, chlorophyll content, and hydrogen peroxide accumulation in carrot (Daucus carota L.). Biocatal. Agric. Biotechnol. 2016, 8, 257–262. [Google Scholar] [CrossRef]
- Moya, C.; Oyanedel, E.; Verdugo, G.; Flores, M.F.; Urrestarazu, M.; Álvaro, J.E. Increased electrical conductivity in nutrient solution management enhances dietary and organoleptic qualities in soilless culture tomato. HortScience 2017, 52, 868–872. [Google Scholar] [CrossRef] [Green Version]
- Basu, S.; Kumar, A.; Benazir, I.; Kumar, G. Reassessing the role of ion homeostasis for improving salinity tolerance in crop plants. Physiol. Plant. 2021, 171, 502–519. [Google Scholar] [CrossRef] [PubMed]
- Gruda, N.; Savvas, D.; Colla, G.; Rouphael, Y. Impacts of genetic material and current technologies on product quality of selected greenhouse vegetables—A review. Eur. J. Hortic. Sci. 2018, 83, 319–328. [Google Scholar] [CrossRef]
- Martínez-Damián, M.; Rodríguez-Pérez, J.; Cruz Álvarez, O.; Colinas-Leon, M. Rendimiento y calidad fisicoquímica en líneas experimentales de Solanum lycopersicum var. cerasiforme cultivadas con diferentes niveles de conductividad eléctrica. Chil. J. Agric. Anim. Sci 2018, 34, 152–164. [Google Scholar] [CrossRef]
- Rodríguez-Ortega, W.M.; Martínez, V.; Nieves, M.; Simón, I.; Lidón, V.; Fernandez-Zapata, J.C.; Martinez-Nicolas, J.J.; Cámara-Zapata, J.M.; García-Sánchez, F. Agricultural and Physiological Responses of Tomato Plants Grown in Different Soilless Culture Systems with Saline Water under Greenhouse Conditions. Sci. Rep. 2019, 9, 6733. [Google Scholar] [CrossRef] [PubMed]
- Anthon, G.E.; LeStrange, M.; Barrett, D.M. Changes in pH, acids, sugars and other quality parameters during extended vine holding of ripe processing tomatoes. J. Sci. Food Agric. 2011, 91, 1175–1181. [Google Scholar] [CrossRef]
- Juarez-Maldonado, A.; Ortega-Ortíz, H.; Pérez-Labrada, F.; Cadenas-Pliego, G.; Benavides-Mendoza, A. Cu Nanoparticles absorbed on chitosan hydrogels positively alter morphological, production, and quality characteristics of tomato. J. Appl. Bot. Food Qual. 2016, 89, 183–189. [Google Scholar] [CrossRef]
- Morales-Díaz, A.B.; Ortega-Ortíz, H.; Juárez-Maldonado, A.; Cadenas-Pliego, G.; González-Morales, S.; Benavides-Mendoza, A. Application of nanoelements in plant nutrition and its impact in ecosystems. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 013001. [Google Scholar] [CrossRef]
- López-Vargas, E.R.; González-García, Y.; Pérez-Álvarez, M.; Cadenas-Pliego, G.; González-Morales, S.; Benavides-Mendoza, A.; Cabrera, R.I.; Juárez-Maldonado, A. Seed Priming with Carbon Nanomaterials to Modify the Germination, Growth, and Antioxidant Status of Tomato Seedlings. Agronomy 2020, 10, 639. [Google Scholar] [CrossRef]
- Steiner, A.A. A universal method for preparing nutrient solutions of a certain desired composition. Plant Soil 1961, 15, 134–154. [Google Scholar] [CrossRef] [Green Version]
- USDA—United States Department of Agriculture. United States Standards for Grades of Fresh Tomatoes; United States Department of Agriculture: Washington, DC, USA, 1997. [Google Scholar]
- López-Vargas, E.; Ortega-Ortíz, H.; Cadenas-Pliego, G.; de Alba Romenus, K.; Cabrera de la Fuente, M.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Foliar application of copper nanoparticles increases the fruit quality and the content of bioactive compounds in tomatoes. Appl. Sci. 2018, 8, 1020. [Google Scholar] [CrossRef] [Green Version]
- Abdel Latef, A.A.; Tran, L.-S.P. Impacts of Priming with Silicon on the Growth and Tolerance of Maize Plants to Alkaline Stress. Front. Plant Sci. 2016, 7, 243. [Google Scholar] [CrossRef] [Green Version]
- Nagata, M.; Yamashita, I. Simple Method for Simultaneous Determination of Chlorophyll and Carotenoids in Tomato Fruit. Nippon Shokuhin Kogyo Gakkaishi 1992, 39, 925–928. [Google Scholar] [CrossRef] [Green Version]
- Hung, C.Y.; Yen, G.C. Antioxidant activity of phenolic compounds isolated from Mesona procumbens Hemsl. J. Agric. Food Chem. 2002, 50, 2993–2997. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Hartikainen, H.; Piironen, V. Antioxidative and growth-promoting effect of selenium on senescing lettuce. Plant Soil 2001, 237, 55–61. [Google Scholar] [CrossRef]
- Yu, Z.; Dahlgren, R.A. Evaluation of methods for measuring polyphenols in conifer foliage. J. Chem. Ecol. 2000, 26, 2119–2140. [Google Scholar] [CrossRef]
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardization of a propolis extract and identification of the main constituents. J. Pharm. Belg. 1994, 49, 462–468. [Google Scholar] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol. 2001, 42, 1265–1273. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 1987, 28, 131–140. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Sykłowska-Baranek, K.; Pietrosiuk, A.; Naliwajski, M.R.; Kawiak, A.; Jeziorek, M.; Wyderska, S.; Łojkowska, E.; Chinou, I. Effect of l-phenylalanine on PAL activity and production of naphthoquinone pigments in suspension cultures of Arnebia euchroma (Royle) Johnst. Vitr. Cell. Dev. Biol. Plant 2012, 48, 555–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-García, Y.; López-Vargas, E.R.; Pérez-Álvarez, M.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Valdés-Reyna, J.; Pérez-Labrada, F.; Juárez-Maldonado, A. Seed Priming with Carbon Nanomaterials Improves the Bioactive Compounds of Tomato Plants under Saline Stress. Plants 2022, 11, 1984. https://doi.org/10.3390/plants11151984
González-García Y, López-Vargas ER, Pérez-Álvarez M, Cadenas-Pliego G, Benavides-Mendoza A, Valdés-Reyna J, Pérez-Labrada F, Juárez-Maldonado A. Seed Priming with Carbon Nanomaterials Improves the Bioactive Compounds of Tomato Plants under Saline Stress. Plants. 2022; 11(15):1984. https://doi.org/10.3390/plants11151984
Chicago/Turabian StyleGonzález-García, Yolanda, Elsy Rubisela López-Vargas, Marissa Pérez-Álvarez, Gregorio Cadenas-Pliego, Adalberto Benavides-Mendoza, Jesús Valdés-Reyna, Fabián Pérez-Labrada, and Antonio Juárez-Maldonado. 2022. "Seed Priming with Carbon Nanomaterials Improves the Bioactive Compounds of Tomato Plants under Saline Stress" Plants 11, no. 15: 1984. https://doi.org/10.3390/plants11151984
APA StyleGonzález-García, Y., López-Vargas, E. R., Pérez-Álvarez, M., Cadenas-Pliego, G., Benavides-Mendoza, A., Valdés-Reyna, J., Pérez-Labrada, F., & Juárez-Maldonado, A. (2022). Seed Priming with Carbon Nanomaterials Improves the Bioactive Compounds of Tomato Plants under Saline Stress. Plants, 11(15), 1984. https://doi.org/10.3390/plants11151984









