Novel Approaches for Species Concepts and Delimitation in Polyploids and Hybrids
Abstract
1. Introduction
2. Hybrid Speciation
2.1. Homoploid Hybridization
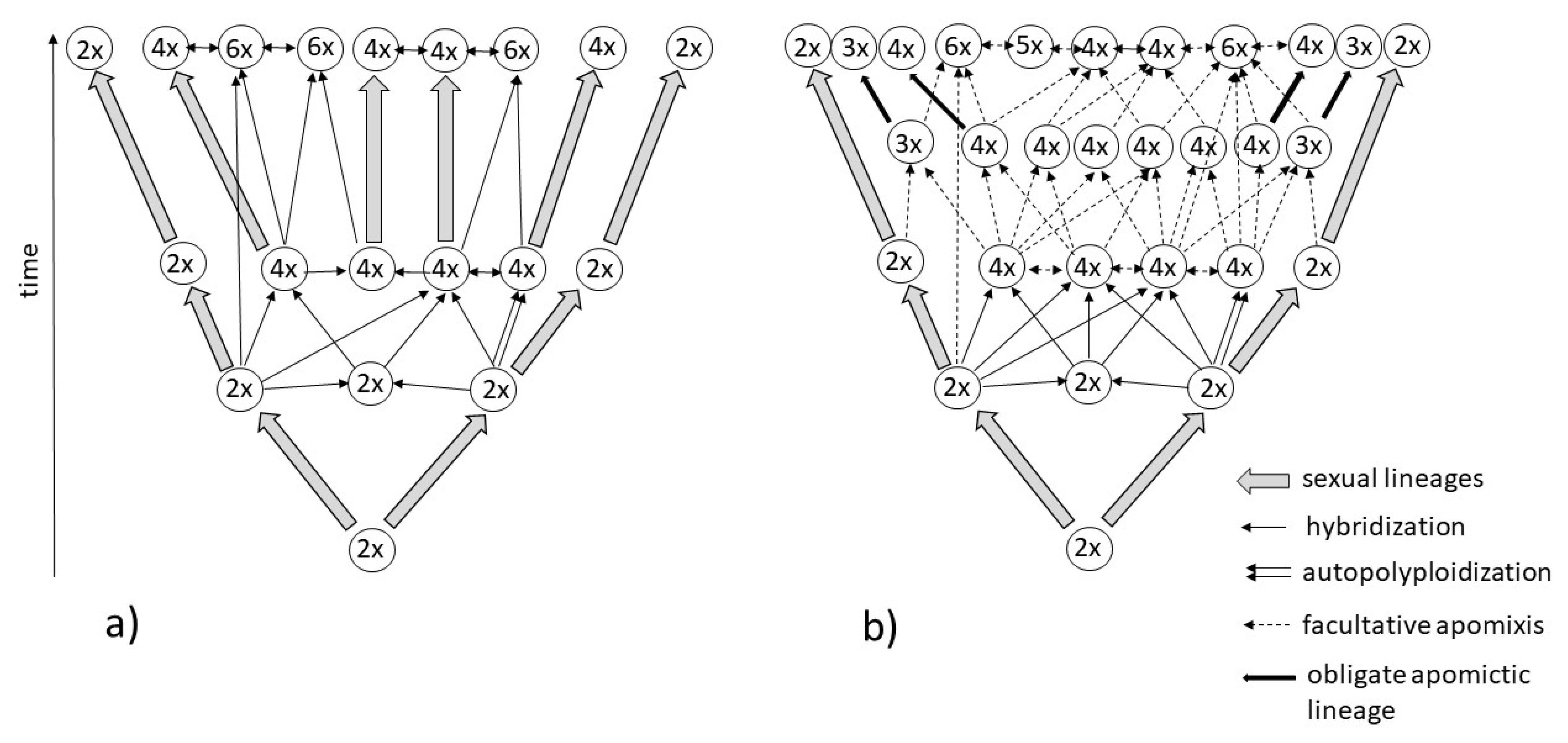
2.2. Polyploidy
2.3. Polyploid Complexes
3. Species Concepts and Their Limitations for Hybrids and Polyploids
3.1. The Biological Species Concept (BSC)
3.2. Problems of Morphological Species Concepts in Hybrids (MSC)
3.3. Cohesion and Genotypic Cluster Concepts in Hybrids (CSC and GCSC)
3.4. Ecological Species Concepts in Hybrids and Polyploids (ESC)
3.5. Evolutionary Lineage Species Concepts (EvoSC): Where to Draw the Borders?
3.6. Phylogenetic Species Concepts (PSCs)
3.7. Uniparental Reproduction and Fitness Concepts
4. Why Species Exist
4.1. How Sex Makes Species in Eukaryotes
4.2. Does Asexuality Form Species?
4.3. Why Humans Want to Classify Species
5. Novel Approaches for Species Delimitation
5.1. Recognition of Existing Lineages: Methodical Advances in the TaxonOMICS Era
5.2. Crossing Experiments, Fitness and Meiosis Studies
5.3. Towards a Broadly Applicable Species Concept: Integrating Morphology, Physiology and Ecology
6. Summary and Outlook
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary
| Allopolyploidy | Multiplication of chromosome sets connected to hybridization |
| Aneuploidy | Abnormal number of chromosomes, mostly uneven numbers |
| Apomixis | Reproduction via asexually formed seeds |
| Automixis | A form of asexual reproduction in animals, whereby meiosis is performed but two meiotic products fuse to restore diploidy |
| Autopolyploidy | Multiplication of chromosome sets within a species |
| Bivalent | Meiotic chromosome pairing of two homologous chromosomes |
| Dobzhansky-Muller-incompatibility | Model for evolution of genetic incompatibility arising from mutations in isolated, diverging lineages that are incompatible when merged again |
| homeolog (=homoeolog) | Duplicated chromosomes in an allopolyploid that are derived from the different parental species |
| Multivalent | Meiotic association of more than two chromosomes, resulting in ring formations or lagging chromosomes |
| Megasporogenesis | The formation of megaspores after female meiosis |
| Mesopolyploid | Polyploid of medium age (around 1–10 My old 1), with more or less stabilized meiosis and genome, with low or high chromosome numbers; intermediate period between neopolyploid and paleopolyploid. 1 age ranges differ between species (and authors) |
| Microsporogenesis | The formation of microspores after male meiosis |
| Minority cytotype disadvantage | Single or few individuals with a different cytotype than the majority in the population suffer from scarcity of homoploid mating partners |
| Neopolyploid | Recently formed polyploid (c. <1 My old 1), with meiotic and genomic instability, usually with low chromosome base number. |
| Nothotaxon | A hybrid taxon, formally indicated by a multiplication sign in the name |
| Paleopolyploid | Ancient polyploid (usually > 10 My old 1), with chromosomal behavior and genomic stability like a diploid; usually with high base chromosome number. |
| Segregation distortion | Departure from the expected gametic ratio of alleles in the progeny of a cross |
References
- Arnold, M.L. Natural Hybridization and Evolution; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Mallet, J. Hybridization as an invasion of the genome. Trends Ecol. Evol. 2005, 20, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Stebbins, G. Variation and Evolution in Plants; Columbia University Press: New York, NY, USA, 1950; p. 643. [Google Scholar]
- Mallet, J. Hybrid speciation. Nature 2007, 446, 279–283. [Google Scholar] [CrossRef]
- Abbott, R.; Albach, D.; Ansell, S.; Arntzen, J.W.; Baird, S.J.E.; Bierne, N.; Boughman, J.; Brelsford, A.; Buerkle, C.A.; Buggs, R.; et al. Hybridization and speciation. J. Evol. Biol. 2013, 26, 229–246. [Google Scholar] [CrossRef]
- Comai, L. The advantages and disadvantages of being polyploid. Nat. Rev. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Van De Peer, Y.; Mizrachi, E.; Marchal, K. The evolutionary significance of polyploidy. Nat. Rev. Genet. 2017, 18, 411–424. [Google Scholar] [CrossRef]
- Leebens-Mack, J.H.; Barker, M.S.; Carpenter, E.J.; Deyholos, M.K.; Gitzendanner, M.A.; Graham, S.W.; One Thousand Plant Transcriptomes Initiative. One thousand plant transcriptomes and the phylogenomics of green plants. Nature 2019, 574, 679. [Google Scholar] [CrossRef]
- Jiao, Y.; Wickett, N.J.; Ayyampalayam, S.; Chanderbali, A.S.; Landherr, L.; Ralph, P.E.; Soltis, P.S. Ancestral polyploidy in seed plants and angiosperms. Nature 2011, 473, 97–100. [Google Scholar] [CrossRef]
- Li, Z.; McKibben, M.T.W.; Finch, G.S.; Blischak, P.D.; Sutherland, B.L.; Barker, M.S. Patterns and Processes of Diploidization in Land Plants. Annu. Rev. Plant Biol. 2021, 72, 387–410. [Google Scholar] [CrossRef]
- Wong, G.K.-S.; Soltis, D.E.; Leebens-Mack, J.; Wickett, N.J.; Barker, M.S.; Van De Peer, Y.; Graham, S.W.; Melkonian, M. Sequencing and Analyzing the Transcriptomes of a Thousand Species Across the Tree of Life for Green Plants. Annu. Rev. Plant Biol. 2020, 71, 741–765. [Google Scholar] [CrossRef] [PubMed]
- Soltis, P.S.; Soltis, D.E. Ancient WGD events as drivers of key innovations in angiosperms. Curr. Opin. Plant Biol. 2016, 30, 159–165. [Google Scholar] [CrossRef]
- Tuskan, G.A.; DiFazio, S.; Jansson, S.; Bohlmann, J.; Grigoriev, I.; Hellsten, U.; Rokhsar, D. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 2006, 313, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Aköz, G.; Nordborg, M. The Aquilegia genome reveals a hybrid origin of core eudicots. Genome Biol. 2019, 20, 12. [Google Scholar] [CrossRef]
- Mandakova, T.; Joly, S.; Krzywinski, M.; Mummenhoff, K.; Lysak, M.A. Fast Diploidization in Close Mesopolyploid Relatives of Arabidopsis. Plant Cell 2010, 22, 2277–2290. [Google Scholar] [CrossRef] [PubMed]
- Carman, J.G. Asynchronous expression of duplicate genes in angiosperms may cause apomixis, bispory, tetraspory, and polyembryony. Biol. J. Linnean Soc. 1997, 61, 51–94. [Google Scholar] [CrossRef]
- Soltis, D.E.; Visger, C.J.; Soltis, P.S. The polyploidy revolution then and now: Stebbins revisited. Am. J. Bot. 2014, 101, 1057–1078. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.B.; Soltis, D.E.; Li, Z.; Marx, H.E.; Barker, M.S.; Tank, D.C.; Soltis, P.S. Impact of whole-genome duplication events on diversification rates in angiosperms. Am. J. Bot. 2018, 105, 348–363. [Google Scholar] [CrossRef]
- Chen, Z.J. Genetic and epigenetic mechanisms for gene expression and phenotypic variation in plant polyploids. Annu. Rev. Plant Biol. 2007, 58, 377–406. [Google Scholar] [CrossRef]
- Van de Peer, Y.; Ashman, T.L.; Soltis, P.S.; Soltis, D.E. Polyploidy: An evolutionary and ecological force in stressful times. Plant Cell 2021, 33, 11–26. [Google Scholar] [CrossRef]
- Bottani, S.; Zabet, N.R.; Wendel, J.F.; Veitia, R.A. Gene Expression Dominance in Allopolyploids: Hypotheses and Models. Trends Plant Sci. 2018, 23, 393–402. [Google Scholar] [CrossRef]
- Boatwright, J.L.; McIntyre, L.M.; Morse, A.M.; Chen, S.; Yoo, M.-J.; Koh, J.; Soltis, P.S.; Soltis, D.E.; Barbazuk, W.B. A Robust Methodology for Assessing Differential Homeolog Contributions to the Transcriptomes of Allopolyploids. Genetics 2018, 210, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, R.; Sun, J.; Hatakeyama, M.; Lischer, H.E.L.; Briskine, R.; Hay, A.; Shimizu Inatsugi, R. Fine-scale empirical data on niche divergence and homeolog expression patterns in an allopolyploid and its diploid progenitor species. New Phytol. 2021, 229, 3587–3601. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.P.; Whitton, J. Polyploid incidence and evolution. Annu. Rev. Genet. 2000, 34, 401–437. [Google Scholar] [CrossRef] [PubMed]
- Rice, A.; Smarda, P.; Novosolov, M.; Drori, M.; Glick, L.; Sabath, N.; Mayrose, I. The global biogeography of polyploid plants. Nat. Ecol. Evol. 2019, 3, 265. [Google Scholar] [CrossRef] [PubMed]
- Te Beest, M.; Le Roux, J.J.; Richardson, D.M.; Brysting, A.K.; Suda, J.; Kubesova, M.; Pysek, P. The more the better? The role of polyploidy in facilitating plant invasions. Ann. Bot. 2012, 109, 19–45. [Google Scholar] [CrossRef]
- Glennon, K.L.; Ritchie, M.E.; Segraves, K.A. Evidence for shared broad-scale climatic niches of diploid and polyploid plants. Ecol. Lett. 2014, 17, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Kirchheimer, B.; Wessely, J.; Gattringer, G.; Hülber, K.; Moser, D.; Schinkel, C.C.F.; Dullinger, S. Reconstructing geographical parthenogenesis: Effects of niche differentiation and reproductive mode on Holocene range expansion of an alpine plant. Ecol. Lett. 2018, 21, 392–401. [Google Scholar] [CrossRef]
- Baniaga, A.; Marx, H.; Arrigo, N.; Barker, M.; Early, R. Polyploid plants have faster rates of multivariate niche differentiation than their diploid relatives. Ecol. Lett. 2020, 23, 68–78. [Google Scholar] [CrossRef]
- Freudenstein, J.V.; Broe, M.B.; Folk, R.A.; Sinn, B.T. Biodiversity and the Species Concept-Lineages are not Enough. Syst. Biol. 2017, 66, 644–656. [Google Scholar] [CrossRef]
- Soltis, P.S.; Soltis, D.E. The Role of Hybridization in Plant Speciation. Annu. Rev. Plant Biol. 2009, 60, 561–588. [Google Scholar] [CrossRef] [PubMed]
- Coyne, J.A.; Orr, H.A. Speciation; Sinauer Ass.: Sunderland, MA, USA, 2004. [Google Scholar]
- Hörandl, E. A combinational theory for maintenance of sex. Heredity 2009, 103, 445–457. [Google Scholar] [CrossRef] [PubMed]
- De Queiroz, K. Species concepts and species delimitation. Syst. Biol. 2007, 56, 879–886. [Google Scholar] [CrossRef]
- Stuessy, T.F. Plant Taxonomy: The Systematic Evaluation of Comparative Data; Columbia University Press: New York, NY, USA, 2009. [Google Scholar]
- Hörandl, E. The classification of asexual organisms: Old myths, new facts, and a novel pluralistic approach. Taxon 2018, 67, 1066–1081. [Google Scholar] [CrossRef]
- Abbott, R.; Brennan, A.C. Altitudinal gradients, plant hybrid zones and evolutionary novelty. Phil. Trans. R. Soc. B 2014, 369, 20130346. [Google Scholar] [CrossRef]
- Brennan, A.C.; Hiscock, S.J.; Abbott, R.J.; Abbott, R.J. Interspecific crossing and genetic mapping reveal intrinsic genomic incompatibility between two Senecio species that form a hybrid zone on Mount Etna, Sicily. Heredity 2014, 113, 195–204. [Google Scholar] [CrossRef]
- Brennan, A.; Bridle, J.; Hiscock, S.; Abbott, R.J.; Wang, A.-L.; Abbott, R. Adaptation and selection in the Senecio (Asteraceae) hybrid zone on Mount Etna, Sicily. New Phytol. 2009, 183, 702–717. [Google Scholar] [CrossRef]
- Milne, R.I.; Abbott, R.J. Reproductive isolation among two interfertile Rhododendron species: Low frequency of post-F-1 hybrid genotypes in alpine hybrid zones. Mol. Ecol. 2008, 17, 1108–1121. [Google Scholar] [CrossRef]
- Bersweden, L.; Viruel, J.; Schatz, B.; Harland, J.; Gargiulo, R.; Cowan, R.; Fay, M. Microsatellites and petal morphology reveal new patterns of admixture in Orchis hybrid zones. Am. J. Bot. 2021, 108, 1388–1404. [Google Scholar] [CrossRef]
- Christe, C.; Stölting, K.; Bresadola, L.; Fussi, B.; Heinze, B.; Wegmann, D.; Stolting, K. Selection against recombinant hybrids maintains reproductive isolation in hybridizing Populus species despite F1 fertility and recurrent gene flow. Mol. Ecol. 2016, 25, 2482–2498. [Google Scholar] [CrossRef]
- Gramlich, S.; Hörandl, E. Fitness of natural willow hybrids in a pioneer mosaic hybrid zone. Ecol. Evol. 2016, 6, 7645–7655. [Google Scholar] [CrossRef] [PubMed]
- Gramlich, S.; Sagmeister, P.; Dullinger, S.; Hadacek, F.; Hörandl, E. Evolution in situ: Hybrid origin and establishment of willows (Salix L.) on alpine glacier forefields. Heredity 2016, 116, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Gramlich, S.; Wagner, N.D.; Hörandl, E. RAD-seq reveals genetic structure of the F-2-generation of natural willow hybrids (Salix L.) and a great potential for interspecific introgression. BMC Plant Biol. 2018, 18. [Google Scholar] [CrossRef]
- Matthews, A.; Emelianova, K.; Hatimy, A.A.; Chester, M.; Pellicer, J.; Ahmad, K.S.; Buggs, R.J.A. 250 years of hybridization between two biennial herb species without speciation. Aob Plants 2015, 7. [Google Scholar] [CrossRef]
- Arnold, M.L.; Ballerini, E.S.; Brothers, A.N.; Brothers, A.N. Hybrid fitness, adaptation and evolutionary diversification: Lessons learned from Louisiana Irises. Heredity 2012, 108, 159–166. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Y.; Chao, L.; Wang, S.; Wu, W.; Dai, S.; Zhou, R. Extensive hybridization and introgression between Melastoma candidum and M. sanguineum. PLoS ONE 2014, 9, e9668. [Google Scholar] [CrossRef]
- Dai, S.P.; Wu, W.; Zhang, R.S.; Liu, T.; Chen, Y.Y.; Shi, S.H.; Zhou, R.C. Molecular evidence for hybrid origin of Melastoma intermedium. Biochem. Syst. Ecol. 2012, 41, 136–141. [Google Scholar] [CrossRef]
- Abbott, R.J.; Hegarty, M.J.; Hiscock, S.J.; Brennan, A.C. Homoploid hybrid speciation in action. Taxon 2010, 59, 1375–1386. [Google Scholar] [CrossRef]
- Buggs, R.J.A.; Soltis, P.S.; Soltis, D.E. Does hybridization between divergent progenitors drive whole-genome duplication? Mol. Ecol. 2009, 18, 3334–3339. [Google Scholar] [CrossRef] [PubMed]
- Rieseberg, L.H.; Raymond, O.; Rosenthal, D.M.; Lai, Z.; Livingstone, K.; Nakazato, T.; Lexer, C. Major ecological transitions in wild sunflowers facilitated by hybridization. Science 2003, 301, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Rieseberg, L.H.; Willis, J.H. Plant speciation. Science 2007, 317, 910–914. [Google Scholar] [CrossRef]
- Karrenberg, S.; Lexer, C.; Rieseberg, L. Reconstructing the History of Selection during Homoploid Hybrid Speciation. Am. Nat. 2007, 169, 725–737. [Google Scholar] [CrossRef]
- Gruenig, S.; Grünig, S.; Fischer, M.; Parisod, C. Recent hybrid speciation at the origin of the narrow endemic Pulmonaria helvetica. Ann. Bot. 2021, 127, 21–31. [Google Scholar] [CrossRef]
- Fabritzek, A.; Griebeler, E.; Kadereit, J.W. Hybridization, ecogeographical displacement and the emergence of new lineages—A genotyping by sequencing (GBS) and ecological niche and species distribution modelling study of Sempervivum tectorum L. (Houseleek). J. Evol. Biol. 2021, 34, 830–844. [Google Scholar] [CrossRef]
- Heyduk, K.; McAssey, E.V.; Grimwood, J.; Shu, S.; Schmutz, J.; McKain, M.R.; Leebens-Mack, J. Hybridization history and repetitive element content in the genome of a homoploid hybrid, Yucca gloriosa (Asparagaceae). Front. Plant Sci. 2021, 11. [Google Scholar] [CrossRef]
- Hegarty, M.; Barker, G.; Brennan, A.; Edwards, K.; Abbott, R.; Hiscock, S. Changes to gene expression associated with hybrid speciation in plants: Further insights from transcriptomic studies in Senecio. Philos. Trans. Biol. Sci. 2008, 363, 3055–3069. [Google Scholar] [CrossRef] [PubMed]
- Schumer, M.; Rosenthal, G.; Andolfatto, P. How common is homoploid hybrid speciation? Evolution 2014, 68, 1553–1560. [Google Scholar] [CrossRef]
- Arnold, M.L.; Martin, N.H. Hybrid fitness across time and habitats. Trends Ecol. Evol. 2010, 25, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Renner, S.S. The relative and absolute frequencies of angiosperm sexual systems: Dioecy, monoecy, gynodioecy, and an updated online database. Am. J. Bot. 2014, 101, 1588–1596. [Google Scholar] [CrossRef]
- Ramsey, J.; Schemske, D.W. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu. Rev. Ecol. Syst. 1998, 29, 467–501. [Google Scholar] [CrossRef]
- Köhler, C.; Dziasek, K.; Del Toro-De Leon, G. Postzygotic reproductive isolation established in the endosperm: Mechanisms, drivers and relevance. Phil. Trans. Roy. Soc. B-Biol. Sci. 2021, 376. [Google Scholar] [CrossRef]
- Cifuentes, M.; Grandont, L.; Moore, G.; Chèvre, A.M.; Jenczewski, E. Genetic regulation of meiosis in polyploid species: New insights into an old question. New Phytol. 2010, 186, 29–36. [Google Scholar] [CrossRef]
- Morgan, C.; Zhang, H.K.; Henry, C.E.; Franklin, F.C.H.; Bomblies, K. Derived alleles of two axis proteins affect meiotic traits in autotetraploid Arabidopsis arenosa. Proc. Natl. Acad. Sci. USA 2020, 117, 8980–8988. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.A. Minority cytotype exclusion in local plant populations. Taxon 1975, 24, 35–43. [Google Scholar] [CrossRef]
- Mable, B.K. Polyploidy and self-compatibility: Is there an association? New Phytol. 2004, 162, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Hojsgaard, D.; Hörandl, E. The Rise of Apomixis in Natural Plant Populations. Front. Plant Sci. 2019, 10, 358. [Google Scholar] [CrossRef] [PubMed]
- Salmon, A.; Ainouche, M.; Wendel, J.F.; Wendel, J.F. Genetic and epigenetic consequences of recent hybridization and polyploidy in Spartina (Poaceae). Mol. Ecol. 2005, 14, 1163–1175. [Google Scholar] [CrossRef]
- Chelaifa, H.; Monnier, A.; Ainouche, M. Transcriptomic changes following recent natural hybridization and allopolyploidy in the salt marsh species Spartina × townsendii and Spartina anglica (Poaceae). New Phytol. 2010, 186, 161–174. [Google Scholar] [CrossRef]
- Ainouche, M.; Baumel, A.; Salmon, A.; Yannic, G.; Yannic, G. Hybridization, polyploidy and speciation in Spartina (Poaceae). New Phytol. 2004, 161, 165–172. [Google Scholar] [CrossRef]
- Vallejo Marín, M.; Buggs, R.J.A.; Cooley, A.; Puzey, J.R.; Puzey, J. Speciation by genome duplication: Repeated origins and genomic composition of the recently formed allopolyploid species Mimulus peregrinus. Evolution 2015, 69, 1487–1500. [Google Scholar] [CrossRef]
- Mandáková, T.; Marhold, K.; Lysak, M.A. The widespread crucifer species Cardamine flexuosa is an allotetraploid with a conserved subgenomic structure. New Phytol. 2014, 201, 982–992. [Google Scholar] [CrossRef]
- Bombarely, A.; Coate, J.E.; Doyle, J.J. Mining transcriptomic data to study the origins and evolution of a plant allopolyploid complex. PeerJ 2014, 2. [Google Scholar] [CrossRef]
- Paterson, A.H.; Wendel, J.F.; Gundlach, H.; Guo, H.; Jenkins, J.; Jin, D.C.; Schmutz, J. Repeated polyploidization of Gossypium genomes and the evolution of spinnable cotton fibres. Nature 2012, 492, 423. [Google Scholar] [CrossRef] [PubMed]
- Grant, V. Plant Speciation, 2nd ed.; Columbia University Press: New York, NY, USA, 1981. [Google Scholar]
- Paun, O.; Forest, F.; Fay, M.F.; Chase, M.W. Hybrid speciation in angiosperms: Parental divergence drives ploidy. New Phytol. 2009, 182, 507–518. [Google Scholar] [CrossRef]
- Wagner, F.; Ott, T.; Zimmer, C.; Reichhart, V.; Vogt, R.; Oberprieler, C. ’At the crossroads towards polyploidy’: Genomic divergence and extent of homoploid hybridization are drivers for the formation of the ox-eye daisy polyploid complex (Leucanthemum, Compositae-Anthemideae). New Phytol. 2019, 223, 2039–2053. [Google Scholar] [CrossRef]
- Šlenker, M.; Kantor, A.; Marhold, K.; Schmickl, R.; Mandáková, T.; Lysak, M.A.; Zozomová-Lihová, J. Allele sorting as a novel approach to resolving the origin of allotetraploids using Hyb-Seq data: A case study of the Balkan Mountain endemic Cardamine barbaraeoides. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef]
- Krak, K.; Vít, P.; Belyayev, A.; Douda, J.; Hreusová, L.; Mandák, B.; Mandak, B. Allopolyploid origin of Chenopodium album s. str. (Chenopodiaceae): A molecular and cytogenetic insight. PLoS ONE 2016, 11, e0161063. [Google Scholar]
- Hewitt, G.M. Some genetic consequences of ice ages, and their role in divergence and speciation. Biol. J. Linnean Soc. 1996, 58, 247–276. [Google Scholar] [CrossRef]
- Hewitt, G.M. Genetic consequences of climatic oscillations in the Quaternary. Phil. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 183–195. [Google Scholar] [CrossRef]
- Hewitt, G.M. Quaternary phylogeography: The roots of hybrid zones. Genetica 2011, 139, 617–638. [Google Scholar] [CrossRef]
- Soltis, D.E.; Soltis, P.S.; Schemske, D.W.; Hancock, J.F.; Thompson, J.N.; Husband, B.C.; Judd, W.S. Autopolyploidy in angiosperms: Have we grossly underestimated the number of species? Taxon 2007, 56, 13–30. [Google Scholar]
- Buono, D.; Khan, G.; von Hagen, K.B.; Kosachev, P.A.; Mayland-Quellhorst, E.; Mosyakin, S.L.; Albach, D.C. Comparative phylogeography of Veronica spicata and V. longifolia (Plantaginaceae) across Europe: Integrating hybridization and polyploidy in phylogeography. Front. Plant Sci. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Sonnleitner, M.; Weis, B.; Flatscher, R.; García, P.; Suda, J.; Krejčíková, J.; Huelber, K. Parental Ploidy Strongly Affects Offspring Fitness in Heteroploid Crosses among Three Cytotypes of Autopolyploid Jacobaea carniolica (Asteraceae). PLoS ONE 2013, 8, e78959. [Google Scholar]
- Pfeiffer, T.; Harter, D.E.V.; Formella, N.; Schnittler, M. Reproductive isolation vs. interbreeding between Gagea lutea (L.) Ker Gawl. and G. pratensis (Pers.) Dumort. (Liliaceae) and their putative hybrids in Mecklenburg-Western Pomerania (Germany). Plant Spec. Biol. 2013, 28, 193–203. [Google Scholar] [CrossRef]
- Melicharkova, A.; Slenker, M.; Zozomova-Lihova, J.; Skokanova, K.; Singliarova, B.; Kacmarova, T.; Marhold, K. So Closely Related and Yet So Different: Strong Contrasts Between the Evolutionary Histories of Species of the Cardamine pratensis Polyploid Complex in Central Europe. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Zozomova Lihova, J.; Mandáková, T.; Kovaříková, A.; Mühlhausen, A.; Mummenhoff, K.; Lysak, M.; Kovarik, A. When fathers are instant losers: Homogenization of rDNAloci in recently formed Cardamine schulzii trigenomic allopolyploid. New Phytol. 2014, 203, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
- Paun, O.; Stuessy, T.F.; Hörandl, E. The role of hybridization, polyploidization and glaciation in the origin and evolution of the apomictic Ranunculus cassubicus complex. New Phytol. 2006, 171, 223–236. [Google Scholar] [CrossRef]
- Hörandl, E.; Greilhuber, J.; Klimova, K.; Paun, O.; Temsch, E.; Emadzade, K.; Hodalova, I. Reticulate evolution and taxonomic concepts in the Ranunculus auricomus complex (Ranunculaceae): Insights from analysis of morphological, karyological and molecular data. Taxon 2009, 58, 1194–1215. [Google Scholar] [CrossRef] [PubMed]
- Köhler, C.; Scheid, O.M.; Erilova, A. The impact of the triploid block on the origin and evolution of polyploid plants. Trends Genet. 2010, 26, 142–148. [Google Scholar] [CrossRef]
- Friedman, W.E.; Madrid, E.N.; Williams, J.H. Origin of the fittest and survival of the fittest: Relating female gametophyte development to endosperm genetics. Int. J. Plant Sci. 2008, 169, 79–92. [Google Scholar] [CrossRef][Green Version]
- Winterfeld, G.; Schneider, J.; Perner, K.; Röser, M. Origin of Highly Polyploid Species: Different Pathways of Auto- and Allopolyploidy in 12x to 18x Species of Avenula (Poaceae). Int. J. Plant Sci. 2012, 173, 399–411. [Google Scholar] [CrossRef]
- Wagner, N.D.; He, L.; Hörandl, E. Phylogenomic relationships and evolution of polyploid Salix species revealed by RAD Sequencing data. Front. Plant Sci. 2020, 11, 1077. [Google Scholar] [CrossRef]
- Xu, L.L.; Li, T.J.; Liao, L.; Deng, H.S.; Han, X.J. Reticulate evolution in Ranunculus cantonensis polyploid complex and its allied species. Plant Syst. Evol. 2013, 299, 603–610. [Google Scholar] [CrossRef]
- Greiner, R.; Vogt, R.; Oberprieler, C. Phylogenetic studies in the polyploid complex of the genus Leucanthemum Mill. (Compositae, Anthemideae) based on cpDNA sequence variation. Plant Syst. Evol. 2012, 298, 1407–1414. [Google Scholar] [CrossRef]
- Mayr, E. Systematics and the Origin of Species; Columbia University Press: Cambridge, UK, 1942. [Google Scholar]
- Knapp, S. Species concepts and floras: What are species for? Biol. J. Linnean Soc. 2008, 95, 17–25. [Google Scholar] [CrossRef]
- Stebbins, G.L. The role of hybridization in evolution. Proc. Am. Phil. Soc. 1959, 103, 145–165. [Google Scholar]
- Barton, N.H.; Hewitt, G.M. Adaptation, speciation and hybrid zones. Nature 1989, 341, 497–503. [Google Scholar] [CrossRef]
- Barke, B.H.; Karbstein, K.; Daubert, M.; Hörandl, E. The relation of meiotic behaviour to hybridity, polyploidy and apomixis in the Ranunculus auricomus complex (Ranunculaceae). BMC Plant Biol. 2020, 20, 1. [Google Scholar] [CrossRef]
- Linnaeus, C. Species Plantarum; Holmiae: Uppsala, Sweden, 1753. [Google Scholar]
- Thompson, K.; Urquhart Cronish, M.; Whitney, K.; Rieseberg, L.; Schluter, D.; Schluter, D. Patterns, Predictors, and Consequences of Dominance in Hybrids. Am. Nat. 2021, 197, E72–E88. [Google Scholar] [CrossRef]
- Hodač, L.; Barke, B.H.; Hörandl, E. Mendelian segregation of leaf phenotypes in experimental F-2 hybrids elucidates origin of morphological diversity of the apomictic Ranunculus auricomus complex. Taxon 2018, 67, 1082–1092. [Google Scholar] [CrossRef]
- Karbstein, K.; Tomasello, S.; Hodac, L.; Dunkel, F.G.; Daubert, M.; Hörandl, E. Phylogenomics supported by geometric morphometrics reveals delimitation of sexual species within the polyploid apomictic Ranunculus auricomus complex (Ranunculaceae). Taxon 2020, 69, 1191–1220. [Google Scholar] [CrossRef]
- Tomasello, S.; Karbstein, K.; Hodač, L.; Paetzold, C.; Hörandl, E. Phylogenomics unravels Quaternary vicariance and allopatric speciation patterns in temperate-montane plant species: A case study on the Ranunculus auricomus species complex. Mol. Ecol. 2020, 29, 2031–2049. [Google Scholar] [CrossRef] [PubMed]
- Bossdorf, O.; Richards, C.L.; Pigliucci, M. Epigenetics for ecologists. Ecol. Lett. 2008, 11, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Giraud, D.; Lima, O.; Rousseau-Gueutin, M.; Salmon, A.; Ainouche, M. Gene and transposable element expression evolution following recent and past polyploidy events in Spartina (Poaceae). Front. Genet. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Syngelaki, E.; Daubert, M.; Klatt, S.; Hörandl, E. Phenotypic responses, reproduction mode and epigenetic patterns under temperature treatments in the alpine plant species Ranunculus kuepferi (Ranunculaceae). Biology 2020, 9, 315. [Google Scholar] [CrossRef]
- Paun, O.; Bateman, R.M.; Fay, M.F.; Hedren, M.; Civeyrel, L.; Chase, M.W. Stable epigenetic effects impact adaptation in allopolyploid Orchids (Dactylorhiza: Orchidaceae). Mol. Biol. Evol. 2010, 27, 2465–2473. [Google Scholar] [CrossRef]
- Verhoeven, K.J.F.; Jansen, J.J.; van Dijk, P.J.; Biere, A. Stress-induced DNA methylation changes and their heritability in asexual dandelions. New Phytol. 2010, 185, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Osabe, K.; Clement, J.D.; Bedon, F.; Pettolino, F.A.; Ziolkowski, L.; Llewellyn, D.J.; Wilson, I.W. Genetic and DNA methylation changes in cotton (Gossypium) genotypes and tissues. PLoS ONE 2014, 9, e86049. [Google Scholar] [CrossRef]
- Stace, C.A. Introductory. In Hybridization and the Flora of the British Islands; Stace, C.A., Ed.; Cambridge University Press: London, UK, 1975; pp. 1–90. [Google Scholar]
- Richards, J.A. Plant Breeding Systems, 2nd ed.; Chapman and Hall: London, UK, 1997; p. 529. [Google Scholar]
- Templeton, A.R. The meaning of species and speciation: A genetic perspective. In Speciation and Its Consequences; Otte, D., Endler, J.A., Eds.; Sinauer Associates: Sunderland, MA, USA, 1989; pp. 3–27. [Google Scholar]
- Mallet, J. A species definition for the modern synthesis. Trends Ecol. Evol. 1995, 10, 294–299. [Google Scholar] [CrossRef]
- Guo, Y.-P.; Saukel, J.; Ehrendorfer, F. AFLP trees versus scatterplots: Evolution and phylogeography of the polyploid complex Achillea millefolium agg. (Asteraceae). Taxon 2008, 57, 153–169. [Google Scholar]
- Van Valen, L. Ecological species, multispecies, and Oaks. Taxon 1976, 25, 233–239. [Google Scholar] [CrossRef]
- Soltis, D.; Visger, C.; Marchant, D.B.; Soltis, P.S.; Soltis, P. Polyploidy: Pitfalls and paths to a paradigm. Am. J. Bot. 2016, 103, 1146–1166. [Google Scholar] [CrossRef] [PubMed]
- Kirchheimer, B.; Schinkel, C.C.F.; Dellinger, A.S.; Klatt, S.; Moser, D.; Winkler, M.; Dullinger, S. A matter of scale: Apparent niche differentiation of diploid and tetraploid plants may depend on extent and grain of analysis. J. Biogeogr. 2016, 43, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Duchoslav, M.; Jandová, M.; Kobrlová, L.; Šafářová, L.; Brus, J.; Vojtěchová, K. Intricate distribution patterns of six cytotypes of Allium oleraceum at a continental scale: Niche expansion and innovation followed by niche contraction with increasing ploidy level. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, K.J.F.; Verbon, E.H.; van Gurp, T.P.; Oplaat, C.; de Carvalho, J.F.; Morse, A.M.; McIntyre, L.M. Intergenerational environmental effects: Functional signals in offspring transcriptomes and metabolomes after parental jasmonic acid treatment in apomictic dandelion. New Phytol. 2018, 217, 871–882. [Google Scholar] [CrossRef]
- Syngelaki, E.; Schinkel, C.C.F.; Klatt, S.; Hörandl, E. Effects of temperature treatments on cytosine-methylation profiles of diploid and autotetraploid plants of the alpine species Ranunculus kuepferi (Ranunculaceae) Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Barton, N.H. What role does natural selection play in speciation? Phil. Trans. R. Soc. B-Biol. Sci. 2010, 365, 1825–1840. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Guerrero, V.; Aguilar, R.; Marten-Rodriguez, S.; Ashworth, L.; Lopezaraiza-Mikel, M.; Bastida, J.M.; Quesada, M. A quantitative review of pollination syndromes: Do floral traits predict effective pollinators? Ecol. Lett. 2014, 17, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Rezende, L.; Suzigan, J.; Amorim, F.; Moraes, A.P.; Moraes, A. Can plant hybridization and polyploidy lead to pollinator shift? Acta Bot. Brasílica 2020, 34, 229–242. [Google Scholar] [CrossRef]
- Wiley, E.O. Evolutionary species concept reconsidered. Syst. Zool. 1978, 27, 17–26. [Google Scholar] [CrossRef]
- Sukumaran, J.; Knowles, L.L. Multispecies coalescent delimits structure, not species. Proc. Natl. Acad. Sci. USA 2017, 114, 1607–1612. [Google Scholar] [CrossRef]
- Hörandl, E. Paraphyletic versus monophyletic taxa-evolutionary versus cladistic classifications. Taxon 2006, 55, 564–570. [Google Scholar] [CrossRef]
- Hörandl, E.; Stuessy, T.F. Paraphyletic groups as natural units of biological classification. Taxon 2010, 59, 1641–1653. [Google Scholar] [CrossRef]
- Goldberg, E.E.; Kohn, J.R.; Lande, R.; Robertson, K.A.; Smith, S.A.; Igić, B. Species selection maintains self-incompatibility. Science 2010, 330, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Majesky, L.; Krahulec, F.; Vasut, R.J. How apomictic taxa are treated in current taxonomy: A review. Taxon 2017, 66, 1017–1040. [Google Scholar] [CrossRef]
- Hausdorf, B. Progress toward a general species concept. Evolution 2011, 65, 923–931. [Google Scholar] [CrossRef]
- Hörandl, E. Species concepts in agamic complexes: Applications in the Ranunculus auricomus complex and general perspectives. Folia Geobot. 1998, 33, 335–348. [Google Scholar] [CrossRef]
- Maynard Smith, J.; Szathmary, E. The Major Transitions in Evolution; W.H. Freeman: Oxford, UK, 1995. [Google Scholar]
- McShea, D.W.; Brandon, R.N. Biology’s First Law; The University of Chicago Press: London, UK, 2010. [Google Scholar]
- Ku, C.; Nelson-Sathi, S.; Roettger, M.; Garg, S.; Hazkani-Covo, E.; Martin, W.F. Endosymbiotic gene transfer from prokaryotic pangenomes: Inherited chimerism in eukaryotes. Proc. Natl. Acad. Sci. USA 2015, 112, 10139–10146. [Google Scholar] [CrossRef]
- Levin, D. The long wait for hybrid sterility in flowering plants. New Phytol. 2012, 196, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Eldredge, N. Unfinished Synthesis. Biological Hierarchies and Modern Evolutionary Thought; Oxford Univ. Press: New York, NY, USA, 1985. [Google Scholar]
- Otto, S.P. The evolutionary enigma of sex. Am. Nat. 2009, 174, S1–S14. [Google Scholar] [CrossRef]
- Birdsell, J.A.; Wills, C. The evolutionary origin and maintenance of sexual recombination: A review of contemporary models. In Evolutionary Biology; Macintyre, R.J., Clegg, M.T., Eds.; Springer US: Boston, MA, USA, 2003; pp. 27–138. [Google Scholar]
- Bernstein, H.; Byerly, H.; Hopf, F.; Michod, R.E. Is meiotic recombination an adaptation for repairing DNA, producing genetic variation, or both? In The Evolution of Sex; Michod, R.E., Levin, B.R., Eds.; Sinauer Ass Inc.: Massachusetts, MA, USA, 1988; pp. 139–160. [Google Scholar]
- Bernstein, C.; Bernstein, H. Aging, Sex and DNA Repair; Academic Press: San Diego, CA, USA, 1991. [Google Scholar]
- Speijer, D.; Lukeš, J.; Eliáš, M. Sex is a ubiquitous, ancient, and inherent attribute of eukaryotic life. Proc. Natl. Acad. Sci. USA 2015, 112, 8827–8834. [Google Scholar] [CrossRef] [PubMed]
- Hörandl, E.; Speijer, D. How oxygen gave rise to eukaryotic sex. Proc. R. Soc. B-Biol. Sci. 2018, 285, 20172706. [Google Scholar] [CrossRef] [PubMed]
- Hörandl, E.; Hadacek, F. The oxidative damage initiation hypothesis for meiosis. Plant Repr. 2013, 26, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Klatt, S.; Hadacek, F.; Hodač, L.; Brinkmann, G.; Eilerts, M.; Hojsgaard, D.; Hörandl, E. Photoperiod extension enhances sexual megaspore formation and triggers metabolic reprogramming in facultative apomictic Ranunculus auricomus. Front. Plant Sci. 2016, 7, 278. [Google Scholar] [CrossRef]
- Mateo de Arias, M.; Gao, L.; Sherwood, D.A.; Dwivedi, K.; Price, B.J.; Jamison, M.; Carman, J.G. Whether gametophytes are reduced or unreduced in angiosperms might be determined metabolically. Genes 2020, 11, 1449. [Google Scholar] [CrossRef]
- Nedelcu, A.; Marcu, O.; Michod, R. Sex as a response to oxidative stress: A twofold increase in cellular reactive oxygen species activates sex genes. Proc. R. Soc. Biol. Sci. 2004, 271, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Nedelcu, A.; Michod, R. Sex as a response to oxidative stress: The effect of antioxidants on sexual induction in a facultatively sexual lineage. Proc. R. Soc. Biol. Sci. 2003, 270 (Suppl. 2), S136–S139. [Google Scholar] [CrossRef] [PubMed]
- Hadany, L.; Otto, S.P. Condition-dependent sex and the rate of adaption. Am. Nat. 2009, 174, S71–S78. [Google Scholar] [CrossRef][Green Version]
- Bernstein, C.; Johns, V. Sexual reproduction as a response to H2O2 damage in Schizosaccharomyces pombe. J. Bacteriol. 1989, 171, 1893–1897. [Google Scholar] [CrossRef]
- Ulum, F.B.; Costa Castro, C.; Hörandl, E. Ploidy-dependent effects of light stress on the mode of reproduction in the Ranunculus auricomus complex (Ranunculaceae). Front. Plant Sci. 2020, 11, 104. [Google Scholar] [CrossRef]
- Schubert, I. Boon and Bane of DNA Double-Strand Breaks. Int. J. Mol. Sci. 2021, 22, 5171. [Google Scholar] [CrossRef]
- Hörandl, E. Meiosis and the paradox of sex in nature. In Meiosis; Bernstein, C., Bernstein, H., Eds.; InTech: Rijeka, Croatia, 2013; pp. 17–39. [Google Scholar] [CrossRef]
- Otto, S.P.; Gerstein, A.C. The evolution of haploidy and diploidy. Curr. Biol. 2008, 18, R1121–R1124. [Google Scholar] [CrossRef]
- Archetti, M. Recombination and loss of complementation: A more than two-fold cost for parthenogenesis. J. Evol. Biol. 2004, 17, 1084–1097. [Google Scholar] [CrossRef]
- Mirzaghaderi, G.; Hörandl, E. The evolution of meiotic sex and its alternatives. Proc. R. Soc. Biol. Sci. 2016, 283, 20161221. [Google Scholar] [CrossRef]
- Brandeis, M. New-age ideas about age-old sex: Separating meiosis from mating could solve a century-old conundrum. Biol. Rev. 2018, 93, 801–810. [Google Scholar] [CrossRef]
- Kraus, D.; Chi, J.; Boenigk, J.; Beisser, D.; Graupner, N.; Dunthorn, M. Putatively asexual chrysophytes have meiotic genes: Evidence from transcriptomic data. PeerJ 2019, 16, e5894. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, M.A.; Malik, S.-B.; Logsdon, J.M. A phylogenomic inventory of meiotic genes: Evidence for sex in Giardia and an early eukaryotic origin of meiosis. Curr. Biol. 2005, 15, 185–191. [Google Scholar]
- Friedberg, E.C.; Wlaker, G.C.; Siede, W.; Wood, R.D.; Schultz, R.A.; Ellenberger, T. DNA Repair and Mutagenesis, 2nd ed.; American Society for Microbiology: Washington, DC, USA, 2006. [Google Scholar]
- Wilkins, A.S.; Holliday, R. The evolution of meiosis from mitosis. Genetics 2009, 181, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Mercier, R.; Mézard, C.; Jenczewski, E.; Macaisne, N.; Grelon, M.; Mezard, C. The Molecular Biology of Meiosis in Plants. Annu. Rev. Plant Biol. 2015, 66, 297–327. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, J.; Bradshaw, H.D.; Schemske, D.W. Components of reproductive isolation between the monkeyflowers Mimulus lewisii and M. cardinalis (Phrymaceae). Evolution 2003, 57, 1520–1534. [Google Scholar] [CrossRef] [PubMed]
- Baack, E.; Melo, M.; Rieseberg, L.; Ortiz Barrientos, D. The origins of reproductive isolation in plants. New Phytol. 2015, 207, 968–984. [Google Scholar] [CrossRef]
- Burt, A. Perspective: Sex, recombination, and the efficacy of selection—Was Weismann right? Evolution 2000, 54, 337–351. [Google Scholar] [PubMed]
- Dyer, P.S.; Kück, U. Sex and the imperfect fungi. In The Fungal Kingdom; Heitman, J., Howlett, B.J., Crous, P.W., Stukenbrock, E.H., James, T.Y., Gow, N.A.R., Eds.; The American Society for Microbiology Press: Washington, DC, USA, 2017. [Google Scholar] [CrossRef]
- Hörandl, E.; Bast, J.; Brandt, A.; Scheu, S.; Bleidorn, C.; Cordellier, M.; Dunthorn, M. Genome evolution of asexual organisms and the paradox of sex in eukaryotes. In Evolutionary Biology: A Transdisciplinary Approach; Pontarotti, P., Ed.; Springer: Basel, Switzerland, 2020. [Google Scholar] [CrossRef]
- Flot, J.-F.; Hespeels, B.; Li, X.; Noel, B.; Arkhipova, I.; Danchin, E.G.; Aury, J.-M. Genomic evidence for ameiotic evolution in the bdelloid rotifer Adineta vaga. Nature 2013, 500, 453–457. [Google Scholar] [CrossRef]
- Fontaneto, D.; Barraclough, T.G. Do Species Exist in Asexuals? Theory and Evidence from Bdelloid Rotifers. Integr. Comp. Biol. 2015, 55, 253–263. [Google Scholar] [CrossRef]
- Bohutinska, M.; Handrick, V.; Yant, L.; Schmickl, R.; Kolar, F.; Bomblies, K.; Paajanen, P. De novo mutation and rapidpProtein (co-)evolution during meiotic adaptation in Arabidopsis arenosa. Mol. Biol. Evol. 2021, 38, 1980–1994. [Google Scholar] [CrossRef] [PubMed]
- Bomblies, K.; Higgins, J.D.; Yant, L. Meiosis evolves: Adaptation to external and internal environments. New Phytol. 2015, 208, 306–323. [Google Scholar] [CrossRef] [PubMed]
- Constable, G.W.A.; Kokko, H. Parthenogenesis and the evolution of anisogamy. Cells 2021, 2467. [Google Scholar] [CrossRef] [PubMed]
- Janko, K. Let us not be unfair to asexuals: Their ephemerality may be explained by neutral models without invoking any evolutionary constraints of asexuality. Evolution 2014, 68, 569–576. [Google Scholar] [CrossRef]
- Gerstein, A.C.; Otto, S.P. Ploidy and the Causes of Genomic Evolution. J. Hered. 2009, 100, 571–581. [Google Scholar] [CrossRef]
- Hodač, L.; Klatt, S.; Hojsgaard, D.; Sharbel, T.; Hörandl, E. A little bit of sex prevents mutation accumulation even in apomictic polyploid plants. BMC Evol. Biol. 2019, 19, 170. [Google Scholar] [CrossRef]
- Hörandl, E.; Hojsgaard, D. The evolution of apomixis in angiosperms: A reappraisal. Plant Biosyst. 2012, 146, 681–693. [Google Scholar] [CrossRef]
- Ozias-Akins, P.; van Dijk, P.J. Mendelian genetics of apomixis in plants. Annu. Rev. Genet. 2007, 41, 509–537. [Google Scholar] [CrossRef]
- Love, A.C. Typology Reconfigured: From the Metaphysics of Essentialism to the Epistemology of Representation. Acta Biotheor. 2009, 57, 51–75. [Google Scholar] [CrossRef]
- Stace, C.A. Species recognition in agamosperms—The need for a pragmatic approach. Folia Geobot. 1998, 33, 319–326. [Google Scholar] [CrossRef]
- Kyriakidou, M.; Tai, H.H.; Anglin, N.L.; Ellis, D.; Strömvik, M.V. Current Strategies of Polyploid Plant Genome Sequence Assembly. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef]
- Greilhuber, J.; Dolezel, J.; Lysák, M.A.; Bennett, M.D. The origin, evolution and proposed stabilization of the terms ’genome size’ and ’C-value’ to describe nuclear DNA contents. Ann. Bot. 2005, 95, 255–260. [Google Scholar] [CrossRef]
- Dolezel, J.; Greilhuber, J.; Suda, J. Estimation of nuclear DNA content in plants using flow cytometry. Nat. Protoc. 2007, 2, 2233–2244. [Google Scholar] [CrossRef]
- Kron, P.; Suda, J.; Husband, B.C. Applications of flow cytometry to evolutionary and population biology. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 847–876. [Google Scholar] [CrossRef]
- Loureiro, J.; Kron, P.; Temsch, E.M.; Koutecky, P.; Lopes, S.; Castro, M.; Castro, S. Isolation of plant nuclei for estimation of nuclear DNA content: Overview and best practices. Cytom. Part A 2021, 99, 318–327. [Google Scholar] [CrossRef]
- Dolezel, J.; Cizkova, J.; Simkova, H.; Bartos, J. One Major Challenge of Sequencing Large Plant Genomes Is to Know How Big They Really Are. Int. J. Mol. Sci. 2018, 19, 3554. [Google Scholar] [CrossRef] [PubMed]
- Matzk, F.; Meister, A.; Schubert, I. An efficient screen for reproductive pathways using mature seeds of monocots and dicots. Plant J. 2000, 21, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Schinkel, C.C.F.; Kirchheimer, B.; Dullinger, S.; Geelen, D.; De Storme, N.; Hörandl, E. Pathways to polyploidy: Indications of a female triploid bridge in the alpine species Ranunculus kuepferi (Ranunculaceae). Plant Syst. Evol. 2017, 303, 1093–1108. [Google Scholar] [CrossRef] [PubMed]
- McDade, L.A. HYBRIDS AND PHYLOGENETIC SYSTEMATICS.1. THE IMPACT OF HYBRIDS ON CLADISTIC-ANALYSIS. Evolution 1992, 46, 1329–1346. [Google Scholar] [CrossRef]
- Wagner, N.D.; Volf, M.; Hörandl, E. Highly diverse shrub willows (Salix L.) share highly similar plastomes. Front. Plant Sci. 2021, 12, 662715. [Google Scholar] [CrossRef]
- Su, N.; Liu, B.; Wang, J.; Tong, R.; Ren, C.; Chang, Z.; Wen, J. On the species delimitation of the Maddenia Group of Prunus (Rosaceae): Evidence from plastome and nuclear Sequences and morphology. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef]
- Michael, T.P.; VanBuren, R. Building near-complete plant genomes. Curr. Opin. Plant Biol. 2020, 54, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Paajanen, P.; Kettleborough, G.; Lopez-Girona, E.; Giolai, M.; Heavens, D.; Baker, D.; Clark, M.D. A critical comparison of technologies for a plant genome sequencing project. Gigascience 2019, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Jeon, M.S.; Hodgett, M.; Waterhouse, P.; Eyun, S.I. Comparative Evaluation of Genome Assemblers from Long-Read Sequencing for Plants and Crops. J. Agricult. Food Chem. 2020, 68, 7670–7677. [Google Scholar] [CrossRef] [PubMed]
- Li, F.W.; Harkess, A. A guide to sequence your favorite plant genomes. Appl. Plant Sci. 2018, 6. [Google Scholar] [CrossRef]
- Hirsch, C.N.; Buell, C.R. Tapping the Promise of Genomics in Species with Complex, Nonmodel Genomes. Annu. Rev. Plant Biol. 2013, 64, 89–110. [Google Scholar] [CrossRef] [PubMed]
- McKain, M.R.; Johnson, M.G.; Uribe-Convers, S.; Eaton, D.; Yang, Y. Practical considerations for plant phylogenomics. Appl. Plant Sci. 2018, 6, 15. [Google Scholar] [CrossRef]
- Davey, J.W.; Hohenlohe, P.A.; Etter, P.D.; Boone, J.Q.; Catchen, J.M.; Blaxter, M.L. Genome-wide genetic marker discovery and genotyping using next-generation sequencing. Nat. Rev. Genet. 2011, 12, 499–510. [Google Scholar] [CrossRef]
- Pease, J.B.; Brown, J.W.; Walker, J.F.; Hinchliff, C.E.; Smith, S.A. Quartet Sampling distinguishes lack of support from conflicting support in the green plant tree of life. Am. J. Bot. 2018, 105, 385–403. [Google Scholar] [CrossRef] [PubMed]
- Bryant, D.; Moulton, V. Neighbor-Net: An agglomerative method for the construction of phylogenetic networks. Mol. Biol. Evol. 2004, 21, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of population structure using multilocus genotype data: Dominant markers and null alleles. Mol. Ecol. Notes 2007, 7, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Frichot, E.; Mathieu, F.; Trouillon, T.; Bouchard, G.; Francois, O. Fast and efficient estimation of individual ancestry coefficients. Genetics 2014, 196, 973. [Google Scholar] [CrossRef]
- Anderson, E.C.; Thompson, E.A. A model-based method for identifying species hybrids using multilocus genetic data. Genetics 2002, 160, 1217–1229. [Google Scholar] [CrossRef]
- Blischak, P.D.; Chifman, J.; Wolfe, A.D.; Kubatko, L.S. HyDe: A Python Package for Genome-Scale Hybridization Detection. Syst. Biol. 2018, 67, 821–829. [Google Scholar] [CrossRef]
- Peralta, M.; Combes, M.-C.; Cenci, A.; Lashermes, P.; Dereeper, A. SNiPloid: A utility to exploit high-throughput SNP data derived from RNA-seq in allopolyploid species. Int. J. Pl. Genom. 2013, 890123. [Google Scholar] [CrossRef]
- Eaton, D.A.R.; Hipp, A.L.; Gonzalez-Rodriguez, A.; Cavender-Bares, J. Historical introgression among the American live oaks and the comparative nature of tests for introgression. Evolution 2015, 69, 2587–2601. [Google Scholar] [CrossRef]
- Malinsky, M.; Trucchi, E.; Lawson, D.J.; Falush, D. RADpainter and fineRADstructure Population Inference from RADseq Data. Mol. Biol. Evol. 2018, 35, 1284–1290. [Google Scholar] [CrossRef]
- Karbstein, K.; Tomasello, S.; Hodac, L.; Lorberg, E.; Daubert, M.; Hörandl, E. Moving beyond assumptions: Polyploidy and environmental effects explain a geographical parthenogenesis scenario in European plants. Mol. Ecol. 2021, 30, 2659–2675. [Google Scholar] [CrossRef] [PubMed]
- Hühn, P.; Dillenberger, M.S.; Gerschwitz-Eidt, M.; Hörandl, E.; Los, J.A.; Messerschmid, T.F.E.; Kadereit, G. How challenging RADseq data turned out to favor coalescent-based species tree inference. A case study in Aichryson (Crassulaceae). Mol. Phy. Evol. 2022, 167, 107342. [Google Scholar] [CrossRef] [PubMed]
- Weitemier, K.; Straub, S.C.K.; Cronn, R.C.; Fishbein, M.; Schmickl, R.; McDonnell, A.; Liston, A. Hyb-Seq: Combining target enrichment and genome skimming for plant phylogenomics. Appl. Plant Sci. 2014, 2. [Google Scholar] [CrossRef]
- Oxelman, B.; Brysting, A.K.; Jones, G.R.; Marcussen, T.; Oberprieler, C.; Pfeil, B.E. Phylogenetics of Allopolyploids. Annu. Rev. Plant Biol. 2017, 48, 543–557. [Google Scholar] [CrossRef]
- Rothfels, C. Polyploid phylogenetics. New Phytol. 2021, 230, 66–72. [Google Scholar] [CrossRef]
- Tiley, G.P.; Crowl, A.A.; Manos, P.S.; Sessa, E.B.; Solís-Lemus, C.; Yoder, A.D.; Burleigh, J.G. Phasing alleles improves network inference with allopolyploids. bioRxiv 2021. [Google Scholar] [CrossRef]
- St Onge, K.R.; Foxe, J.P.; Li, J.R.; Li, H.P.; Holm, K.; Corcoran, P.; Wright, S.I. Coalescent-based analysis distinguishes between allo- and autopolyploid origin in Shepherd’s Purse (Capsella bursa-pastoris). Mol. Biol. Evol. 2012, 29, 1721–1733. [Google Scholar] [CrossRef]
- Brandrud, M.K.; Baar, J.; Lorenzo, M.T.; Athanasiadis, A.; Bateman, R.; Chase, M.W.; Paun, O. Phylogenomic relationships of diploids and the origins of allotetraploids in Dactylorhiza (Orchidaceae). Syst. Biol. 2020, 69, 91–109. [Google Scholar] [CrossRef]
- Pellino, M.; Hojsgaard, D.; Schmutzer, T.; Scholz, U.; Hörandl, E.; Vogel, H.; Sharbel, T.F. Asexual genome evolution in the apomictic Ranunculus auricomus complex: Examining the effects of hybridization and mutation accumulation. Mol. Ecol. 2013, 22, 5908–5921. [Google Scholar] [CrossRef]
- Lyu, R.; He, J.; Luo, Y.; Lin, L.; Yao, M.; Cheng, J.; Li, L. Natural hybrid origin of the controversial “species” Clematis × pinnata (Ranunculaceae) based on multidisciplinary evidence. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef]
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Smith, G.F. International Code of Nomenclature for Algae, Fungi, and Plants (Shenzhen Code) Adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017; Koeltz Botanical Books: Glashütten, Germany, 2018; Volume 159. [Google Scholar]
- Ballere, C.L.; Trewavas, A. Plant Behaviour Special Issue. Plant Cell Environ. 2009, 32, 605. [Google Scholar]
- Ulum, F.B.; Hadacek, F.; Hörandl, E. Polyploidy improves photosynthesis regulation within the Ranunculus auricomus complex (Ranunculaceae). Biology 2021, 10, 811. [Google Scholar] [CrossRef] [PubMed]
- Coate, J.E.; Powell, A.F.; Owens, T.G.; Doyle, J.J. Transgressive physiological and transcriptomic responses to light stress in allopolyploid Glycine dolichocarpa (Leguminosae). Heredity 2013, 110, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.T.; Hu, G.J.; Yu, J.W.; Thu, S.W.; Grover, C.E.; Zhu, S.J.; Wendel, J.F. Salt-tolerance diversity in diploid and polyploid cotton (Gossypium) species. Plant J. 2020, 101, 1135–1151. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hörandl, E. Novel Approaches for Species Concepts and Delimitation in Polyploids and Hybrids. Plants 2022, 11, 204. https://doi.org/10.3390/plants11020204
Hörandl E. Novel Approaches for Species Concepts and Delimitation in Polyploids and Hybrids. Plants. 2022; 11(2):204. https://doi.org/10.3390/plants11020204
Chicago/Turabian StyleHörandl, Elvira. 2022. "Novel Approaches for Species Concepts and Delimitation in Polyploids and Hybrids" Plants 11, no. 2: 204. https://doi.org/10.3390/plants11020204
APA StyleHörandl, E. (2022). Novel Approaches for Species Concepts and Delimitation in Polyploids and Hybrids. Plants, 11(2), 204. https://doi.org/10.3390/plants11020204





