Natural Variation of Seed Tocopherol Composition in Diverse World Soybean Accessions from Maturity Group 0 to VI Grown in China
Abstract
:1. Introduction
2. Results and Discussion
2.1. Variation in Seed Tocopherol Composition in Diverse Soybean Accessions
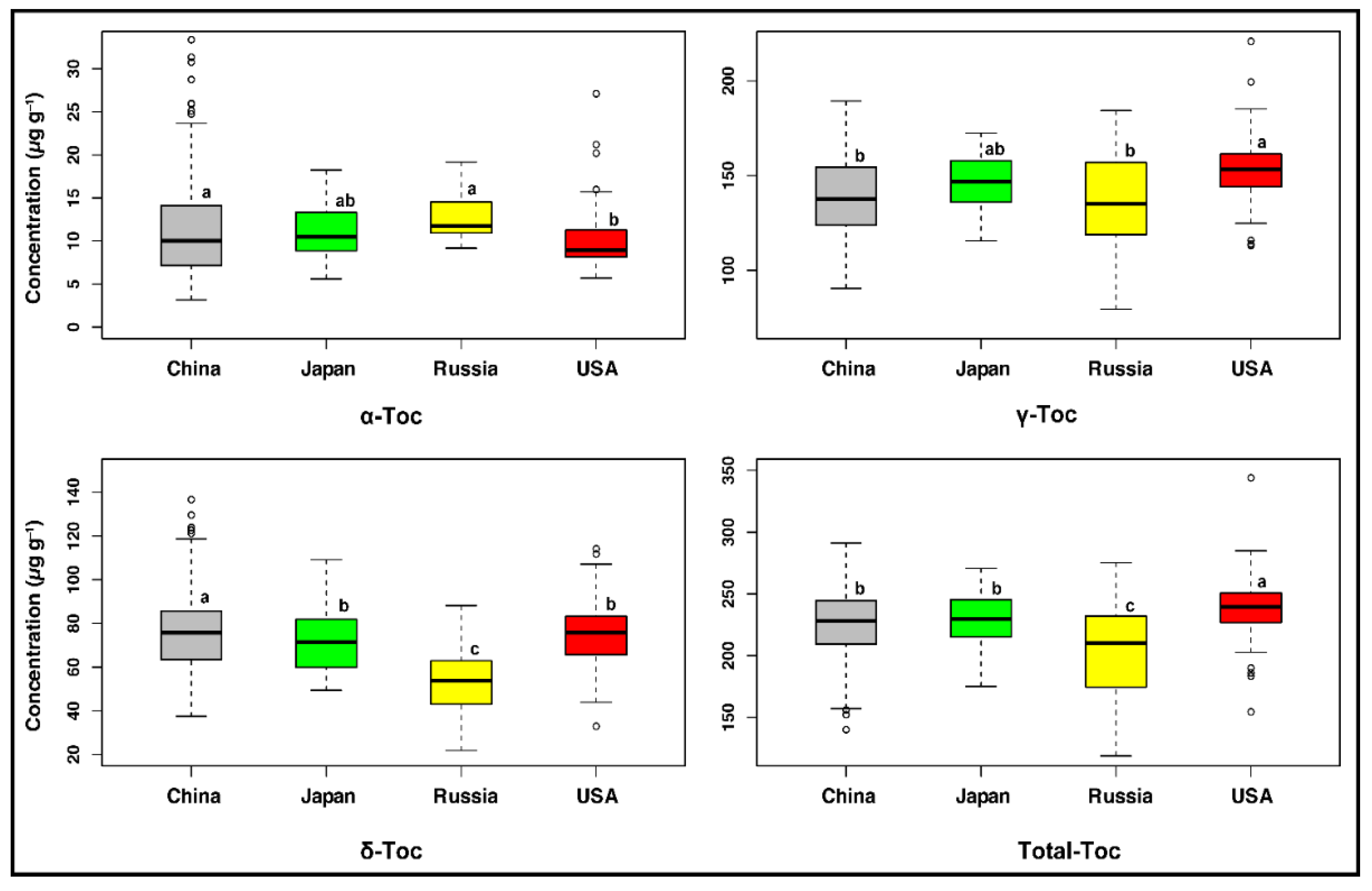
2.2. Germplasm Origins Had a Significant Influence on Seed Tocopherol Composition
2.3. Maturity Groups Had a Pronounced Impact on Seed Tocopherol Composition
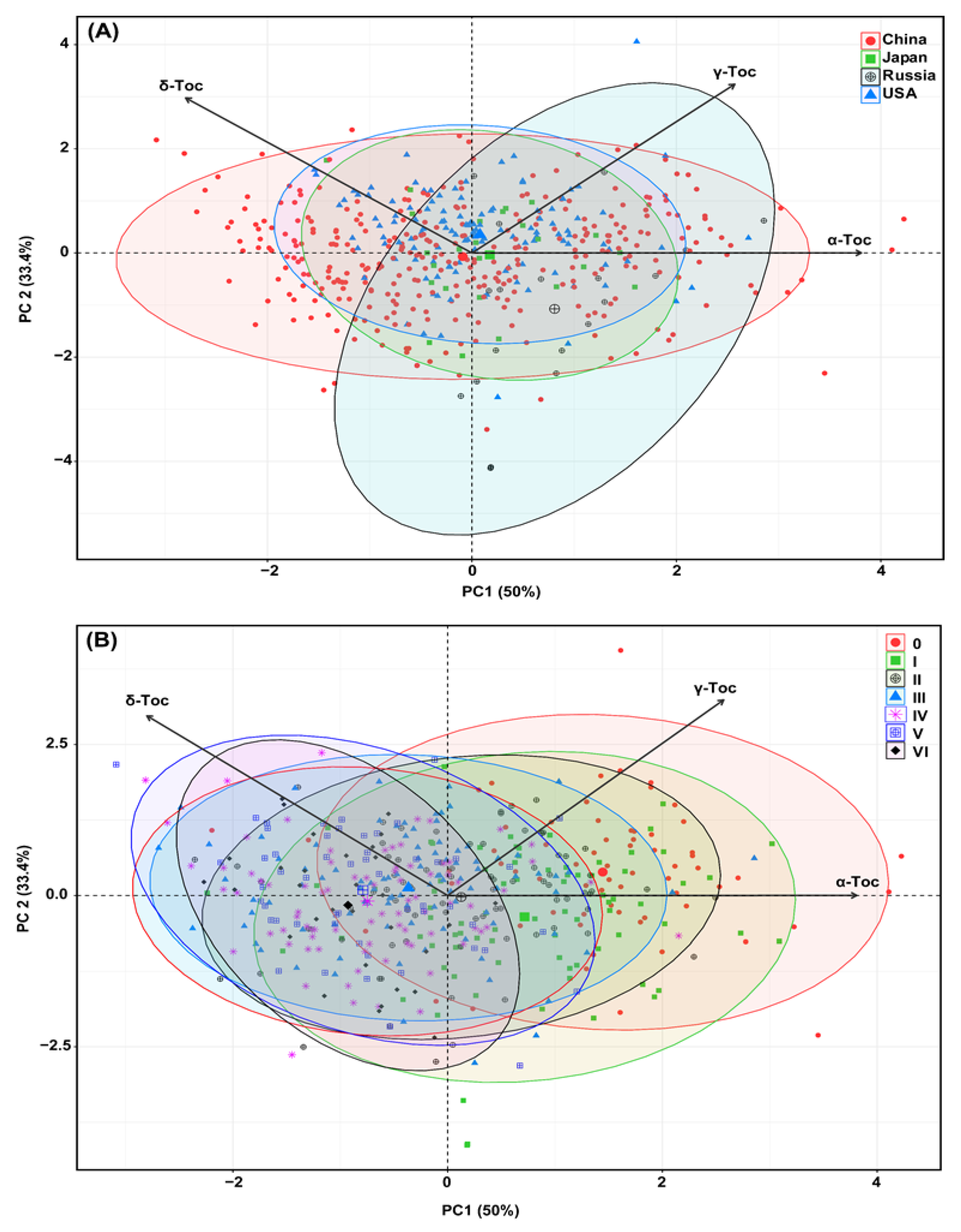
2.4. Principal Component Analysis (PCA) Based on Origin and Maturity Groups
2.5. Geographical Distribution of Soybean Seed Tocopherol Components
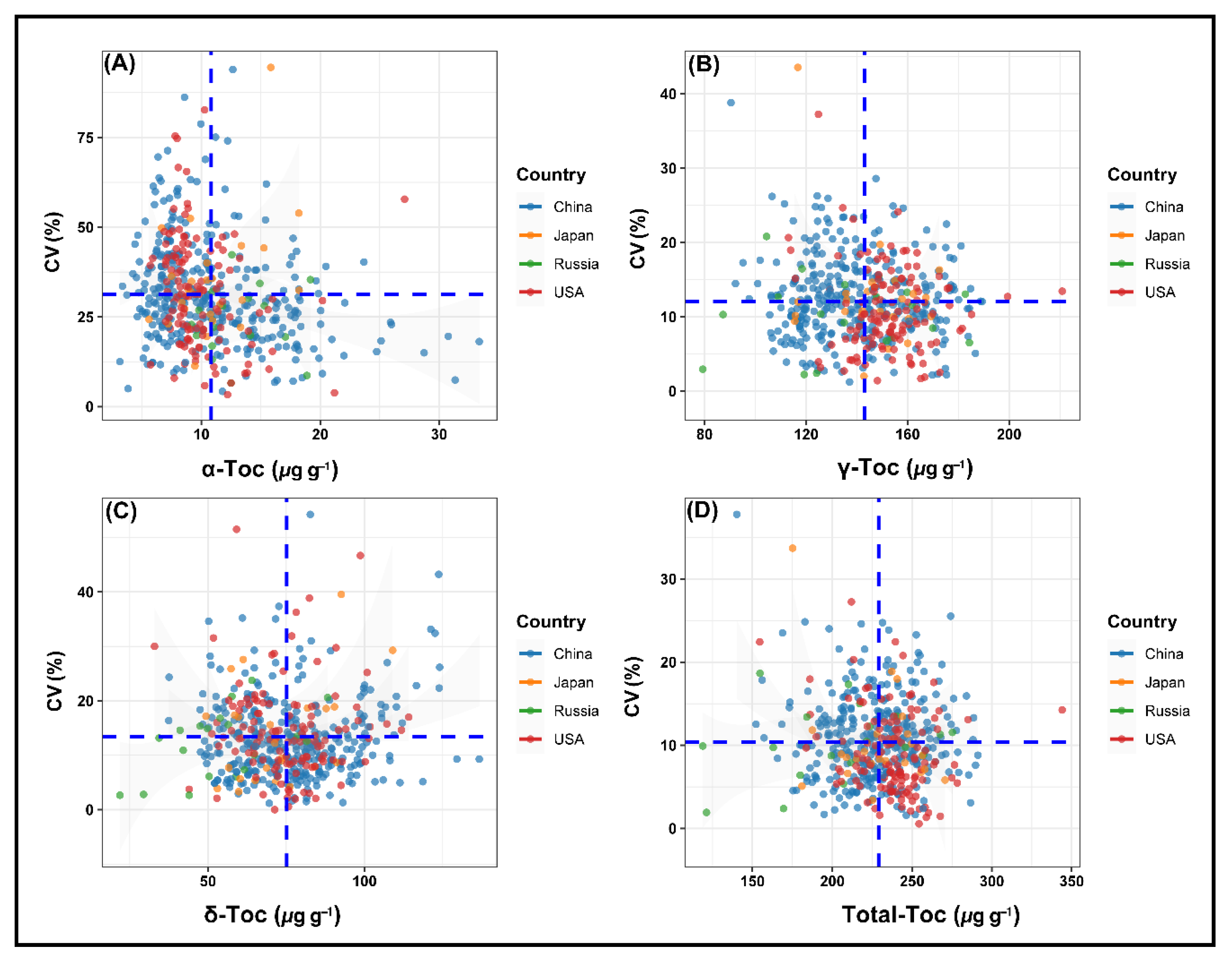
2.6. Stability Performance of Soybean Accessions for Tocopherols across Environments
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Materials
3.3. Field Experiments
3.4. Tocopherol Extraction and Determination
3.5. Statistical Analysis
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahidi, F.; De Camargo, A.C. Tocopherols and tocotrienols in common and emerging dietary sources: Occurrence, applications, and health benefits. Int. J. Mol. Sci. 2016, 17, 1745. [Google Scholar] [CrossRef]
- Boschin, G.; Arnoldi, A. Legumes are valuable sources of tocopherols. Food Chem. 2011, 127, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Bramley, P.; Elmadfa, I.; Kafatos, A.; Kelly, F.; Manios, Y.; Roxborough, H.; Schuch, W.; Sheehy, P.; Wagner, K.-H. Review vitamin E. J. Sci. Food Agric. 2000, 80, 913–938. [Google Scholar] [CrossRef]
- Buring, J.E.; Gaziano, J.M. Antioxidant vitamins and cardiovascular disease. In Preventive Nutrition; Springer: Berlin/Heidelberg, Germany, 1997; pp. 171–180. [Google Scholar]
- Shintani, D.; DellaPenna, D. Elevating the vitamin E content of plants through metabolic engineering. Science 1998, 282, 2098–2100. [Google Scholar] [CrossRef]
- Tangolar, S.G.; Özogul, F.; Tangolar, S.; Yağmur, C. Tocopherol content in fifteen grape varieties obtained using a rapid HPLC method. J. Food Compos. Anal. 2011, 24, 481–486. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Appelqvist, L.Å. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef] [PubMed]
- Warner, K.A. Effects of antioxidants in frying oils. In Chemistry of Frying Oils; AOCS: Champaign, IL, USA, 2003; pp. 210–227. [Google Scholar]
- Britz, S.J.; Kremer, D.F. Warm temperatures or drought during seed maturation increase free α-tocopherol in seeds of soybean (Glycine max [L.] Merr.). J. Agric. Food Chem. 2002, 50, 6058–6063. [Google Scholar] [CrossRef]
- Britz, S.J.; Kremer, D.F.; Kenworthy, W.J. Tocopherols in soybean seeds: Genetic variation and environmental effects in field-grown crops. J. Am. Oil Chem. Soc. 2008, 85, 931–936. [Google Scholar] [CrossRef]
- Carrera, C.; Martínez, M.J.; Dardanelli, J.; Balzarini, M. Environmental variation and correlation of seed components in nontransgenic soybeans: Protein, oil, unsaturated fatty acids, tocopherols, and isoflavones. Crop Sci. 2011, 51, 800–809. [Google Scholar] [CrossRef]
- Almonor, G.; Fenner, G.; Wilson, R. Temperature effects on tocopherol composition in soybeans with genetically improved oil quality. J. Am. Oil Chem. Soc. 1998, 75, 591–596. [Google Scholar] [CrossRef]
- Carrao-Panizzi, M.C.; Erhan, S.Z. Environmental and genetic variation of soybean tocopherol content under Brazilian growing conditions. J. Am. Oil Chem. Soc. 2007, 84, 921–928. [Google Scholar] [CrossRef]
- Carrera, C.S.; Dardanelli, J.L.; Soldini, D.O. Genotypic and environmental variation in seed nutraceutical and industrial composition of non-transgenic soybean (Glycine max) genotypes. Crop Pasture Sci. 2014, 65, 1311–1322. [Google Scholar] [CrossRef]
- Chennupati, P.; Seguin, P.; Liu, W. Effects of high temperature stress at different development stages on soybean isoflavone and tocopherol concentrations. J. Agric. Food Chem. 2011, 59, 13081–13088. [Google Scholar] [CrossRef]
- Ujiie, A.; Yamada, T.; Fujimoto, K.; Endo, Y.; Kitamura, K. Identification of soybean varieties with high α-tocopherol content. Breed. Sci. 2005, 55, 123–125. [Google Scholar] [CrossRef] [Green Version]
- Whent, M.; Hao, J.; Slavin, M.; Zhou, M.; Song, J.; Kenworthy, W.; Yu, L.L. Effect of genotype, environment, and their interaction on chemical composition and antioxidant properties of low-linolenic soybeans grown in Maryland. J. Agric. Food Chem. 2009, 57, 10163–10174. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Li, Y.; Guan, R.; Liu, Z.; Wang, L.; Chang, R. Establishment, representative testing and research progress of soybean core collection and mini core collection. Acta Agron. Sin. 2009, 35, 571–579. [Google Scholar] [CrossRef]
- Dwiyanti, M.S.; Yamada, T.; Sato, M.; Abe, J.; Kitamura, K. Genetic variation of γ-tocopherol methyltransferase gene contributes to elevated α-tocopherol content in soybean seeds. BMC Plant Biol. 2011, 11, 152. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, P.; Weihrauch, J.L. Vitamin E content of foods. J. Am. Diet. Assoc. 1979, 75, 647–665. [Google Scholar] [CrossRef]
- Ghosh, S.; Zhang, S.; Azam, M.; Qi, J.; Abdelghany, A.M.; Shaibu, A.S.; Gebregziabher, B.S.; Feng, Y.; Huai, Y.; Htway, H.T.P.; et al. Seed tocopherol assessment and geographical distribution of 1151 Chinese soybean accessions from diverse ecoregions. J. Food Compos. Anal. 2021, 100, 103932. [Google Scholar] [CrossRef]
- Seguin, P.; Turcotte, P.; Tremblay, G.; Pageau, D.; Liu, W. Tocopherols concentration and stability in early maturing soybean genotypes. Agron. J. 2009, 101, 1153–1159. [Google Scholar] [CrossRef]
- Grilo, E.C.; Costa, P.N.; Gurgel, C.S.S.; Beserra, A.F.D.L.; Almeida, F.N.D.S.; Dimenstein, R. Alpha-tocopherol and gamma-tocopherol concentration in vegetable oils. Food Sci. Technol. 2014, 34, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.; Christen, S.; Shigenaga, M.K.; Ames, B.N. γ-Tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am. J. Clin. Nutr. 2001, 74, 714–722. [Google Scholar] [CrossRef] [Green Version]
- Scherder, C.W.; Fehr, W.R.; Welke, G.A.; Wang, T. Tocopherol content and agronomic performance of soybean lines with reduced palmitate. Crop Sci. 2006, 46, 1286–1290. [Google Scholar] [CrossRef]
- Kim, H.; Lee, H.; Min, D. Effects and prooxidant mechanisms of oxidized α-tocopherol on the oxidative stability of soybean oil. J. Food Sci. 2007, 72, C223–C230. [Google Scholar] [CrossRef] [PubMed]
- Dolde, D.; Vlahakis, C.; Hazebroek, J. Tocopherols in breeding lines and effects of planting location, fatty acid composition, and temperature during development. J. Am. Oil Chem. Soc. 1999, 76, 349–355. [Google Scholar] [CrossRef]
- Song, J.Y.; Piao, X.M.; Choi, Y.M.; Lee, G.A.; Chung, J.W.; Lee, J.R.; Jung, Y.; Park, H.J.; Lee, M.C. Evaluation of genetic diversity and comparison of biochemical traits of soybean (Glycine max L.) germplasm collections. Plant Breed. Biotechnol. 2013, 1, 374–384. [Google Scholar] [CrossRef] [Green Version]
- Maras, J.E.; Bermudez, O.I.; Qiao, N.; Bakun, P.J.; Boody-Alter, E.L.; Tucker, K.L. Intake of α-tocopherol is limited among US adults. J. Am. Diet. Assoc. 2004, 104, 567–575. [Google Scholar] [CrossRef]
- Nedoluzhko, A.; Dorokhov, D. Study of the biosafety of genetically modified soybean in the center of its origin and diversity in the Far East of the Russian Federation. Cytol. Genet. 2007, 41, 190–198. [Google Scholar] [CrossRef]
- Dardanelli, J.L.; Balzarini, M.; Martínez, M.J.; Cuniberti, M.; Resnik, S.; Ramunda, S.F.; Herrero, R.; Baigorri, H. Soybean maturity groups, environments, and their interaction define mega-environments for seed composition in Argentina. Crop Sci. 2006, 46, 1939–1947. [Google Scholar] [CrossRef]
- Mourtzinis, S.; Conley, S.P. Delineating soybean maturity groups across the United States. Agron. J. 2017, 109, 1397–1403. [Google Scholar] [CrossRef] [Green Version]
- Rodiño, A.P.; Santalla, M.; De Ron, A.M.; Singh, S.P. A core collection of common bean from the Iberian Peninsula. Euphytica 2003, 131, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Ge, Y.; Han, F.; Li, B.; Yan, S.; Sun, J.; Wang, L. Isoflavone content of soybean cultivars from maturity group 0 to VI grown in northern and southern China. J. Am. Oil Chem. Soc. 2014, 91, 1019–1028. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo, N.; Nolasco, S.; Mateo, C.; Santos, D.; Aguirrezábal, L. Relationship between oil tocopherol concentration and oil weight per grain in several crop species. Crop Pasture Sci. 2012, 62, 1088–1097. [Google Scholar] [CrossRef]
- Wu, T.T.; Li, J.Y.; Wu, C.X.; Sun, S.; Mao, T.T.; Jiang, B.J.; Hou, W.S.; Han, T.F. Analysis of the independent-and interactive-photo-thermal effects on soybean flowering. J. Integr. Agric. 2015, 14, 622–632. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Wu, T.; Zhang, X.; Song, W.; Xu, C.; Sun, S.; Hou, W.; Jiang, B.; Han, T.; Wu, C. Critical photoperiod measurement of soybean genotypes in different maturity groups. Crop Sci. 2019, 59, 2055–2061. [Google Scholar] [CrossRef]
- Riggs, R.D.; Wrather, J.A.; Mauromoustakos, A.; Rakes, L. Planting date and soybean cultivar maturity group affect population dynamics of Heterodera glycines, and all affect yield of soybean. J. Nematol. 2000, 32, 334–342. [Google Scholar] [PubMed]
- Schneider, C. Chemistry and biology of vitamin E. Mol. Nutr. Food Res. 2005, 49, 7–30. [Google Scholar] [CrossRef]
- Ramadan, Z.; Jacobs, D.; Grigorov, M.; Kochhar, S. Metabolic profiling using principal component analysis, discriminant partial least squares, and genetic algorithms. Talanta 2006, 68, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Cober, E.; Tanner, J.; Voldeng, H. Genetic control of photoperiod response in early-maturing, near-isogenic soybean lines. Crop Sci. 1996, 36, 601–605. [Google Scholar] [CrossRef]
- Liu, X.; Wu, J.A.; Ren, H.; Qi, Y.; Li, C.; Cao, J.; Zhang, X.; Zhang, Z.; Cai, Z.; Gai, J. Genetic variation of world soybean maturity date and geographic distribution of maturity groups. Breed. Sci. 2017, 67, 221–232. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Sun, S.; Ibrahim, S.E.; Xu, Z.; Wu, H.; Hu, X.; Jia, H.; Cheng, Y.; Yang, Z.; Jiang, S.; et al. Standard cultivar selection and digital quantification for precise classification of maturity groups in soybean. Crop Sci. 2019, 59, 1997–2006. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Yang, R.; Wu, T.; Wu, C.; Sun, S.; Zhang, S.; Jiang, B.; Tian, S.; Liu, X.; Han, T. Analyzing the effects of climate factors on soybean protein, oil contents, and composition by extensive and high-density sampling in China. J. Agric. Food Chem. 2016, 64, 4121–4130. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Liu, Z.X.; Yang, C.Y.; Xu, R.; Lu, W.G.; Zhang, L.F.; Wang, Q.; Wei, S.-H.; Yang, C.M.; Wang, H.C.; et al. Stability of growth periods traits for soybean cultivars across multiple locations. J. Integr. Agric. 2016, 15, 963–972. [Google Scholar] [CrossRef]
- Demurin, Y.; Skoric, D.; Karlovic, D. Genetic variability of tocopherol composition in sunflower seeds as a basis of breeding for improved oil quality. Plant Breed. 1996, 115, 33–36. [Google Scholar] [CrossRef]
- Li, G.-X.; Lee, M.-J.; Liu, A.B.; Yang, Z.; Lin, Y.; Shih, W.J.; Yang, C.S. δ-tocopherol is more active than α-or γ-tocopherol in inhibiting lung tumorigenesis in vivo. Cancer Prev. Res. 2011, 4, 404–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsubokura, Y.; Matsumura, H.; Xu, M.; Liu, B.; Nakashima, H.; Anai, T.; Kong, F.; Yuan, X.; Kanamori, H.; Katayose, Y. Genetic variation in soybean at the maturity locus E4 is involved in adaptation to long days at high latitudes. Agronomy 2013, 3, 117–134. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Jiang, B.; Wu, C.; Lu, W.; Hou, W.; Sun, S.; Yan, H.; Han, T. Maturity group classification and maturity locus genotyping of early-maturing soybean varieties from high-latitude cold regions. PLoS ONE 2014, 9, e94139. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, R.; Hesketh, J.D. Effects of photoperiod on growth and development of soybean floral bud in different maturity. Agron. J. 2001, 93, 944–948. [Google Scholar] [CrossRef]
- You, F.M.; Jia, G.; Xiao, J.; Duguid, S.D.; Rashid, K.Y.; Booker, H.M.; Cloutier, S. Genetic variability of 27 traits in a core collection of flax (Linum usitatissimum L.). Front. Plant Sci. 2017, 8, 1636. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S.; Harada, K.; Abe, J. Genetic and molecular bases of photoperiod responses of flowering in soybean. Breed. Sci. 2012, 61, 531–543. [Google Scholar] [CrossRef] [Green Version]
- Abdelghany, A.M.; Zhang, S.; Azam, M.; Shaibu, A.S.; Feng, Y.; Li, Y.; Tian, Y.; Hong, H.; Li, B.; Sun, J. Profiling of seed fatty acid composition in 1025 Chinese soybean accessions from diverse ecoregions. Crop J. 2020, 8, 635–644. [Google Scholar] [CrossRef]
- Dwiyanti, M.S.; Ujiie, A.; Le, T.B.T.; Yamada, T.; Kitamura, K. Genetic analysis of high α-tocopherol content in soybean seeds. Breed. Sci. 2007, 57, 23–28. [Google Scholar] [CrossRef] [Green Version]
- SAS Institute. The SAS System for Windows, Release 9.2; SAS Inst. Inc.: Cary, NC, USA, 2009. [Google Scholar]

| Tocopherol Isomers | Minimum (μg g−1) | Maximum (μg g−1) | Mean (μg g−1) | Std. | CV (%) |
|---|---|---|---|---|---|
| α-Tocopherol | 3.15 | 33.38 | 10.84 | 4.56 | 42.04 |
| γ-Tocopherol | 79.31 | 220.91 | 143.15 | 20.64 | 14.41 |
| δ-Tocopherol | 21.87 | 136.67 | 75.11 | 16.90 | 22.50 |
| Total-Tocopherol | 118.92 | 344.02 | 229.12 | 27.22 | 11.88 |
| Tocopherol Isomers | ID Number | Name | Mean (μg g−1) | CV (%) | Origin |
|---|---|---|---|---|---|
| Higher α-Toc | ZDD00041 | Heihe1 | 33.38 | 18.14 | China |
| ZDD23615 | Hefeng47 | 31.35 | 7.42 | China | |
| ZDD22657 | Hefeng35 | 30.77 | 19.59 | China | |
| ZDD24346 | Kenfeng22 | 28.74 | 15.01 | China | |
| ZDD22798 | Dongnong42E | 25.99 | 22.89 | China | |
| Higher γ-Toc | ZDD06815 | Heihe4 | 186.69 | 5.08 | China |
| WDD02019 | 9234 | 185.16 | 10.32 | USA | |
| ZDD24376 | Hefeng52 | 184.33 | 11.14 | China | |
| WDD02599 | C∏691 | 184.33 | 6.52 | Russia | |
| ZDD24342 | Kenfeng13 | 183.86 | 7.37 | China | |
| Higher δ-Toc | ZDD06638 | Baishuidou | 136.67 | 9.29 | China |
| ZDD06595 | Maojihui | 129.54 | 9.30 | China | |
| ZDD06361 | Dapudou | 118.63 | 5.14 | China | |
| ZDD13149 | Qingpidou | 111.35 | 4.95 | China | |
| ZDD14232 | Huangdou | 108.15 | 9.02 | China | |
| Higher Tot-Toc | ZDD24336 | Huajiang4 | 291.24 | 8.80 | China |
| ZDD24157 | Quxian1 | 289.75 | 7.61 | China | |
| ZDD01629 | Liaodou26 | 286.65 | 3.08 | China | |
| ZDD06815 | Heihe4 | 285.45 | 6.43 | China | |
| ZDD24685 | Jidou17 | 279.22 | 6.73 | China |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, S.; Zhang, S.; Azam, M.; Gebregziabher, B.S.; Abdelghany, A.M.; Shaibu, A.S.; Qi, J.; Feng, Y.; Agyenim-Boateng, K.G.; Liu, Y.; et al. Natural Variation of Seed Tocopherol Composition in Diverse World Soybean Accessions from Maturity Group 0 to VI Grown in China. Plants 2022, 11, 206. https://doi.org/10.3390/plants11020206
Ghosh S, Zhang S, Azam M, Gebregziabher BS, Abdelghany AM, Shaibu AS, Qi J, Feng Y, Agyenim-Boateng KG, Liu Y, et al. Natural Variation of Seed Tocopherol Composition in Diverse World Soybean Accessions from Maturity Group 0 to VI Grown in China. Plants. 2022; 11(2):206. https://doi.org/10.3390/plants11020206
Chicago/Turabian StyleGhosh, Suprio, Shengrui Zhang, Muhammad Azam, Berhane S. Gebregziabher, Ahmed M. Abdelghany, Abdulwahab S. Shaibu, Jie Qi, Yue Feng, Kwadwo Gyapong Agyenim-Boateng, Yitian Liu, and et al. 2022. "Natural Variation of Seed Tocopherol Composition in Diverse World Soybean Accessions from Maturity Group 0 to VI Grown in China" Plants 11, no. 2: 206. https://doi.org/10.3390/plants11020206
APA StyleGhosh, S., Zhang, S., Azam, M., Gebregziabher, B. S., Abdelghany, A. M., Shaibu, A. S., Qi, J., Feng, Y., Agyenim-Boateng, K. G., Liu, Y., Feng, H., Li, Y., Li, J., Li, B., & Sun, J. (2022). Natural Variation of Seed Tocopherol Composition in Diverse World Soybean Accessions from Maturity Group 0 to VI Grown in China. Plants, 11(2), 206. https://doi.org/10.3390/plants11020206











