The Alleviation of Metal Stress Nuisance for Plants—A Review of Promising Solutions in the Face of Environmental Challenges
Abstract
1. Introduction
2. Functional Traits of Plants Developed in Response to Severe External Pressures
2.1. Specific Characteristics of Metal-Tolerant Species and Their Application in Soil Remediation
- (1)
- ‘excluders’ that detoxify most of the toxic ions in roots and minimize their translocation to shoots; for these plants the accumulation coefficient, i.e., the ratio of metal concentration in the shoot to the soil, is always lower than one;
- (2)
- ‘indicators’, whose shoots contain a similar concentration of metals as the soil (the accumulation coefficient is close to one);
- (3)
- ‘accumulators’, which are characterized by effective metal uptake, transport, and storage in shoots (the accumulation coefficient is higher than one); among them, approximately 720 species are considered to be hyperaccumulators that are able to accumulate extraordinary amounts of metallic ions without suffering any phytotoxic effects [43].
2.2. Relationship between Chosen Metal Tolerance Traits and Other Stresses
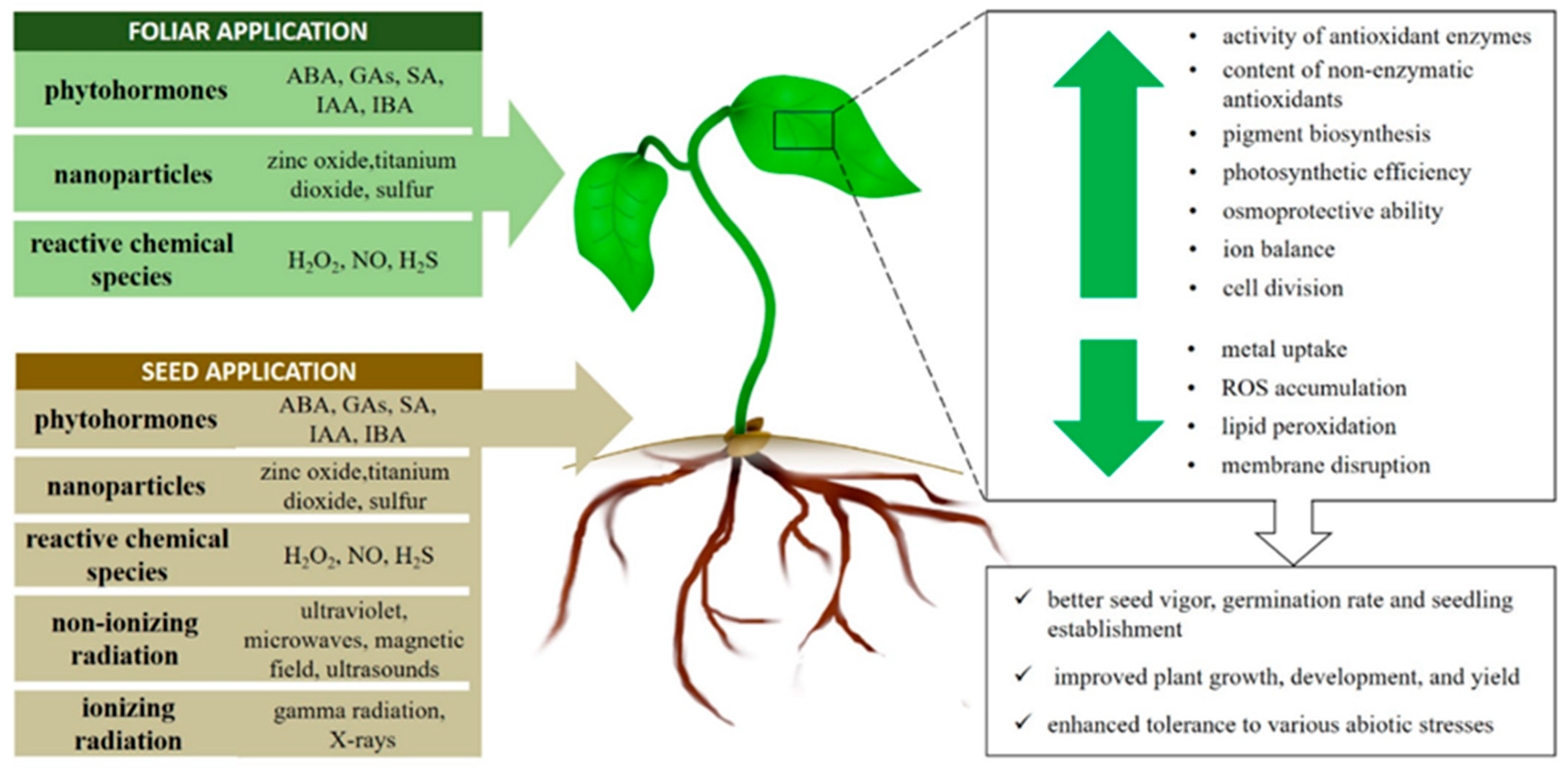
3. Chemical and Physical Agents for Enhancing Plant Resistance to Abiotic Stress
3.1. Chemical Priming Agents
3.1.1. Phytohormonal Priming
3.1.2. Nanoparticle Priming
3.1.3. Priming by Reactive Chemical Species
3.2. Physical Priming
3.2.1. Priming with Non-Ionizing Radiation
3.2.2. Priming with Ionizing Radiation
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chakraborti, S.; Bera, K.; Sadhukhan, S.; Dutta, P. Bio-priming of seeds: Plant stress management and its underlying cellular, biochemical and molecular mechanisms. Plant Stress 2022, 3, 100052. [Google Scholar] [CrossRef]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical priming of plants against multiple abiotic stresses: Mission possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Zinta, G.; Hamed, B.A.; Selim, S.; Beemster, G.; Hozzein, W.N.; Wadaan, M.A.M.; Asard, H.; Abuelsoud, W. Maize roots and shoots show distinct profiles of oxidative stress and antioxidant defense under heavy metal toxicity. Environ. Pollut. 2020, 258, 113705. [Google Scholar] [CrossRef]
- García-Caparrós, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative stress and antioxidant metabolism under adverse environmental conditions: A review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
- Liu, D.; Gao, Z.; Li, J.; Yao, Q.; Tan, W.; Xing, W.; Lu, Z. Effects of cadmium stress on the morphology, physiology, cellular ultrastructure, and BvHIPP24 gene expression of sugar beet (Beta vulgaris L.). Int. J. Phytoremediation 2022, 30, 1–11. [Google Scholar] [CrossRef]
- Farooq, M.; Usman, M.; Nadeem, F.; Rehman, H.; Wahid, A.; Basra, S.M.A.; Siddique, K.H.M. Seed priming in field crops: Potential benefits, adoption and challenges. Crop Pasture Sci. 2019, 70, 731–771. [Google Scholar] [CrossRef]
- He, G.; Tian, W.; Qin, L.; Meng, L.; Wu, D.; Huang, Y.; Li, D.; Zhao, D.; He, T. Identification of novel heavy metal detoxification proteins in Solanum tuberosum: Insights to improve food security protection from metal ion stress. Sci. Total Environ. 2021, 779, 146197. [Google Scholar] [CrossRef]
- Sytar, O.; Kumari, P.; Yadav, S.; Brestic, M.; Rastogi, A. Phytohormone priming: Regulator for heavy metal stress in plants. J. Plant Growth Regul. 2019, 38, 739–752. [Google Scholar] [CrossRef]
- Chandra, R.; Kumar, V. Phytoextraction of heavy metals by potential native plants and their microscopic observation of root growing on stabilised distillery sludge as a prospective tool for in situ phytoremediation of industrial waste. Environ. Sci. Pollut. Res. 2017, 24, 2605–2619. [Google Scholar] [CrossRef] [PubMed]
- Zeiner, M.; Juranović Cindrić, I.; Nemet, I.; Franjković, K.; Salopek Sondi, B. Influence of soil salinity on selected element contents in different Brassica species. Molecules 2022, 27, 1878. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Linić, I.; Salopek-Sondi, B. Salinity stress as an elicitor for phytochemicals and minerals accumulation in selected leafy vegetables of Brassicaceae. Agronomy 2021, 11, 361. [Google Scholar] [CrossRef]
- Tan, B.; Wang, H.; Wang, X.; Ma, C.; Zhou, J.; Dai, X. Health risks and source analysis of heavy metal pollution from dust in Tianshui, China. Minerals 2021, 11, 502. [Google Scholar] [CrossRef]
- Bi, C.; Zhou, Y.; Chen, Z.; Jia, J.; Bao, X. Heavy metals and lead isotopes in soil, road dust and leafy vegetables and health risks via vegetable consumption in the industrial areas of Shanghai, China. Sci. Total Environ. 2018, 619–620, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Muszyńska, E.; Labudda, M.; Kamińska, I.; Górecka, M.; Bederska-Błaszczyk, M. Evaluation of heavy metal-induced responses in Silene vulgaris ecotypes. Protoplasma 2019, 256, 1279–1297. [Google Scholar] [CrossRef]
- Giannakoula, A.; Therios, I.; Chatzissavvidis, C. Effect of lead and copper on photosynthetic apparatus in citrus (Citrus aurantium L.) plants. The role of antioxidants in oxidative damage as a response to heavy metal stress. Plants 2021, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Li, Y.; Ma, Y.-H.; He, L.-F.; Li, S.-W. Abscisic acid modulates differential physiological and biochemical responses of roots, stems, and leaves in mung bean seedlings to cadmium stress. Environ. Sci. Pollut. Res. 2021, 28, 6030–6043. [Google Scholar] [CrossRef]
- Sitko, K.; Opała-Owczarek, M.; Jemioła, G.; Gieroń, Z.; Szopiński, M.; Owczarek, P.; Rudnicka, M.; Małkowski, E. Effect of drought and heavy metal contamination on growth and photosynthesis of silver birch trees growing on post-industrial heaps. Cells 2022, 11, 53. [Google Scholar] [CrossRef]
- Muszyńska, E.; Labudda, M.; Hanus-Fajerska, E. Changes in proteolytic activity and protein carbonylation in shoots of Alyssum montanum ecotypes under multi-metal stress. J. Plant Physiol. 2019, 232, 61–64. [Google Scholar] [CrossRef]
- Muszyńska, E.; Tokarz, K.; Dziurka, M.; Labudda, M.; Dziurka, K.; Piwowarczyk, B. Photosynthetic apparatus efficiency, phenolic acid profiling and pattern of chosen phytohormones in metal-tolerant and intolerant Alyssum montanum ecotypes. Sci. Rep. 2021, 11, 4135. [Google Scholar] [CrossRef]
- Shahid, M.; Khalid, S.; Abbas, G.; Shahid, N.; Nadeem, M.; Sabir, M.; Aslam, M.; Dumat, C. Heavy Metal Stress and Crop Productivity. In Crop Production and Global Environmental Issues; Hakeem, K., Ed.; Springer: Cham, Switzerland, 2015; pp. 1–25. [Google Scholar]
- Muszyńska, E.; Labudda, M. Dual role of metallic trace elements in stress biology—From negative to beneficial impact on plants. Int. J. Mol. Sci. 2019, 20, 3117. [Google Scholar] [CrossRef]
- Hilker, M.; Schwachtje, J.; Baier, M.; Balazadeh, S.; Bäurle, I.; Geiselhardt, S.; Hincha, D.K.; Kunze, R.; Mueller-Roeber, B.; Rillig, M.C.; et al. Priming and memory of stress responses in organisms lacking a nervous system. Biol. Rev. 2016, 91, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Leuendorf, J.E.; Frank, M.; Schmülling, T. Acclimation, priming and memory in the response of Arabidopsis thaliana seedlings to cold stress. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Dumlao, M.R.; Herzenach, M.K.; Adams, W.W. Acclimation. In Encyclopedia of Ecology; Jørgensen, S.E., Fath, B.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 15–23. [Google Scholar]
- Ernst, W.H.O. Evolution of metal tolerance in higher plants. For. Snow Landsc. Res. 2006, 80, 251–274. [Google Scholar]
- Sharma, S.S.; Dietz, K.-J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, H.; Philippe, F.; Domon, J.M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Sujkowska-Rybkowska, M.; Muszyńska, E.; Labudda, M. Structural adaptation and physiological mechanisms in the leaves of Anthyllis vulneraria L. from metallicolous and non-metallicolous populations. Plants 2020, 9, 662. [Google Scholar] [CrossRef]
- Guerinot, M.L. The ZIP family of metal transporters. Biochim. Biophys. Acta (BBA)-Biomembr. 2000, 1465, 190–198. [Google Scholar] [CrossRef]
- Krämer, U. Metal hyperaccumulation in plants. Annu. Rev. Plant Biol. 2010, 61, 517–534. [Google Scholar] [CrossRef]
- Kajala, K.; Walker, K.L.; Mitchell, G.S.; Krämer, U.; Cherry, S.R.; Brady, S.M. Real-time whole-plant dynamics of heavy metal transport in Arabidopsis halleri and Arabidopsis thaliana by gamma-ray imaging. Plant Direct 2019, 3, e00131. [Google Scholar] [CrossRef]
- Wójcik, M.; Gonnelli, C.; Selvi, F.; Dresler, S.; Rostański, A.; Vangronsveld, J. Metallophytes of serpentine and calamine soils—Their unique ecophysiology and potential for phytoremediation. Adv. Bot. Res. 2017, 83, 1–42. [Google Scholar]
- Wierzbicka, M.; Potocka, A. Lead tolerance in plants growing on dry and moist soils. Acta Biol. Crac. Ser. Bot. 2002, 44, 21–28. [Google Scholar]
- Saraswat, S.; Rai, J.P.N. Complexation and detoxification of Zn and Cd in metal accumulating plants. Rev. Environm. Sci. Biotechnol. 2011, 10, 327–339. [Google Scholar] [CrossRef]
- Ricachenevsky, F.K.; Punshon, T.; Salt, D.E.; Fett, J.P.; Guerinot, M.L. Arabidopsis thaliana zinc accumulation in leaf trichomes is correlated with zinc concentration in leaves. Sci. Rep. 2021, 11, 5278. [Google Scholar] [CrossRef] [PubMed]
- Muszyńska, E.; Labudda, M.; Różańska, E.; Hanus-Fajerska, E.; Znojek, E. Heavy metal tolerance in contrasting ecotypes of Alyssum montanum. Ecotoxicol. Environ. Saf. 2018, 161, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, M.; Pielichowska, M.; Kalabun, O.B.; Wąsowicz, P. Microevolution on anthropogenically changed areas on the example of Biscutella laevigata plants from calamine waste heap in Poland. J. Environ. Anal. Toxicol. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Woźniak, A.; Bednarski, W.; Dancewicz, K.; Gabryś, B.; Borowiak-Sobkowiak, B.; Bocianowski, J.; Samardakiewicz, S.; Rucińska-Sobkowiak, R.; Morkunas, I. Oxidative stress links response to lead and Acyrthosiphon pisum in Pisum sativum L. J. Plant Physiol. 2019, 240, 152996. [Google Scholar] [CrossRef]
- Ahmad, P.; Raja, V.; Ashraf, M.; Wijaya, L.; Bajguz, A.; Alyemeni, M.N. Jasmonic acid (JA) and gibberellic acid (GA3) mitigated Cd-toxicity in chickpea plants through restricted Cd uptake and oxidative stress management. Sci. Rep. 2021, 11, 19768. [Google Scholar] [CrossRef]
- Muszyńska, E.; Labudda, M. Effects of lead, cadmium and zinc on protein changes in Silene vulgaris shoots cultured in vitro. Ecotoxicol. Environ. Saf. 2020, 204, 111086. [Google Scholar] [CrossRef]
- Muszyńska, E.; Labudda, M.; Kral, A. Ecotype-specific pathways of reactive oxygen species deactivation in facultative metallophyte Silene vulgaris (Moench) Garcke treated with heavy metals. Antioxidants 2020, 9, 102. [Google Scholar] [CrossRef]
- Baker, A.J.M. Accumulators and excluders strategies in the response of plants to heavy metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- Reeves, R.D.; Baker, A.J.M.; Jaffré, T.; Erskine, P.D.; Echevarria, G.; van der Ent, A. A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol. 2018, 218, 407–411. [Google Scholar] [CrossRef]
- Muszyńska, E.; Hanus-Fajerska, E.; Ciarkowska, K. Phytoremediation as an antidote to environmental pollution. In Buckler Mustard (Biscutella laevigata L.) an Extraordinary Plant on Ordinary Mine Heaps Near Olkusz; Szarek-Łukaszewska, G., Ed.; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2020; pp. 231–259. [Google Scholar]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Drozdova, I.; Alekseeva-Popova, N.; Kalimova, I.; Bech, J.; Roca, N. Research of reclamation of polluted mine soils by native metallophytes: Some cases. Geochem. Explor. Environ. Anal. 2019, 19, 164–170. [Google Scholar] [CrossRef]
- Bhatia, N.P.; Baker, A.J.M.; Walsh, K.B.; Midmore, D.J. A role for nickel in osmotic adjustment in drought-stressed plants of the nickel hyperaccumulator Stackhousia tryonii Bailey. Planta 2005, 223, 134–139. [Google Scholar] [CrossRef]
- Chaney, R.L.; Angle, J.S.; Broadhurst, C.L.; Peters, C.A.; Tappero, R.V.; Sparks, D.L. Improved understanding of hyperaccumulation yields commercial phytoextraction and phytomining technologies. J. Environ. Qual. 2007, 36, 1429–1443. [Google Scholar] [CrossRef]
- Ciarkowska, K.; Hanus-Fajerska, E.; Gambuś, F.; Muszyńska, E.; Czech, T. Phytostabilization of Zn-Pb ore flotation tailings with Dianthus carthusianorum and Biscutella laevigata after amending with mineral fertilizers or sewage sludge. J. Environ. Manag. 2017, 189, 75–83. [Google Scholar] [CrossRef]
- Gadgil, R.L. Tolerance of heavy metals and the reclamation of industrial waste. J. Appl. Ecol. 1969, 6, 247–259. [Google Scholar] [CrossRef]
- Mummey, D.L.; Stahl, P.D.; Buyer, J.S. Soil microbiological properties 20 years after surface mine reclamation: Spatial analysis of reclaimed and undisturbed sites. Soil Biol. Biochem. 2002, 34, 1717–1725. [Google Scholar] [CrossRef]
- Conesa, H.M.; Robinson, B.H.; Schulin, R.; Nowack, B. Growth of Lygeum spartum in acid mine tailings: Response of plants developed from seedlings, rhizomes and at field conditions. Environ. Pollut. 2007, 145, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Jamali Hajiani, N.; Ghaderian, S.M.; Karimi, N.; Schat, H. A comparison of antimony accumulation and tolerance among Achillea wilhelmsii, Silene vulgaris and Thlaspi arvense. Plant Soil 2017, 412, 267–281. [Google Scholar] [CrossRef]
- Muszyńska, E.; Hanus-Fajerska, E.; Piwowarczyk, B.; Augustynowicz, J.; Ciarkowska, K.; Czech, T. From laboratory to field studies—The assessment of Biscutella laevigata suitability to biological reclamation of areas contaminated with lead and cadmium. Ecotoxicol. Environ. Saf. 2017, 142, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Muszyńska, E.; Hanus-Fajerska, E.; Koźmińska, A. Differential tolerance to lead and cadmium of micropropagated Gypsophila fastigiata ecotype. Water Air Soil Pollut. 2018, 229, 42. [Google Scholar] [CrossRef]
- Hanus-Fajerska, E.; Ciarkowska, K.; Muszyńska, E. Long-term field study on stabilization of contaminated wastes by growing clonally reproduced Silene vulgaris calamine ecotype. Plant Soil 2019, 439, 431–445. [Google Scholar] [CrossRef]
- Akram, N.A.; Hafeez, N.; Farid-ul-Haq, M.; Ahmad, A.; Sadiq, M.; Ashraf, M. Foliage application and seed priming with nitric oxide causes mitigation of salinity-induced metabolic adversaries in broccoli (Brassica oleracea L.) plants. Acta Physiol. Plant 2020, 42, 155. [Google Scholar] [CrossRef]
- Nazir, F.; Fariduddin, Q.; Hussain, A.; Khan, T.A. Brassinosteroid and hydrogen peroxide improve photosynthetic machinery, stomatal movement, root morphology and cell viability and reduce Cu-triggered oxidative burst in tomato. Ecotoxicol. Environ. Saf. 2021, 207, 111081. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Pereira, M.P.; de Almeida Rodrigues, L.C.; Corręa, F.F.; de Castro, E.M.; Ribeiro, V.E.; Pereira, F.J. Cadmium tolerance in Schinus molle trees is modulated by enhanced leaf anatomy and photosynthesis. Trees 2016, 30, 807–814. [Google Scholar] [CrossRef]
- Muszyńska, E.; Labudda, M.; Różańska, E.; Hanus-Fajerska, E.; Koszelnik-Leszek, A. Structural, physiological and genetic diversification of Silene vulgaris ecotypes from heavy metal-contaminated areas and their synchronous in vitro cultivation. Planta 2019, 249, 1761–1778. [Google Scholar] [CrossRef] [PubMed]
- Nikalje, G.C.; Saini, N.; Suprasanna, P. Halophytes and heavy metals: Interesting partnerships. In Plant-Metal Interactions; Srivastava, S., Srivastava, A., Suprasanna, P., Eds.; Springer: Cham, Switzerland, 2019; pp. 99–118. [Google Scholar]
- Nawaz, I.; Iqbal, M.; Bliek, M.; Schat, H. Salt and heavy metal tolerance and expression levels of candidate tolerance genes among four extremophile Cochlearia species with contrasting habitat preferences. Sci. Total Environ. 2017, 584, 731–741. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Y.; Jiang, B.; Liu, G.; Yu, L.; Wei, Z.; Yang, C. A novel vacuolar membrane H+-ATPase c subunit gene (ThVHAc1) from Tamarix hispida confers tolerance to several abiotic stresses in Saccharomyces cerevisiae. Mol. Biol. Rep. 2011, 38, 957–963. [Google Scholar] [CrossRef]
- Neumann, D.; Nieden, U.Z.; Lichtenberger, O.; Leopold, I. How Does Armeria maritima tolerate high heavy metal concentrations? J. Plant Physiol. 1995, 146, 704–717. [Google Scholar] [CrossRef]
- Manousaki, E.; Kosmoula, G.; Lamprini, P.; Kalogerakis, N. Metal phytoremediation by the halophyte Limoniastrum monopetalum (L.) Boiss: Two contrasting ecotypes. Int. J. Phytorem. 2014, 16, 755–769. [Google Scholar] [CrossRef] [PubMed]
- Manousaki, E.; Kalogerakis, N. Phytoextraction of Pb and Cd by the Mediterranean saltbush (Atriplex halimus L.): Metal uptake in relation to salinity. Eviron. Pollut. Res. 2009, 16, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Mazharia, M.; Homaeed, M. Annual halophyte Chenopodium botrys can phytoextract cadmium from contaminated soils. J. Basic Appl. Sci. Res. 2012, 2, 1415–1422. [Google Scholar]
- Wang, L.; Wang, X.; Jiang, L.; Zhang, K.; Tanveer, M.; Tian, C.; Zhao, Z. Reclamation of saline soil by planting annual euhalophyte Suaeda salsa with drip irrigation: A three-year field experiment in arid northwestern China. Ecol. Eng. 2021, 159, 106090. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Maggio, A. Functional biology of halophytes in the phytoremediation of heavy metal contaminated soils. Environ. Exp. Bot. 2015, 111, 135–146. [Google Scholar] [CrossRef]
- Nikalje, G.C.; Suprasanna, P. Coping with metal toxicity—Cues from halophytes. Front. Plant Sci. 2018, 9, 777. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Öztürk, M. Ecophysiology, Abiotic Stress Responses and Utilization of Halophytes; Springer Nature: Singapore, 2019. [Google Scholar]
- Milić, D.; Luković, J.; Ninkov, J.; Zeremski-Škorić, T.; Zorić, L.; Vasin, J.; Milić, S. Heavy metal content in halophytic plants from inland and maritime saline areas. Cent. Eur. J. Biol. 2012, 7, 307–317. [Google Scholar] [CrossRef]
- Li, B.; Wang, J.; Yao, L.; Meng, Y.; Ma, X.; Si, E.; Ren, P.; Yang, K.; Shang, X.; Wang, H. Halophyte Halogeton glomeratus, a promising candidate for phytoremediation of heavy metal-contaminated saline soils. Plant Soil 2019, 442, 323–331. [Google Scholar] [CrossRef]
- Ayyappan, D.; Sathiyaraj, G.; Ravindran, K.G. Phytoextraction of heavy metals by Sesuvium portulacastrum L. A salt marsh halophyte from tannery effluent. Int. J. Phytorem. 2016, 18, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Sghaier, D.B.; Pedro, S.; Diniz, M.S.; Duarte, B.; Caçador, I.; Sleimi, N. Tissue localization and distribution of As and Al in the halophyte Tamarix gallica under controlled conditions. Front. Mar. Sci. 2016, 3, 274. [Google Scholar] [CrossRef]
- Anwar, A.; Kim, J.-K. Transgenic breeding approaches for improving abiotic stress tolerance: Recent progress and future perspectives. Int. J. Mol. Sci. 2020, 21, 2695. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.; Bernal, P.; Almela, C.; Vélez, D.; García-Agustín, P.; Serrano, R.; Navarro-Aviñó, J. An engineered plant that accumulates higher levels of heavy metals than Thlaspi caerulescens, with yields of 100 times more biomass in mine soils. Chemosphere 2006, 64, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, Y.; Cheng, Z.; Cheng, Z.; Xia, G.; Wang, M. A wheat histone variant gene TaH2A.7 enhances drought tolerance and promotes stomatal closure in Arabidopsis. Plant Cell Rep. 2016, 35, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.; Khan, A.R.; Liu, L.; Yang, S.; Azhar, W.; Ulhassan, Z.; Zeeshan, M.; Wu, J.; Fan, X.; Gan, Y. Seed priming with zinc oxide nanoparticles downplayed ultrastructural damage and improved photosynthetic apparatus in maize under cobalt stress. J. Hazard. Mater. 2022, 423, 127021. [Google Scholar] [CrossRef]
- Waqas, M.; Korres, N.E.; Khan, M.D.; Nizami, A.-S.; Deeba, F.; Ali, I.; Hussain, H. Advances in the concept and methods of seed priming. In Priming and Pretreatment of Seeds and Seedlings; Hasanuzzaman, M., Fotopoulos, V., Eds.; Springer: Singapore, 2019; pp. 11–41. [Google Scholar]
- Rezk, A.A.; Al-Khayri, J.M.; Al-Bahrany, A.M.; El-Beltagi, H.S.; Mohamed, H.I. X-ray irradiation changes germination and biochemical analysis of two genotypes of okra (Hibiscus esculentus L.). J. Radiat. Res. Appl. Sci. 2019, 12, 393–402. [Google Scholar] [CrossRef]
- Hussein, H.-A.A. Influence of radio-grain priming on growth, antioxidant capacity, and yield of barley plants. Biotechnol. Rep. 2022, 34, e00724. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, S.; Biswas, B.; Chakraborty, D.; Timsina, J.; Pal, S.; Tarafdar, J.C.; Banerjee, S.; Hossain, A.; Roy, S. Seed priming with selenium and zinc nanoparticles modifies germination, growth, and yield of direct-seeded rice (Oryza sativa L.). Sci. Rep. 2022, 12, 7103. [Google Scholar] [CrossRef]
- Mir, A.R.; Alam, P.; Hayat, S. Auxin regulates growth, photosynthetic efficiency and mitigates copper induced toxicity via modulation of nutrient status, sugar metabolism and antioxidant potential in Brassica juncea. Plant Physiol. Biochem. 2022, 185, 244–259. [Google Scholar] [CrossRef]
- Zafar, S.; Perveen, S.; Khan, M.K.; Shaheen, M.R.; Hussain, R.; Sarwar, N.; Rashid, S.; Nafees, M.; Farid, G.; Alamri, S.; et al. Effect of zinc nanoparticles seed priming and foliar application on the growth and physio-biochemical indices of spinach (Spinacia oleracea L.) under salt stress. PLoS ONE 2022, 17, e0263194. [Google Scholar] [CrossRef]
- Sako, K.; Nguyen, H.M.; Seki, M. Advances in chemical priming to enhance abiotic stress tolerance in plants. Plant Cell Physiol. 2021, 61, 1995–2003. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, U.; Field, B. Molecular mechanisms controlling plant growth during abiotic stress. J. Exp. Bot. 2018, 69, 2753–2758. [Google Scholar] [CrossRef] [PubMed]
- Saha, I.; Hasanuzzaman, M.; Adak, M.K. Abscisic acid priming regulates arsenite toxicity in two contrasting rice (Oryza sativa L.) genotypes through differential functioning of Sub1A quantitative trait loci. Environ. Pollut. 2021, 287, 117586. [Google Scholar] [CrossRef]
- Rehman, S.; Abbas, G.; Shahid, M.; Saqib, M.; Umer Farooq, A.B.; Hussain, M.; Murtaza, B.; Amjad, M.; Naeem, M.A.; Farooq, A. Effect of salinity on cadmium tolerance, ionic homeostasis and oxidative stress responses in conocarpus exposed to cadmium stress: Implications for phytoremediation. Ecotoxicol. Environ. Saf. 2019, 171, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Fidler, J.; Graska, J.; Gietler, M.; Nykiel, M.; Prabucka, B.; Rybarczyk-Płońska, A.; Muszyńska, E.; Morkunas, I.; Labudda, M. PYR/PYL/RCAR receptors play a vital role in the abscisic-acid-dependent responses of plants to external or internal stimuli. Cells 2022, 11, 1352. [Google Scholar] [CrossRef] [PubMed]
- Gietler, M.; Fidler, J.; Labudda, M.; Nykiel, M. Abscisic acid—Enemy or savior in the response of cereals to abiotic and biotic stresses? Int. J. Mol. Sci. 2020, 21, 4607. [Google Scholar] [CrossRef]
- Wei, T.-J.; Wang, M.-M.; Jin, Y.-Y.; Zhang, G.-H.; Liu, M.; Yang, H.-Y.; Jiang, C.-J.; Liang, Z.-W. Abscisic acid priming creates alkaline tolerance in alfalfa seedlings (Medicago sativa L.). Agriculture 2021, 11, 608. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Farhangi-Abriz, S. Foliar sprays of salicylic acid and jasmonic acid stimulate H+-ATPase activity of tonoplast, nutrient uptake and salt tolerance of soybean. Ecotoxicol. Environ. Saf. 2018, 166, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Akar, M.; Atis, I. The effects of priming pretreatments on germination and seedling growth in perennial ryegrass exposed to heavy metal stress. Fresenius Environ. Bull. 2018, 27, 6677–6685. [Google Scholar]
- Nouri, M.; Haddioui, A. Improving seed germination and seedling growth of Lepidium sativum with different priming methods under arsenic stress. Acta Ecol. Sin. 2021, 41, 64–71. [Google Scholar] [CrossRef]
- Mazumder, M.K.; Sharma, P.; Moulick, D.; Tata, S.K.; Choudhury, S. Salicylic acid ameliorates zinc and chromium-induced stress responses in wheat seedlings: A biochemical and computational analysis. Cereal Res. Commun. 2021, 50, 407–418. [Google Scholar] [CrossRef]
- Huang, Y.T.; Cai, S.Y.; Ruan, X.L.; Chen, S.Y.; Mei, G.F.; Ruan, G.H.; Cao, D.D. Salicylic acid enhances sunflower seed germination under Zn2+ stress via involvement in Zn2+ metabolic balance and phytohormone interactions. Sci. Hortic. 2021, 275, 109702. [Google Scholar] [CrossRef]
- Gupta, S.; Seth, C.S. Salicylic acid alleviates chromium (VI) toxicity by restricting its uptake, improving photosynthesis and augmenting antioxidant defense in Solanum lycopersicum L. Physiol. Mol. Biol. Plants 2021, 27, 2651–2664. [Google Scholar] [CrossRef] [PubMed]
- Demecsová, L.; Zelinová, V.; Liptáková, Ľ.; Valentovičová, K.; Tamás, L. Indole-3-butyric acid priming reduced cadmium toxicity in barley root tip via NO generation and enhanced glutathione peroxidase activity. Planta 2020, 252, 46. [Google Scholar] [CrossRef] [PubMed]
- Santo Pereira, A.E.; Caixeta Oliveira, H.; Fernandes Fraceto, L.; Santaella, C. Nanotechnology potential in seed priming for sustainable agriculture. Nanomaterials 2021, 11, 267. [Google Scholar] [CrossRef]
- Sridharan, K.; Puthur, J.T.; Dhankher, O.P. Priming with nanoscale materials for boosting abiotic stress tolerance in crop plants. J. Agric. Food Chem. 2021, 69, 10017–10035. [Google Scholar]
- Rai-Kalal, P.; Jajoo, A. Priming with zinc oxide nanoparticles improve germination and photosynthetic performance in wheat. Plant Physiol. Biochem. 2021, 160, 341–351. [Google Scholar] [CrossRef]
- Shah, T.; Latif, S.; Saeed, F.; Ali, I.; Ullah, S.; Abdullah Alsahli, A.; Jan, S.; Ahmad, P. Seed priming with titanium dioxide nanoparticles enhances seed vigor, leaf water status, and antioxidant enzyme activities in maize (Zea mays L.) under salinity stress. J. King Saud Univ. Sci. 2021, 33, 101207. [Google Scholar] [CrossRef]
- Ragab, G.; Saad-Allah, K. Seed priming with greenly synthesized sulfur nanoparticles enhances antioxidative defense machinery and restricts oxidative injury under manganese stress in Helianthus annuus (L.) seedlings. J. Plant Growth Regul. 2021, 40, 1894–1902. [Google Scholar] [CrossRef]
- Ellouzi, H.; Sghayar, S.; Abdelly, C. H2O2 seed priming improves tolerance to salinity; drought and their combined effect more than mannitol in Cakile maritima when compared to Eutrema salsugineum. J. Plant Physiol. 2017, 210, 38–50. [Google Scholar] [CrossRef]
- Habib, N.; Ali, Q.; Ali, S.; Javed, M.T.; Zulqurnain Haider, M.; Perveen, R.; Shahid, M.R.; Rizwan, M.; Abdel-Daim, M.M.; Elkelish, A.; et al. Use of nitric oxide and hydrogen peroxide for better yield of wheat (Triticum aestivum L.) under water deficit conditions: Growth, osmoregulation, and antioxidative defense mechanism. Plants 2020, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Majeed, S.; Nawaz, F.; Naeem, M.; Ashraf, M.Y. Effect of exogenous nitric oxide on sulfur and nitrate assimilation pathway enzymes in maize (Zea mays L.) under drought stress. Acta Physiol. Plant. 2018, 40, 206. [Google Scholar] [CrossRef]
- Silva, P.C.C.; de Azevedo Neto, A.D.; Gheyi, H.R.; Ribas, R.F.; dos Reis Silva, C.R.; Cova, A.M.W. Salt tolerance induced by hydrogen peroxide priming on seed is related to improvement of ion homeostasis and antioxidative defense in sunflower plants. J. Plant Nutr. 2021, 44, 1207–1221. [Google Scholar] [CrossRef]
- Ellouzi, H.; Oueslati, S.; Hessini, K.; Rabhi, M.; Abdelly, C. Seed-priming with H2O2 alleviates subsequent salt stress by preventing ROS production and amplifying antioxidant defense in cauliflower seeds and seedlings. Sci. Hortic. 2021, 288, 110360. [Google Scholar] [CrossRef]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.-M.; Qian, P.; Xin, W.; Li, H.-Y.; Burritt, D.J.; Fujita, M.; Tran, L.-S.P. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef]
- Cuypers, A.; Hendrix, S.; dos Reis, R.A.; De Smet, S.; Deckers, J.; Gielen, H.; Jozefczak, M.; Loix, C.; Vercampt, H.; Vangronsveld, J.; et al. Hydrogen peroxide, signaling in disguise during metal phytotoxicity. Front. Plant Sci. 2016, 7, 470. [Google Scholar] [CrossRef]
- Singh, S.; Husain, T.; Kushwaha, B.K.; Suhel, M.; Fatima, A.; Mishra, V.; Singh, S.K.; Tripathi, D.K.; Rai, M.; Prasad, S.M.; et al. Regulation of ascorbate-glutathione cycle by exogenous nitric oxide and hydrogen peroxide in soybean roots under arsenate stress. J. Hazard. Mater. 2020, 409, 123686. [Google Scholar] [CrossRef]
- Bai, X.-J.; Liu, L.-J.; Zhang, C.-H.; Ge, Y.; Cheng, W.-D. Effect of H2O2 pretreatment on Cd tolerance of different rice cultivars. Rice Sci. 2011, 18, 29–35. [Google Scholar] [CrossRef]
- Yıldız, M.; Terzi, H.; Bingül, N. Protective role of hydrogen peroxide pretreatment on defense systems and BnMP1 gene expression in Cr(VI)-stressed canola seedlings. Ecotoxicology 2013, 22, 1303–1312. [Google Scholar] [CrossRef]
- Verna, N.; Prasad, S.M. Regulation of redox homeostasis in cadmium stressed rice field cyanobacteria by exogenous hydrogen peroxide and nitric oxide. Sci. Rep. 2021, 11, 2893. [Google Scholar] [CrossRef]
- dos Santos Araújo, G.; de Oliveira Paula-Marinho, S.; de Paiva Pinheiro, S.K.; de Castro Miguel, E.; de Sousa Lopes, L.; Camelo Marques, E.; de Carvalho, H.H.; Gomes-Filho, E. H2O2 priming promotes salt tolerance in maize by protecting chloroplasts ultrastructure and primary metabolites modulation. Plant Sci. 2021, 303, 110774. [Google Scholar] [CrossRef] [PubMed]
- Jira-anunkul, W.; Pattanagul, W. Effects of hydrogen peroxide application on agronomic traits of rice (Oryza sativa L.) under drought stress. Plant Soil Environ. 2021, 67, 221–229. [Google Scholar] [CrossRef]
- Alnusairi, G.S.H.; Mazrou, Y.S.A.; Qari, S.H.; Elkelish, A.A.; Soliman, M.H.; Eweis, M.; Abdelaal, K.; El-Samad, G.A.; Ibrahim, M.F.M.; ElNahhas, N. Exogenous nitric oxide reinforces photosynthetic efficiency, osmolyte, mineral uptake, antioxidant, expression of stress-responsive genes and ameliorates the effects of salinity stress in wheat. Plants 2021, 10, 1693. [Google Scholar] [CrossRef]
- Basit, F.; Ulhassan, Z.; Mou, Q.; Nazir, M.M.; Hu, J.; Hu, W.; Song, W.; Sheteiwy, M.S.; Zhou, W.; Bhat, J.A.; et al. Seed priming with nitric oxide and/or spermine mitigate the chromium toxicity in rice (Oryza sativa) seedlings by improving the carbon-assimilation and minimising the oxidative damages. Funct. Plant Biol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Alyemeni, M.N.; Wijaya, L.; Ahanger, M.A.; Ashraf, M.; Alam, P.; Paray, B.A.; Rinklebe, J. Nitric oxide donor, sodium nitroprusside, mitigates mercury toxicity in different cultivars of soybean. J. Hazard. Mater. 2021, 408, 124852. [Google Scholar] [CrossRef] [PubMed]
- Samet, H. Alleviation of cobalt stress by exogenous sodium nitroprusside in iceberg lettuce. Chil. J. Agric. Res. 2020, 80, 161–170. [Google Scholar] [CrossRef]
- Kopyra, M.; Gwóźdź, E.A. Nitric oxide stimulates seed germination and counteracts the inhibitory effect of heavy metals and salinity on root growth of Lupinus luteus. Plant Physiol. Biochem. 2003, 41, 1011–1017. [Google Scholar] [CrossRef]
- Hassanein, A.; Esmail, N.; Hashem, H. Sodium nitroprusside mitigates the inhibitory effect of salt and heavy metal stress on lupine yield and downregulates antioxidant enzyme activities. Acta Agrobot. 2020, 73, 7336. [Google Scholar] [CrossRef]
- He, H.; Oo, T.L.; Huang, W.; He, L.F.; Gu, M. Nitric oxide acts as an antioxidant and inhibits programmed cell death induced by aluminum in the root tips of peanut (Arachis hypogaea L.). Sci. Rep. 2019, 9, 9516. [Google Scholar] [CrossRef]
- Li, Z.-G.; Min, X.; Zhou, Z.-H. Hydrogen sulfide: A signal molecule in plant cross-adaptation. Front. Plant Sci. 2016, 7, 1621. [Google Scholar] [CrossRef]
- Zanganeh, R.; Jamei, R.; Rahmani, F. Pre-sowing seed treatment with salicylic acid and sodium hydrosulfide confers Pb toxicity tolerance in maize (Zea mays L.). Ecotoxicol. Environ. Saf. 2020, 206, 111392. [Google Scholar] [CrossRef] [PubMed]
- Valivand, M.; Amooaghaie, R.; Ahadi, A. Seed priming with H2S and Ca2+ trigger signal memory that induces cross-adaptation against nickel stress in zucchini seedlings. Plant Physiol. Biochem. 2019, 143, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Hossain, M.S.; Mahmud, J.; Rahman, A.; Inafuku, M.; Oku, H.; Fujita, M. Coordinated actions of glyoxalase and antioxidant defense systems in conferring abiotic stress tolerance in plants. Int. J. Mol. Sci. 2017, 18, 200. [Google Scholar] [CrossRef] [PubMed]
- Christou, A.; Manganaris, G.A.; Papadopoulos, I.; Fotopoulos, V. Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J. Exp. Bot. 2013, 64, 1953–1966. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, J.; Wang, Z.; Zhang, W.; Yang, H. H2S pretreatment mitigates the alkaline salt stress on Malus hupehensis roots by regulating Na+/K+ homeostasis and oxidative stress. Plant Physiol. Biochem. 2020, 156, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Chen, Y.; Zhai, F.; Zhang, J.; Zhang, F.; Yuan, X.; Xie, Y. Hydrogen sulfide promotes rice drought tolerance via reestablishing redox homeostasis and activation of ABA biosynthesis and signaling. Plant Physiol. Biochem. 2020, 155, 213–220. [Google Scholar] [CrossRef]
- Antoniou, C.; Xenofontos, R.; Chatzimichail, G.; Christou, A.; Kashfi, K.; Fotopoulos, V. Exploring the potential of nitric oxide and hydrogen sulfide (NOSH)-releasing synthetic compounds as novel priming agents against drought stress in Medicago sativa plants. Biomolecules 2020, 10, 120. [Google Scholar] [CrossRef]
- Bera, K.; Dutta, P.; Sadhukhan, S. Seed priming with non-ionizing physical agents: Plant responses and underlying physiological mechanisms. Plant Cell Rep. 2022, 41, 53–73. [Google Scholar] [CrossRef]
- Rao, G.; Huang, S.; Ashraf, U.; Mo, Z.; Duan, M.; Pan, S.; Tang, X. Ultrasonic seed treatment improved cadmium (Cd) tolerance in Brassica Napus L. Ecotoxicol. Environ. Saf. 2019, 185, 109659. [Google Scholar] [CrossRef]
- Dutta, P. Seed priming: New vistas and contemporary perspectives. In Advances in Seed Priming; Rakshit, A., Singh, H., Eds.; Springer: Singapore, 2018; pp. 3–22. [Google Scholar]
- Xia, Q.; Tao, H.; Li, Y.; Pan, D.; Cao, J.; Liu, L.; Zhou, X.; Barba, F.J. Characterizing physicochemical, nutritional and quality attributes of wholegrain Oryza sativa L. subjected to high intensity ultrasound-stimulated pre-germination. Food Control 2020, 108, 106827. [Google Scholar] [CrossRef]
- Thomas, T.T.D.; Dinakar, C.; Puthur, J.T. Effect of UV-B priming on the abiotic stress tolerance of stress-sensitive rice seedlings: Priming imprints and cross-tolerance. Plant Physiol. Biochem. 2020, 147, 21–30. [Google Scholar] [CrossRef]
- Sen, A.; Challabathula, D.; Puthur, J.T. UV-B priming of Oryza sativa seeds augments the innate tolerance potential in a tolerant variety more effectively toward NaCl and PEG stressors. J. Plant Growth Regul. 2021, 40, 1166–1180. [Google Scholar] [CrossRef]
- Xu, Y.; Charles, M.T.; Luo, Z.; Mimee, B.; Tong, Z.; Véronneau, P.-Y.; Roussel, D.; Rolland, D. Ultraviolet-C priming of strawberry leaves against subsequent Mycosphaerella fragariae infection involves the action of reactive oxygen species, plant hormones, and terpenes. Plant Cell Environ. 2019, 42, 815–831. [Google Scholar] [CrossRef]
- Sen, A.; Puthur, J.T.; Challabathula, D.; Brestič, M. Transgenerational effect of UV-B priming on photochemistry and associated metabolism in rice seedlings subjected to PEG-induced osmotic stress. Photosynthetica 2022, 60, 219–229. [Google Scholar] [CrossRef]
- Xiong, Y.; Xing, Q.; Müller-Xing, R. A novel UV-B priming system reveals an UVR8-depedent memory, which provides resistance against UV-B stress in Arabidopsis leaves. Plant Signal. Behav. 2021, 16, 1879533. [Google Scholar] [CrossRef]
- Kanwal, S.; Tariq, M.; Dawar, S. Effect of microwave radiation on plants infected with root rot pathogens. Pak. J. Bot. 2018, 50, 2389–2393. [Google Scholar]
- Araújo, S.S.; Paparella, S.; Dondi, D.; Bentivoglio, A.; Carbonera, D.; Balestrazzi, A. Physical methods for seed invigoration: Advantages and challenges in seed technology. Front. Plant Sci. 2016, 7, 646. [Google Scholar] [CrossRef]
- Bian, Z.-X.; Wang, J.-F.; Ma, H.; Wang, S.-M.; Luo, L.; Wang, S.-M. Effect of microwave radiation on antioxidant capacities of tartary buckwheat sprouts. J. Food Sci. Technol. 2020, 57, 3913–3919. [Google Scholar] [CrossRef]
- Maswada, H.F.; Sunoj, V.S.J.; Prasad, P.V.V. A comparative study on the effect of seed pre-sowing treatments with microwave radiation and salicylic acid in alleviating the drought-induced damage in wheat. J. Plant Growth Regul. 2021, 40, 48–66. [Google Scholar] [CrossRef]
- Farid, M.; Ali, S.; Rizwan, M.; Saeed, R.; Tauqeer, H.M.; Sallah-Ud-Din, R.; Azam, A.; Raza, N. Microwave irradiation and citric acid assisted seed germination and phytoextraction of nickel (Ni) by Brassica napus L.: Morpho-physiological and biochemical alterations under Ni stress. Environ. Sci. Pollut. Res. 2017, 24, 21050–21064. [Google Scholar] [CrossRef]
- Radhakrishnan, R. Magnetic field regulates plant functions, growth and enhances tolerance against environmental stresses. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2019, 25, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, R.; Roshandel, P. Ameliorative effects of a static magnetic field on hyssop (Hyssopus officinalis L.) growth and phytochemical traits under water stress. Bioelectromagnetics 2020, 41, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Kataria, S.; Baghel, L.; Jain, M.; Guruprasad, K.N. Magnetopriming regulates antioxidant defense system in soybean against salt stress. Biocatal. Agric. Biotechnol. 2019, 18, 101090. [Google Scholar] [CrossRef]
- Baghel, L.; Kataria, S.; Jain, M. Mitigation of adverse effects of salt stress on germination, growth, photosynthetic efficiency and yield in maize (Zea mays L.) through magnetopriming. Acta Agrobot. 2019, 72, 1757. [Google Scholar] [CrossRef]
- Dashab, S.; Omidi, H. Effect of intensity, duration and power of ultrasonic waves on germination indices and photosynthetic pigments of canola seedling. Agroecol. J. 2020, 15, 13–24. [Google Scholar]
- Chen, Y.P.; Liu, Q.; Yue, X.Z.; Meng, Z.W.; Liang, J. Ultrasonic vibration seeds showed improved resistance to cadmium and lead in wheat seedling. Environ. Sci. Pollut. Res. 2013, 20, 4807–4816. [Google Scholar] [CrossRef]
- Hanafy, R.S.; Akladious, S.A. Physiological and molecular studies on the effect of gamma radiation in fenugreek (Trigonella foenum-graecum L.) plants. J. Genet. Eng. Biotechnol. 2018, 16, 683–692. [Google Scholar] [CrossRef]
- Pradhan, B.; Patra, S.; Nayak, R.; Swain, S.S.; Jit, B.P.; Behera, C.; Ragusa, A.; Ki, J.-S.; Jena, M. Low-dose priming of gamma radiation enhanced cadmium tolerance in Chlamydomonas reinhardtii by modulating physio-biochemical pathways. Environ. Sci. Pollut. Res. 2022. [Google Scholar] [CrossRef]
- Al-Enezi, N.A.; Al-Bahrany, A.M.; Al-Khayri, J.M. Effect of X-irradiation on date palm seed germination and seedling growth. Emir. J. Food Agric. 2012, 24, 415–424. [Google Scholar]

| Technique | Halophyte Species | Accumulated Metal (s) | References |
|---|---|---|---|
| phytostabilization | Atriplex atacamensis | As | [62] |
| Atriplex halimus | Cd, Pb | [67] | |
| Cochearia species | Zn, Pb | [63] | |
| Halimione portulacoides | Zn, Cu, Ni, Co | [73] | |
| Tamarix hispida | Zn, Pb | [64] | |
| phytoextraction | Chenopodium botrys | Cd | [68] |
| Halogeton glomeratus | Cr, Ni, Cu, Zn, As, Cd, Hg | [74] | |
| Limoniastrum monopetalum | Cd, Pb | [66] | |
| Sesuvium portulacastrum | Cr, Cd, Cu, Zn | [75] | |
| Tamarix gallica | As | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labudda, M.; Dziurka, K.; Fidler, J.; Gietler, M.; Rybarczyk-Płońska, A.; Nykiel, M.; Prabucka, B.; Morkunas, I.; Muszyńska, E. The Alleviation of Metal Stress Nuisance for Plants—A Review of Promising Solutions in the Face of Environmental Challenges. Plants 2022, 11, 2544. https://doi.org/10.3390/plants11192544
Labudda M, Dziurka K, Fidler J, Gietler M, Rybarczyk-Płońska A, Nykiel M, Prabucka B, Morkunas I, Muszyńska E. The Alleviation of Metal Stress Nuisance for Plants—A Review of Promising Solutions in the Face of Environmental Challenges. Plants. 2022; 11(19):2544. https://doi.org/10.3390/plants11192544
Chicago/Turabian StyleLabudda, Mateusz, Kinga Dziurka, Justyna Fidler, Marta Gietler, Anna Rybarczyk-Płońska, Małgorzata Nykiel, Beata Prabucka, Iwona Morkunas, and Ewa Muszyńska. 2022. "The Alleviation of Metal Stress Nuisance for Plants—A Review of Promising Solutions in the Face of Environmental Challenges" Plants 11, no. 19: 2544. https://doi.org/10.3390/plants11192544
APA StyleLabudda, M., Dziurka, K., Fidler, J., Gietler, M., Rybarczyk-Płońska, A., Nykiel, M., Prabucka, B., Morkunas, I., & Muszyńska, E. (2022). The Alleviation of Metal Stress Nuisance for Plants—A Review of Promising Solutions in the Face of Environmental Challenges. Plants, 11(19), 2544. https://doi.org/10.3390/plants11192544













