Salinity Tolerance, Ion Accumulation Potential and Osmotic Adjustment In Vitro and In Planta of Different Armeria maritima Accessions from a Dry Coastal Meadow
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. In Vitro Experiment
2.3. In Planta Experiments
2.4. Measurements
2.5. Data Analysis
3. Results
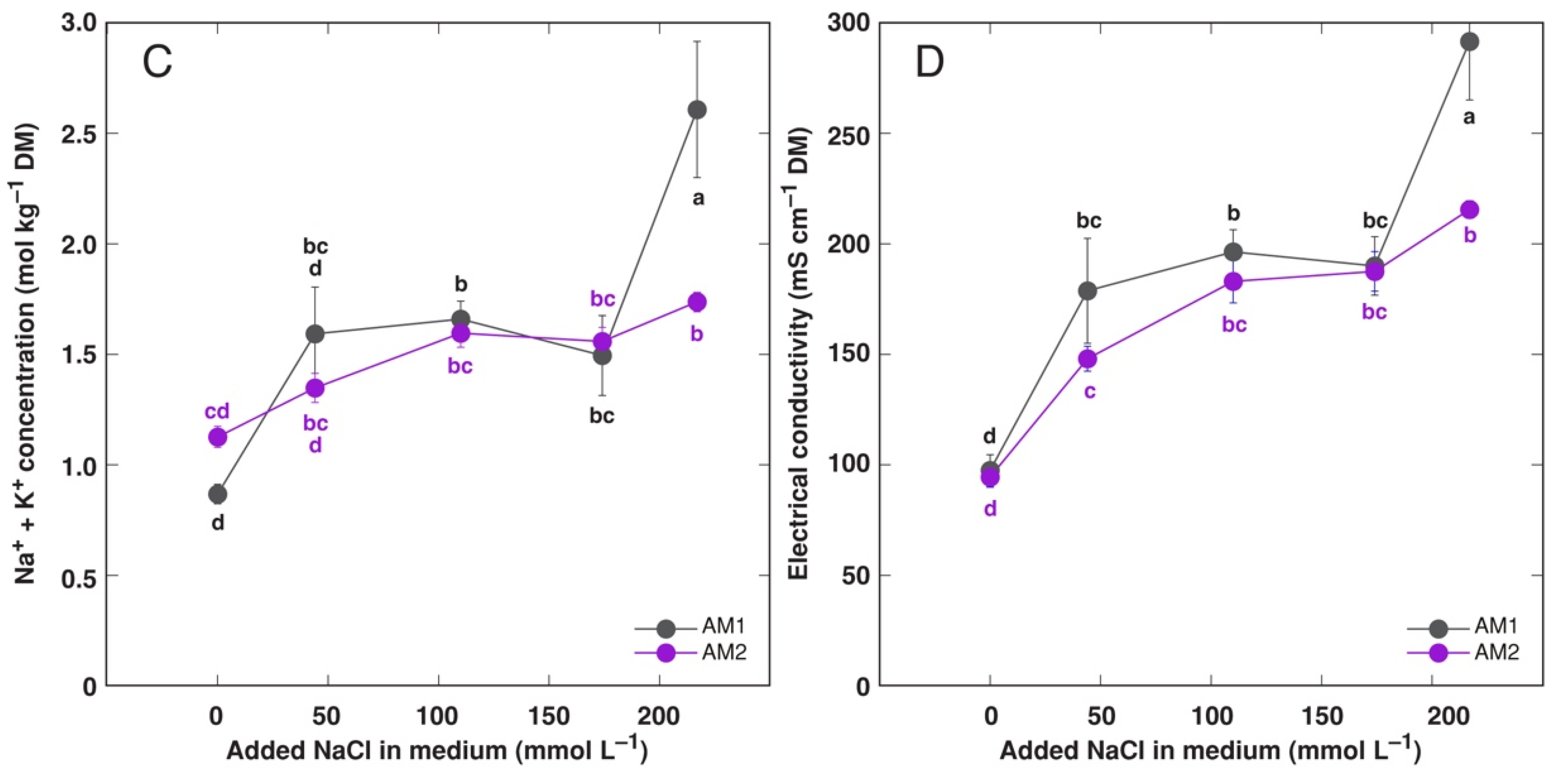
3.1. In Vitro Experiment
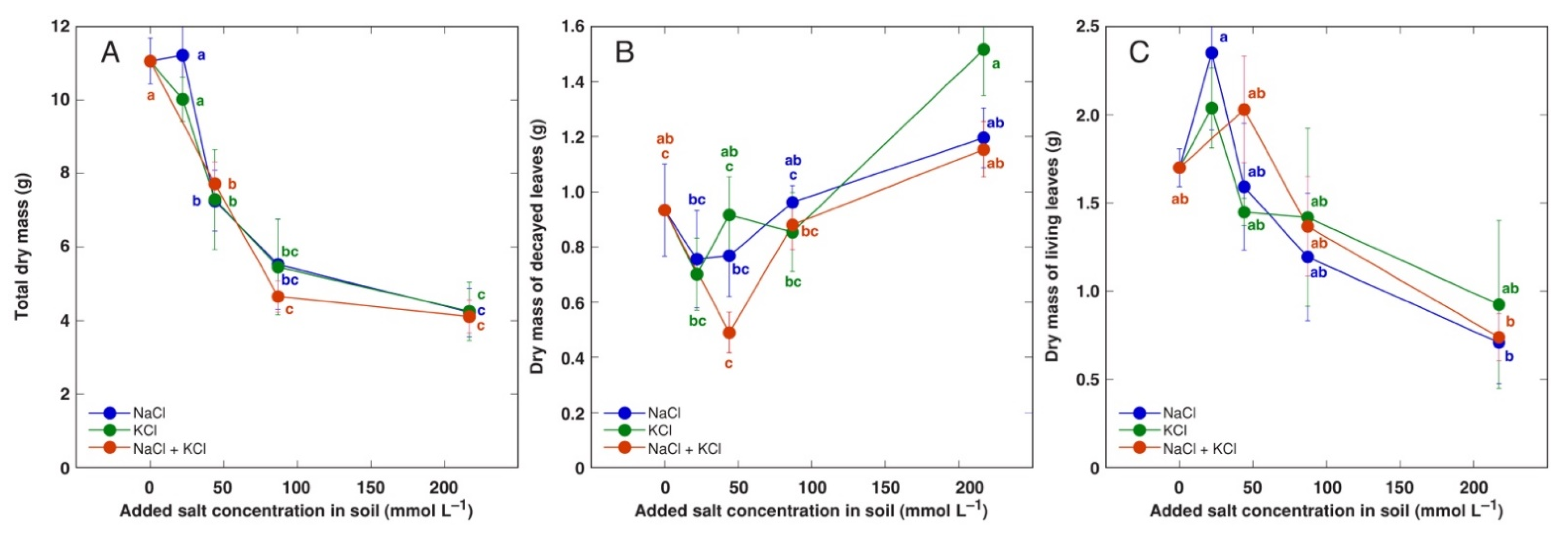
3.2. In Planta Experiments: Effect of Salinity on Growth
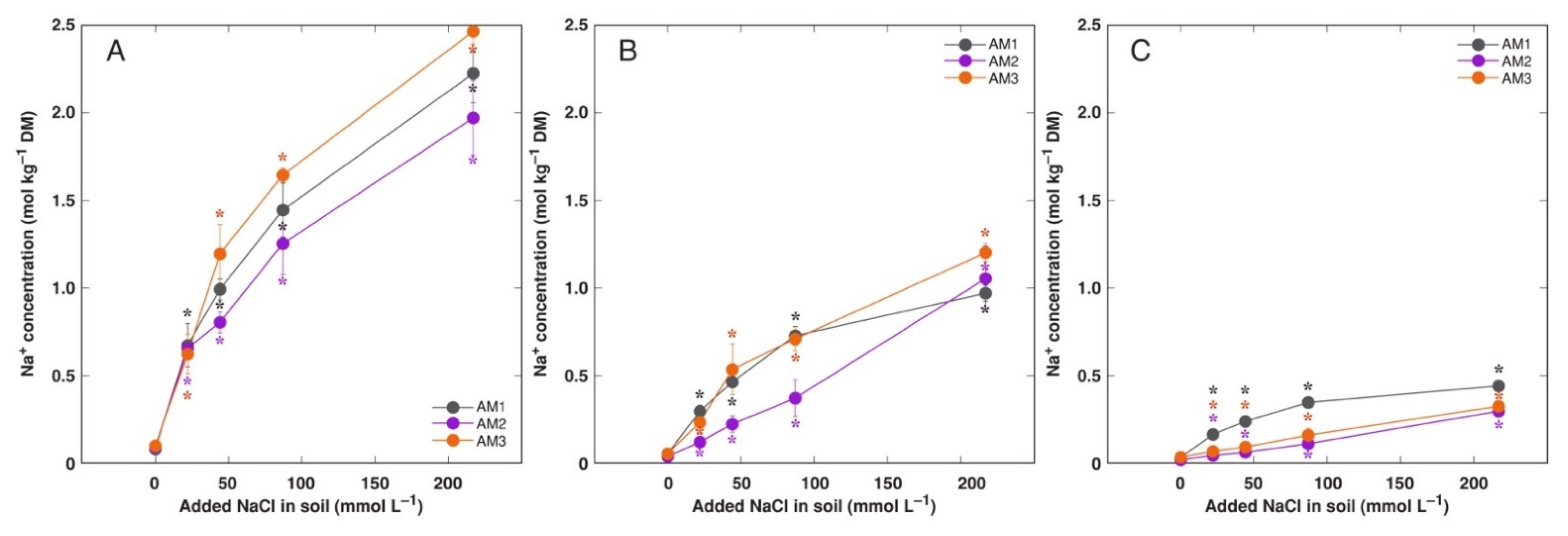
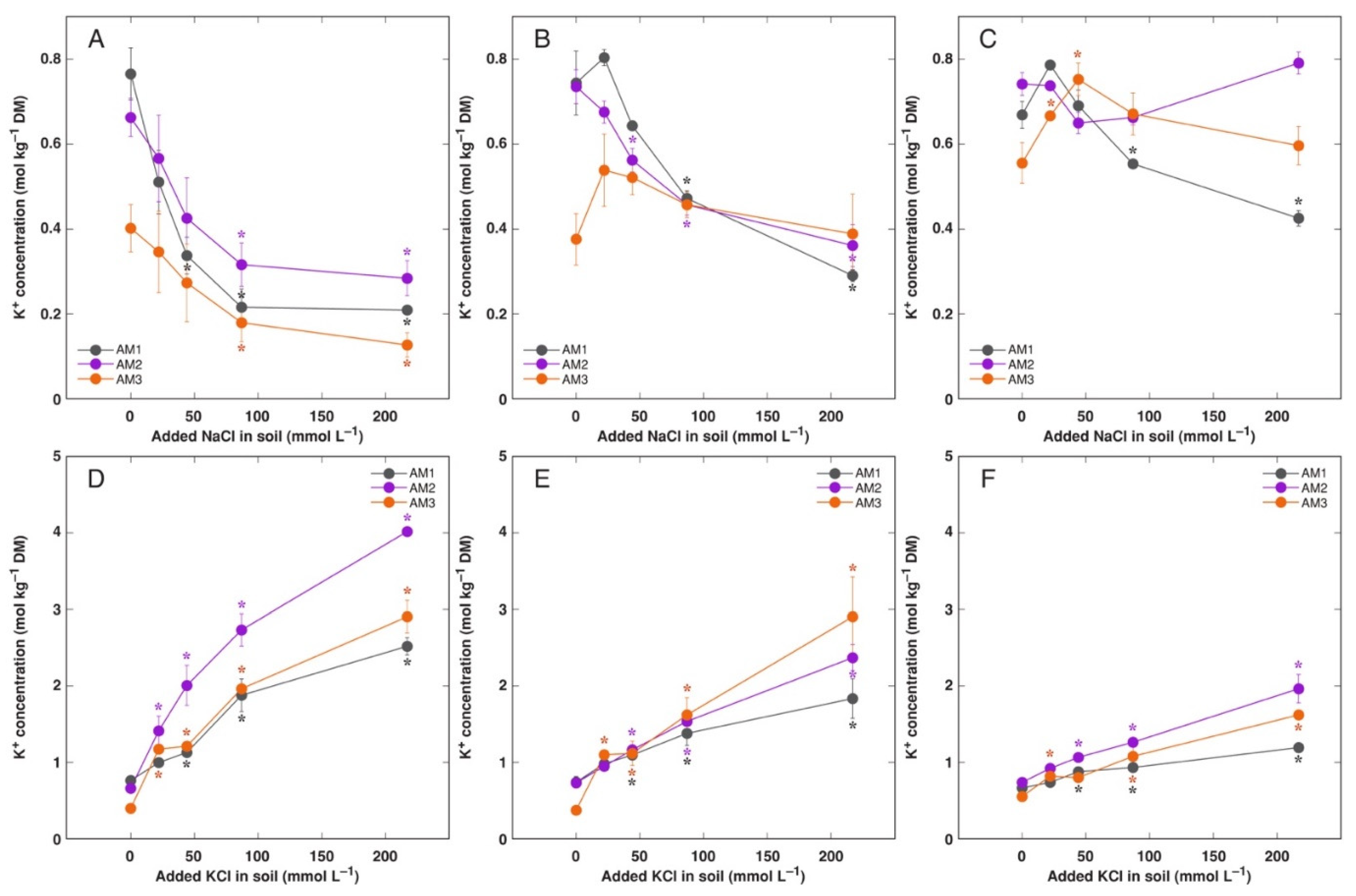
3.3. In Planta Experiments: Effect of Salinity on Ion Accumulation and Osmotic Adjustment
4. Discussion
4.1. Salinity Tolerance of A. maritima
4.2. Differences between Accessions
4.3. Effect of Na+ vs. K+
4.4. Osmotic Adjustment
4.5. In Vitro vs. In Planta Effects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daliakopoulos, I.N.; Tsanis, I.K.; Koutroulis, A.; Kourgialas, N.N.; Varouchakis, A.E.; Karatzas, G.P.; Ritsema, C.J. The threat of soil salinity: A European scale review. Sci. Total Envrion. 2016, 573, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of salt-induced land degradation and restoration. Nat. Resour. Forum 2018, 38, 282–295. [Google Scholar] [CrossRef]
- Ondrasek, G.; Rengel, Z. Environmental salinization processes: Detection, implications & solutions. Sci. Total Environ. 2021, 754, 142432. [Google Scholar] [PubMed]
- Meng, X.; Zhou, J.; Sui, N. Mechanisms of salt tolerance in halophytes: Current understanding and recent advances. Open Life Sci. 2018, 13, 149–154. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef]
- Maathuis, F.J.M.; Amtmann, A. K+ nutrition and Na+ toxicity: The basis of cellular K+/Na+ ratios. Ann. Bot. 1999, 84, 123–133. [Google Scholar] [CrossRef]
- Rubio, F.; Nieves-Cordones, M.; Horie, T.; Shabala, S. Doing ‘business as usual’ comes with a cost: Evaluating energy cost of maintaining plant intracellular K+ homeostasis under saline conditions. New Phytol. 2019, 225, 1097–1104. [Google Scholar] [CrossRef]
- Ievinsh, G.; Ieviņa, S.; Andersone-Ozola, U.; Samsone, I. Leaf sodium, potassium and electrolyte accumulation capacity of plant species from salt-affected coastal habitats of the Baltic Sea: Towards a definition of Na hyperaccumulation. Flora 2021, 274, 151748. [Google Scholar] [CrossRef]
- Ramos, J.; López, M.J.; Benlloch, M. Effect of NaCl and KCl salts on the growth and solute accumulation of the halophyte Atriplex nummularia. Plant Soil 2004, 259, 163–168. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Yan, X.; Guo, J. Physiological and transcriptomic analyses of yellow horn (Xanthoceras sorbifiolia) provide important insights into salt and saline-alkali stress tolerance. PLoS ONE 2012, 15, e0244365. [Google Scholar]
- Rahman, M.M.; Mostofa, M.G.; Keya, S.S.; Siddiqui, M.N.; Ansary, M.M.U.; Das, A.K.; Rahman, M.A.; Tran, L.S.-P. Adaptive mechanisms of halophytes and their potential in improving salinity tolerance in plants. Int. J. Mol. Sci. 2021, 22, 10733. [Google Scholar] [CrossRef]
- Lombardi, T.; Bertacchi, A.; Pistelli, L.; Pardossi, A.; Pecchia, S.; Toffanin, A.; Sanmartin, C. Biological and agronomic traits of the main halophytes widespread in the Mediterranean region as potential new vegetable crops. Horticulturae 2022, 8, 195. [Google Scholar] [CrossRef]
- Ellouzi, H.; Ben Hamed, K.; Cela, J.; Munné-Bosch, S.; Abdelly, C. Early effects of salt stress on the physiological and oxidative status of Cakile maritima (halophyte) and Arabidopsis thaliana (glycophyte). Physiol. Plant. 2011, 142, 128–143. [Google Scholar] [CrossRef]
- Bueno, M.; Lendinez, M.L.; Aparicio, C.; Cordovilla, M.P. Germination and growth of Atriplex prostrata and Plantago coronopus: Two strategies to survive in saline habitats. Flora 2017, 227, 56–63. [Google Scholar] [CrossRef]
- Tabot, P.T.; Adams, J.B. Ecophysiology of salt marsh plants and predicted responses to climate change in South Africa. Ocean. Coast. Manag. 2013, 80, 89–99. [Google Scholar] [CrossRef]
- Rilke, S.; Reimans, C. Morphological and ecophysiological differences between the subspecies of Salsola kali L. in Europe: Results of culture experiments. Flora 1996, 191, 363–376. [Google Scholar] [CrossRef]
- Ghoulam, C.; Foursy, A.; Fares, K. Effects of salt stress om growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Envrion. Exp. Bot. 2002, 47, 39–50. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Y.; Mi, P.; Wang, B.; Yuan, F. Salt-tolerance screening in Limonium sinuatum varieties with different flower colors. Sci. Rep. 2021, 11, 14562. [Google Scholar] [CrossRef]
- Jēkabsone, A.; Andersone-Ozola, U.; Karlsons, A.; Romanovs, M.; Ievinsh, G. Effect of salinity on growth, ion accumulation and mineral nutrition of different accessions of a crop wild relative legume species, Trifolium fragiferum. Plants 2022, 11, 797. [Google Scholar] [CrossRef]
- Winicov, I. Characterization of rice (Oryza sativa L.) plants regenerated from salt-tolerant cell lines. Plant Sci. 1996, 113, 105–111. [Google Scholar] [CrossRef]
- Hossain, Z.; Mandal, A.K.A.; Datta, S.K.; Biswas, A.K. Development of NaCl-tolerant line in Chrysanthemum morifolium Ramat. through shoot organogenesis of selected callus line. J. Biotechnol. 2007, 129, 658–667. [Google Scholar] [CrossRef]
- Rattana, K.; Bunnag, S. Differential salinity tolerance in calli and shoots of four rice cultivars. Asian J. Crop Sci. 2015, 7, 48–60. [Google Scholar] [CrossRef][Green Version]
- Winicov, I.; Bastola, D.R. Salt tolerance in crop plants: New approaches through tissue culture and gene regulation. Acta Physiol. Plant. 1997, 19, 435–449. [Google Scholar] [CrossRef]
- Aldahhak, O.; Zaid, S.; Teixeira da Silva, J.A.; Abdul-Kader, A.M. In vitro approach to the multiplication of a halophyte species forage shrub Atriplex halimus L. and in vitro selection for salt tolerance. Int. J. Plant Dev. Biol. 2010, 4, 8–14. [Google Scholar]
- Lokhande, V.H.; Nikam, T.D.; Patade, V.Y.; Ahire, M.L.; Suprasanna, P. Effects of optimal and supra-optimal salinity stress on antioxidative defence, osmolytes and in vitro growth response on Sesuvium portulacastrum L. Plant Cell Tissue Organ Cult. 2011, 104, 41–49. [Google Scholar] [CrossRef]
- Shi, X.L.; Han, H.P.; Shi, W.L.; Li, Y.X. NaCl and TDZ are two key factors for the improvement of in vitro regeneration rate of Salicornia europaea L. J. Integr. Plant Biol. 2006, 48, 1185–1189. [Google Scholar] [CrossRef]
- Joshi, M.; Mishra, A.; Jha, B. NaCl plays a key role for in vitro micropropagation of Salicornia brachiata, an extreme halophyte. Industr. Crops Prod. 2012, 35, 313–316. [Google Scholar] [CrossRef]
- Martini, A.N.; Papafotiou, M. In vitro propagation and NaCl tolerance of the multipurpose medicinal halophyte Limoniastrum monopetalum. HortScience 2020, 55, 436–443. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Maloupa, E. Microporpagation and salt tolerance of in vitro grown Crithmum maritimum L. Plant Cell Tissue Organ Cult. 2008, 94, 209–217. [Google Scholar] [CrossRef]
- Aghaleh, M.; Niknam, V.; Ebrahimzadeh, H.; Razavi, K. Salt stress effects on growth, pigments, proteins and lipid peroxidation in Salicorrnia persica and S. europaea. Biol. Plant. 2008, 53, 243–248. [Google Scholar] [CrossRef]
- Samiei, L.; Pahnehkilayi, M.D.; Karimian, Z.; Nabati, J. Morpho-physiological responses of halophyte Climacoptera crassa to salinity and heavy metal stresses in in vitro condition. S. Afr. J. Bot. 2021, 131, 468–474. [Google Scholar] [CrossRef]
- Xiong, Y.; Liang, H.; Yan, H.; Guo, B.; Niu, M.; Chen, S.; Jian, S.; Ren, H.; Zhang, X.; Li, Y.; et al. NaCl-induced stress: Physiological responses of six halophytic species in in vitro and in vivo culture. Plant Cell Tissue Organ Cult. 2019, 139, 531–546. [Google Scholar] [CrossRef]
- Woodell, S.R.J.; Dale, A. Armeria maritima (Mill.) Willd. (Statice armeria L.; S. maritima Mill.). J. Ecol. 1993, 81, 573–588. [Google Scholar] [CrossRef]
- Goldsmith, F.B. Interaction (competition) studies as a step towards the synthesis of sea-cliff vegetation. J. Ecol. 1978, 66, 921–931. [Google Scholar] [CrossRef]
- Köhl, K.I. The effect of NaCl on growth, dry matter allocation and ion uptake in salt marsh and inland populations of Armeria maritima. New Phytol. 1997, 135, 213–225. [Google Scholar] [CrossRef]
- Purmale, l.; Jēkabsone, A.; Andersone-Ozola, U.; Karlsons, A.; Osvalde, A.; Ievinsh, G. Comparison of in vitro and in planta heavy metal tolerance and accumulation potential of different Armeria maritima accessions from a dry coastal meadow. Plants 2022, 11, 2104. [Google Scholar] [CrossRef] [PubMed]
- Köhl, K.I. NaCl homeostasis as a factor for the survival of the evergreen halophyte Armeria maritima (Mill.) Willd. under salt stress in winter. Plant Cell Environ. 1997, 20, 1253–1263. [Google Scholar] [CrossRef]
- Jennings, D.H. Halophytes, succulence and sodium in plants—A unified theory. New Phytol. 1968, 67, 899–911. [Google Scholar] [CrossRef]
- Belkheiri, O.; Mulas, M. The effects of salt stress on growth, water relations and ion accumulation in two halophyte Atriplex species. Environ. Exp. Bot. 2013, 86, 17–28. [Google Scholar] [CrossRef]
- Rozema, J.; Gude, H.; Pollak, G. An ecophysiological study of the salt secretion of four halophytes. New Phytol. 1981, 89, 201–217. [Google Scholar] [CrossRef]
- Wingler, A.; Stangberg, E.J.; Saxena, T.; Mistry, R. Interactions between temperature and sugars in the regulation of leaf senescence in the perennial herb Arabis alpina L. J. Integr. Plant Biol. 2012, 54, 595–605. [Google Scholar] [CrossRef]
- Dahmani-Muller, H.; van Ort, F.; Gélie, B.; Balabane, M. Strategies of heavy metal uptake by three plant species growing near a metal smelter. Envrion. Pollut. 2000, 109, 231–238. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Coskun, D.; Schulze, L.M.; Wong, J.R.; Britto, D.T. Sodium as nutrient and toxicant. Plant Soil 2013, 369, 1–23. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.C.; Martínez, V.; Carvajal, M. Osmotic adjustment, water relations and gas exchange in pepper plants grown under NaCl and KCl. Environ. Exp. Bot. 2004, 52, 161–174. [Google Scholar] [CrossRef]
- Wang, D.; Wang, H.; Han, B.; Wang, B.; Guo, A.; Zheng, D.; Liu, C.; Chang, L.; Peng, M.; Wang, X. Sodium instead of potassium and chloride is an important macronutrient to improve leaf succulence and shoot development for halophyte Sesuvium portulacastrum. Plant Physiol. Biochem. 2012, 51, 53–62. [Google Scholar] [CrossRef]
- Ben-Gal, A.; Borochov-Neori, H.; Yermiyahu, U.; Shani, U. Is osmotic potential a more appropriate property than electrical conductivity for evaluating whole plant response to salinity? Environ. Exp. Bot. 2009, 65, 232–237. [Google Scholar] [CrossRef]
- Nada, R.M.; Abogadallah, G.M. Developmental acquisition of salt tolerance in the halophyte Atriplex halimus L. is related to differential regulation of salt inducible genes. Plant Growth Regul. 2015, 75, 165–178. [Google Scholar] [CrossRef]
- Song, J.; Feng, G.; Tian, C.; Zhang, F. Strategies for adaptation of Suaeda physophora, Haloxylon ammodendron and Haloxylon persicum to a saline environment during seed-germination stage. Ann. Bot. 2005, 96, 399–405. [Google Scholar] [CrossRef]
- Li, J.; Hussain, T.; Feng, X.; Guo, K.; Chen, H.; Yang, C.; Liu, X. Comparative study on the resistance of Suaeda glauca and Suaeda salsa to drought, salt, and alkali stresses. Ecol. Eng. 2019, 140, 105593. [Google Scholar] [CrossRef]
- Bazihizina, N.; Colmer, T.D.; Cuin, T.A.; Mancuso, S.; Shabala, S. Friend or foe? Chloride patterning in halophytes. Trends Plant Sci. 2019, 24, 142–151. [Google Scholar] [CrossRef]
- Flowers, T.J.; Munns, R.; Colmer, T.D. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann. Bot. 2015, 115, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Ugarte, R.M.; Escudero, A.; Gavilán, R.G. Assessing the role of selected osmolytes in Mediterranean high-mountain specialists. Front. Ecol. Evol. 2021, 9, 576122. [Google Scholar] [CrossRef]
- Stewart, C.R.; Lee, J.A. The role of proline accumulation in halophytes. Planta 1974, 120, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a multifaceted signalling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Adrian-Romero, M.; Wilson, S.J.; Blunden, G.; Yang, M.-H.; Carabot-Cuervvo, A.; Bashir, A.K. Betaines in coastal plants. Biochem. Syst. Ecol. 1998, 26, 535–543. [Google Scholar] [CrossRef]
- Shabala, S.; Shabala, L. Ion transport and osmotic adjustment in plants and bacteria. BioMol. Concepts 2011, 2, 407–419. [Google Scholar] [CrossRef]
- Yamada, M.; Kuroda, C.; Fujiyama, H. Function of sodium and potassium in growth of sodium-lowing Amaranthaceae species. Soil Sci. Plant Nutr. 2016, 62, 20–26. [Google Scholar] [CrossRef]
- Ragel, P.; Raddatz, N.; Leidi, E.O.; Quintero, F.J.; Pardo, J.M. Regulation of K+ nutrition in plants. Front. Plant Sci. 2019, 10, 281. [Google Scholar] [CrossRef]
- Skeffington, M.J.; Jeffrey, D.W. Response of Armeria maritima (MIll.) Willd. and Plantago maritima L. from an Irish salt marsh to nitrogen and salinity. New Phytol. 1988, 110, 399–408. [Google Scholar] [CrossRef]
- Chen, M.; Yang, Z.; Liu, J.; Zhu, T.; Wei, X.; Fan, H.; Wang, B. Adaptation mechanisms of salt excluders under saline conditions and its applications. Int. J. Mol. Sci. 2018, 19, 3668. [Google Scholar] [CrossRef]
- Chen, T.; Cai, X.; Wu, X.; Karahara, I.; Schreiber, L.; Lin, J. Casparian strip development and its potential function in salt tolerance. Plant Signal. Behav. 2011, 6, 1499–1502. [Google Scholar] [CrossRef]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef]




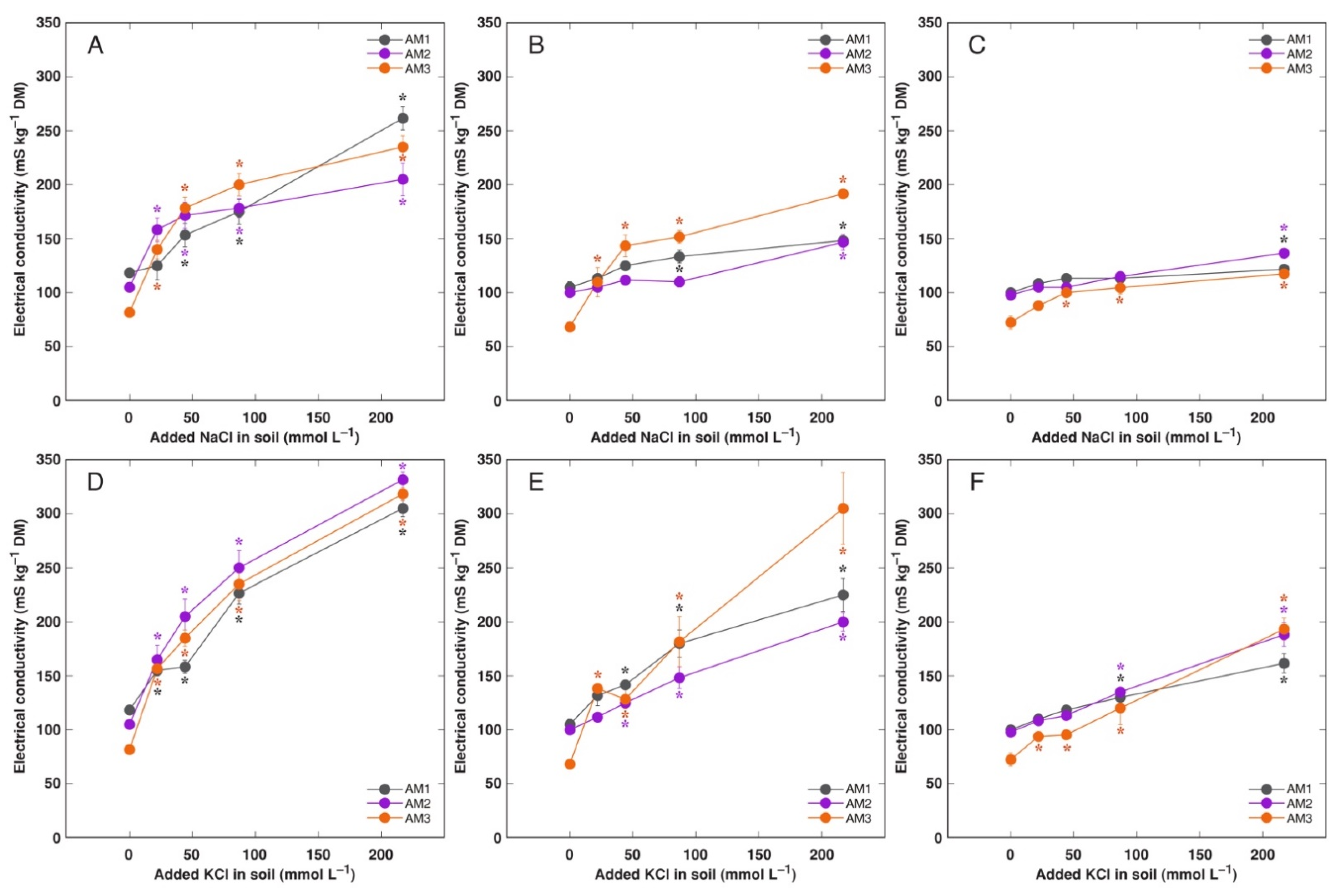
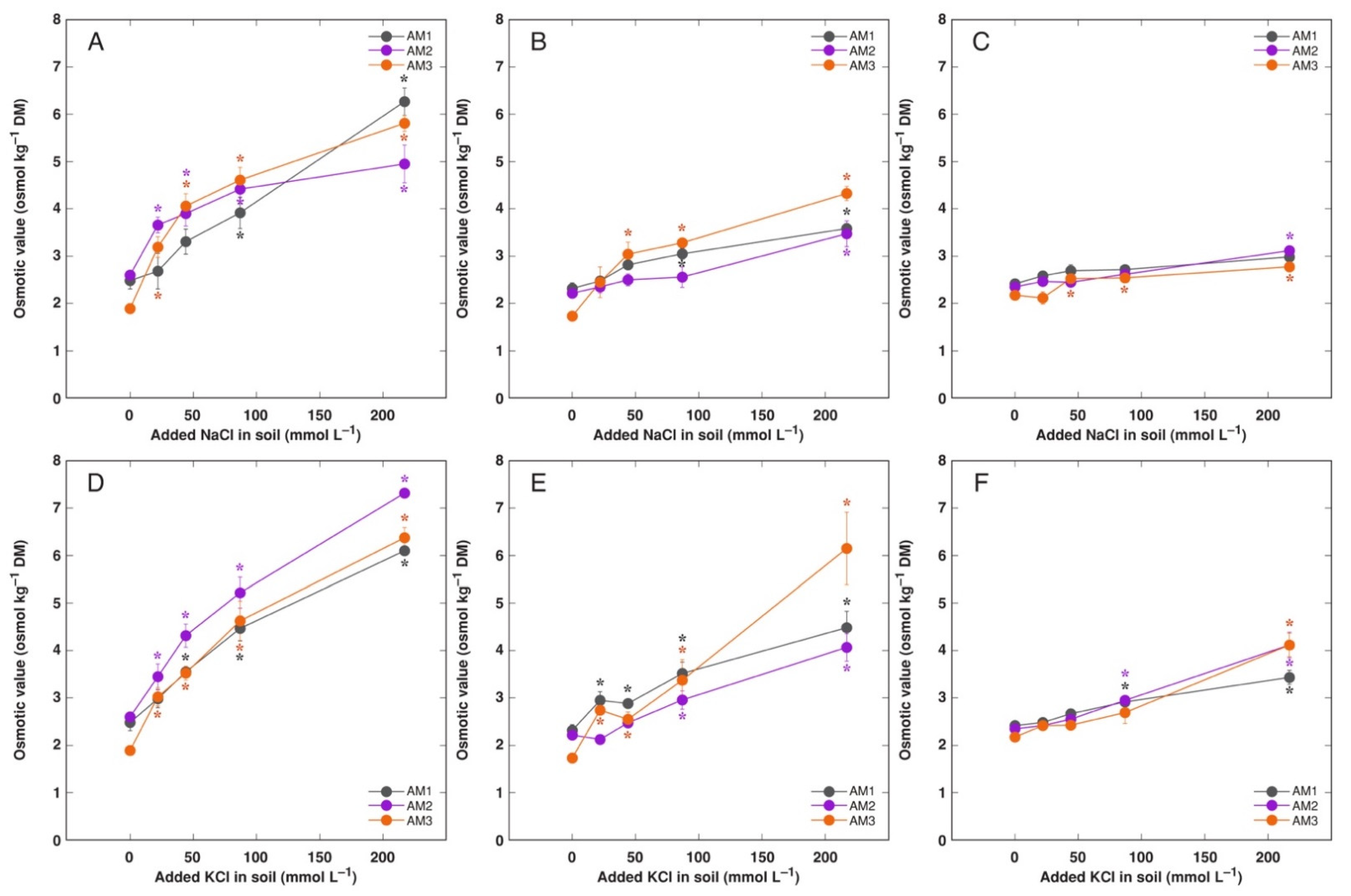
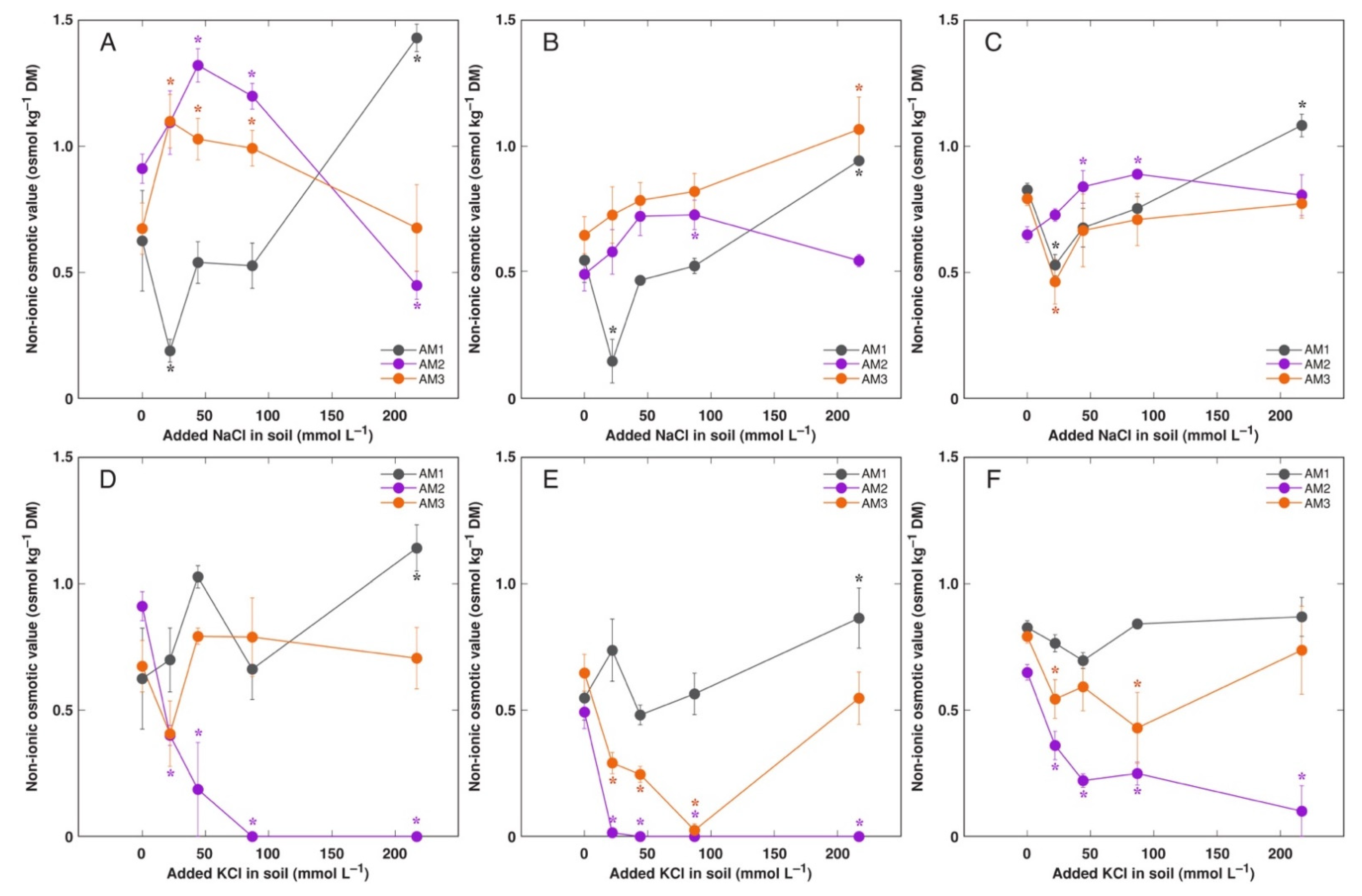
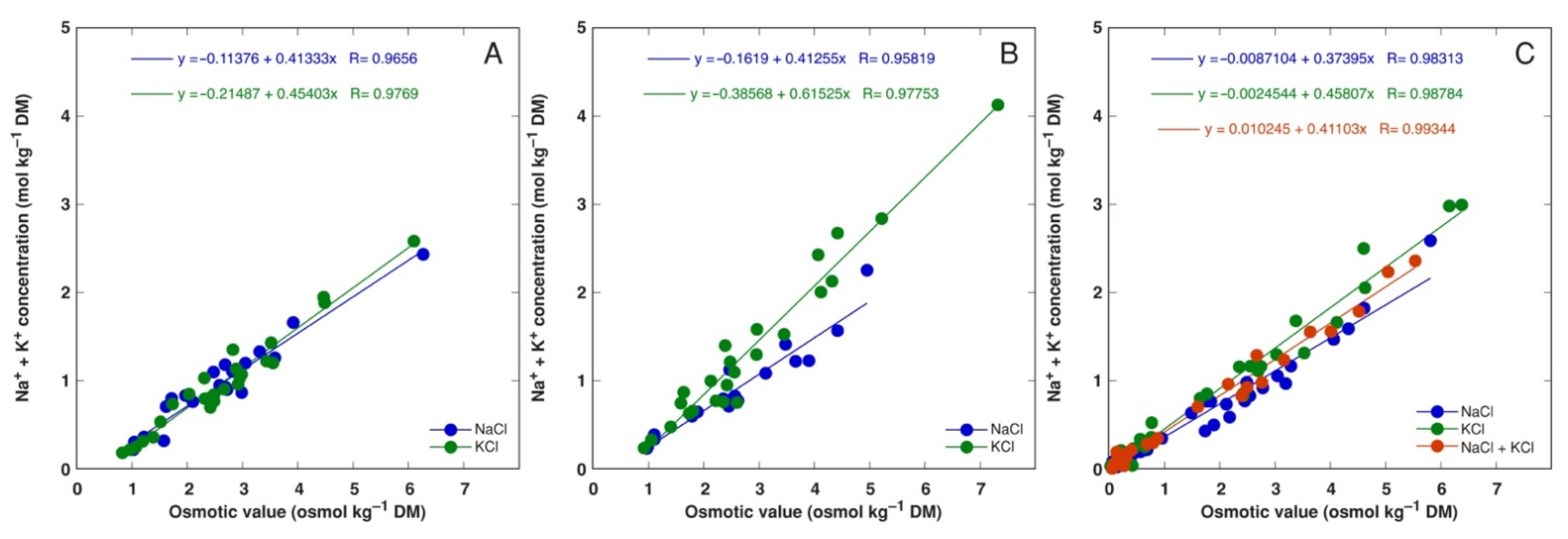
| Code | Associated Water Reservoir | Habitat | Electrical Conductivity (mS m−1) | Location | Coordinates | Performed Experiments (Treatments) |
|---|---|---|---|---|---|---|
| AM1 | River Vecdaugava | Dry shore meadow | 97 ± 5 c | City of Riga, Ziemeļu District, Vecdaugava, Latvia | 57°03′29″ N 24°05′47′′ E | In vitro (NaCl 44, 110, 174, 217 mol L−1).In planta (NaCl 22, 44, 87, 217 mol L−1; KCl 22, 44, 87, 217 mol L−1) |
| AM2 | River Buļļupe | Dry shore meadow | 127 ± 4 b | City of Riga, Kurzeme District, Island of Buļļu Sala, Vakarbuļļi, Latvia | 56°59′54″ N 23°57′31″ E | In vitro (NaCl 44, 110, 174, 217 mol L−1).In planta (NaCl 22, 44, 87, 217 mol L−1; KCl 22, 44, 87, 217 mol L−1) |
| AM3 | The Baltic Sea | Dry coastal meadow | 223 ± 11 a | Nybrostrand, Ystad Municipality, Skåne County, Sweden | 55°25′40″ N 13°57′27″ E | In planta (NaCl 22, 44, 87, 217 mol L−1; KCl 22, 44, 87, 217 mol L−1); NaCl + KCl 44, 87, 217 mol L−1) |
| Salt | Concentration (mmol L−1) | Flower Stalks (n) | Total Length of Flower Stalks (m Plant−1) | Dry Mass of Leaves (g) | |||
|---|---|---|---|---|---|---|---|
| AM1 | AM2 | AM1 | AM2 | AM1 | AM2 | ||
| Control | 0 | 8.0 ± 0.7 abc | 8.4 ± 1.0 ab | 1.77 ± 0.14 abc | 2.00 ± 0.25 ab | 6.26 ± 0.47 ab | 3.77 ± 0.52 a |
| NaCl | 22 | 13.2 ± 1.2 a | 9.8 ± 1.3 a | 2.52 ± 0.30 a | 2.13 ± 0.25 ab | 7.64 ± 0.56 a | 3.70 ± 0.35 a |
| 44 | 10.4 ± 0.5 ab | 7.6 ± 0.6 ab | 1.91 ± 0.09 ab | 1.71 ± 0.17 abc | 6.88 ± 0.55 ab | 3.58 ± 0.40 a | |
| 87 | 9.8 ± 0.9 abc | 7.8 ± 0.5 ab | 1.76 ± 0.12 abc | 1.61 ± 0.13 abc | 6.65 ± 0.61 ab | 3.36 ± 0.27 a | |
| 217 | 6.2 ± 0.6 c | 6.6 ± 0.6 ab | 1.02 ± 0.06 c | 1.18 ± 0.16 bc | 5.59 ± 0.30 ab | 3.75 ± 0.37 a | |
| KCl | 22 | 10.8 ± 0.6 ab | 10.0 ± 0.7 a | 2.07 ± 0.15 a | 2.26 ± 0.15 a | 7.61 ± 0.75 a | 3.66 ± 0.23 a |
| 44 | 9.8 ± 1.2 abc | 9.6 ± 0.7 ab | 2.07 ± 0.17 a | 2.16 ± 0.20 a | 6.73 ± 0.62 ab | 3.66 ± 1.02 a | |
| 87 | 9.4 ± 1.3 abc | 8.4 ± 1.3 ab | 1.86 ± 0.26 ab | 1.89 ± 0.32 abc | 6.48 ± 0.20 ab | 4.13 ± 0.69 a | |
| 217 | 7.6 ± 0.7 bc | 5.4 ± 1.0 b | 1.16 ± 0.13 bc | 1.00 ± 0.21 c | 5.06 ± 0.28 b | 3.61 ± 0.26 a | |
| Salt | Concentration (mmol L−1) | Dry Mass of Flower Stalks (g) | Dry Mass of Flowers (g) | Dry Mass of Roots (g) | |||
|---|---|---|---|---|---|---|---|
| AM1 | AM2 | AM1 | AM2 | AM1 | AM2 | ||
| Control | 0 | 0.70 ± 0.03 abc | 0.97 ± 0.06 abc | 0.83 ± 0.08 ab | 1.01 ± 0.05 ab | 1.79 ± 0.20 a | 1.18 ± 0.14 ab |
| NaCl | 22 | 1.01 ± 0.13 a | 1.13 ± 0.11 a | 1.30 ± 0.17 a | 1.08 ± 0.12 ab | 1.93 ± 0.10 a | 1.01 ± 0.19 ab |
| 44 | 0.80 ± 0.05 ab | 0.89 ± 0.13 abc | 1.03 ± 0.06 ab | 0.95 ± 0.16 ab | 1.73 ± 0.15 a | 1.18 ± 0.14 ab | |
| 87 | 0.68 ± 0.05 abc | 0.89 ± 0.09 abc | 0.99 ± 0.08 ab | 1.04 ± 0.11 ab | 1.19 ± 0.09 bc | 0.93 ± 0.11 ab | |
| 217 | 0.43 ± 0.01 c | 0.57 ± 0.09 bc | 0.79 ± 0.06 b | 0.69 ± 0.05 b | 0.75 ± 0.07 c | 0.60 ± 0.03 b | |
| KCl | 22 | 0.91 ± 0.06 ab | 1.33 ± 0.10 a | 1.11 ± 0.08 ab | 1.46 ± 0.11 a | 1.44 ± 0.18 ab | 1.45 ± 0.22 a |
| 44 | 0.87 ± 0.08 ab | 1.07 ± 0.04 ab | 1.13 ± 0.08 ab | 1.14 ± 0.04 ab | 1.51 ± 0.11 ab | 1.12 ± 0.20 ab | |
| 87 | 0.82 ± 0.12 ab | 0.96 ± 0.20 abc | 1.13 ± 0.16 ab | 0.98 ± 0.14 ab | 1.16 ± 0.07 bc | 0.88 ± 0.03 ab | |
| 217 | 0.58 ± 0.07 bc | 0.49 ± 0.10 c | 1.06 ± 0.08 ab | 0.63 ± 0.13 b | 0.77 ± 0.06 c | 0.56 ± 0.05 b | |
| Salt | Concentration (mol L−1) | Flower Stalks (n) | Total Length of Flower Stalks (m) | Dry Mass of Leaves (g) | Dry Mass of Flower Stalks (g) | Dry Mass of Flowers (g) | Dry Mass of Roots (g) |
|---|---|---|---|---|---|---|---|
| Control | 0 | 10.6 ± 0.7 a | 4.34 ± 0.26 a | 2.63 ± 0.16 a | 3.50 ± 0.14 a | 2.64 ± 0.11 a | 2.28 ± 0.34 ab |
| NaCl | 22 | 8.2 ± 0.9 ab | 3.80 ± 0.33 ab | 3.11 ± 0.60 a | 2.80 ± 0.31 ab | 2.39 ± 0.18 abc | 2.93 ± 0.49 a |
| 44 | 7.2 ± 0.6 abc | 2.49 ± 0.24 bc | 2.36 ± 0.33 a | 1.77 ± 0.17 bcd | 1.74 ± 0.18 abcde | 1.38 ± 0.34 bcd | |
| 87 | 5.4 ± 0.5 bc | 1.81 ± 0.27 cd | 2.16 ± 0.38 a | 1.07 ± 0.24 cde | 1.29 ± 0.40 abcde | 1.02 ± 0.22 bcd | |
| 217 | 4.5 ± 1.2 bc | 1.19 ± 0.29 d | 1.91 ± 0.20 a | 0.65 ± 0.19 de | 0.83 ± 0.26 de | 1.13 ± 0.21 bcd | |
| KCl | 22 | 10.6 ± 1.1 a | 4.03 ± 0.44 ab | 2.74 ± 0.11 a | 2.83 ± 0.23 ab | 2.59 ± 0.35 ab | 1.87 ± 0.39 abc |
| 44 | 7.4 ± 1.8 ab | 2.51 ± 0.68 bc | 2.36 ± 0.20 a | 1.69 ± 0.44 cd | 1.97 ± 0.60 abcde | 1.23 ± 0.20 bcd | |
| 87 | 5.4 ± 0.9 bc | 1.69 ± 0.40 cd | 2.27 ± 0.61 a | 1.05 ± 0.27 cde | 1.23 ± 0.29 bcde | 0.90 ± 0.20 cd | |
| 217 | 2.8 ± 0.7 c | 0.64 ± 0.22 d | 2.44 ± 0.64 a | 0.39 ± 0.12 e | 0.60 ± 0.18 e | 0.82 ± 0.17 cd | |
| NaCl + KCl | 44 | 6.6 ± 0.8 abc | 2.44 ± 0.17 bc | 2.52 ± 0.26 a | 1.97 ± 0.12 bc | 2.18 ± 0.23 abcd | 1.05 ± 0.12 bcd |
| 87 | 4.4 ± 0.3 bc | 1.44 ± 0.37 cd | 2.25 ± 0.26 a | 0.92 ± 0.03 cde | 1.00 ± 0.16 cde | 0.61 ± 0.11 cd | |
| 217 | 4.4 ± 0.7 bc | 1.26 ± 0.22 d | 1.89 ± 0.15 a | 0.74 ± 0.14 de | 0.86 ± 0.14 de | 0.48 ± 0.03 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purmale, L.; Jēkabsone, A.; Andersone-Ozola, U.; Ievinsh, G. Salinity Tolerance, Ion Accumulation Potential and Osmotic Adjustment In Vitro and In Planta of Different Armeria maritima Accessions from a Dry Coastal Meadow. Plants 2022, 11, 2570. https://doi.org/10.3390/plants11192570
Purmale L, Jēkabsone A, Andersone-Ozola U, Ievinsh G. Salinity Tolerance, Ion Accumulation Potential and Osmotic Adjustment In Vitro and In Planta of Different Armeria maritima Accessions from a Dry Coastal Meadow. Plants. 2022; 11(19):2570. https://doi.org/10.3390/plants11192570
Chicago/Turabian StylePurmale, Līva, Astra Jēkabsone, Una Andersone-Ozola, and Gederts Ievinsh. 2022. "Salinity Tolerance, Ion Accumulation Potential and Osmotic Adjustment In Vitro and In Planta of Different Armeria maritima Accessions from a Dry Coastal Meadow" Plants 11, no. 19: 2570. https://doi.org/10.3390/plants11192570
APA StylePurmale, L., Jēkabsone, A., Andersone-Ozola, U., & Ievinsh, G. (2022). Salinity Tolerance, Ion Accumulation Potential and Osmotic Adjustment In Vitro and In Planta of Different Armeria maritima Accessions from a Dry Coastal Meadow. Plants, 11(19), 2570. https://doi.org/10.3390/plants11192570








