Micromorphology and Histology of the Secretory Apparatus of Diospyros villosa (L.) de Winter Leaves and Stem Bark
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Collection
2.2. Stereomicroscopy
2.3. Electron Microscopy
2.4. Scanning Electron Microscopy
2.5. Transmission Electron Microscopy
2.6. Light Microscopy
2.7. Histochemistry
2.8. Fluorescence Microscopy
2.9. Energy Dispersive X-ray Microanalysis (EDX)
2.10. Trichome Density, Length and Statistical Analysis
3. Results
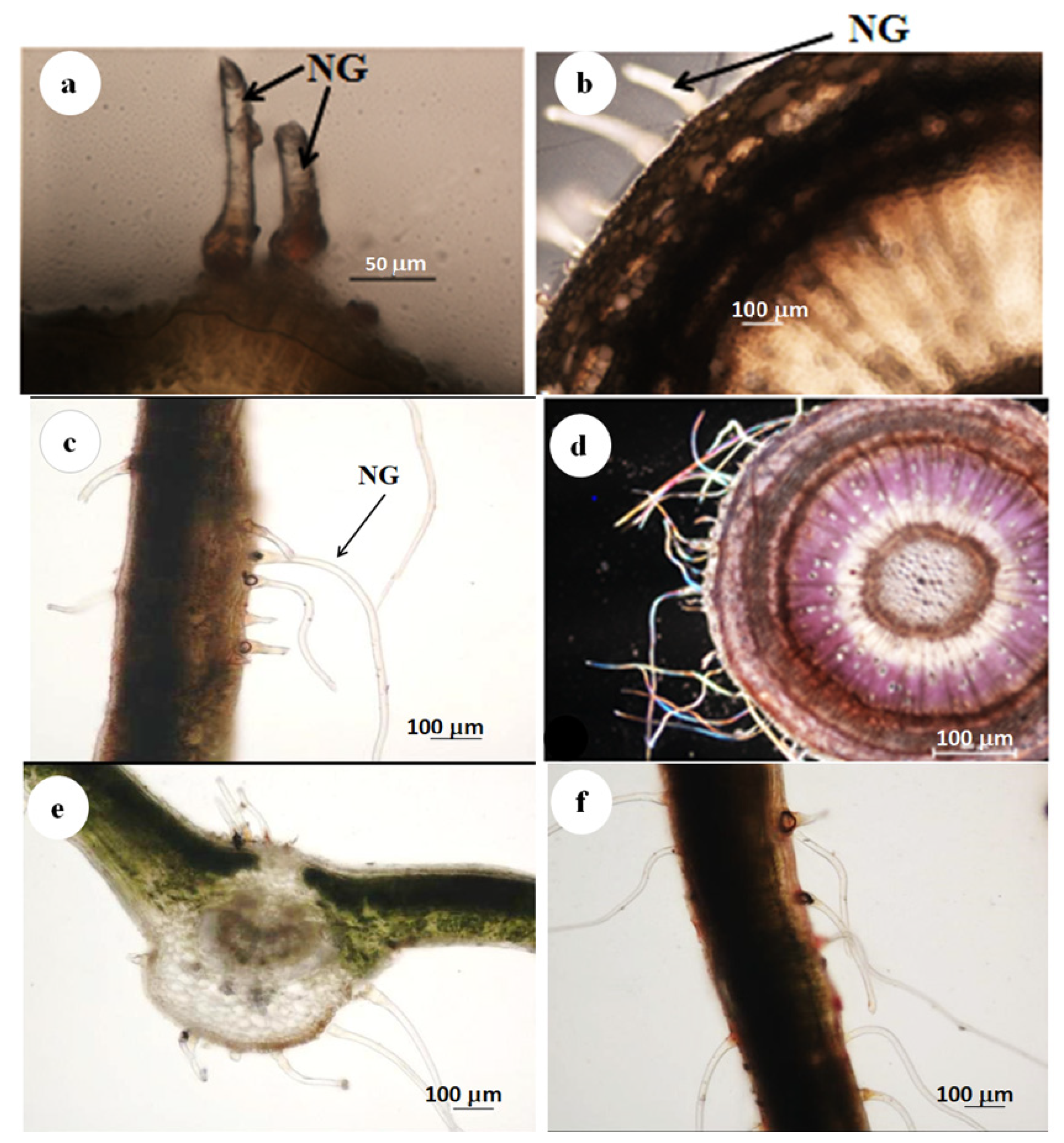
3.1. Stereomicroscopy
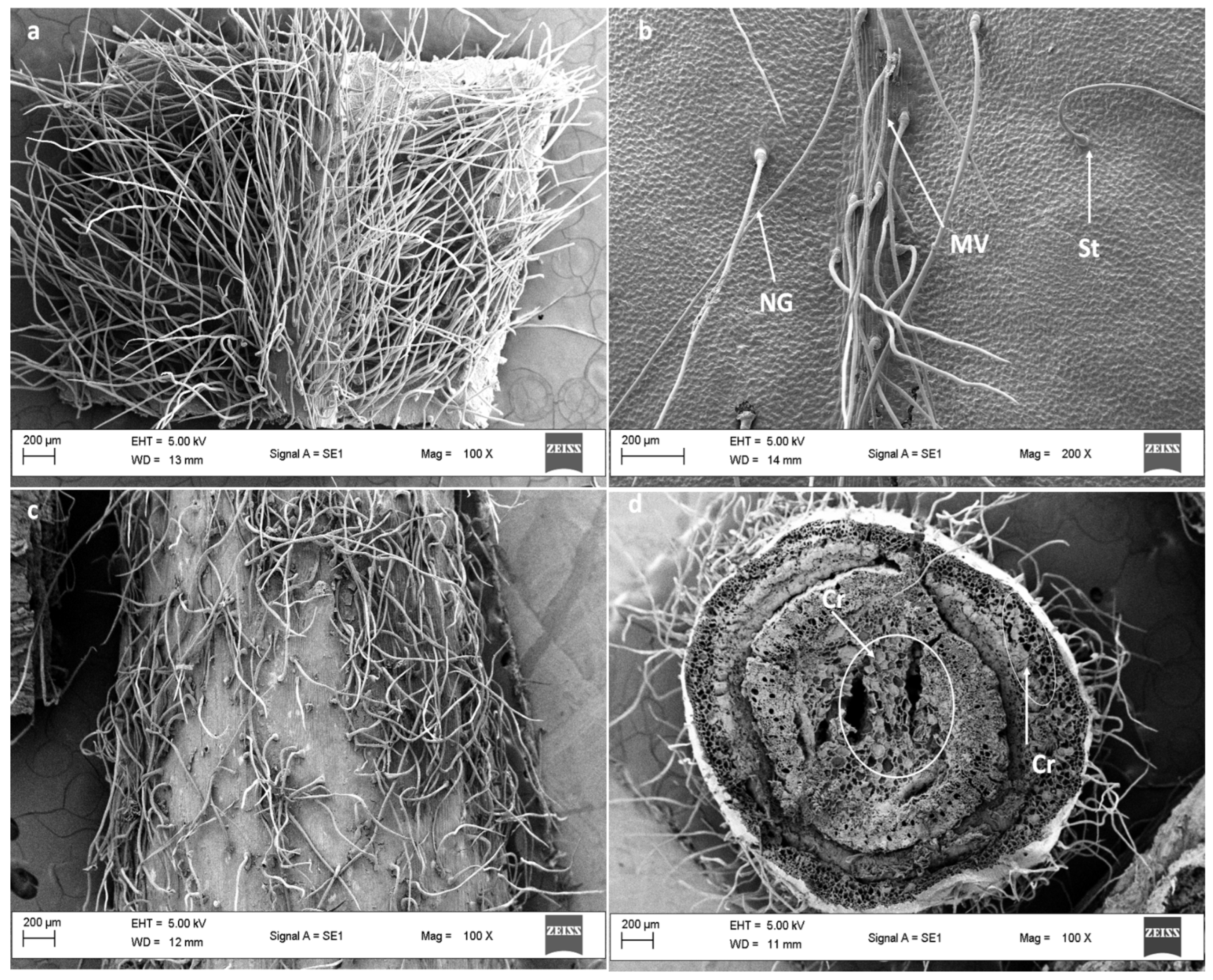
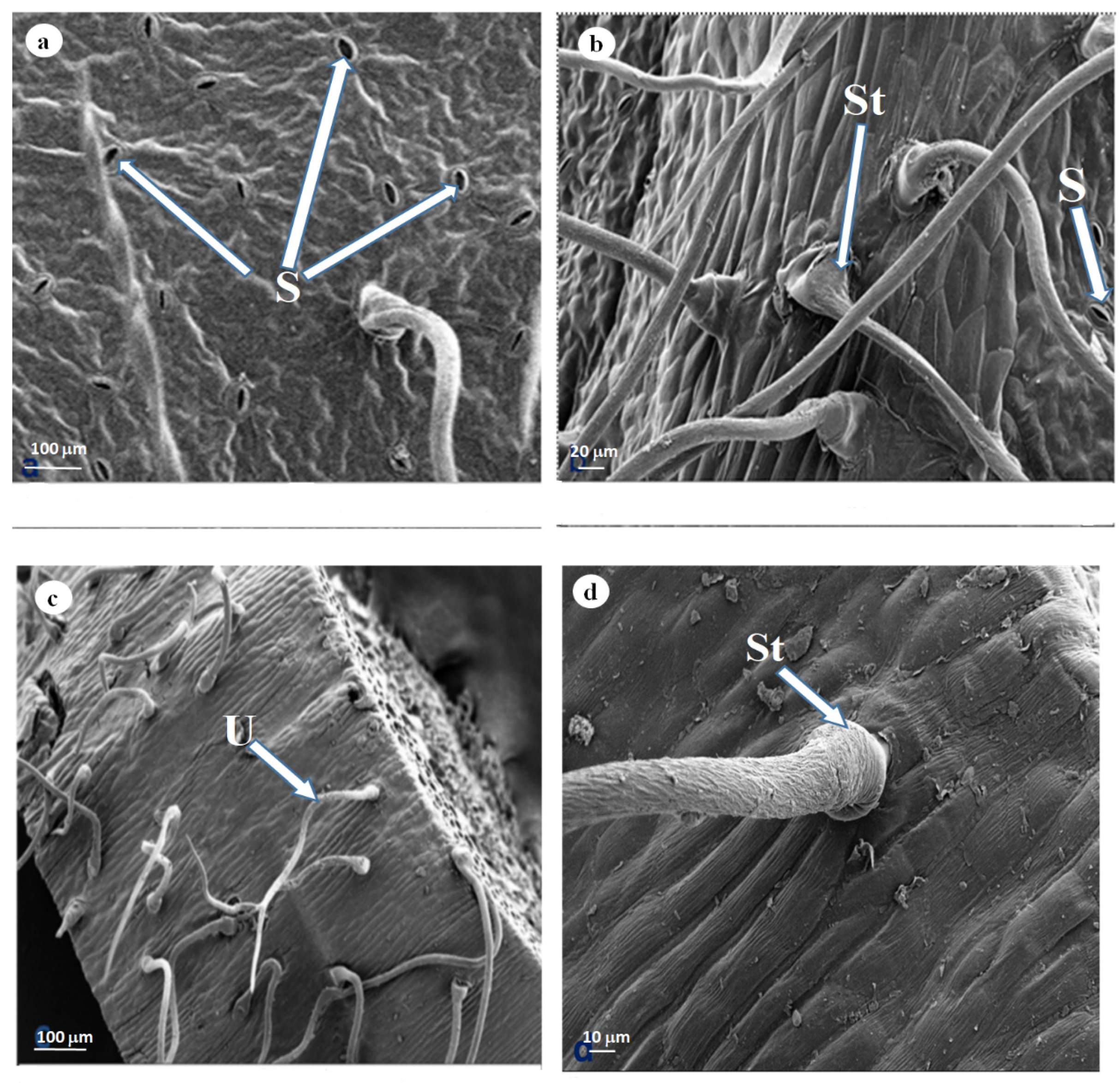
3.2. Scanning Electron Microscopy
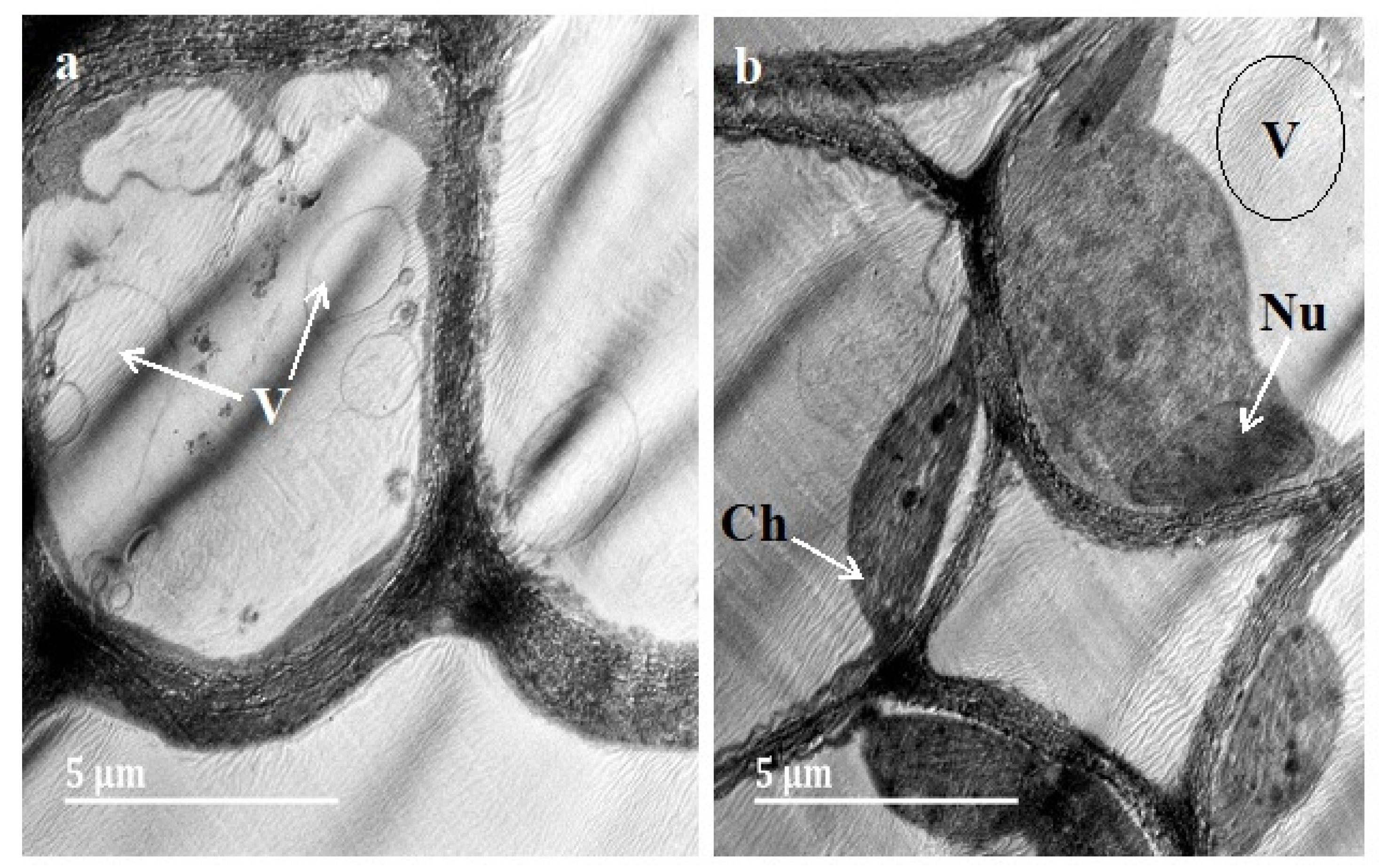
3.3. Transmission Electron Microscopy (TEM)
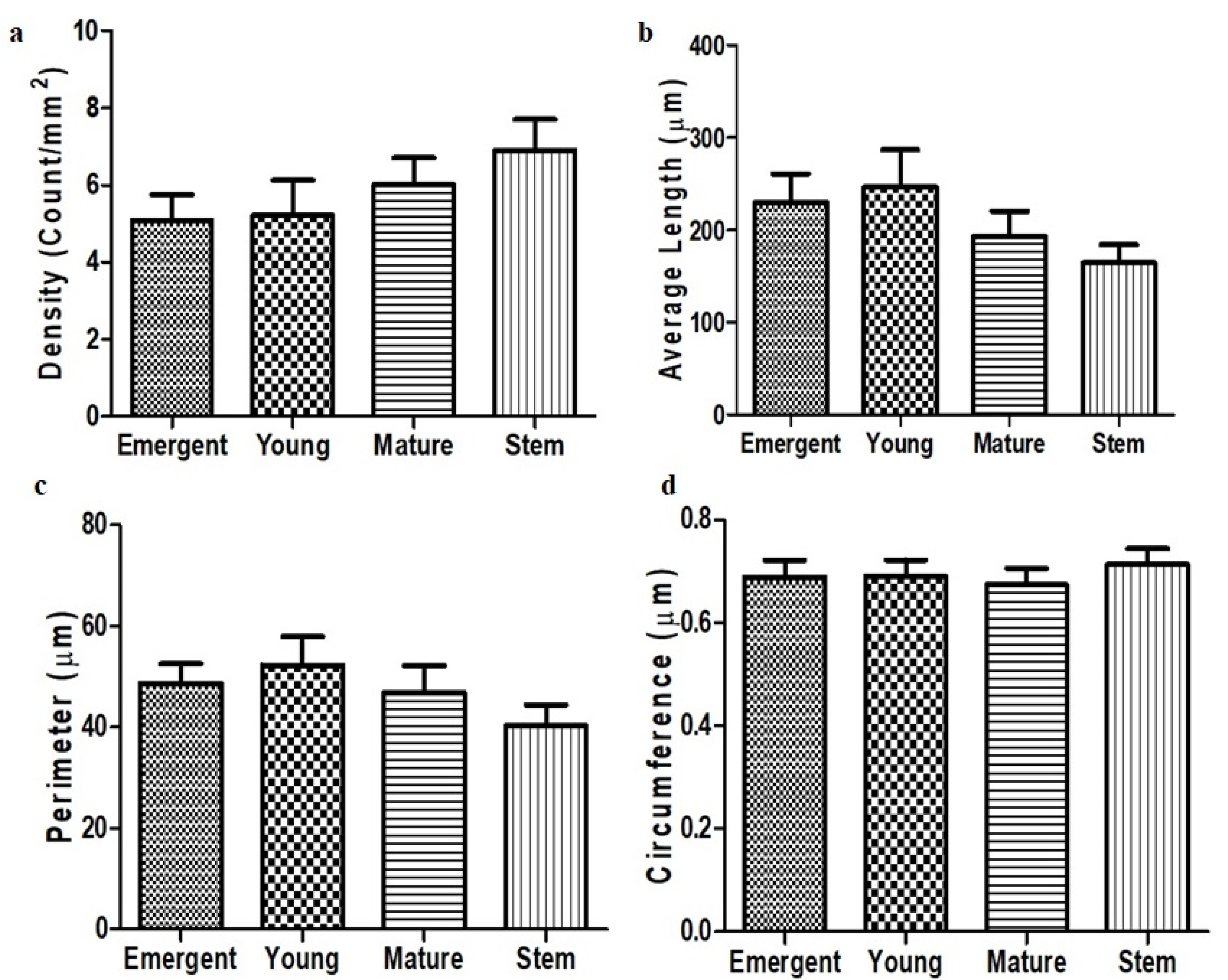
3.4. Trichome Density, Length, Perimeter and Circumference
3.5. Histochemistry
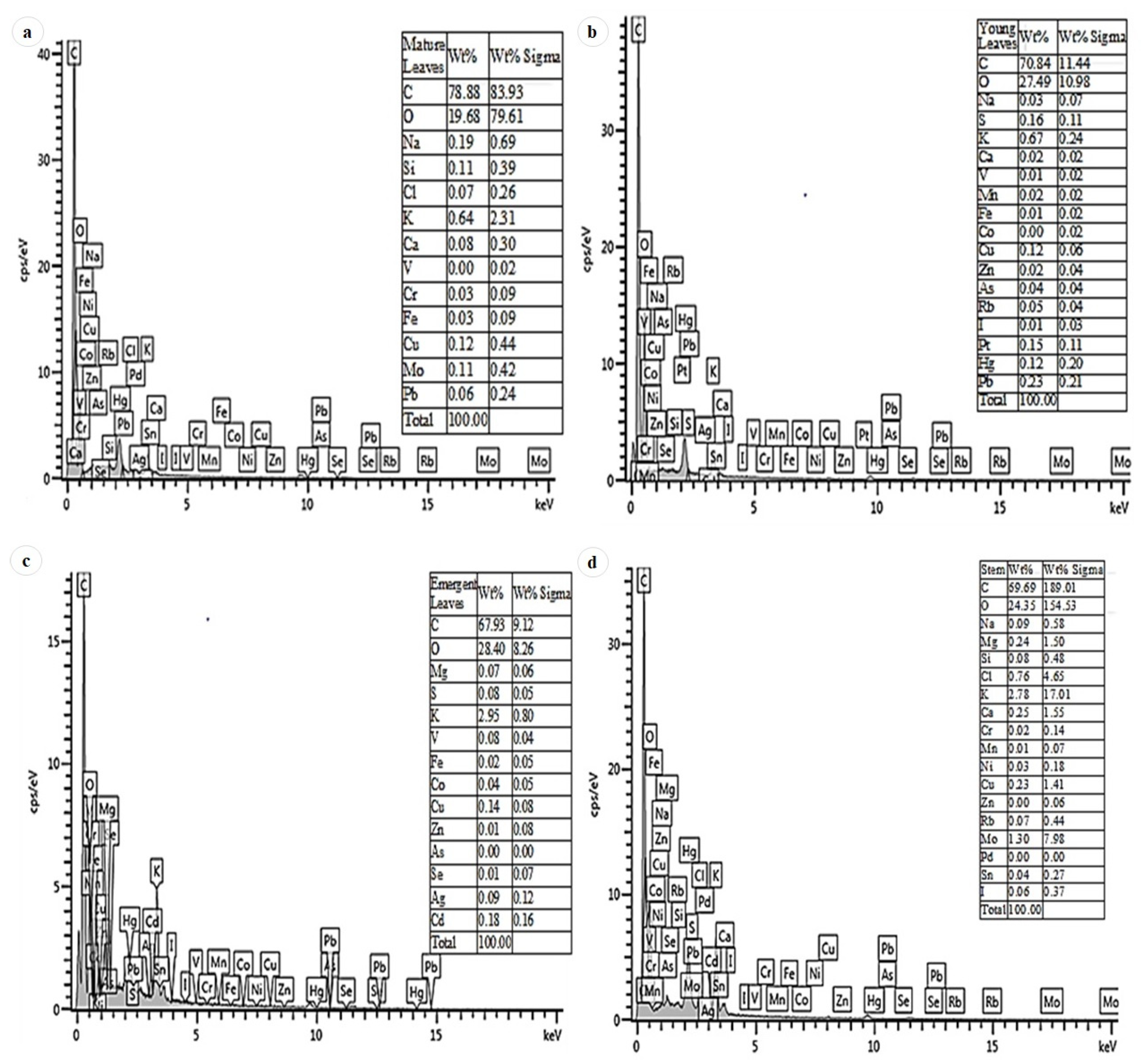
3.6. EDX
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mallavadhani, U.; Panda, A.K.; Rao, Y. Review article number 134 pharmacology and chemotaxonomy of diospyros. Phytochemistry 1998, 49, 901–951. [Google Scholar] [CrossRef]
- El-Hawary, S.S.E.-D.; Tadros, S.H.; Taha, H.; Abdelmohsen, M.; Nazif, N.M.; EL Sheikh, I.; El-Nasr, M.S. Biotechnological and chemical analysis of Egyptian Diospyros kaki L. cv. Costata grown in Egypt. Bull. Natl. Res. Cent. 2020, 44, 1–7. [Google Scholar] [CrossRef]
- Ganapaty, S.; Thomas, P.S.; Karagianis, G.; Waterman, P.G.; Brun, R. Antiprotozoal and cytotoxic naphthalene derivatives from Diospyros assimilis. Phytochemistry 2006, 67, 1950–1956. [Google Scholar] [CrossRef]
- Nematollahi, A.; Aminimoghadamfarouj, N.; Wiart, C. Reviews on 1, 4-naphthoquinones from Diospyros L. J. Asian Nat. Prod. Res. 2012, 14, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Lachenaud, O.; Schatz, G.E.; Dauby, G.; Stévart, T. Two new species of Diospyros (Ebenaceae) from Central Africa. Plant Ecol. Evol. 2017, 150, 217–224. [Google Scholar] [CrossRef]
- Cirera, J.; Da Silva, G.; Serrano, R.; Gomes, E.; Duarte, A.; Silva, O. Antimicrobial activity of Diospyros villosa root. Planta Med. 2010, 76, P454. [Google Scholar] [CrossRef]
- Cirera, J.; Da Silva, G.; Gomes, E.; Serrano, R.; Silva, O. Diospyros villosa root botanical identification. Planta Med. 2010, 76, P012. [Google Scholar] [CrossRef]
- Werker, E. Trichome diversity and development. Adv. Bot. Res 2000, 31, 1–35. [Google Scholar]
- Li, S.; Tosens, T.; Harley, P.C.; Jiang, Y.; Kanagendran, A.; Grosberg, M.; Jaamets, K.; Niinemets, Ü. Glandular trichomes as a barrier against atmospheric oxidative stress: Relationships with ozone uptake, leaf damage, and emission of LOX products across a diverse set of species. Plant Cell Environ. 2018, 41, 1263–1277. [Google Scholar] [CrossRef]
- Xiao, C.J.; Liu, Y.C.; Luo, S.H.; Hua, J.; Liu, Y.; Li, S.H. Localisation of two bioactive labdane diterpenoids in the peltate glandular trichomes of Leonurus japonicus by laser microdissection coupled with UPLC-MS/MS. Phytochem. Anal. 2017, 28, 404–409. [Google Scholar] [CrossRef]
- Glas, J.J.; Schimmel, B.C.; Alba, J.M.; Escobar-bravo, R.; Schuurink, R.C.; Kant, M.R. Plant glandular trichomes as targets for breeding or engineering of resistance to herbivores. Int. J. Mol. Sci. 2012, 13, 17077–17103. [Google Scholar] [CrossRef]
- Mahadevan, V. Report of the literature review committee. J. Am. Oil Chem. Soc. 1964, 41, 559–584. [Google Scholar] [CrossRef]
- Levy, R.S.; Mazia, D. Partial purification of renal alkaline phosphatase by electrophoresis on paper. Arch. Biochem. Biophys. 1953, 44, 280–283. [Google Scholar] [CrossRef]
- Demarco, D. Histochemical analysis of plant secretory structures. In Histochemistry of Single Molecules; Humana Press: New York, NY, USA, 2017; pp. 313–330. [Google Scholar]
- Shalini, S.; Sampathkumar, P. Phytochemical screening and antimicrobial activity of plant extracts for disease management. Int. J. Curr. Sci. 2012, 1, 209–218. [Google Scholar]
- Ascensão, L.; Pais, M. Glandular trichomes of Artemisia campestris (ssp. Maritima): Ontogeny and histochemistry of the secretory product. Bot. Gaz. 1987, 148, 221–227. [Google Scholar] [CrossRef]
- Flores-Félix, J.D.; Menéndez, E.; Marcos-garcía, M.; Celador-lera, L.; Rivas, R. Calcofluor white, an alternative to propidium iodide for plant tissues staining in studies of root colonization by fluorescent-tagged rhizobia. J. Adv. Biol. Biotechnol. 2015, 2, 65–70. [Google Scholar] [CrossRef]
- Marguí, E.; Queralt, I.; Hidalgo, M. Application of X-ray fluorescence spectrometry to determination and quantitation of metals in vegetal material. Trace Trends Anal. Chem. 2009, 28, 362–372. [Google Scholar] [CrossRef]
- Beenken, L. Redetermination of host plants reveals that the rust fungi Aecidium annonae, Aecidium chrysophaeum and Cerotelium xylopiae occur on Diospyros species (Ebenaceae) instead of Annonaceae. Phytotaxa 2017, 313, 249–258. [Google Scholar] [CrossRef]
- Wagner, G.J. Secreting glandular trichomes: More than just hairs. Plant Physiol. 1991, 96, 675–679. [Google Scholar] [CrossRef]
- Gonzáles, W.L.; Negritto, M.A.; Suarez, L.H.; Gianoli, E. Induction of glandular and non-glandular trichomes by damage in leaves of Madia sativa under contrasting water regimes. Acta Oecol. 2008, 33, 128–132. [Google Scholar] [CrossRef]
- Dutta Banik, D.; Benfey, E.D.; Martin, L.E.; Kay, K.E.; Loney, G.C.; Nelson, A.R.; Medler, K.F. A subset of broadly responsive type III taste cells contribute to the detection of bitter, sweet and umami stimuli. PLoS Genet. 2020, 16, e1008925. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-K.; Huang, B.-H.; Ho, S.-W.; Huang, M.-Y.; Wang, J.-C.; Gao, J.; Liao, P.-C. Molecular genetic and biochemical evidence for adaptive evolution of leaf abaxial epicuticular wax crystals in the genus Lithocarpus (Fagaceae). BMC Plant Biol. 2018, 18, 196. [Google Scholar] [CrossRef] [PubMed]
- Jou, Y.; Wang, Y.-L.; Yen, H.E. Vacuolar acidity, protein profile, and crystal composition of epidermal bladder cells of the halophyte Mesembryanthemum crystallinum. Funct. Plant Biol. 2007, 34, 353–359. [Google Scholar] [CrossRef]
- Gao, Z.; Gao, S.; Li, P.; Zhang, Y.; MA, B.; Wang, Y. Exogenous methyl jasmonate promotes salt stress-induced growth inhibition and prioritizes defense response of Nitraria tangutorum Bobr. Physiol. Plant. 2021, 172, 162–175. [Google Scholar] [CrossRef]
- Ghasemi-Omran, V.O.; Ghorbani, A.; Sajjadi-Otaghsara, S.A. Melatonin alleviates NaCl-induced damage by regulating ionic homeostasis, antioxidant system, redox homeostasis, and expression of steviol glycosides-related biosynthetic genes in in vitro cultured Stevia rebaudiana Bertoni. In Vitro Cell. Dev. Biol.-Plant 2021, 57, 319–331. [Google Scholar] [CrossRef]
- Nakata, P.A. An assessment of Engineered calcium oxalate crystal formation on plant growth and development as a step toward evaluating its use to enhance plant defense. PLoS ONE 2015, 10, e0141982. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Oliveira, R.S. Biotic Influences: Ecological Biochemistry: Allelopathy and Defense Against Herbivores. In Plant Physiological Ecology; Springer: Cham, Switzerland, 2019; pp. 541–581. [Google Scholar]
- Mhinana, Z.; Mayekiso, B.; Magwa, M.L. Anatomy and morphology of Nicotiana glauca with regard to its crystals characterization. Afr. J. Plant Sci. 2010, 4, 172–178. [Google Scholar]
- Navarro-Leon, E.; Ruiz, J.M.; Graham, N.; Blasco, B. Physiological profile of CAX1a tilling mutant of Brassica rapa exposed to different calcium doses. Plant Sci. 2018, 272, 164–172. [Google Scholar] [CrossRef]
- Kasim, N. The Micromorphological and Essential Oil Status of the Foliar Secretory Structures of Ocimum obovatum E. Mey. ex Benth. subsp. Obovatum (Lamiaceae). Ph.D. Thesis, University of KwaZulu-Natal, Durban, South Africa, 2011. [Google Scholar]
- Dai, X.; Wang, G.; Yang, D.S.; Tang, Y.; Broun, P.; Marks, M.D.; Sumner, L.W.; Dixon, R.A.; Zhao, P.X. Trichome: A comparative omics database for plant trichomes. Plant Physiol. 2010, 152, 44–54. [Google Scholar] [CrossRef]
- Hameed, I.; Hussain, F. Stomatal studies of some selected medicinal plants of family Solanaceae. J. Med. Plants Res. 2011, 5, 4525–4529. [Google Scholar]
- Soni, U.; Brar, S.; Gauttam, V.K. Effect of seasonal variation on secondary metabolites of medicinal plants. Int. J. Pharm. Sci. Res. 2015, 6, 3654–3662. [Google Scholar]
- Aslam, N.; Janbaz, K.H. Studies on antidiarrheal and laxative activities of aqueous-ethanol extract of Asphodelus tenuifolius and underlying mechanisms. BMC Complement. Altern. Med. 2019, 19, 307. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, D.; Bah, M.; Garduño, M.; Mendoza, S.; Serrano, V. Anti-inflammatory and antioxidant activities of methanol extracts and alkaloid fractions of four Mexican medicinal plants of Solanaceae. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 259–267. [Google Scholar] [CrossRef]
- Agu, K.C.; Okolie, P.N. Proximate composition, phytochemical analysis, and in vitro antioxidant potentials of extracts of Annona muricata (Soursop). Food Sci. Nutr. 2017, 5, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Ashihara, H.; Mizuno, K.; Yokota, T.; Crozier, A. Xanthine Alkaloids: Occurrence, Biosynthesis, and Function in Plants. In Progress in the Chemistry of Organic Natural Products; Springer: Cham, Switzerland, 2017; Volume 105, pp. 1–88. [Google Scholar]
- Kortbeek, R.W.; Van Der Gragt, M.; Bleeker, P.M. Endogenous plant metabolites against insects. Eur. J. Plant Pathol. 2019, 154, 67–90. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Junior, M.R.M. Phenolic compounds: Structure, classification, and antioxidant power. In Bioactive Compounds: Health Benefits and Potential Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–50. [Google Scholar]
- Lee, J.Y.; Aguilar, L.E.; Park, C.H.; Kim, C.S. UV light assisted coating method of polyphenol caffeic acid and mediated immobilization of metallic silver particles for antibacterial implant surface modification. Polymers 2019, 11, 1200. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]



| Compounds | Stains | Leaves/Stem | Trichomes | Reactions Observed |
|---|---|---|---|---|
| Alkaloids | Dittmar’s | + | + | Brownish colouration in the stem as well as the trichomes |
| Lipids | Sudan III and IV | + | + | Cells in the leaf and stem sections stained black, the trichomes stained black as well |
| Nile Blue | + | + | Black colouration in the leaf sections and non-glandular trichomes | |
| Phenols | Ferric trichloride | + | + | Brown deposits on the cells of the leaf sections, the non-glandular cells further stained brown |
| Acidic Polysaccharides | Ruthenium red | + | + | Leaf and non-glandular trichomes stained purplish red |
| Total protein | Mercuric bromophenol blue | + | + | Stem cells and non-glandular trichomes stained purple |
| Polyphenols (lignin, tannins) | Toluidine blue | + | + | Leaf cells and non-glandular trichomes stained brown |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adu, O.T.; Naidoo, Y.; Adu, T.S.; Sivaram, V.; Dewir, Y.H.; Rihan, H. Micromorphology and Histology of the Secretory Apparatus of Diospyros villosa (L.) de Winter Leaves and Stem Bark. Plants 2022, 11, 2498. https://doi.org/10.3390/plants11192498
Adu OT, Naidoo Y, Adu TS, Sivaram V, Dewir YH, Rihan H. Micromorphology and Histology of the Secretory Apparatus of Diospyros villosa (L.) de Winter Leaves and Stem Bark. Plants. 2022; 11(19):2498. https://doi.org/10.3390/plants11192498
Chicago/Turabian StyleAdu, Oluwatosin Temilade, Yougasphree Naidoo, Temitope Samson Adu, Venkataramegowda Sivaram, Yaser Hassan Dewir, and Hail Rihan. 2022. "Micromorphology and Histology of the Secretory Apparatus of Diospyros villosa (L.) de Winter Leaves and Stem Bark" Plants 11, no. 19: 2498. https://doi.org/10.3390/plants11192498
APA StyleAdu, O. T., Naidoo, Y., Adu, T. S., Sivaram, V., Dewir, Y. H., & Rihan, H. (2022). Micromorphology and Histology of the Secretory Apparatus of Diospyros villosa (L.) de Winter Leaves and Stem Bark. Plants, 11(19), 2498. https://doi.org/10.3390/plants11192498







