Identification of Soil Properties Associated with the Incidence of Banana Wilt Using Supervised Methods
Abstract
:1. Introduction
2. Results
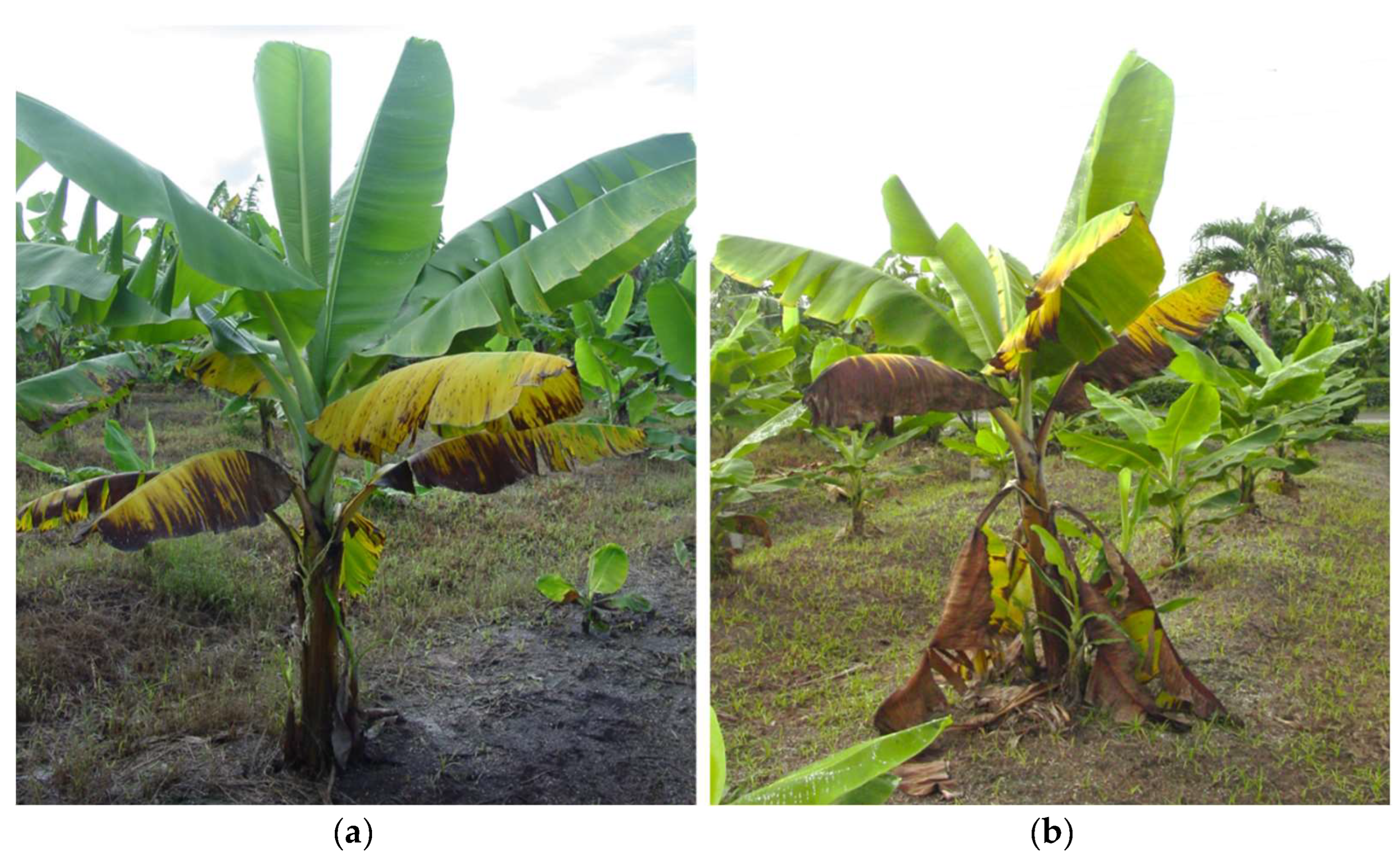
2.1. Incidence of BW in Experimental Lots
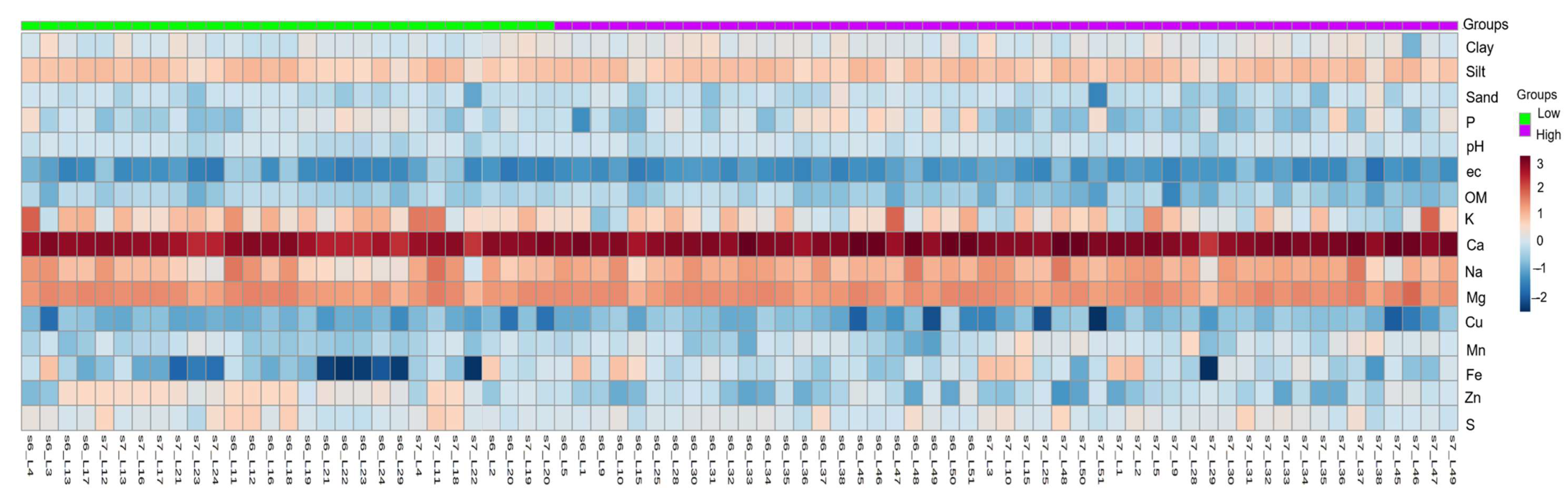
2.2. Description of Soil Properties in Experimental Lots
2.3. Wilcoxon Rank Test
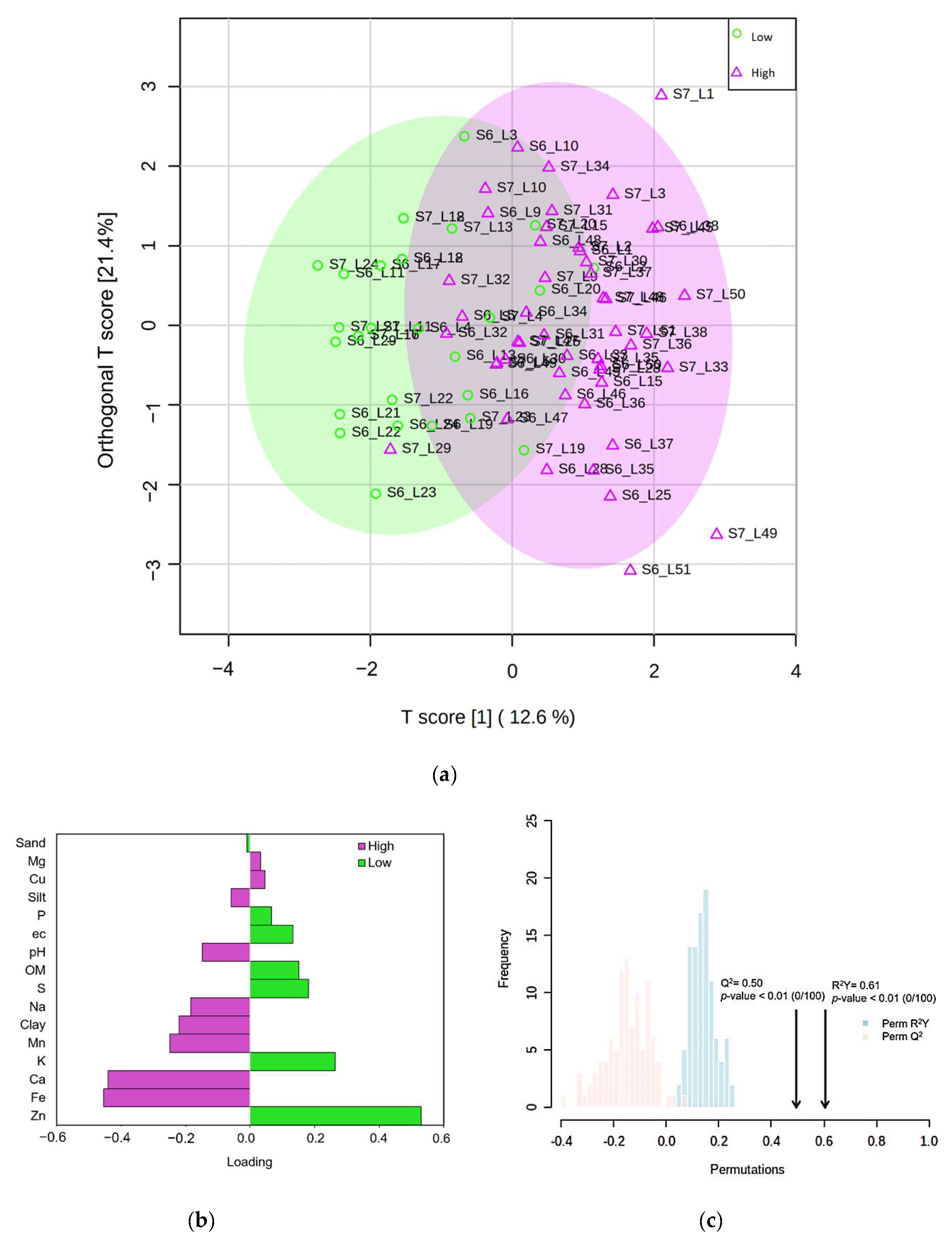
2.4. Identification of Important Soil Variables
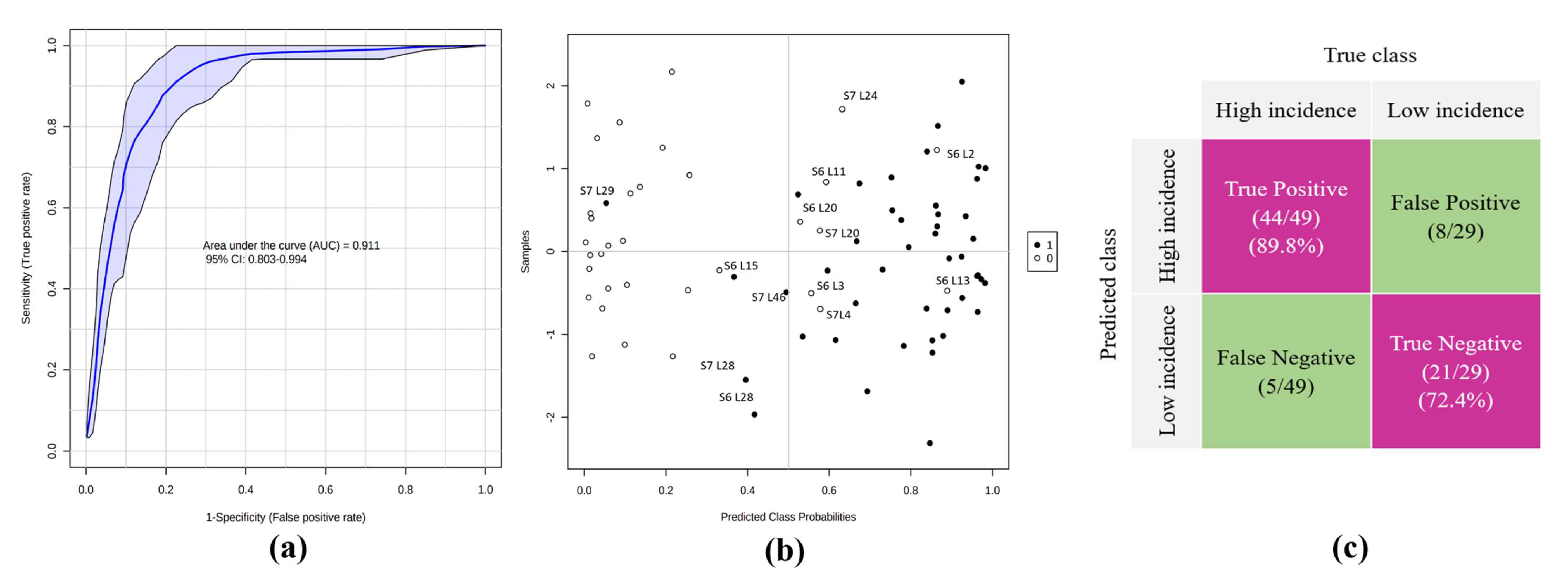
2.5. Classifier Performance and Accuracy Assessment
3. Discussion
4. Materials and Methods
4.1. Study Area
4.2. Soil Sampling
4.3. Banana Wilt Incidence
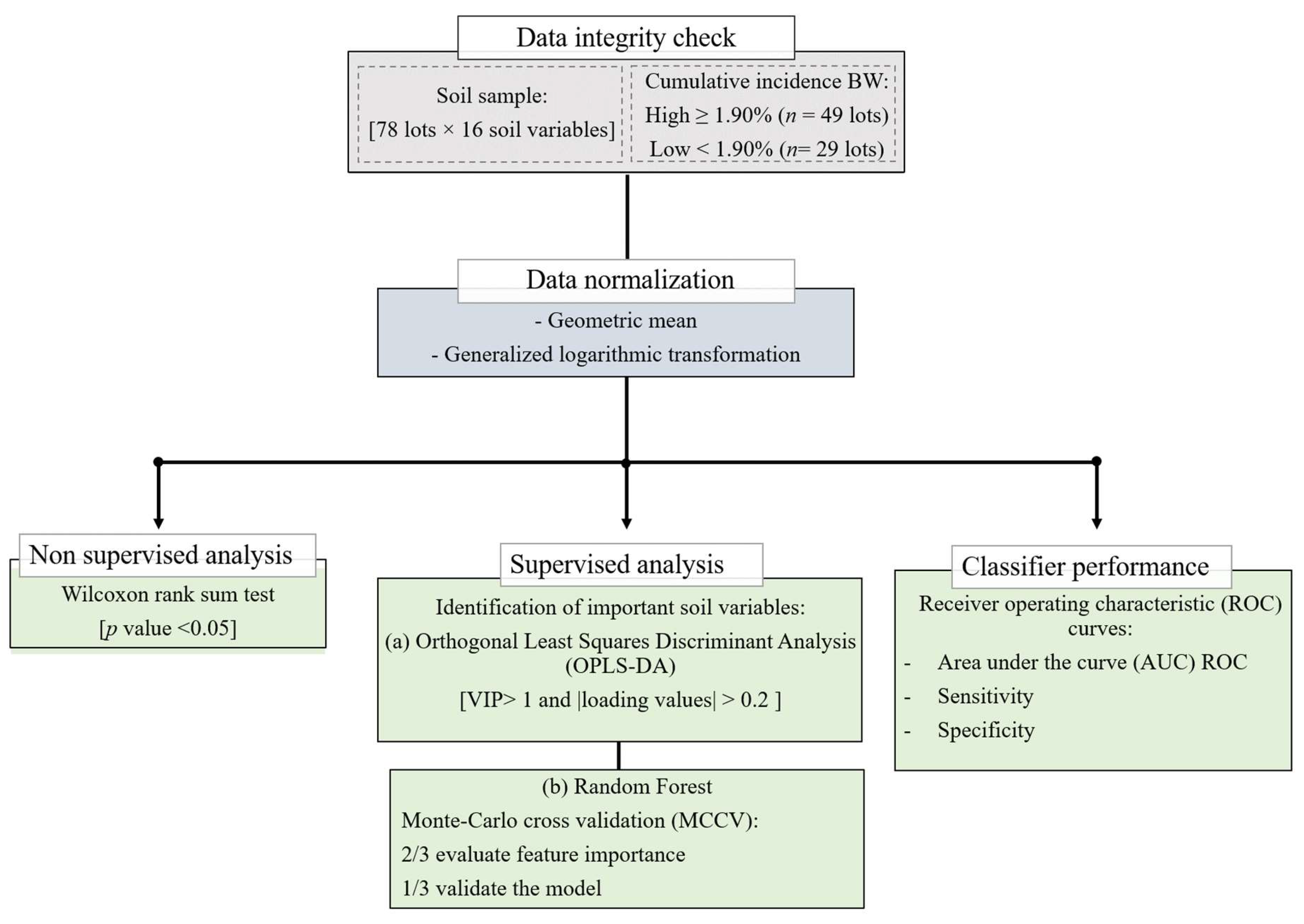
4.4. Data Analysis
4.4.1. Identification of Important Soil Variables
4.4.2. Classifier Performance and Accuracy Assessment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT Database. Food and Agriculture Organization Statistic. Available online: https://bit.ly/34z8QN8 (accessed on 14 March 2022).
- Deacon, J.W.; Herbert, J.A.; Dames, J. Fals Panama disorder of bananas. Citrus Subtrop Fruit Inst. Inf. Bul. 1985, 149, 15–18. [Google Scholar]
- Rey, J.C.; Martínez, G.; Pizzo, N.; Micale, E.; Fernández, N. Susceptible areas to false Panama disease in Cavendish bananas, in the Central Region of Venezuela. In Proceedings of the Reunión Internacional ACORBAT, Miami, FL, USA, 19–22 April 2016. [Google Scholar]
- Domínguez, J.; Negrin, M.A.; Rodriguez, C.M. Soil potassium indices and clay-sized particles affecting banana-wilt expression caused by soil fungus in banana plantation development on transported volcanic soils. Commun. Soil Sci. Plant Anal. 2008, 39, 397–412. [Google Scholar] [CrossRef]
- Dita, M.; Barquero, M.; Heck, D.; Mizubuti, E.S.G.; Staver, C.P. Fusarium wilt of banana: Current knowledge on epidemiology and research needs toward sustainable disease management. Front. Plant Sci. 2018, 9, 1468. [Google Scholar] [CrossRef] [Green Version]
- Olivares, B.; Rey, J.C.; Lobo, D.; Navas-Cortés, J.A.; Gómez, J.A.; Landa, B.B. Fusarium Wilt of Bananas: A review of agro-environmental factors in the Venezuelan production system affecting its development. Agronomy 2021, 11, 986. [Google Scholar] [CrossRef]
- Martínez, G.E.; Rey-Brina, J.C.; Rodríguez, D.; Jiménez, C.; Rodríguez, Y.; Rumbos, R.; Pargas-Pichardo, R.; Martínez, E. Analysis of the current phytopathological situation of edible Musaceae in Venezuela. Agron. Trop. 2020, 70, e4323273. [Google Scholar] [CrossRef]
- Rey, J.C.; Martínez-Solórzano, G.; Ramírez, H.; Pargas-Pichardo, R. Relationship of the agroecological conditions of a plot of lacustrine plain with the wilting of Cavendish banana in Aragua, Venezuela. Agron. Trop. 2020, 70, 1–12. [Google Scholar] [CrossRef]
- Olivares, B.; Paredes, F.; Rey, J.; Lobo, D.; Galvis-Causil, S. The relationship between the normalized difference vegetation index, rainfall, and potential evapotranspiration in a banana plantation of Venezuela. STJSSA—J. Soil Sci. Agroclimatol. 2021, 18, 58–64. [Google Scholar] [CrossRef]
- Hou, J.C.; Hu, Y.H.; Hou, L.X.; Guo, K.Q.; Satake, T. Classification of ripening stages of bananas based on support vector machine. Int. J. Agric. Biol. Eng. 2015, 8, 99–103. [Google Scholar] [CrossRef]
- Ye, H.C.; Huang, W.J.; Huang, S.Y.; Cui, B.; Dong, Y.Y.; Guo, A.T.; Ren, Y.; Jin, Y. Identification of banana fusarium wilt using supervised classification algorithms with UAV-based multi-spectral imagery. Int. J. Agric. Biol. 2020, 13, 136–142. [Google Scholar] [CrossRef]
- Gomez-Selvaraj, M.; Vergara, A.; Montenegro, F.; Alonso Ruiz, H.; Safari, N.; Raymaekers, D.; Ocimati, W.; Ntamwira, J.; Tits, L.; Omondi, A.B.; et al. Detection of banana plants and their major diseases through aerial images and machine learning methods: A case study in DR Congo and Republic of Benin. ISPRS J. Photogramm. Remote Sens. 2020, 169, 110–124. [Google Scholar] [CrossRef]
- Yuan, J.; Wen, T.; Zhang, H.; Zhao, M.; Penton, C.R.; Thomashow, L.S.; Shen, Q. Predicting disease occurrence with high accuracy based on soil macroecological patterns of Fusarium wilt. ISME J. 2020, 14, 2936–2950. [Google Scholar] [CrossRef] [PubMed]
- Sabadell, S. Etiología y Epidemiologia del Falso mal de Panamá en Canarias. Ph.D. Thesis, Universidad Autónoma de Barcelona, Tenerife, Spain, 2003. Available online: https://bit.ly/3nBp3cx (accessed on 1 August 2022).
- Beer, Z.; Hernández, J.; Sabadell, S. False Panama disease in banana. Musa Dis. 2001, 9, 1–4. Available online: https://bit.ly/386KkUS (accessed on 1 August 2022).
- Lichtemberg, P.S.; Pocasangre, L.E.; Sikora, R.A.; Staver, C. Current status of Fusarium Wilt (Fusarium oxysporum f. sp. cubense) in bananas Gros Michel (AAA) at smallholder level of Costa Rica. In Proceedings of the XIX Reunión Internacional ACORBAT, Medellín, Colombia, 8–12 November 2010; Available online: https://n9.cl/kzu6y (accessed on 1 August 2022).
- Merchán, V.M. Manejo Integrado de plagas del plátano y banano. In Proceedings of the ACORBAT. Asociación de Bananeros de Colombia AUGURA, Medellín, Colombia; 2002; pp. 354–361. [Google Scholar]
- Hermanto, C.; Sutanto, A.; Jumjunidang, H.S.; Daniells, J.W.; O’Neill, W.T.; Sinohin, V.G.O.; Molina, A.B.; Taylor, P. Incidence and distribution of fusarium wilt disease of banana in Indonesia. Acta Hortic. 2011, 897, 313–322. [Google Scholar] [CrossRef]
- Martínez, G.; Rey, J.C.; Castro, L.; Micale, E.; López, O.; Pargas, R.; Manzanilla, E. Cavendish banana wilt in the Central Region of Venezuela associated with a fungus-bacteria complex. In Proceedings of the Reunión Internacional ACORBAT, Miami, FL, USA, 19–22 April 2016. [Google Scholar]
- Ramírez, H.; Rey, J.C.; Martinez, G. Relationship between the incidence of banana wilt and properties of a lacustrine soil. In Proceedings of the Reunión Internacional ACORBAT, Miami, FL, USA, 19–22 April 2016. [Google Scholar]
- Gutiérrez-Boem, F.H. Micronutrientes. In Manejo de la Fertilidad del Suelo en Planteos Orgánicos; Carina, R., Álvarez, C.R., Rimski-Korsakov, H., Eds.; Editorial Facultad de Agronomía, Universidad de Buenos Aires: Buenos Aires, Argentina, 2016; pp. 106–113. [Google Scholar]
- Borges-Pérez, A.F.; Bravo, J.J.; Perez, J.F.; Lopez, I. Enhanced resistance of banana plants (Dwarf Cavendish) to Fusarium oxysporum f.sp. cubense by controlled Zn nutrition under field conditions. Banan. Newsl. 1991, 14, 24–26. [Google Scholar]
- Dorel, M. Banana development in an andosol in Guadeloupe: Effect of soil compaction. Fruits 1993, 48, 83–88. Available online: https://n9.cl/vahwkz (accessed on 1 August 2022).
- Deltour, P.; Franca, S.C.; Pereira, O.L.; Cardoso, I.; De Neve, S.; Debode, J.; Hofte, M. Disease suppressiveness to Fusarium wilt of banana in an agroforestry system: Influence of soil characteristics and plant community. Agric. Ecosyst. Environ. 2017, 239, 173–181. [Google Scholar] [CrossRef]
- Lahav, E.; Israeli, Y. Mineral deficiencies of banana. In Diseases of Banana; Jones, D., Ed.; CAB International Publishing: Wallingford, UK, 2019; pp. 475–486. [Google Scholar] [CrossRef]
- Owomugisha, G.; Quinn, J.; Mwebaze, E.; Lwasa, J. Automated Vision-Based Diagnosis of Banana Bacterial Wilt Disease and Black Sigatoka Disease. In Proceedings of the 1rst International Conference on the use of Mobile Information and Communication Technology (ICT) in Africa, Stellembosh, South Africa, 9–10 December 2014; Available online: https://n9.cl/6bmyj (accessed on 1 August 2022).
- Ma, L.; Fu, T.; Blaschke, T.; Li, M.; Tiede, D.; Zhou, Z.; Ma, X.; Chen, D. Evaluation of feature selection methods for object-based land cover mapping of unmanned aerial vehicle imagery using Random Forest and Support Vector Machine classifiers. ISPRS Int. J. Geo-Inf. 2017, 6, 51. [Google Scholar] [CrossRef]
- Sangeetha, T.; Lavanya, G.; Jeyabharathi, D.; Rajesh Kumar, T.; Mythili, K. Detection of pest and disease in banana leaf using convolution Random Forest. Test Eng. Manag. 2020, 83, 3727–3735. Available online: https://n9.cl/mevdug (accessed on 1 August 2022).
- Vipinadas, M.J.; Thamizharasi, A. Banana leaf disease identification technique. Int. J. Adv. Eng. Res. Sci. 2016, 3, 120–124. [Google Scholar]
- Aruraj, A.; Alex, A.; Subathra, M.S.P.; Sairamya, N.J.; George, S.T.; Ewards, S.E.V. Detection and Classification of Diseases of Banana Plant Using Local Binary Pattern and Support Vector Machine. In Proceedings of the 2nd International Conference on Signal Processing and Communication (ICSPC), Coimbatore, India, 29–30 March 2019. [Google Scholar] [CrossRef]
- Olivares, B.O.; Araya-Alman, M.; Acevedo-Opazo, C.; Rey, J.C.; Cañete-Salinas, P.; Kurina, F.G.; Balzarini, M.; Lobo, D.; Navas- Cortés, J.A.; Landa, B.B.; et al. Relationship between soil properties and banana productivity in the two main cultivation areas in Venezuela. J. Soil Sci. Plant Nutr. 2020, 20, 2512–2524. [Google Scholar] [CrossRef]
- Olivares, B.O. Tropical rainfall conditions in rainfed agriculture in Carabobo, Venezuela. LA GRANJA Revista Ciencias Vida 2018, 27, 86–102. [Google Scholar] [CrossRef] [Green Version]
- Delgado, E.; Trejos, J.; Villalobos, M.; Martínez, G.; Lobo, D.; Carlos Rey, J.; Rodríguez, G.; Rosales, F.E.; Pocasangre, L.E. Determination of a soil quality and health index for banana plantations in Venezuela. Interciencia 2010, 35, 927–933. [Google Scholar]
- Olivares, B.; Calero, J.; Rey, J.C.; Lobo, D.; Landa, B.B.; Gómez, J.A. Correlation of banana productivity levels and soil morphological properties using Regularized Optimal Scaling Regression. Catena 2022, 208, 105718. [Google Scholar] [CrossRef]
- Lozano, P.Z.; Bravo, C.; Ovalles, F.; Hernández, R.M.; Moreno, B.; Piñango, L.; Villanueva, J.G. Selección de un diseño de muestreo en parcelas experimentales a partir del estudio de la variabilidad espacial de los suelos. Bioagro 2004, 16, 61–72. Available online: https://n9.cl/oxb45 (accessed on 1 August 2022).
- Gee, G.W.; Or, D. Particle-Size Analysis. In Methods of Soil Analysis: Part 4 Physical Methods; Dane, J.H., Clarke Topp, G., Eds.; Soil Science Society of America: Madison, WI, USA, 2002; pp. 255–293. [Google Scholar] [CrossRef]
- Soil Survey Staff. Soil Survey Manual Hadbook 18; Publisher United States Department of Agriculture: Washington, DC, USA, 2017; pp. 154–196. [Google Scholar]
- Heanes, D.L. Determination of total organic-C in soils by an improved chromic acid digestion and spectrophotometric procedure. Commun. Soil Sci. Plant Anal. 1984, 15, 1191–1213. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Bosman, M. Role of the Environment on the Incidence of Panama Disease in Bananas. Master’s Thesis, Soil Geography and Landscape, Wageningen University, Wageningen, The Netherlands, 7 August 2016. Available online: https://edepot.wur.nl/390872 (accessed on 1 August 2022).
- Macchiavelli, R.E. Non-linear mixed models for disease incidence and severity: Modeling plant diseases in tropical crops. In Proceedings of the 59th World Statistics Congress of the International Statistical Institute, Hong Kong, China, 25–30 August 2013. [Google Scholar]
- Rocha, A.D.J.; Ferreira, M.d.S.; Rocha, L.d.S. Interaction between Fusarium oxysporum f. sp. cubense and Radopholus similis can lead to changes in the resistance of banana cultivars to Fusarium wilt. Eur. J. Plant Pathol. 2020, 158, 403–417. [Google Scholar] [CrossRef]
- Bornmann, L.; Leydesdorff, L.; Mutz, R. The use of percentiles and percentile rank classes in the analysis of bibliometric data: Opportunities and limits. J. Informetr. 2013, 7, 158–165. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, C.; Cheng, S.; Li, G.; Griebel, J.; Neuhaus, J. Novel Metabolic Signatures of Prostate Cancer Revealed by 1H-NMR Metabolomics of Urine. Diagnostics 2021, 11, 149. [Google Scholar] [CrossRef]
- Bylesjö, M.; Rantalainen, M.; Cloarec, O.; Nicholson, J.K.; Holmes, E.; Trygg, J. OPLS discriminant analysis: Combining the strengths of PLS-DA and SIMCA classification. J. Chemom. 2006, 20, 341–351. [Google Scholar] [CrossRef]
- Szymańska, E.; Saccenti, E.; Smilde, A.K.; Westerhuis, J.A. Double-check: Validation of diagnostic statistics for PLS-DA models in metabolomics studies. Metabolomics 2012, 8, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Triba, M.N.; Le Moyec, L.; Amathieu, R.; Goossens, C.; Bouchemal, N.; Nahon, P.; Rutledge, D.N.; Savarin, P. PLS/OPLS models in metabolomics: The impact of permutation of dataset rows on the K-fold cross-validation quality parameters. Mol. Biosyst. 2015, 11, 13–19. [Google Scholar] [CrossRef]
- Breiman, L.; Last, M.; Rice, J. Random forests: Finding quasars. In Statistical Challenges in Astronomy; Feigelson, E., Jogesh, G., Eds.; Springer: New York, NY, USA, 2003; pp. 243–254. [Google Scholar] [CrossRef]
- Xu, Q.S.; Liang, Y.Z. Monte Carlo cross validation. Chemom. Intell. Lab. Syst. 2001, 56, 1–11. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for comprehensive metabolomics data analysis. Curr. Protoc. Bioinform. 2016, 55, 14.10.1–14.10.91. [Google Scholar] [CrossRef] [PubMed]
- Paraskevaidi, M.; Cameron, S.J.; Whelan, E.; Bowden, S.; Tzafetas, M.; Mitra, A.; Semertzidou, A.; Athanasiou, A.; Bennett, P.R.; MacIntyre, D.A.; et al. Laser-assisted rapid evaporative ionisation mass spectrometry (LA-REIMS) as a metabolomics platform in cervical cancer screening. EBioMedicine 2020, 60, 103017. [Google Scholar] [CrossRef]
- Garosi, Y.; Sheklabadi, M.; Conoscenti, C.; Pourghasemi, H.R.; Van Oost, K. Assessing the performance of GIS- based machine learning models with different accuracy measures for determining susceptibility to gully erosion. Sci. Total Environ. 2019, 664, 1117–1132. [Google Scholar] [CrossRef]

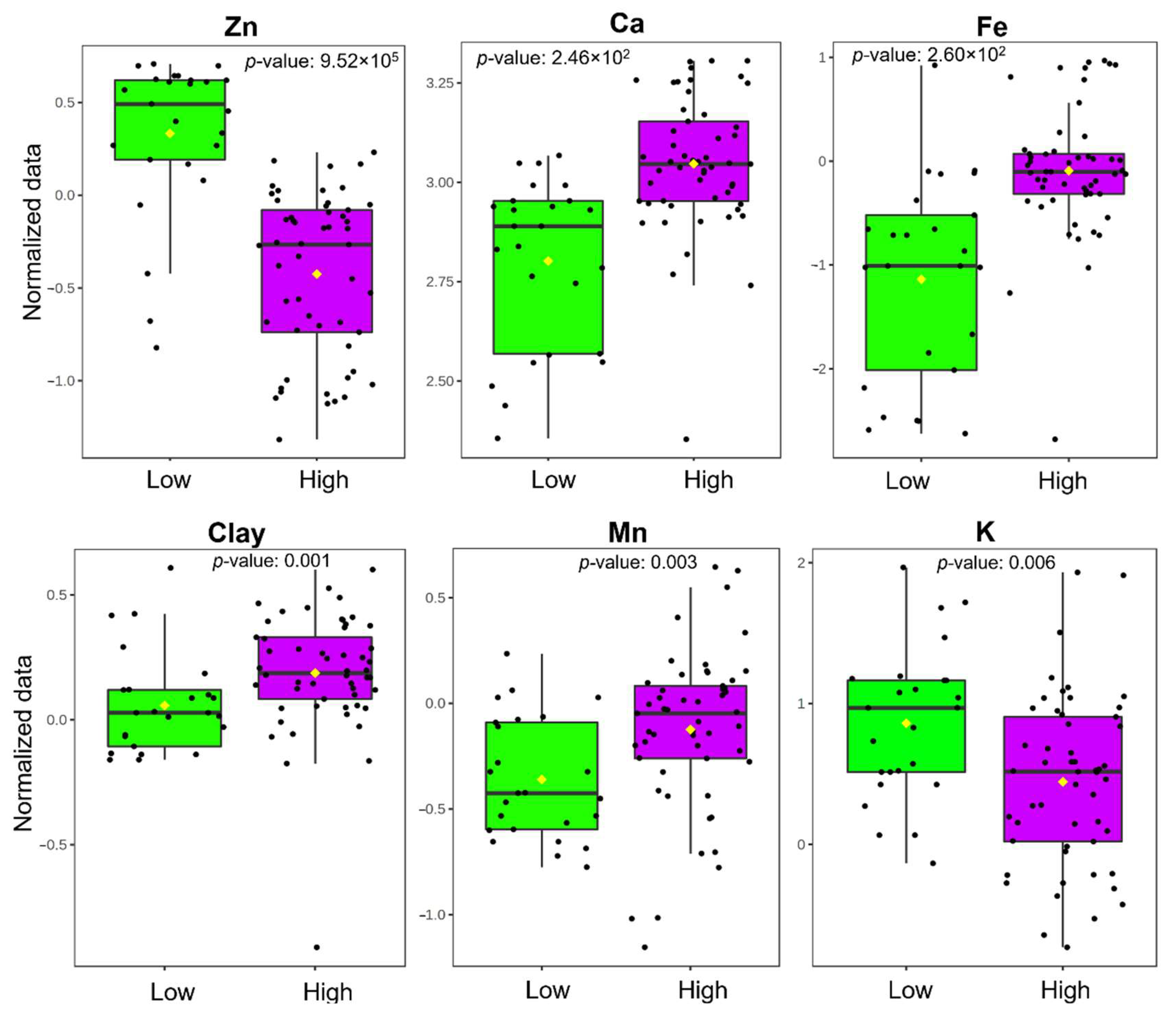

| Variable | V | p Value | −log10(p) | FDR |
|---|---|---|---|---|
| Zn | 1199 | 9.52 × 105 | 80.21 | 1.52 × 103 |
| Ca | 222 | 2.46 × 102 | 56.08 | 1.38 × 101 |
| Fe | 223 | 2.60 × 102 | 55.85 | 1.38 × 101 |
| Clay | 357 | 0.001 | 29.61 | 0.004 |
| Mn | 386 | 0.003 | 25.05 | 0.009 |
| K | 921 | 0.005 | 22.41 | 0.015 |
| Variable | Mean ± SD | Median | CV (%) | Min | Max | VIP |
|---|---|---|---|---|---|---|
| Clay (%) | 16.10 ± 7.86 | 15.00 | 48.78 | 1.00 | 40.00 | 0.36 |
| Silt (%) | 76.82 ± 9.75 | 78.30 | 12.70 | 39.93 | 90.84 | 0.08 |
| Sand (%) | 7.08 ± 4.98 | 6.27 | 70.33 | 0.38 | 38.07 | 0.01 |
| pH | 7.86 ± 0.22 | 7.85 | 2.74 | 7.41 | 8.55 | 0.15 |
| EC (dS m−1) | 0.65 ± 0.53 | 0.45 | 82.51 | 0.21 | 2.58 | 0.28 |
| OM (%) | 3.39 ± 1.52 | 3.50 | 44.86 | 0.25 | 6.33 | 0.32 |
| P (mg kg−1) | 12.98 ± 15.48 | 5.67 | 119.32 | 0.35 | 54.97 | 0.24 |
| K (mg kg−1) | 110.57 ± 229.26 | 35.60 | 207.34 | 1.48 | 1336.00 | 1.16 |
| Ca (mg kg−1) | 9704.51 ± 2968.46 | 8892.00 | 30.59 | 4936.00 | 16,648.00 | 0.68 |
| Na (mg kg−1) | 152.80 ± 97.07 | 132.40 | 62.33 | 10.72 | 472.00 | 0.55 |
| Mg (mg kg−1) | 300.47 ± 55.34 | 296.00 | 18.65 | 216.00 | 640.00 | 0.06 |
| Cu (mg kg−1) | 1.41 ± 0.87 | 1.60 | 61.20 | 0.03 | 3.20 | 0.15 |
| Mn (mg kg−1) | 9.47 ± 10.81 | 5.60 | 114.12 | 0.80 | 58.40 | 0.66 |
| Fe (mg kg−1) | 13.39 ± 22.59 | 5.20 | 168.72 | 0.04 | 78.40 | 2.91 |
| Zn (mg kg−1) | 13.21 ± 13.28 | 7.60 | 100.57 | 0.36 | 36.80 | 2.11 |
| S (mg kg−1) | 17.09 ± 11.86 | 11.84 | 69.43 | 6.47 | 48.80 | 0.34 |
| Variables | Frequencies of Being Selected (%) | Mean Decrease Accuracy | Low Incidence Group (n = 29) | High Incidence Group (n = 49) | ||
|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | |||
| Zn (mg kg−1) | 1.00 | 0.18 | 26.03 ± 12.98 | (1.60–38.00) | 5.66 ± 5.41 | (0.36–30.40) |
| Fe (mg kg−1) | 1.00 | 0.05 | 7.04 ± 16.84 | (0.04–69.60) | 17.14 ± 24.79 | (0.06–78.40) |
| Ca (mg kg−1) | 0.97 | 0.04 | 7326.34 ± 1675.99 | (4936.00–11,496.00) | 11,111.18 ± 2657.83 | (6472.0–16,648.0) |
| Clay (%) | 0.88 | 0.01 | 15.66 ± 8.70 | (5.00–31.00) | 16.37 ± 7.39 | (1.00–40.00) |
| K (mg kg−1) | 0.65 | 0.01 | 142.20 ± 199.53 | (5.60–984.00) | 91.83 ± 245.22 | (1.50–1336.0) |
| Mn (mg kg−1) | 0.50 | 0.01 | 6.38 ± 6.19 | (1.60–33.60) | 11.30 ± 12.49 | (0.80–58.40) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivares, B.O.; Vega, A.; Calderón, M.A.R.; Rey, J.C.; Lobo, D.; Gómez, J.A.; Landa, B.B. Identification of Soil Properties Associated with the Incidence of Banana Wilt Using Supervised Methods. Plants 2022, 11, 2070. https://doi.org/10.3390/plants11152070
Olivares BO, Vega A, Calderón MAR, Rey JC, Lobo D, Gómez JA, Landa BB. Identification of Soil Properties Associated with the Incidence of Banana Wilt Using Supervised Methods. Plants. 2022; 11(15):2070. https://doi.org/10.3390/plants11152070
Chicago/Turabian StyleOlivares, Barlin O., Andrés Vega, María A. Rueda Calderón, Juan C. Rey, Deyanira Lobo, José A. Gómez, and Blanca B. Landa. 2022. "Identification of Soil Properties Associated with the Incidence of Banana Wilt Using Supervised Methods" Plants 11, no. 15: 2070. https://doi.org/10.3390/plants11152070
APA StyleOlivares, B. O., Vega, A., Calderón, M. A. R., Rey, J. C., Lobo, D., Gómez, J. A., & Landa, B. B. (2022). Identification of Soil Properties Associated with the Incidence of Banana Wilt Using Supervised Methods. Plants, 11(15), 2070. https://doi.org/10.3390/plants11152070











