Synergism in Antiplasmodial Activities of Artemether and Lumefantrine in Combination with Securidaca longipedunculata Fresen (Polygalaceae)
Abstract
1. Introduction
2. Results
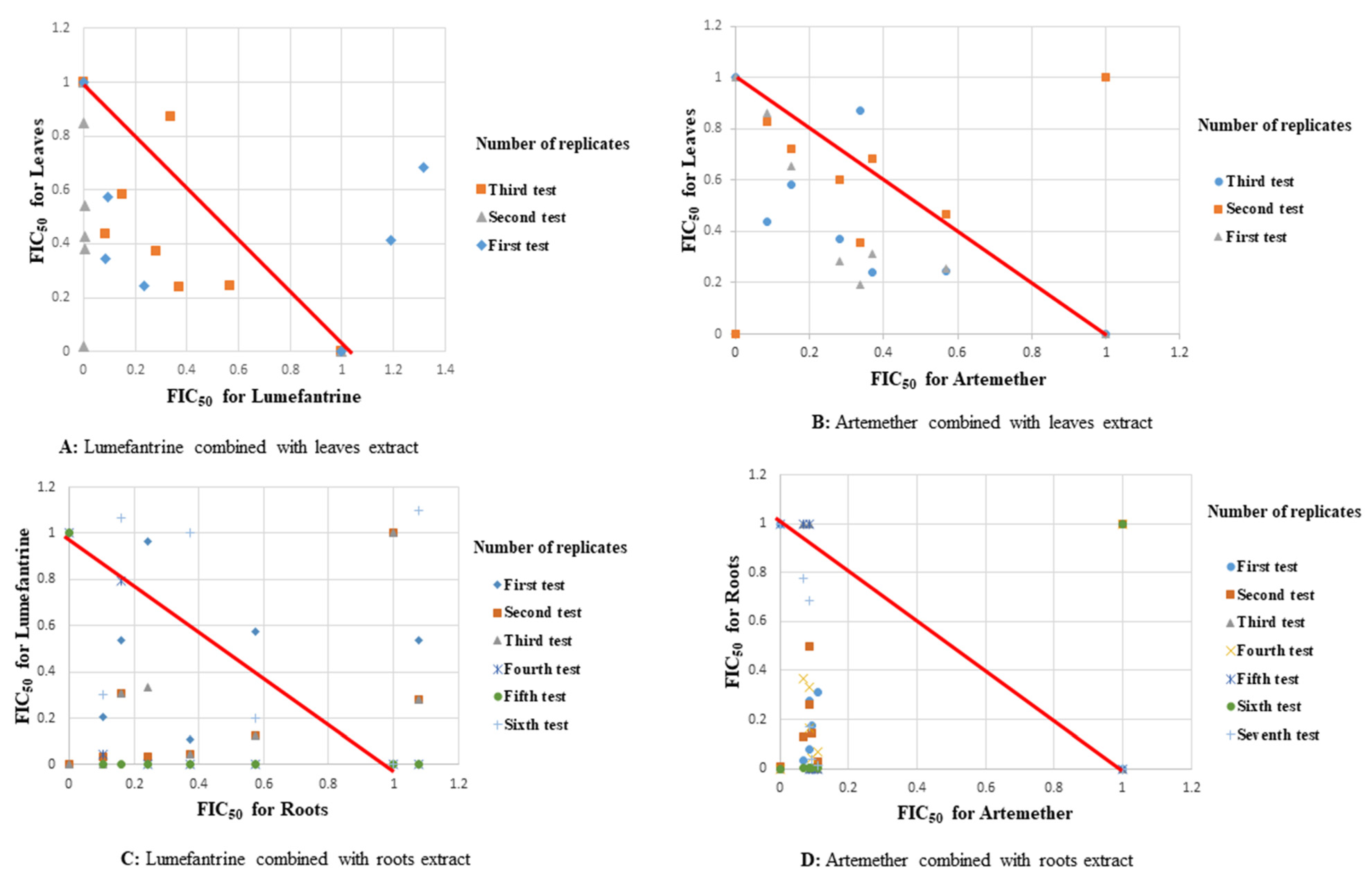
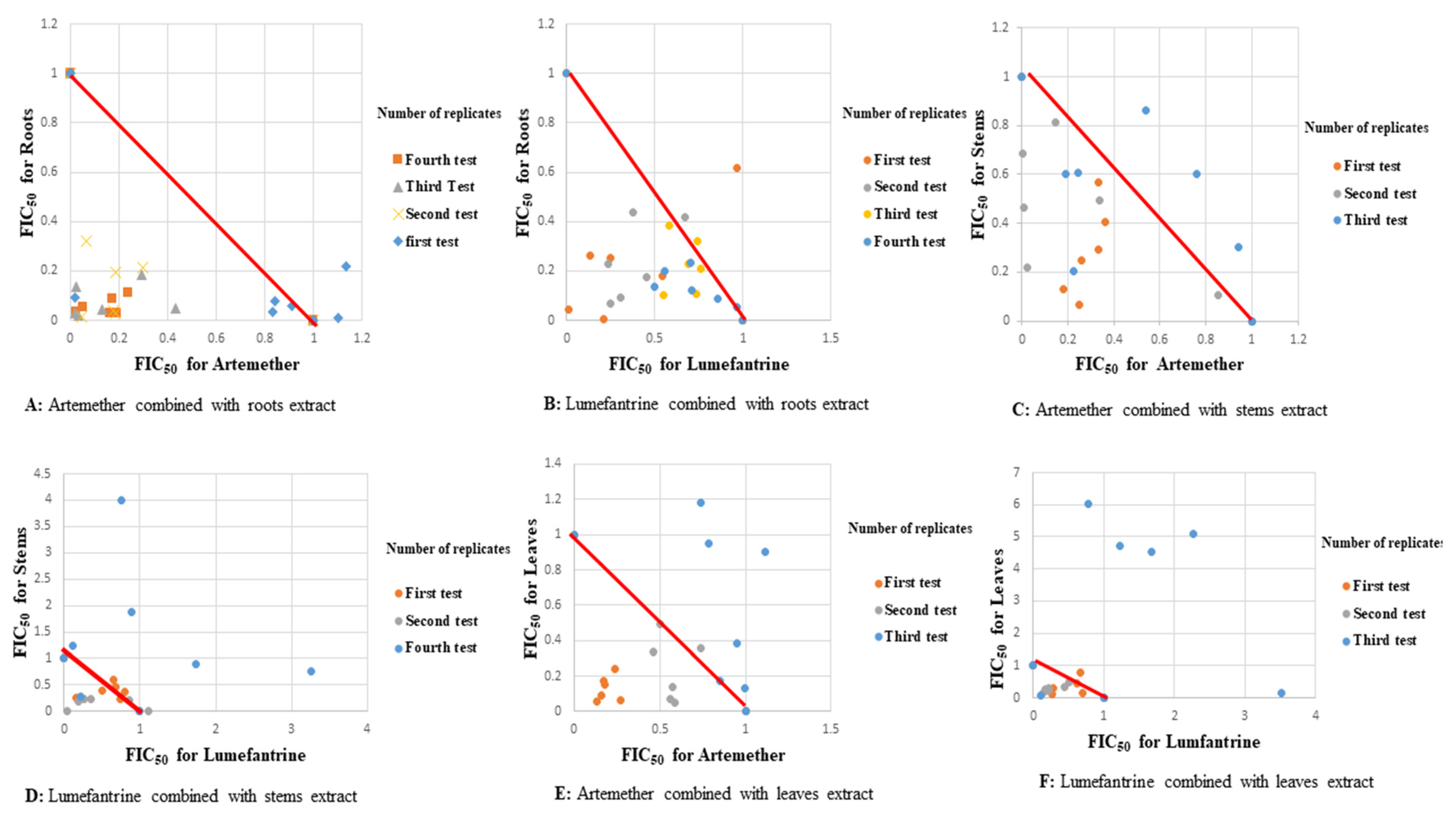
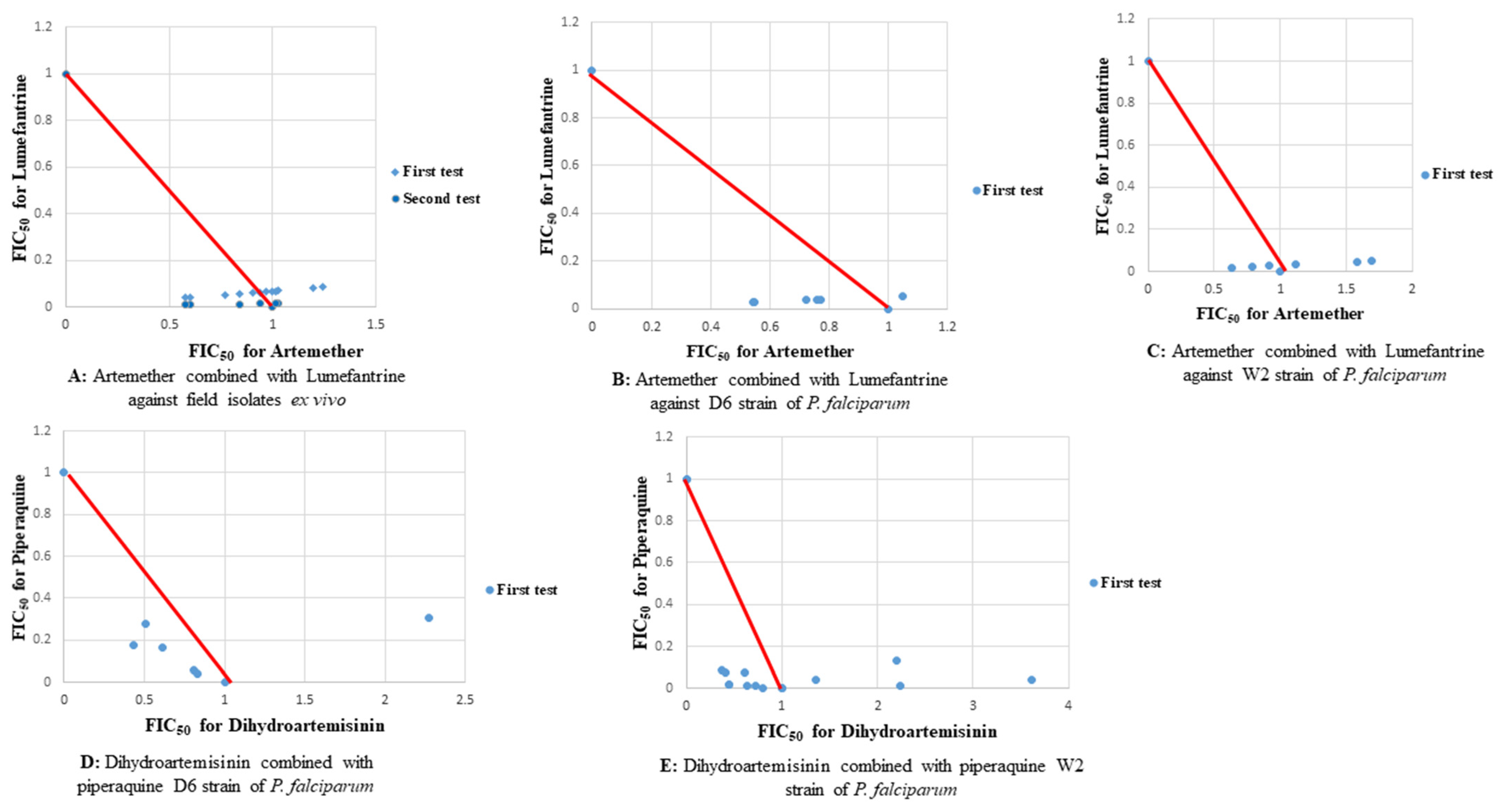
2.1. Antiplasmodial Activities
2.2. Molecular Analysis
3. Discussion
4. Materials and Methods
4.1. Collection of Plant Materials
4.2. Preparation of Test Extracts and Drugs
Reference P. falciparum Strains and Reference Antimalarial Drugs
4.3. Drug Combinations
4.4. Antiplasmodial Activity Assays
4.4.1. In Vitro Antiplasmodial Activity
4.4.2. Ex Vivo Antiplasmodial Activity
4.5. Molecular Characterization of Field Isolates
4.5.1. DNA (Deoxyribonucleic Acid) Extraction
4.5.2. Species Determination
4.5.3. Single Nucleotide Polymorphism (SNP) Analysis
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Malaria Report; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Zaw, M.T.; Emran, N.A.; Lin, Z. Updates on k13 mutant alleles for artemisinin resistance in Plasmodium falciparum. J. Microbiol. Immunol. Infect. 2018, 51, 159–165. [Google Scholar] [CrossRef]
- Menard, D.; Dondorp, A. Antimalarial drug resistance: A threat to malaria elimination. Cold Spring Harb. Perspect. Med. 2017, 7, a025619. [Google Scholar] [CrossRef] [PubMed]
- Saifi, M.A.; Beg, T.; Harrath, A.H.; Altayalan, F.S.H.; Qurais, S.A. Antimalarial drugs: Mode of action and status of resistance. Afr. J. Pharm. Pharmacol. 2013, 7, 148–156. [Google Scholar] [CrossRef]
- Tse, E.G.; Korsik, M.; Todd, M.H. The past, present and future of anti-malarial medicines. Malar. J. 2019, 18, 93. [Google Scholar] [CrossRef]
- Lu, F.; He, X.L.; Richard, C.; Cao, J. A brief history of artemisinin: Modes of action and mechanisms of resistance. Chin. J. Nat. Med. 2019, 17, 331–336. [Google Scholar] [CrossRef]
- Smith, C.S.; Aerts, A.; Saunderson, P.; Kawuma, J.; Kita, E.; Virmond, M. Review Multidrug therapy for leprosy: A game changer on the path to elimination. Lancet Infect. Dis. 2017, 17, 3099–3105. [Google Scholar] [CrossRef]
- Kerantzas, C.A.; William, R.J. Origins of Combination Therapy for Tuberculosis: Lessons for Future Antimicrobial Development and Application. Am. Soc. Microbiol. 2017, 8, 1586–1602. [Google Scholar] [CrossRef]
- Chesney, M.A.; Morin, M.; Sherr, L. Adherence to HIV Combination Therapy. Soc. Sci. Med. 2000, 50, 1599–1605. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for Malaria; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Bhatt, S.; Weiss, D.J.; Cameron, E.; Bisanzio, D.; Mappin, B.; Dalrymple, U.; Battle, K.E.; Moyes, C.L.; Henry, A.; Eckhoff, P.A.; et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015, 526, 207–211. [Google Scholar] [CrossRef]
- Banda, C.G.; Chaponda, M.; Mukaka, M.; Mulenga, M.; Hachizovu, S.; Kabuya, J.B.; Mulenga, J.; Sikalima, J.; Phiri, L.K.; Terlouw, D.J.; et al. Efficacy and safety of artemether–lumefantrine as treatment for Plasmodium falciparum uncomplicated malaria in adult patients on efavirenz-based antiretroviral therapy in Zambia: An open label non-randomized interventional trial. Malar. J. 2019, 18, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Kaur, H. Plant Derived Antimalarial Agents. J. Med. Plants Stud. 2017, 5, 346–363. [Google Scholar]
- Mongalo, N.I.; McGaw, L.J.; Finnie, J.F.; Staden, J.V. Securidaca longipedunculata Fresen (Polygalaceae): A review of its ethnomedicinal uses, phytochemistry, pharmacological properties and toxicology. J. Ethnopharmacol. 2015, 165, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Ochora, D.O.; Saifudin, F.D.; Nguta, J.M.; Akunda, E.M. Antimalarial activity and acute toxicity of four plants traditionally used in treatment of malaria in Msambweni District of Kenya. Eur. Int. J. Sci. Technol. 2014, 3, 31–40. [Google Scholar]
- Hamill, F.A.; Apio, S.; Mubiru, N.K.; Bukenya-Ziraba, R.; Mosango, M.; Maganyi, O.W.; Soejarto, D.D. Traditional herbal drugs of Southern Uganda, II: Literature analysis and antimicrobial assays. J. Ethnopharmacol. 2003, 84, 57–78. [Google Scholar] [CrossRef]
- Opio, D.; Andama, E.; Kureh, G. Ethnobotanical Survey of Antimalarial Plants in Areas of: Abukamola, Angeta, Oculokori and Omarari of Alebtong District in Northern Uganda. Eur. J. Med. Plants 2018, 21, 1–14. [Google Scholar] [CrossRef]
- Joseph, C.C.; Moshi, M.J.; Sempombe, J.; Nkunya, M.H.H. (4-methoxy-benzo[1,3]dioxol-5-yl)-phenylmethanone: An antibacterial benzophenone from Securidaca longepedunculata. Afr. J. Tradit. Complement. Altern. Med. 2006, 3, 80–86. [Google Scholar] [CrossRef][Green Version]
- Nadembega, P.; Boussim, J.I.; Nikiema, J.B.; Poli, F.; Antognoni, F. Medicinal plants in Baskoure, Kourittenga Province, Burkina Faso: An ethnobotanical study. J. Ethnopharmacol. 2011, 133, 378–395. [Google Scholar] [CrossRef]
- Mustapha, A.A. Ethno-medico-botanical uses of Securidaca longepedunculata Fresen (family-polygalaceae) from Keffi local government, Nasarawa state, Nigeria. J. Nat. Remedies 2013, 13, 133–137. [Google Scholar]
- Ochora, D.O.; Kakudidi, E.; Namukobe, J.; Heydenreich, M.; Coghi, P.; Yang, L.J.; Mwakio, E.W.; Andagalu, B.; Roth, A.; Akala, H.M.; et al. A new benzophenone, and the antiplasmodial activities of the constituents of Securidaca longipedunculata fresen (Polygalaceae). Nat. Prod. Res. 2021, 1, 1–9. [Google Scholar] [CrossRef]
- Ocloo, A.; Okpattah, W.E.; Quasie, W.O.; Sakyiamah, M.M.; Okine, K.N. Concurrent administration of aqueous extract of Cryptolepis sanguinolenta reduces the effectiveness of ART against Plasmodium berghei in rats. J. Appl. Pharm. Sci. 2014, 4, 24–28. [Google Scholar]
- Akala, H.M.; Waters, C.N.; Yenesew, A.; Wanjala, C.; Akenga, T.A. In vitro antiplasmodial and cyclin-dependent protein kinase (pfmrk) inhibitory activities of selected flavonoids in combination with chloroquine (CQ) and artemisinin. Res. Pharm. Biotechnol. 2010, 2, 40–50. [Google Scholar]
- Ohrt, C.; Willingmyre, G.D.; Lee, P.; Knirsch, C.; Milhous, W. Assessment of Azithromycin in Combination with Other Antimalarial Drugs against Plasmodium falciparum In Vitro. Antimicrob. Agents Chemother. 2002, 46, 2518–2524. [Google Scholar] [CrossRef]
- Gathirwa, J.W.; Rukunga, G.M.; Njagi, E.N.M.; Omar, S.A.; Mwitari, P.G.; Guantai, A.N.; Tolo, F.M.; Kimani, C.W.; Muthaura, C.N.; Kirira, P.G.; et al. The in vitro anti-plasmodial and in vivo anti-malarial efficacy of combinations of some medicinal plants used traditionally for treatment of malaria by the Meru community in Kenya. J. Ethnopharmacol. 2008, 115, 223–231. [Google Scholar] [CrossRef]
- Adegbolagun, O.M.; Emikpe, B.O.; Woranola, I.O.; Ogunremi, Y. Synergistic effect of aqueous extract of Telfaria occidentalis on the biological activities of artesunate in Plasmodium berghei infected mice. Afr. Health Sci. 2013, 13, 970–976. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ukwe, C.V.; Ekwunife, O.I.; Epueke, E.A.; Ubaka, C.M. Antimalarial activity of Ageratum conyzoides in combination with chloroquine and artesunate. Asian Pac. J. Trop. Med. 2010, 3, 943–947. [Google Scholar]
- Mukungu, N.; Abuga, K.; Okalebo, F.; Ingwela, R.; Mwangi, J. Medicinal plants used for management of malaria among the Luhya community of Kakamega East sub-County, Kenya. J. Ethnopharmacol. 2016, 194, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Erhirhie, E.O.; Ikegbune, C.; Okeke, A.I.; Onwuzuligbo, C.C.; Madubuogwu, N.U.; Chukwudulue, U.M.; Okonkwo, O.B. Antimalarial herbal drugs: A review of their interactions with conventional antimalarial drugs. Clin. Phytosci. 2021, 7, 4. [Google Scholar] [CrossRef]
- Lourens, C.; Watkins, W.M.; Barnes, K.I.; Sibley, C.H.; Guerin, P.J.; White, N.J.; Lindegardh, N. Implementation of a reference standard and proficiency testing programme by the World Wide Antimalarial Resistance Network (WWARN). Malar. J. 2010, 9, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Yenesew, A.; Akala, H.M.; Twinomuhwezi, H.; Chepkirui, C.; Irungu, B.N.; Eyase, F.L.; Kamatenesi-Mugisha, M.; Kiremire, B.T.; Johnson, J.D.; Waters, N.C. The antiplasmodial and radical scavenging activities of flavonoids of Erythrina burttii. Acta Trop. 2012, 123, 123–127. [Google Scholar] [CrossRef]
- Smilkstein, M.; Sriwilaijaroen, N.; Kelly, J.X.; Wilairat, P.; Riscoe, M. Simple and Inexpensive Fluorescence-Based Technique for High-Throughput Antimalarial Drug Screening. Antimicrob. Agents Chemother. 2004, 48, 1803–1806. [Google Scholar] [CrossRef]
- Johnson, J.D.; Dennull, R.A.; Gerena, L.; Lopez-Sanchez, M.; Roncal, N.E.; Waters, N.C. Assessment and continued validation of the malaria SYBR Green I-based fluorescence assay for use in malaria drug screening. Antimicrob. Agents Chemother. 2007, 51, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Chulay, J.D.; Haynes, J.D.; Diggs, C.L. Plasmodium falciparum: Assessment of in Vitro Growth by [3H] Hypoxanthine Incorporation. Exp. Para. 1983, 55, 138–146. [Google Scholar] [CrossRef]
- Akala, H.M.; Eyase, F.L.; Cheruiyot, A.C.; Omondi, A.A.; Ogutu, B.R.; Waters, N.C.; Johnson, J.D.; Polhemus, M.E.; Schnabel, D.C.; Walsh, D.S. Antimalarial drug sensitivity profile of western Kenya Plasmodium falciparum field isolates determined by a SYBR green I in vitro assay and molecular analysis. Am. J. Trop. Med. Hyg. 2011, 85, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Kamau, E.; Tolbert, L.S.; Kortepeter, L.; Pratt, M.; Nyakoe, N.; Muringo, L.; Ogutu, B.; Waitumbi, J.N.; Ockenhouse, C.F. Development of a highly sensitive genus-specific quantitative reverse transcriptase real-time PCR assay for detection and quantitation of plasmodium by amplifying RNA and DNA of the 18S rRNA genes. J. Clin. Microbiol. 2011, 49, 2946–2953. [Google Scholar] [CrossRef]
- Akala, H.M.; Watson, O.J.; Mitei, K.K.; Juma, D.W.; Verity, R.; Opot, B.H.; Okoth, R.O.; Chemwor, G.C.; Juma, J.A.; Mwakio, E.W.; et al. Plasmodium interspecies interactions during a period of increasing prevalence of Plasmodium ovale in symptomatic individuals seeking treatment: An observational study. Lancet Microbe 2021, 2, 141–150. [Google Scholar] [CrossRef]
- Maraka, M.; Akala, H.M.; Amolo, A.S.; Juma, D.; Omariba, D.; Cheruiyot, A.; Opot, B.; Okudo, C.O.; Mwakio, E.; Chemwor, G.; et al. A seven-year surveillance of epidemiology of malaria reveals travel and gender are the key drivers of dispersion of drug resistant genotypes in Kenya. Peer J. 2020, 8, 1–27. [Google Scholar] [CrossRef]


| Drugs and MeOH Extracts | Ex Vivo | W2 | D6 | DD2 |
|---|---|---|---|---|
| Root extracts | 9.8 ± 1.3 | 1.4 ± 0.07 | 2.9 ± 0.5 | NT |
| Stem extracts | 45.5 ± 6.2 | NT | 14.6 ± 2.6 | 16.7 ± 5.3 |
| Leaf extracts | 13.1 ± 1.8 | 20.4 ± 4.7 | 18.6 ± 4.8 | NT |
| Artemether | 0.8 ± 0.5 | 1.4 ± 0.1 | 0.8 ± 0.2 | 1.7 ± 0.3 |
| Lumefantrine | 61.1 ± 21.9 | 33.8 ± 7.6 | 40.1 ± 11.1 | 65.0 ± 21.2 |
| Mefloquine | 37.2 ± 10.6 | 0.8 ± 0.2 | 1.5 ± 0.4 | 1.8 ± 0.6 |
| Chloroquine | 120.1 ± 14.1 | 207.1 ± 77.8 | 7.4 ± 1.5 | 9.6 ± 1.3 |
| Fixed Ratios Drug:Extract | W2 (p = 0.0007, R2 = 0.5648) | DD2 (p = 0.0002, R2 = 0.6165) | ||||||
|---|---|---|---|---|---|---|---|---|
| ART-Roots | LU-Roots | ART-Leaves | LU-Leaves | ART-Roots | LU-Roots | ART-Stems | LU-Stems | |
| 4:1 | 1.801 ± 1.899 | 0.691 ± 0.253 | 1.291 ± 0.0898 | 0.651 ± 0.405 | 0.875 ± 0.158 | 0.829 ± 0.292 | 0.371 ± 0.293 | 0.531 ± 0.574 |
| 3:1 | 0.546 ± 0.695 | 1.08 ± 0.847 | 1.277 ± 0.625 | 0.649 ± 0.481 | 1.078 ± 0.577 | 0.588 ± 0.334 | 0.219 ± 0.082 | 0.306 ± 0.198 |
| 1:1 | 0.705 ± 0.83 | 0.711 ± 0.41 | 1.444 ± 0.898 | 0.542 ± 0.252 | 0.713 ± 0.366 | 0.655 ± 0.477 | 0.775 ± 0.451 | 0.483 ± 0.476 |
| 1:2 | 0.759 ± 0.436 | 0.946 ± 0.648 | 2.046 ± 2.392 | 0.262 ± 0.122 | 0.802 ± 0.211 | 0.831 ± 0.504 | 0.424 ± 0.296 | 0.272 ± 0.23 |
| 1:3 | 0.428 ± 0.222 | 0.448 ± 0.354 | 2.351 ± 1.945 | 0.275 ± 0.319 | 0.796 ± 0.322 | 0.302 ± 0.147 | 0.524 ± 0.333 | 0.344 ± 0.157 |
| 1:4 | 0.897 ± 0.614 | 0.629 ± 0.488 | 3.978 ± 4.028 | 0.354 ± 0.119 | 1.11 ± 0.089 | 0.57 ± 0.343 | 0.596 ± 0.364 | 0.32 ± 0.309 |
| Fixed Ratios Drug:Extract | ART-Roots | ART-Stems | ART-Leaves | LU-Roots | LU-Stems | LU-Leaves |
|---|---|---|---|---|---|---|
| 4:1 | 0.425 ± 0.347 | 0.9 ± 0.255 | 0.65 ± 0.383 | 0.677 ± 0.251 | 1.435 ± 0.892 | 0.292 ± 0.133 |
| 3:1 | 0.468 ± 0.278 | 0.892 ± 0.446 | 0.664 ± 0.28 | 0.916 ± 0.427 | 0.956 ± 0.335 | 0.856 ± 0.307 |
| 1:1 | 0.43 ± 0.536 | 0.787 ± 0.434 | 0.763 ± 0.443 | 0.684 ± 0.173 | 2.27 ± 1.762 | 0.554 ± 0.161 |
| 1:2 | 0.342 ± 0.333 | 0.763 ± 0.238 | 1.147 ± 0.688 | 0.631 ± 0.213 | 0.613 ± 0.549 | 0.936 ± 0.523 |
| 1:3 | 0.471 ± 0.377 | 0.534 ± 0.251 | 0.959 ± 0.577 | 0.735 ± 0.306 | 1.924 ± 1.497 | 1.379 ± 0.641 |
| 1:4 | 0.284 ± 0.159 | 0.678 ± 0.145 | 1.132 ± 0.595 | 0.785 ± 0.427 | 1.351 ± 1.03 | 1.993 ± 1.094 |
| Fixed Ratios Drug:Extract | ART-Roots | LU-Roots | ART-Stems | LU-Stems | ART-Leaves | LU-Leaves |
|---|---|---|---|---|---|---|
| 4:1 | 0.665 ± 0.294 | 0.548 ± 0.362 | 0.584 ± 0.336 | 0.765 ± 0.169 | 1.265 ± 0.32 | 0.791 ± 0.449 |
| 3:1 | 0.505 ± 0.426 | 3.179 ± 2.546 | 0.771 ± 0.514 | 0.483 ± 0.187 | 1.124 ± 0.313 | 0.365 ± 0.178 |
| 1:1 | 0.585 ± 0.39 | 0.86 ± 0.815 | 0.685 ± 0.066 | 1.168 ± 1.17 | 0.638 ± 0.276 | 0.72 ± 0.324 |
| 1:2 | 0.988 ± 0.464 | 0.916 ± 0.536 | 0.574 ± 0.149 | 0.985 ± 0.849 | 0.74 ± 0.343 | 1.149 ± 0.654 |
| 1:3 | 0.973 ± 0.536 | 0.352 ± 0.365 | 0.636 ± 0.093 | 0.752 ± 0.627 | 0.289 ± 0.155 | 0.88 ± 0.637 |
| 1:4 | 1.111 ± 0.664 | 0.526 ± 0.775 | 0.683 ± 0.425 | 0.6894 ± 0.28 | 0.174 ± 0.032 | 1.044 ± 0.526 |
| ART-Roots | ART-Stems | ART-Leaves | LU-Roots | LU-Stems | LU-Leaves | |
|---|---|---|---|---|---|---|
| D6 | 0.403 ± 0.068 a | 0.756 ± 0.126 b | 0.886 ± 0.206 | 0.738 ± 0.093 c | 1.425 ± 0.555 a,b,c | 1.001 ± 0.556 |
| W2 | 0.856 ± 0.448 a | NT | 2.065 ± 0.944 a,b,c | 0.75 ± 0.207 b | NT | 0.456 ± 0.165 c |
| DD2 | 0.896 ± 0.148 a,b | 0.485 ± 0.176 a | NT | 0.629 ± 0.18 | 0.376 ± 0.096 b | NT |
| Field isolates * | 0.805 ± 0.229 | 0.656 ± 0.067 | 0.705 ± 0.398 | 1.064 ± 0.996 | 0.807 ± 0.218 | 0.825 ± 0.252 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochora, D.O.; Kakudidi, E.K.; Namukobe, J.; Ipulet, P.; Wakoli, D.M.; Okore, W.; Mwakio, E.W.; Yeda, R.A.; Cheruiyot, A.C.; Juma, D.W.; et al. Synergism in Antiplasmodial Activities of Artemether and Lumefantrine in Combination with Securidaca longipedunculata Fresen (Polygalaceae). Plants 2022, 11, 47. https://doi.org/10.3390/plants11010047
Ochora DO, Kakudidi EK, Namukobe J, Ipulet P, Wakoli DM, Okore W, Mwakio EW, Yeda RA, Cheruiyot AC, Juma DW, et al. Synergism in Antiplasmodial Activities of Artemether and Lumefantrine in Combination with Securidaca longipedunculata Fresen (Polygalaceae). Plants. 2022; 11(1):47. https://doi.org/10.3390/plants11010047
Chicago/Turabian StyleOchora, Douglas O., Esezah K. Kakudidi, Jane Namukobe, Perpetua Ipulet, Dancan M. Wakoli, Winnie Okore, Edwin W. Mwakio, Redempthah A. Yeda, Agnes C. Cheruiyot, Dennis W. Juma, and et al. 2022. "Synergism in Antiplasmodial Activities of Artemether and Lumefantrine in Combination with Securidaca longipedunculata Fresen (Polygalaceae)" Plants 11, no. 1: 47. https://doi.org/10.3390/plants11010047
APA StyleOchora, D. O., Kakudidi, E. K., Namukobe, J., Ipulet, P., Wakoli, D. M., Okore, W., Mwakio, E. W., Yeda, R. A., Cheruiyot, A. C., Juma, D. W., Andagalu, B., Roth, A. L., Ogutu, B. R., Yenesew, A., & Akala, H. M. (2022). Synergism in Antiplasmodial Activities of Artemether and Lumefantrine in Combination with Securidaca longipedunculata Fresen (Polygalaceae). Plants, 11(1), 47. https://doi.org/10.3390/plants11010047






