Contrasting Dihydronaphthoquinone Patterns in Closely Related Drosera (Sundew) Species Enable Taxonomic Distinction and Identification
Abstract
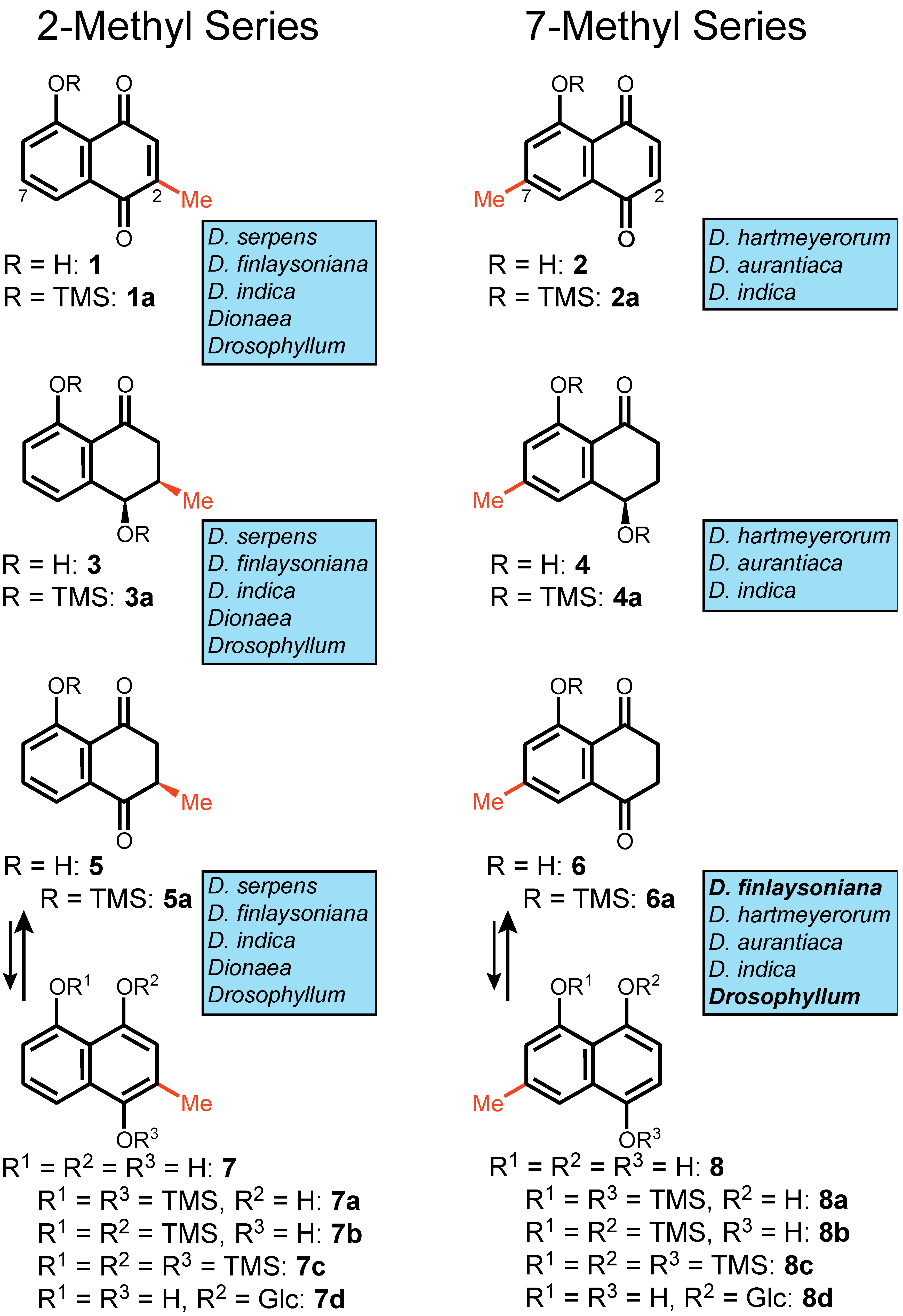
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation
- Plumbagin (1): C11H8O3, EIMS m/z 188 [M]+ (100), 173 [M-CH3]+ (30), 160 [M-CO]+ (25), 132 (30), 131 [M-C3H5O]+ (50), 121 [M-C4H3O]+ (15), 120 [M-C4H4O]+ (25), 103 [M-C4H5O2]+ (10), 92 [M-C5H4O2]+ (40), 77 (15), 63 (40).
- Ramentaceone (2): C11H8O3, EIMS m/z 188 [M]+ (100), 187 [M − H]+ (30), 173 [M − CH3]+ (10), 160 [M − CO]+ (15), 134 [M − C3H2O]+ (20), 132 (30), 131 [M − C3H5O]+ (30), 106 [M − C4H2O2]+ (20), 104 (10), 103 [M − C4H5O2]+ (10), 78 (15), 77 (25), 63 (15), 62 (10), 51 (25).
- Isoshinanolone (3): C11H12O3, EIMS m/z 192 [M]+ (70), 177 [M − CH3]+ (20), 174 [M − H2O]+ (10), 150 [M − C3H6]+ (40), 149 [M − C2H3O]+ (25), 131 [M − C2H5O2]+ (20), 122 [M − C3H2O2]+ (45), 121 [M − C4H7O]+ (100), 115 (10), 93 (25), 77 (20), 65 (30), 51 (20).
- Shinanolone (4): C11H12O3, EIMS m/z 192 [M]+ (70), 177 [M − CH3]+ (10), 174 [M − H2O]+ (10), 164 [M − C2H4]+ (20), 149 [M − C2H3O]+ (15), 135 [M − C3H5O]+ (100), 107 [M − C4H5O2]+ (20).
- Dihydroplumbagin (5): C11H10O3, EIMS m/z 190 [M]+ (85), 175 [M − CH3]+ (100), 162 [M − CO]+ (20), 147 [M − C2H3O]+ (20), 120 [M − C4H6O]+ (60), 92 [M − C5H6O2]+ (40), 63 (15) (Figure S1).
- Dihydroramentaceone (6): C11H10O3, EIMS m/z 190 [M]+ (100), 175 [M − CH3]+ (10), 162 [M − CO]+ (15), 134 [M − C3H4O]+ (55), 106 [M − C4H4O2]+ (20) (Figure S2).
- 5-O-Trimethylsilyl-plumbagin (1a): C14H16O3Si, EIMS m/z 245 [M − CH3]+ (100), 217 [M − CH3 − CO]+ (30), 186 (10), 115 (10) (Figure S3).
- 5-O-Trimethylsilyl-ramentaceone (2a): C14H16O3Si, EIMS m/z 245 [M − CH3]+ (100), 217 [M − CH3 − CO]+ (20), 187 (10), 115 (10) (Figure S4).
- 4,8-Di-(O-trimethylsilyl)-isoshinanolone (3a): C17H28O3Si2, EIMS m/z 321 [M − CH3]+ (5), 231 [M − CH3 − C3H10OSi]+ (100), 216 [M − 2CH3 − C3H10OSi]+ (20), 201 [M − 3CH3 − C3H10OSi]+ (10), 186 [M − 4CH3 − C3H10OSi]+ (5) (Figure S5).
- 4,8-Di-(O-trimethylsilyl)-shinanolone (4a): C17H28O3Si2, EIMS m/z 321 [M − CH3]+ (5), 231 [M − CH3 − C3H10OSi]+ (100), 216 [M − 2CH3 − C3H10OSi]+ (20), 201 [M − 3CH3 − C3H10OSi]+ (10), 186 [M − 4CH3 − C3H10OSi]+ (5) (Figure S6).
- 5-O-Trimethylsilyl-dihydroplumbagin (5a): C14H18O3Si, EIMS m/z 247 [M − CH3]+ (100), 219 [M − CH3 − CO]+ (10) (Figure S7).
- 5-O-Trimethylsilyl-dihydroramentaceone (6a): C14H18O3Si, EIMS m/z 247 [M − CH3]+ (100), 219 [M − CH3 − CO]+ (10) (Figure S8).
- 1,5-Di-(O-trimethylsilyl)-2-methyl-naphtho-1,4,5-triol (7a): C17H26O3Si2, EIMS m/z 318 [M − CH4]+ (100), 288 [M − CH4 − 2CH3]+ (20), 273 [M − CH4 − 3CH3]+ (10) (Figure S9).
- 4,5-Di-(O-trimethylsilyl)-2-methyl-naphtho-1,4,5-triol (7b): C17H26O3Si2, EIMS m/z 319 [M − CH3]+ (100), 245 (15), 217 (10).
- 2-Methyl-1,4,5-tri-(O-trimethylsilyl)-naphtho-1,4,5-triol (7c): C20H34O3Si3, EIMS m/z 406 [M]+ (100).
- 1,5-Di-(O-trimethylsilyl)-7-methyl-naphtho-1,4,5-triol (8a): C17H26O3Si2, EIMS m/z 318 [M − CH4]+ (100), 288 [M − CH4 − 2CH3]+ (20), 273 [M − CH4 − 3CH3]+ (10) (Figure S10).
- 4,5-Di-(O-trimethylsilyl)-7-methyl-naphtho-1,4,5-triol (8b): C17H26O3Si2, EIMS m/z 319 [M − CH3]+ (100), 245 (15), 217 (10).
- 7-Methyl-1,4,5-tri-(O-trimethylsilyl)-naphtho-1,4,5-triol (8c): C20H34O3Si3, EIMS m/z 406 [M]+ (100).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleischmann, A.; Gonella, P.M. Species of carnivorous plants. In Carnivorous Plants; Ellison, A., Adamec, L., Eds.; University Press: Oxford, UK, 2017; p. 415. [Google Scholar]
- Culham, A.; Gornall, R.J. The taxonomic significance of naphthoquinones in the Droseraceae. Biochem. Syst. Ecol. 1994, 22, 507–515. [Google Scholar] [CrossRef]
- Schlauer, J.; Nerz, J.; Rischer, H. Carnivorous plant chemistry. Acta Bot. Gall. 2005, 152, 187–195. [Google Scholar] [CrossRef][Green Version]
- Schlauer, J.; Fleischmann, A. Chemical evidence for hybridity in Drosera (Droseraceae). Biochem. Syst. Ecol. 2016, 66, 33–36. [Google Scholar] [CrossRef]
- Schlauer, J.; Hartmeyer, S.R.H.; Hartmeyer, I. Unexpected Discovery of 7-Methyljuglone (Ramentaceone) in Several Australian Sundews; Carnival Corporation & plc: Miami, FL, USA, 2017; Volume 46, pp. 20–22. [Google Scholar]
- Schlauer, J.; Hartmeyer, S.R.H.; Hartmeyer, I.; Hennern, H.; Hennern, A. Sundew Chemistry and Emergence Updates; Carnival Corporation & plc: Miami, FL, USA, 2018; Volume 47, pp. 10–17. [Google Scholar]
- Schlauer, J.; Hartmeyer, S.R.H.; Hartmeyer, I.; Hennern, H.; Hennern, A. New Sundew Quinone and Emergence Data; Carnival Corporation & plc: Miami, FL, USA, 2019; Volume 48, pp. 6–12. [Google Scholar]
- Schlauer, J.; Hartmeyer, S.R.H.; Hartmeyer, I. Quinone Patterns and Identification of Japanese Spider Leg Sundews (Drosera Sect. Arachnopus); Carnival Corporation & plc: Miami, FL, USA, 2019; Volume 48, pp. 161–163. [Google Scholar]
- Budzianowski, J.; Budzianowska, A.; Kromer, K. Naphthalene glucoside and other phenolics from the shoot and callus cultures of Drosophyllum lusitanicum. Phytochemistry 2002, 61, 421–425. [Google Scholar] [CrossRef]
- Bringmann, G.; Hamm, A.; Günther, C.; Michel, M.; Brun, R.; Mudogo, V. Ancistroealaines A and B, two new bioactive naphthylisoquinolines, and related naphthoic acids from Ancistrocladus ealaensis. J. Nat. Prod. 2000, 63, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- Binder, R.G.; Benson, M.E.; Flath, R.A. Eight 1,4-naphthoquinones from Juglans. Phytochemistry 1989, 28, 2799–2801. [Google Scholar] [CrossRef]
- Higa, M.; Takashima, Y.; Yokaryo, H.; Harie, Y.; Suzuka, T.; Ogihara, K. Naphthoquinone derivatives from Diospyros maritima. Chem. Pharm. Bull. 2017, 65, 739–745. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Revirego, F.; Alkorta, I.; Elguero, J. Desmotropy in reduced plumbagins: α- and β-dihydroplumbagins. J. Mol. Struct. 2008, 891, 325–328. [Google Scholar] [CrossRef]
- Budzianowski, J. Naphthohydroquinone glucosides of Drosera rotundifolia and D. intermedia from in vitro cultures. Phytochemistry 1996, 42, 1145–1147. [Google Scholar] [CrossRef]
- Schlauer, J.; Hartmeyer, S.R.H.; Hartmeyer, I. Chemistry and Surface Micromorphology of the Queensland Sundews (Drosera Section Prolifera); Carnival Corporation & plc: Miami, FL, USA, 2019; Volume 48, pp. 111–116. [Google Scholar]
- Schlauer, J.; Carow, T.; Fleischmann, A. Quinones from “Gondwanan” Sundews; Carnival Corporation & plc: Miami, FL, USA, 2019; Volume 48, pp. 13–17. [Google Scholar]

| Species (Provenance, Accession No.) | 2-Methyl Series | 7-Methyl Series | |||||
|---|---|---|---|---|---|---|---|
| Plumbagin (1) | Dihydroplumbagin (5)/(7) | Isoshinanolone (3) | Ramentaceone (2) | Dihydroramentaceone (6)/(8) | Shinanolone (4) | ||
| D. serpens (Australia, 2020_101) | + | + | + | - | - | - | |
| D. finlaysoniana (Tropical Asia, 2020_102) | + | + | + | - | + * | - | |
| D. hartmeyerorum (Australia, 2020_103) | - | - | - | + | + | + | |
| D. aurantiaca (Australia, 2020_104) | - | - | - | + | + | + | |
| D. indica (Tropical Asia, 2020_105) | + | + | + | + | + | + | |
| Dionaea muscipula (USA, 2020_106) | + | + | + | - | - | - | |
| Drosophyllum lusitanicum (SW Europe, 2020_107) | + | + | + | - | + * | - | |
| Rt (GC) [min] | 14.49 | 14.47 | 15.46 | 14.70 | 15.56 | 15.97? | |
| M+ [m/z] | 188.0 | 190.1 | 192.1 | 188.0 | 190.1 | 192.1 | |
| Rt (GC) [min]; characteristic MS signal [m/z] of TMS-Derivatives | TMS | 16.15; 245.1 (1a) | 15.88; 247.1 (5a) | - | 16.13; 245.1 (2a) | 16.54; 247.1 (6a) | |
| TMS2 | - | 17.65; 318.2 (7a) 17.43; 319.1 (7b) | 16.52; 231.1 (3a) | - | 17.52; 318.2 (8a) 17.37; 319.1 (8b) | 17.10; 231.1 (4a) | |
| TMS3 | - | 18.41; 406.3 (7c) | - | - | 18.18; 406.3 (8c) | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlauer, J.; Hartmeyer, S.R.H.; Hartmeyer, I.; Seppänen-Laakso, T.; Rischer, H. Contrasting Dihydronaphthoquinone Patterns in Closely Related Drosera (Sundew) Species Enable Taxonomic Distinction and Identification. Plants 2021, 10, 1601. https://doi.org/10.3390/plants10081601
Schlauer J, Hartmeyer SRH, Hartmeyer I, Seppänen-Laakso T, Rischer H. Contrasting Dihydronaphthoquinone Patterns in Closely Related Drosera (Sundew) Species Enable Taxonomic Distinction and Identification. Plants. 2021; 10(8):1601. https://doi.org/10.3390/plants10081601
Chicago/Turabian StyleSchlauer, Jan, Siegfried R. H. Hartmeyer, Irmgard Hartmeyer, Tuulikki Seppänen-Laakso, and Heiko Rischer. 2021. "Contrasting Dihydronaphthoquinone Patterns in Closely Related Drosera (Sundew) Species Enable Taxonomic Distinction and Identification" Plants 10, no. 8: 1601. https://doi.org/10.3390/plants10081601
APA StyleSchlauer, J., Hartmeyer, S. R. H., Hartmeyer, I., Seppänen-Laakso, T., & Rischer, H. (2021). Contrasting Dihydronaphthoquinone Patterns in Closely Related Drosera (Sundew) Species Enable Taxonomic Distinction and Identification. Plants, 10(8), 1601. https://doi.org/10.3390/plants10081601








