Yield, Physiological Performance, and Phytochemistry of Basil (Ocimum basilicum L.) under Temperature Stress and Elevated CO2 Concentrations
Abstract
1. Introduction
2. Results
2.1. Gas Exchange Parameters
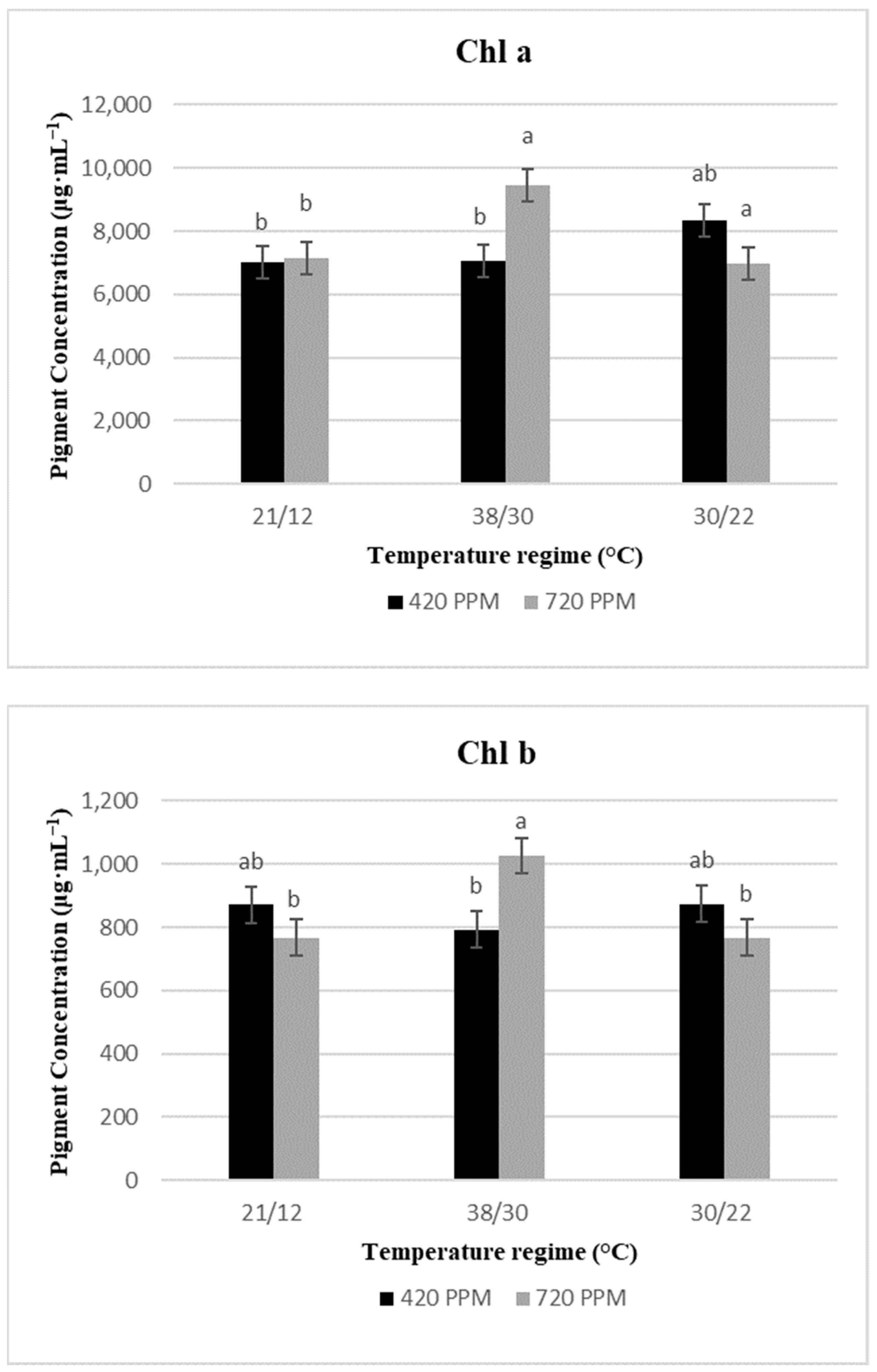
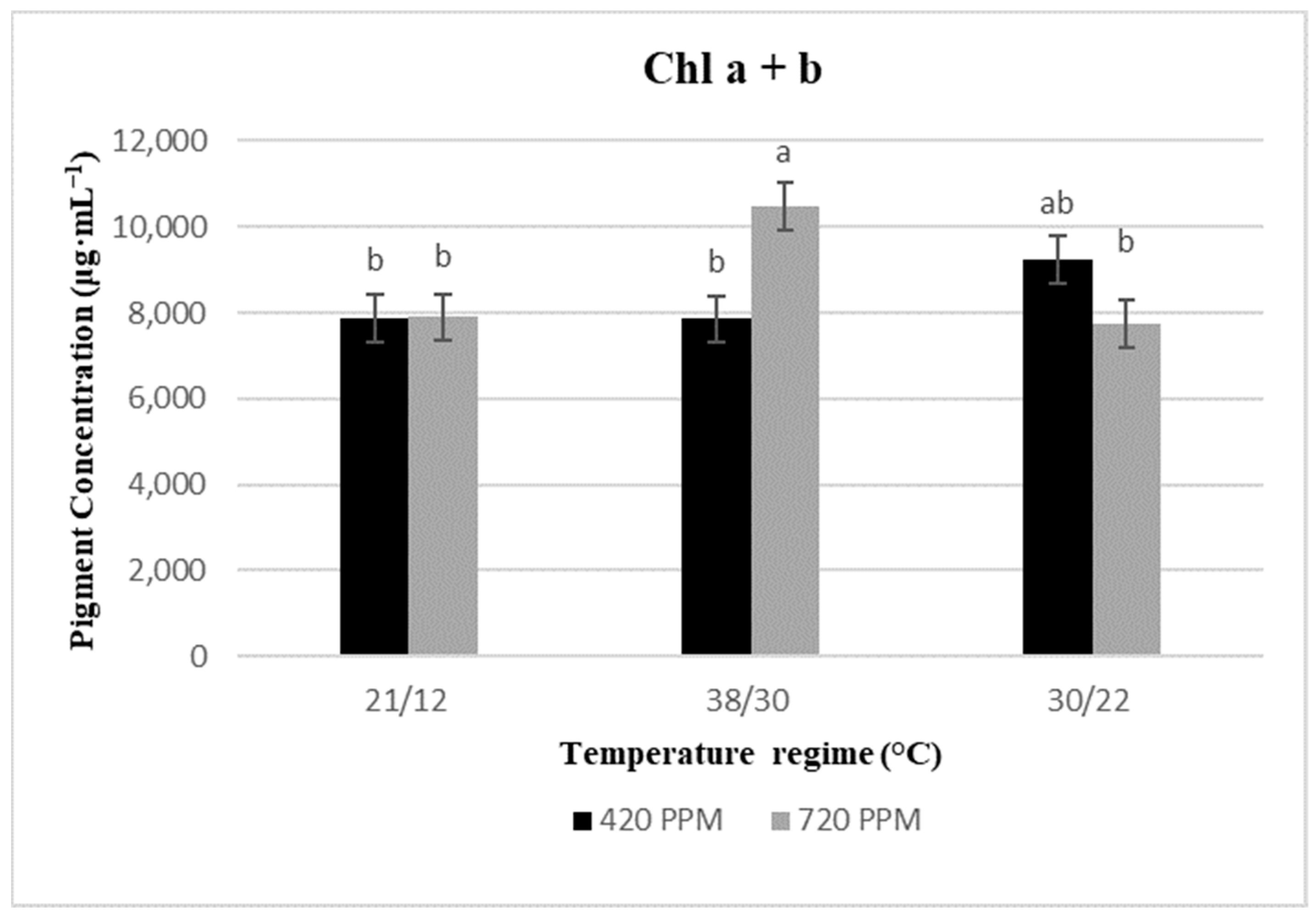
2.2. Chlorophyll Content and Total Carotenoids
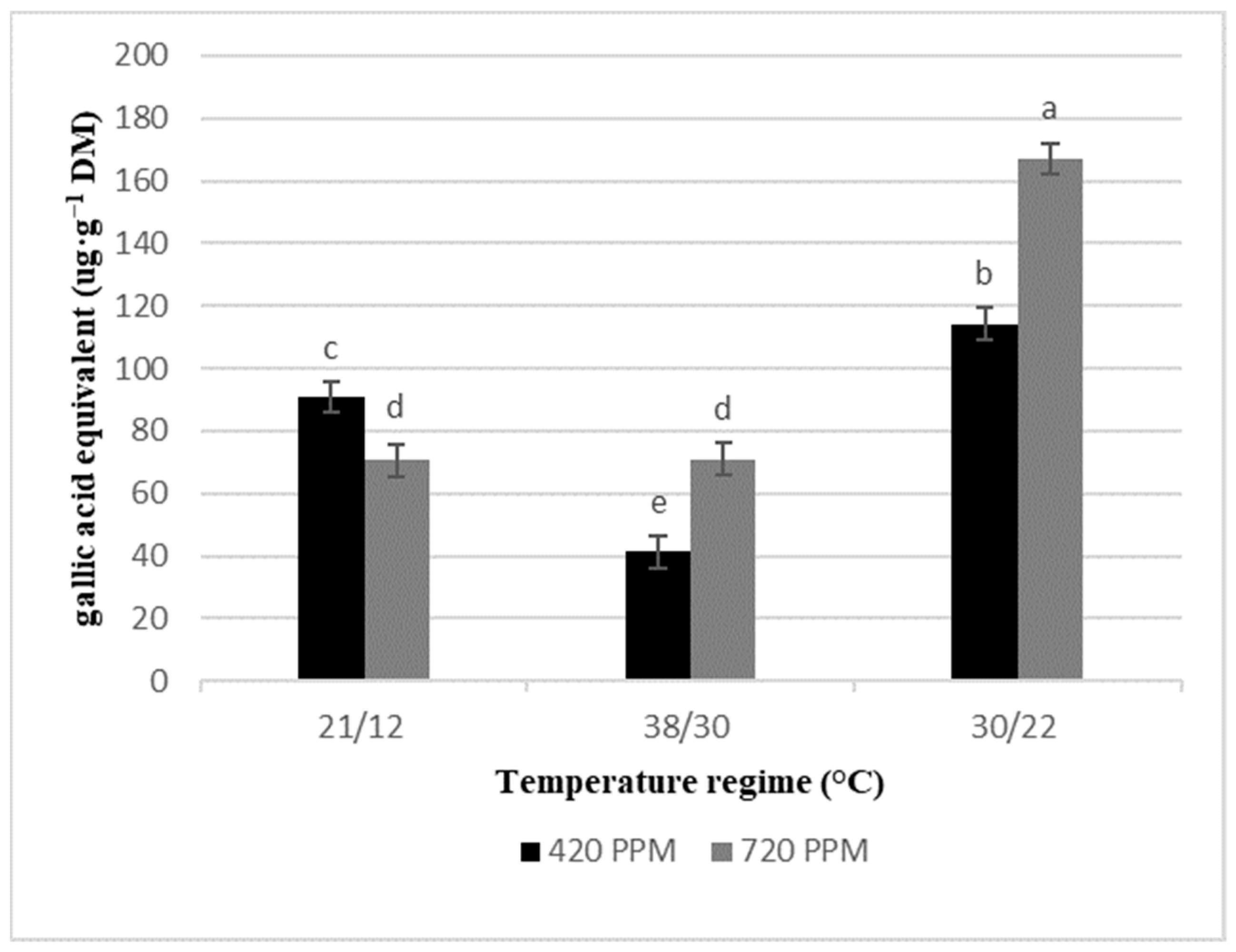
2.3. Total Phenolics
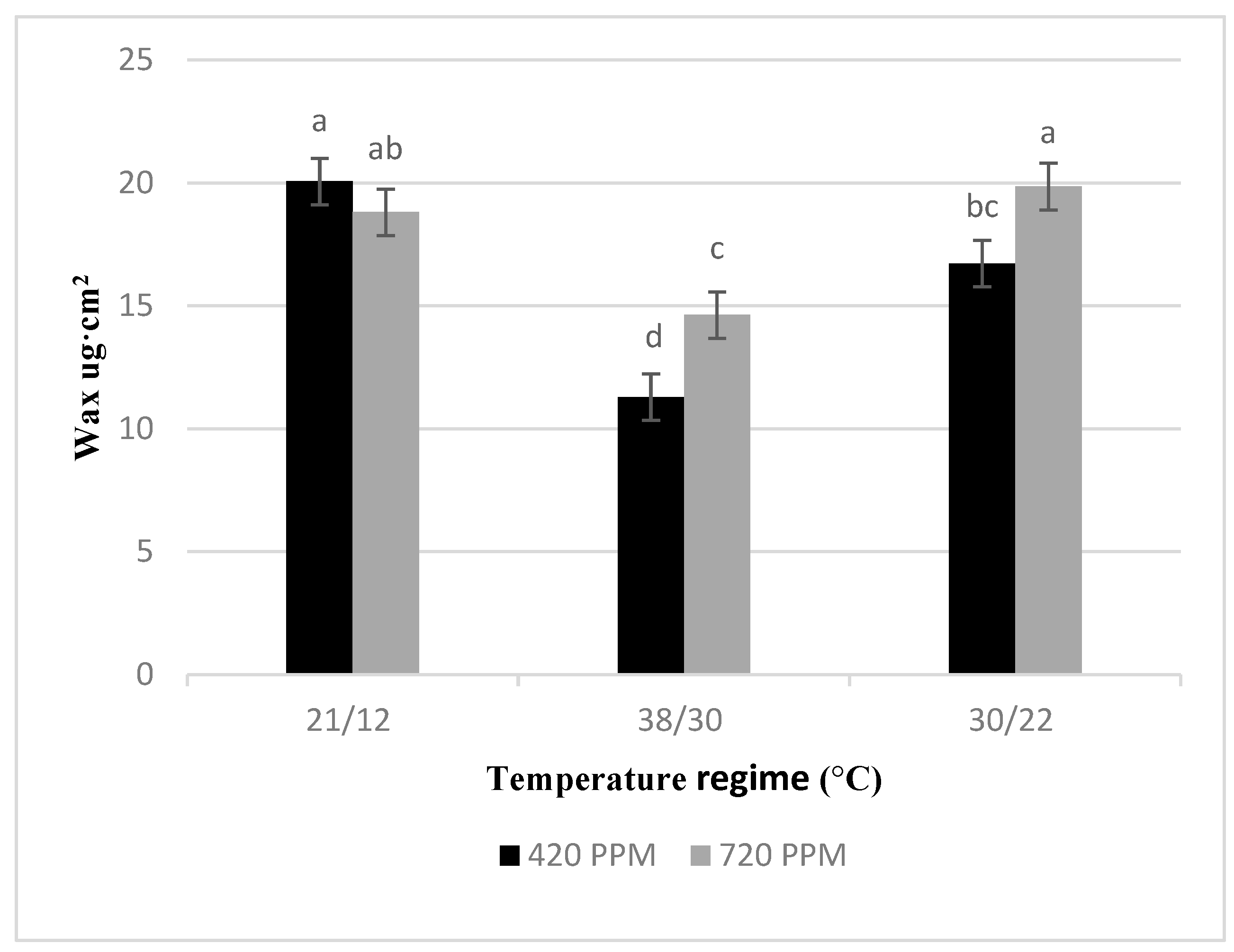
2.4. Epiticular Wax
2.5. Antioxidant and Oxidative Parameters
3. Discussion
4. Materials and Methods
4.1. Growth Condition and Plant Material
4.2. Temperature and CO2 Treatments
4.3. Physiology and Gas Exchange Measurements
4.4. Carotenoid and Chlorophyll Analysis
4.5. Epicuticular Wax Content Determination
4.6. Antioxidant and Oxidative Analysis
4.6.1. Malondialdehyde (MDA)
4.6.2. Hydrogen Peroxide (H2O2)
4.6.3. Superoxide Dismutase (SOD)
4.6.4. Ascorbic Acid (ASC)
4.6.5. Glutathione (GSH)
4.6.6. Trehalose (TRE)
5. Data Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Field, C.B.; Barros, V.R.; Dokken, D.J.; Mach, K.J.; Mastrandrea, M.D.; Bilir, T.E.; Chatterjee, M.; Ebi, K.L.; Estrada, Y.O.; Genova, R.C.; et al. IPCC. Climate Change 2014: Impacts, Adaptation, and Vulnerability; Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Field, C.B., Barros, V.R., Dokken, D.J., Mach, K.J., Mastrandrea, M.D., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; p. 1132. [Google Scholar]
- Al Jaouni, S.; Saleh, A.M.; Wadaan, M.A.M.; Hozzein, W.N.; Selim, S.; AbdElgawad, H. Elevated CO2 induces a global metabolic change in basil (Ocimum Basilicum L.) and peppermint (Mentha Piperita L.) and improves their biological activity. J. Plant Physiol. 2018, 224–225, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Prentice, I.C.; Farquhar, G.D.; Fasham, M.J.R.; Goulden, M.L.; Heimann, M.; Jaramillo, V.J.; Kheshgi, H.S.; Le Quéré, C.; Scholes, R.J.; Wallace, D.W.R. The carbon cycle and atmospheric carbon dioxide. In Climate Change 2001: The Scientific Basis; Cambridge University Press: Cambridge, UK, 2001; pp. 183–237. [Google Scholar]
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. Climate Change 2013: The Physical Science Basis; Intergovernmental Panel on Climate Change, Working Group I Contribution to the IPCC Fifth Assessment Report (AR5); Cambridge University Press: New York, NY, USA, 2013; p. 25. [Google Scholar]
- USGCRP. Impacts, Risks, and Adaptation in the United States: Fourth National Climate Assessment, Volume II; Reidmiller, D.R., Avery, C.W., Easterling, D.R., Kunkel, K.E., Lewis, K.L.M., Maycock, T.K., Stewart, B.C., Eds.; U.S. Global Change Research Program: Washington, DC, USA, 2018; p. 1515. [Google Scholar] [CrossRef]
- Reddy, A.R.; Reddy, K.R.; Hodges, H.F. Interactive effects of elevated carbon dioxide and growth temperature on photosynthesis in cotton leaves. Plant Growth Regul. 1998, 26, 33–40. [Google Scholar] [CrossRef]
- Dong, J.; Gruda, N.; Li, X.; Tang, Y.; Zhang, P.; Duan, Z. Sustainable vegetable production under changing climate: The impact of elevated co2 on yield of vegetables and the interactions with environments-A review. J. Clean. Prod. 2020, 253, 119920. [Google Scholar] [CrossRef]
- Solomon, S.; Manning, M.; Marquis, M.; Qin, D. Climate Change 2007—The Physical Science Basis: Working Group I Contribution to the Fourth Assessment Report of the IPCC; Cambridge University Press: Cambridge, UK, 2007; Volume 4. [Google Scholar]
- Simon, J.E.; Quinn, J.; Murray, R.G. Basil: A Source of Essential Oils. In Adv. New Crops; Janick, J., Simon, J.E., Eds.; Timber Press: Portland, OR, USA, 1990; pp. 484–489. [Google Scholar]
- Kopsell, D.A.; Kopsell, D.E.; Curran-Celentano, J. Carotenoid and chlorophyll pigments in sweet basil grown in the field and greenhouse. Hort. Sci. 2005, 40, 1230–1233. [Google Scholar] [CrossRef]
- Chang, X.; Alderson, P.; Wright, C. Effect of temperature integration on the growth and volatile oil content of basil (Ocimum Basilicum L.). J. Hortic. Sci. Biotechnol. 2005, 80, 593–598. [Google Scholar] [CrossRef]
- Hiltunen, R.; Holm, Y. Essential of oil of Ocimum. In Basil: The Genus Ocimum; Hiltunen, R., Holm, Y., Eds.; Harwood Academic Publishers: Amsterdam, The Netherlands, 1999; pp. 113–135. [Google Scholar]
- Kumar, B.; Gupta, E.; Yadav, R.; Singh, S.C.; Lal, R.K. Temperature effects on seed germination potential of holy basil (Ocimum tenuiflorum). Seed Technol. 2014, 36, 75–79. [Google Scholar]
- Mortensen, L.M. The effect of air temperature on growth of eight herb species. Am. J. Plant Sci. 2014, 5, 1542–1546. [Google Scholar] [CrossRef][Green Version]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2015; ISBN 978-1-60535-255-8. [Google Scholar]
- Ribeiro, P.; Simon, J.E. Breeding sweet basil for chilling tolerance. In Issues in New Crops and New Uses; Janick, J., Whipkey, A., Eds.; ASHS Press: Alexandria, VA, USA, 2007; pp. 302–305. [Google Scholar]
- Kalisz, A.; Jezdinský, A.; Pokluda, R.; Sękara, A.; Grabowska, A.; Gil, J. Impacts of chilling on photosynthesis and chlorophyll pigment content in juvenile basil cultivars. Hortic. Environ. Biotechnol. 2016, 57, 330–339. [Google Scholar] [CrossRef]
- Al-Huqail, A.; El-Dakak, R.M.; Sanad, M.N.; Badr, R.H.; Ibrahim, M.M.; Soliman, D.; Khan, F. Effects of climate temperature and water stress on plant growth and accumulation of antioxidant compounds in sweet basil (Ocimum basilicum L.) leafy vegetable. Scientifica 2020, 3808909. [Google Scholar] [CrossRef]
- Singh, H.; Poudel, M.R.; Dunn, B.L.; Fontanier, C.; Kakani, G. Effect of greenhouse CO2 supplementation on yield and mineral element concentrations of leafy greens grown using nutrient film technique. Agronomy 2020, 10, 323. [Google Scholar] [CrossRef]
- Watanabe, C.K.; Sato, S.; Yanagisawa, S.; Uesono, Y.; Terashima, I.; Noguchi, K. Effects of elevated CO2 on levels of primary metabolites and transcripts of genes encoding respiratory enzymes and their diurnal patterns in Arabidopsis thaliana: Possible relationships with respiratory rates. Plant Cell Physiol. 2014, 55, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Gillig, S.; Heinemann, R.; Hurd, G.; Pittore, K.; Powell, D. Response of basil (Ocimum basilicum) to increased CO2 levels. In E&ES359 Global Climate Change, Johan Varekamp; Wesleyan University: Middletown, CT, USA, 2008. [Google Scholar]
- Walters, K.J.; Currey, C.J. Growth and development of basil species in response to temperature. HortScience 2019, 54, 1915–1920. [Google Scholar] [CrossRef]
- Brand, D.; Wijewardana, C.; Gao, W.; Reddy, K.R. Interactive effects of carbon dioxide, low temperature, and ultraviolet-b radiation on cotton seedling root and shoot morphology and growth. Front. Earth Sci. 2016, 10, 607–620. [Google Scholar] [CrossRef]
- Balasooriya, H.N.; Dassanayake, K.B.; Seneweera, S.; Ajlouni, S. Interaction of elevated carbon dioxide and temperature on strawberry (Fragaria × Ananassa) growth and fruit yield. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. World Acad. Sci. Eng. Technol. Int. Sci. Index 2018, 12, 279–287. [Google Scholar] [CrossRef]
- Saibo, N.J.M.; Lourenço, T.; Oliveira, M.M. Transcription factors and regulation of photosynthetic and related metabolism under environmental stresses. Ann. Bot. 2009, 103, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Barickman, T.C.; Olorunwa, O.J.; Sehgal, A.; Walne, C.H.; Reddy, K.R.; Gao, W. Interactive impacts of temperature and elevated CO2 on Basil (Ocimum Basilicum L.) root and shoot morphology and growth. Horticulturae 2021, 7, 112. [Google Scholar] [CrossRef]
- Yuan, L.; Yuan, Y.; Liu, S.; Wang, J.; Zhu, S.; Chen, G.; Hou, J.; Wang, C. Influence of high temperature on photosynthesis, antioxidative capacity of chloroplast, and carbon assimilation among heat-tolerant and heat-susceptible genotypes of nonheading chinese cabbage. HortScience 2017, 52, 1464–1470. [Google Scholar] [CrossRef]
- Camejo, D.; Rodríguez, P.; Angeles Morales, M.; Miguel Dell’Amico, J.; Torrecillas, A.; Alarcón, J.J. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol. 2005, 162, 281–289. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.; Li, X.; Mendanha dos Santos, T.; Rosenqvist, E.; Ottosen, C.-O. Combined high light and heat stress induced complex response in tomato with better leaf cooling after heat priming. Plant Physiol. Biochem. 2020, 151, 1–9. [Google Scholar] [CrossRef]
- Liu, W.; Yu, K.; He, T.; Li, F.; Zhang, D.; Liu, J. The low temperature induced physiological responses of avena nuda l., a cold-tolerant plant species. Sci. World J. 2013, 2013, 658793. [Google Scholar] [CrossRef]
- Loladze, I.; Nolan, J.M.; Ziska, L.H.; Knobbe, A.R. Rising atmospheric CO2 lowers concentrations of plant carotenoids essential to human health: A Meta-Analysis. Mol. Nutr. Food Res. 2019, 63, 1801047. [Google Scholar] [CrossRef]
- Shamloo, M.; Babawale, E.A.; Furtado, A.; Henry, R.J.; Eck, P.K.; Jones, P.J.H. Effects of genotype and temperature on accumulation of plant secondary metabolites in canadian and australian wheat grown under controlled environments. Sci. Rep. 2017, 7, 9133. [Google Scholar] [CrossRef]
- Sublett, W.L.; Barickman, T.C.; Sams, C.E. Effects of elevated temperature and potassium on biomass and quality of dark red ‘lollo rosso’ lettuce. Horticulturae 2018, 4, 11. [Google Scholar] [CrossRef]
- Jumrani, K.; Bhatia, V.S. Interactive effect of temperature and water stress on physiological and biochemical processes in soybean. Physiol. Mol. Biol. Plants 2019, 25, 667–681. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Feng, H.-L.; Wang, L.-Y.; Li, D.; Meng, Q.-W. Overexpression of thylakoidal ascorbate peroxidase shows enhanced resistance to chilling stress in tomato. J. Plant Physiol. 2012, 169, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.; Read, J.J.; McKinion, J.M. Soil-Plant-Atmosphere-Research (SPAR) facility: A tool for plant research and modeling. Biotronics 2001, 30, 27–50. [Google Scholar]
- Wijewardana, C.; Hock, M.; Henry, B.; Reddy, K.R. Screening corn hybrids for cold tolerance using morphological traits for early-season seeding. Crop Sci. 2015, 55, 851–867. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil, 2nd ed.; Circular 347; California Agricultural Experiment Station: Berkeley, CA, USA, 1950; p. 347. [Google Scholar]
- Kopsell, D.A.; Kopsell, D.E.; Lefsrud, M.G.; Curran-Celentano, J.; Dukach, L.E. Variation in lutein, β-carotene, and chlorophyll concentrations among brassica oleracea cultigens and seasons. HortScience 2004, 39, 361–364. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Kopsell, D.E.; Curran-Celentano, J. carotenoid pigments in kale are influenced by nitrogen concentration and form. J. Sci. Food Agric. 2007, 87, 900–907. [Google Scholar] [CrossRef]
- Barickman, T.C.; Kopsell, D.A.; Sams, C.E. Abscisic acid impacts tomato carotenoids, soluble sugars, and organic acids. HortScience 2016, 51, 370–376. [Google Scholar] [CrossRef]
- Ebercon, A.; Blum, A.; Jordan, W.R. A rapid colorimetric method for epicuticular wax contest of sorghum leaves. Crop Sci. 1977, 17, 179–180. [Google Scholar] [CrossRef]
- Singh, S.K.; Reddy, K.R. Regulation of photosynthesis, fluorescence, stomatal conductance and water-use efficiency of cowpea (Vigna Unguiculata [L.] Walp.) under drought. J. Photochem. Photobiol. B Biol. 2011, 105, 40–50. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophs. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in vigna seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Awasthi, R.; Gaur, P.; Turner, N.C.; Vadez, V.; Siddique, K.H.M.; Nayyar, H. Effects of individual and combined heat and drought stress during seed filling on the oxidative metabolism and yield of chickpea (Cicer Arietinum) genotypes differing in heat and drought tolerance. Crop Past. Sci. 2017, 68, 823–841. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Trevelyan, W.E.; Harrison, J.S. Studies on yeast metabolism. Fractionation and microdetermination of cell carbohydrates. Biochem. J. 1952, 50, 298–303. [Google Scholar] [CrossRef]
- Brin, M. [89] Transketolase: Clinical aspects. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1966; Volume 9, pp. 506–514. ISBN 0076-6879. [Google Scholar]
- Pramanik, H.R.; Imai, R. Functional identification of a trehalose 6-phosphate phosphatase gene that is involved in transient induction of trehalose biosynthesis during chilling stress in rice. Plant Mol. Biol. 2005, 58, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Hottiger, T.; Boller, T.; Wiemken, A. Rapid changes of heat and desiccation tolerance correlated with changes of trehalose content in saccharomyces cerevisiae cells subjected to temperature shifts. FEBS Lett. 1987, 220, 113–115. [Google Scholar] [CrossRef]
- Klutts, S.; Pastuszak, I.; Edavana, V.K.; Thampi, P.; Pan, Y.-T.; Abraham, E.C.; Carroll, J.D.; Elbein, A.D. Purification, cloning, expression, and properties of mycobacterial trehalose-phosphate phosphatase. J. Biol. Chem. 2003, 278, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Einig, W.; Hampp, R. Carbon partitioning in Norway spruce: Amounts of Fructose 2, 6-bisphosphate and of intermediates of starch/sucrose synthesis in relation to needle age and degree of needle loss. Trees 1990, 4, 9–15. [Google Scholar] [CrossRef]
| Pn | gs | Ci | ETR | E | Ci/Ca 1 | Fv’/Fm’ | |
|---|---|---|---|---|---|---|---|
| Treatment | (μmol·m2·s−1) | (mol·m2·s−1) | (μmol·m2·s−1) | (μmol m−2·s−1) | (mmol·m2·s−1) | ||
| 420 PPM | |||||||
| Control | 24.48 c | 0.38 b | 295.09 d | 187.33 ab | 6.79 c | 0.70 b | 0.47 b |
| High Temperature | 42.22 a | 0.71 a | 303.47 d | 205.94 a | 15.66 a | 0.72 b | 0.52 a |
| Low Temperature | 15.20 d | 0.14 c | 237.64 e | 146.46 bc | 1.91 d | 0.54 c | 0.42 c |
| 720 PPM | |||||||
| Control | 31.51 b | 0.31 b | 530.71 b | 184.97 ab | 6.67 c | 0.74 b | 0.51 a |
| High Temperature | 35.51 b | 0.63 a | 597.80 a | 183.19 abc | 14.39 b | 0.83 a | 0.49 ab |
| Low Temperature | 19.46 d | 0.13 c | 444.50 c | 130.16 c | 1.63 d | 0.62 c | 0.47 b |
| Treatment 2,3 | *** | *** | *** | * | *** | *** | ** |
| CO2 | NS | NS | *** | NS | NS | ** | NS |
| Trt *CO2 | *** | NS | * | NS | NS | NS | * |
| Concentration (μg·g−1 Dry Mass) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Neo a | Viol | Anth | Zea | Lut | β-car | Total Xan | ZA/ZAV b |
| 420 ppm | ||||||||
| Control | 276.43 a | 204.25 a | 68.76 ab | 163.94 a | 793.09 a | 509.66 a | 436.95 a | 0.53 a |
| High Temperature | 252.16 abc | 215.89 a | 58.23 b | 101.84 bc | 710.64 ab | 464.48 ab | 375.97 bc | 0.43 c |
| Low Temperature | 264.59 ab | 239.62 a | 74.12 a | 161.19 a | 669.04 b | 506.33 a | 474.93 a | 0.50 ab |
| 720 ppm | ||||||||
| Control | 222.36 c | 208.03 a | 74.29 a | 157.75 a | 561.09 c | 384.95 bc | 440.08 a | 0.53 a |
| High Temperature | 265.46 ab | 226.79 a | 43.14 c | 78.38 c | 687.31 b | 390.65 bc | 348.31 c | 0.35 d |
| Low Temperature | 235.20 bc | 236.26 a | 62.88 ab | 119.79 b | 552.75 c | 342.73 c | 418.93 ab | 0.44 bc |
| Treatment c,d | NS | NS | *** | *** | NS | NS | *** | *** |
| CO2 | * | NS | NS | * | *** | *** | NS | * |
| Trt *CO2 | * | NS | * | NS | * | NS | NS | NS |
| Concentration | ||||||
|---|---|---|---|---|---|---|
| nmol·g−1 DM | µmol·g−1 DM | Units/mg Protein | nmol·g−1 DM | µmol·g−1 DM | nmol·g−1 DM | |
| Treatment | MDA a | H202 | SOD | ASC | TRE | GSH |
| 420 ppm | ||||||
| Control | 0.0080 b | 0.191 a | 0.0304 c | 0.101 b | 0.0892 bc | 0.192 b |
| High Temperature | 0.0076 b | 0.206 a | 0.0306 c | 0.123 ab | 0.103 abc | 0.188 b |
| Low Temperature | 0.0074 b | 0.198 a | 0.0396 ab | 0.106 b | 0.0834 c | 0.189 b |
| 720 ppm | ||||||
| Control | 0.0078 b | 0.183 a | 0.0392 ab | 0.191 a | 0.126 ab | 0.177 b |
| High Temperature | 0.0046 b | 0.206 a | 0.0330 bc | 0.150 ab | 0.116 abc | 0.275 a |
| Low Temperature | 0.0200 a | 0.266 a | 0.0466 a | 0.175 a | 0.133 a | 0.188 b |
| Treatment b,c | ** | NS | ** | NS | NS | * |
| CO2 | NS | NS | * | ** | ** | NS |
| Trt *CO2 | ** | NS | NS | NS | NS | * |
| Treatments | Measured Temperature (°C) | CO2 (ppm) | VPD (kPa) | Mean ET (H2O L·d−1) | |
|---|---|---|---|---|---|
| Day/night | Day | Day/night | Day/night | ||
| Control | 30/22 °C, 420 ppm | 26.27 ± 0.02 | 430.47 ± 0.98 | 1.82 ± 0.01 | 14.64 ± 1.41 |
| Control + High CO2 | 30/22 °C, 720 ppm | 26.34 ± 0.01 | 731.21 ± 1.52 | 1.98 ± 0.01 | 12.60 ± 1.27 |
| High Temperature | 38/30 °C, 420 ppm | 32.16 ± 0.49 | 434.19 ± 1.21 | 2.80 ± 0.07 | 8.74 ± 0.64 |
| Low Temperature | 20/12 °C, 420 ppm | 19.53 ± 0.56 | 431.08 ± 0.66 | 0.89 ± 0.08 | 8.59 ± 0.47 |
| High Temperature + High CO2 | 38/30 °C, 720 ppm | 32.09 ± 0.49 | 728.79 ± 0.83 | 2.87 ± 0.07 | 18.41 ± 1.86 |
| Low Temperature + High CO2 | 20/12 °C, 720 ppm | 19.56 ± 0.57 | 724.78 ± 0.35 | 0.95 ± 0.09 | 6.39 ± 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barickman, T.C.; Olorunwa, O.J.; Sehgal, A.; Walne, C.H.; Reddy, K.R.; Gao, W. Yield, Physiological Performance, and Phytochemistry of Basil (Ocimum basilicum L.) under Temperature Stress and Elevated CO2 Concentrations. Plants 2021, 10, 1072. https://doi.org/10.3390/plants10061072
Barickman TC, Olorunwa OJ, Sehgal A, Walne CH, Reddy KR, Gao W. Yield, Physiological Performance, and Phytochemistry of Basil (Ocimum basilicum L.) under Temperature Stress and Elevated CO2 Concentrations. Plants. 2021; 10(6):1072. https://doi.org/10.3390/plants10061072
Chicago/Turabian StyleBarickman, T. Casey, Omolayo J. Olorunwa, Akanksha Sehgal, C. Hunt Walne, K. Raja Reddy, and Wei Gao. 2021. "Yield, Physiological Performance, and Phytochemistry of Basil (Ocimum basilicum L.) under Temperature Stress and Elevated CO2 Concentrations" Plants 10, no. 6: 1072. https://doi.org/10.3390/plants10061072
APA StyleBarickman, T. C., Olorunwa, O. J., Sehgal, A., Walne, C. H., Reddy, K. R., & Gao, W. (2021). Yield, Physiological Performance, and Phytochemistry of Basil (Ocimum basilicum L.) under Temperature Stress and Elevated CO2 Concentrations. Plants, 10(6), 1072. https://doi.org/10.3390/plants10061072







