Climate Change, Crop Yields, and Grain Quality of C3 Cereals: A Meta-Analysis of [CO2], Temperature, and Drought Effects
Abstract
1. Introduction
2. Results
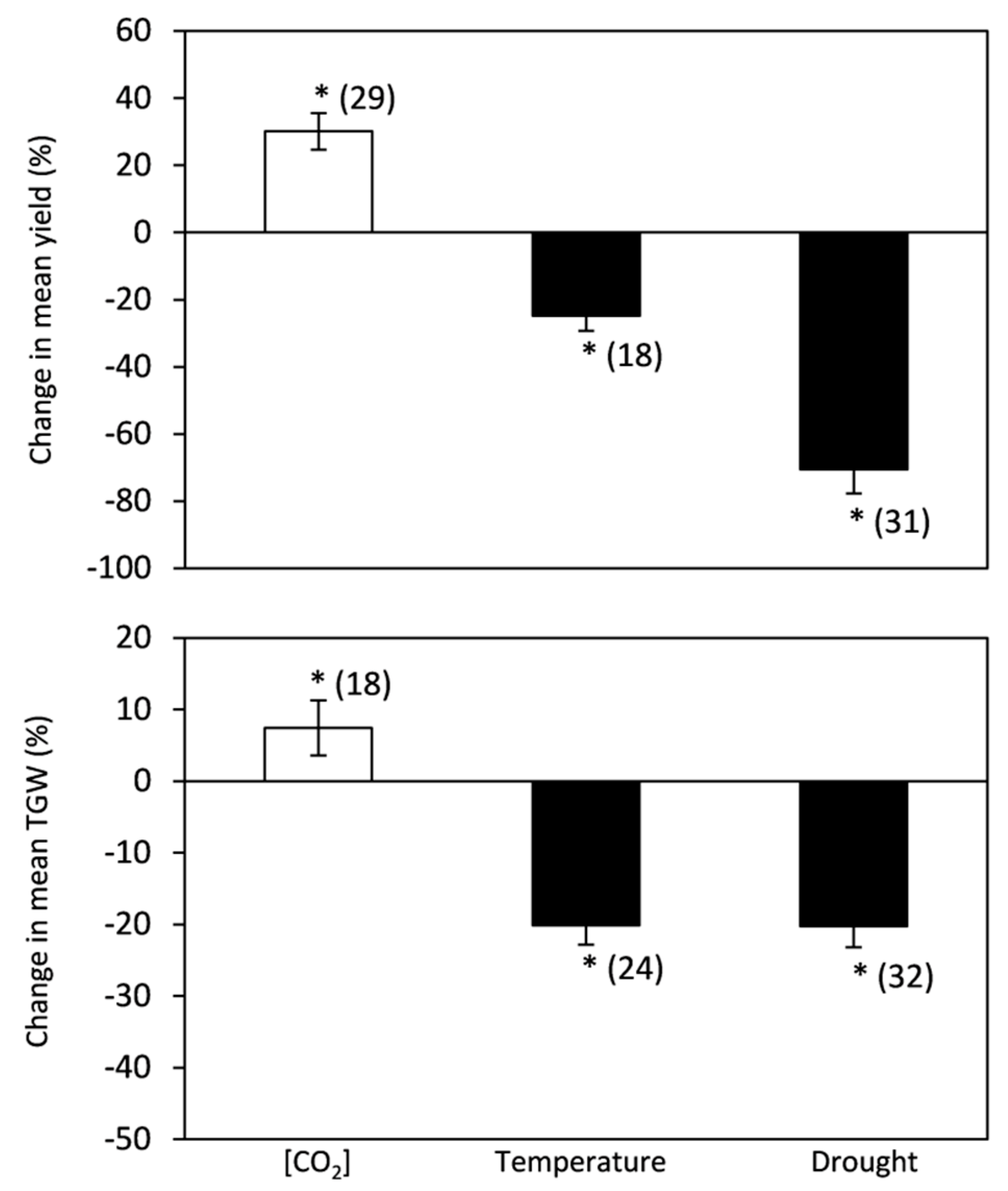
2.1. [CO2], Temperature and Drought Stress Effects on Grain Yield Components
2.2. [CO2], Temperature and Drought Stress Effects on Grain Quality
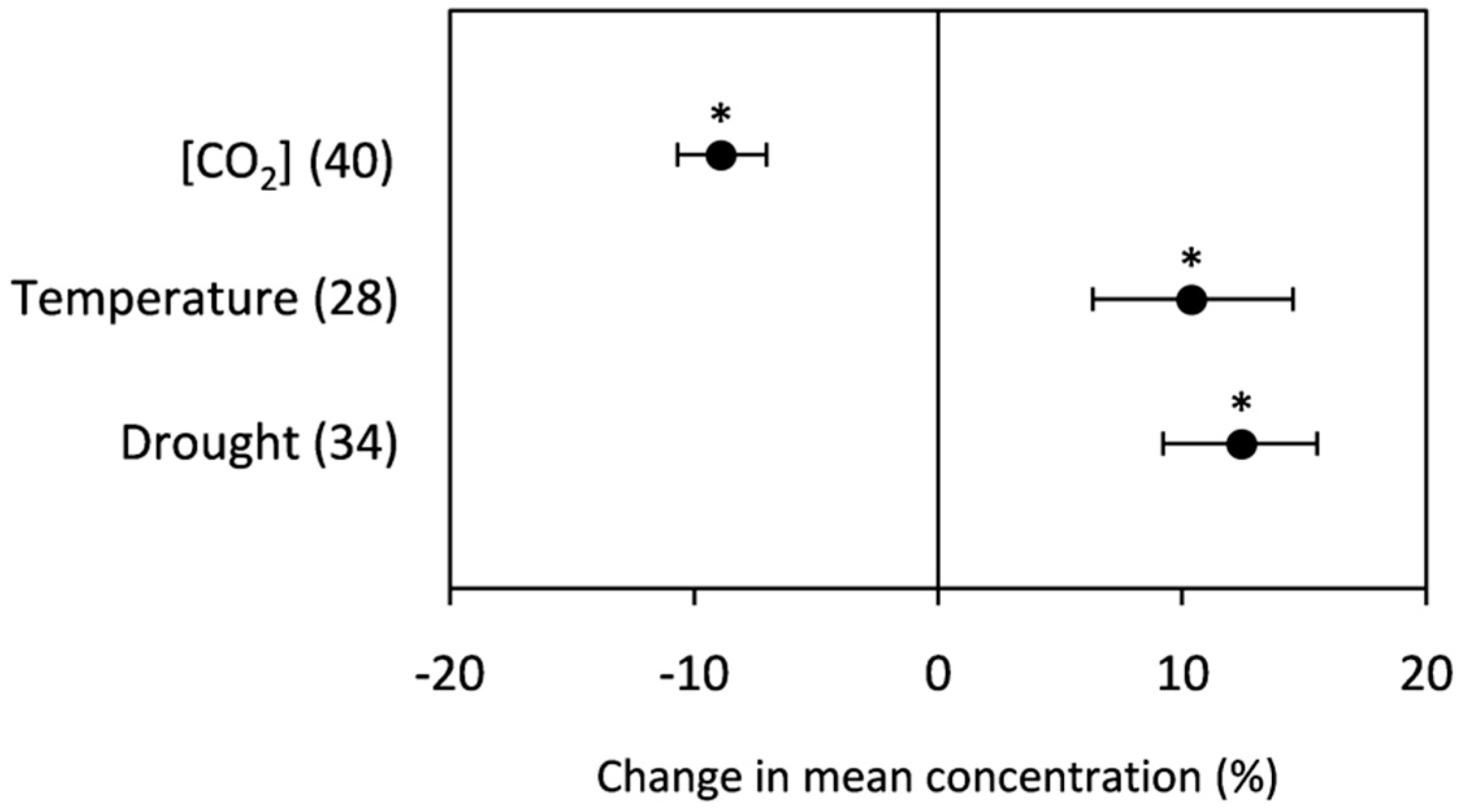
2.2.1. Starch
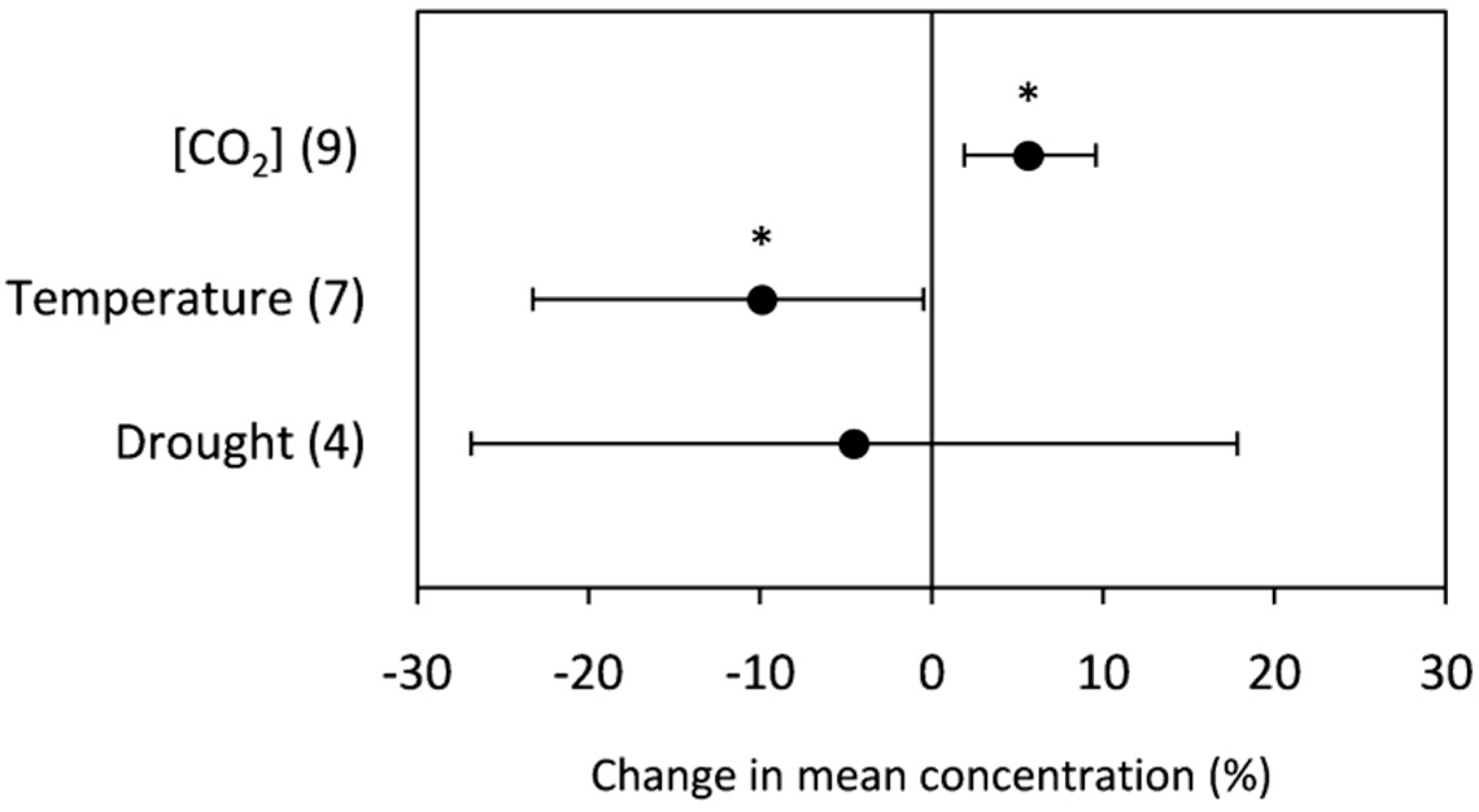
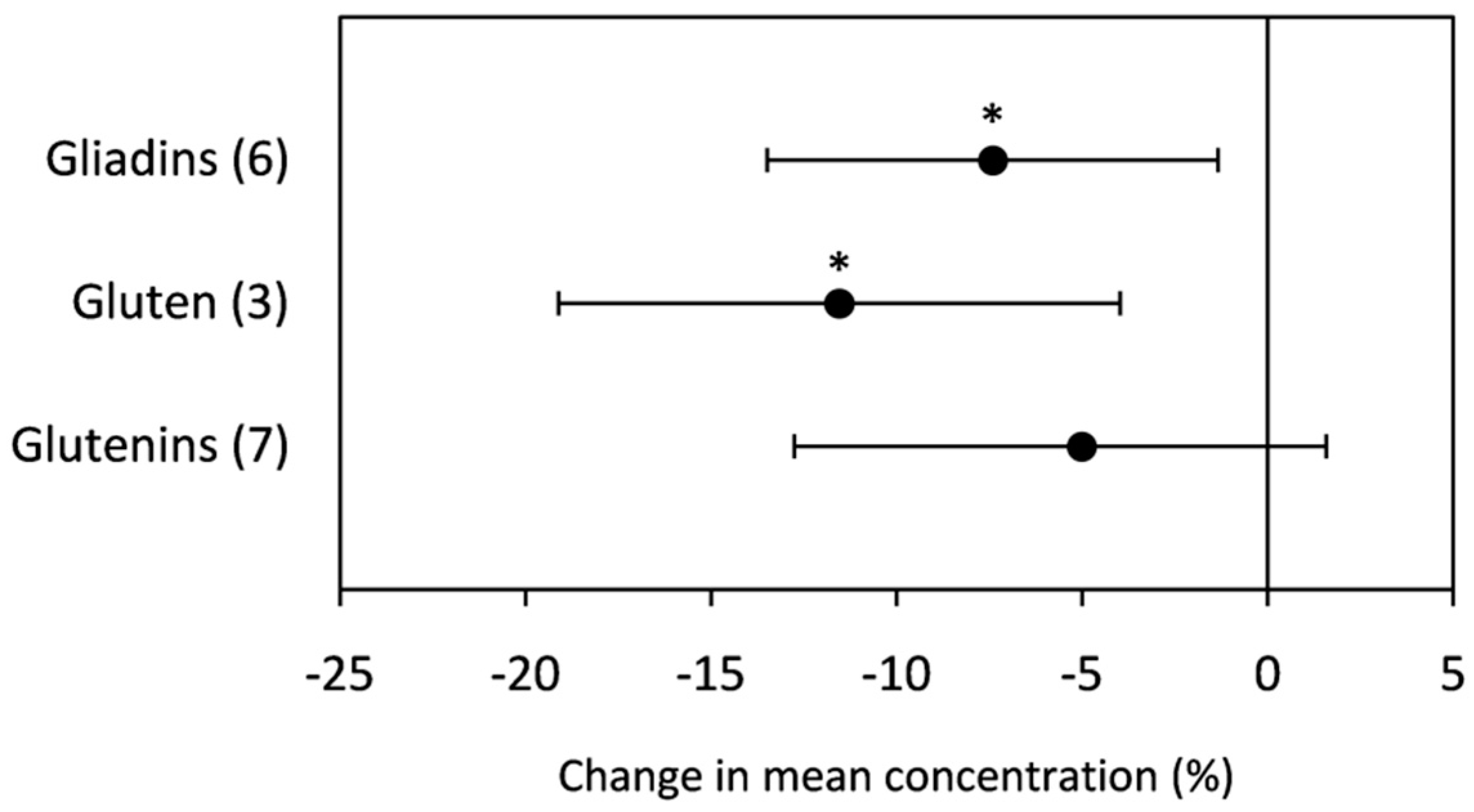
2.2.2. Total Protein
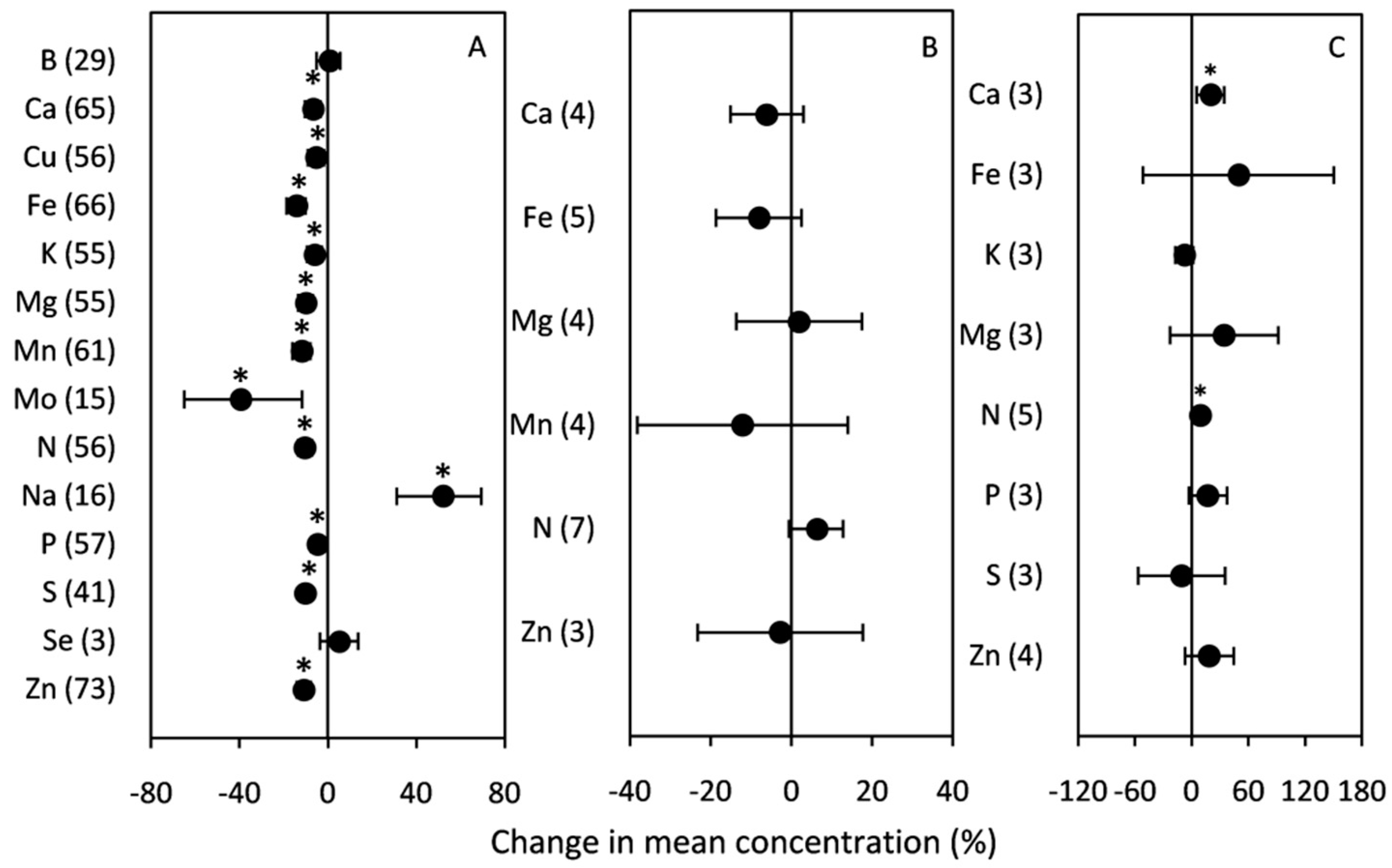
2.2.3. Mineral Composition
3. Discussion
3.1. [CO2], Temperature and Drought Stress Effects on Grain Yield Components
3.2. Effects of [CO2], Temperature and Drought Stress on Grain Quality
3.2.1. Starch
3.2.2. Total Protein
3.2.3. Mineral Composition
4. Materials and Methods
4.1. Data Search and Selection Criteria
4.2. Data Analysis
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Papers | Species |
|---|---|
| High [CO2] | |
| [92] | Triticum durum L. |
| [113] | Triticum aestivum L. |
| [17] | Triticum aestivum L. |
| [114] | Triticum aestivum L. |
| [115] | Triticumaestivum L./Hordeumvulgare |
| [26] | Oryza sativa L. |
| [116] | Oryza sativa L. |
| [73] | Triticum aestivum L. |
| [22] | Triticumdurum L./Oryza sativa L. |
| [117] | Triticum aestivum L. |
| [20] | Triticum aestivum L. |
| [118] | Triticum aestivum L. |
| [119] | Triticum aestivum L. |
| [120] | Triticum aestivum L. |
| [121] | Triticum aestivum L. |
| [122] | Triticum aestivum L. |
| [84] | Triticum aestivum L. |
| [123] | Triticum aestivum L. |
| [124] | Triticum aestivum L. |
| [125] | Triticum aestivum L. |
| [126] | Triticum aestivum L. |
| [127] | Triticum aestivum L. |
| [74] | Triticum aestivum L. |
| [128] | Triticum aestivum L. |
| [129] | Triticum durum L. |
| [38] | Oryza sativa L. |
| [80] | Triticum durum L. |
| [18] | Triticum durum L. |
| [19] | Triticum aestivum L. |
| [130] | Triticum aestivum L. |
| [131] | Oryza sativa L. |
| [132] | Triticum aestivum L. |
| [133] | Triticum aestivum L. |
| [134] | Triticum durum L. |
| [35] | Oryza sativa L. |
| [135] | Oryza sativa L. |
| Drought stress | |
| [136] | Triticum aestivum L. |
| [57] | Triticum durum L. |
| [137] | Triticum aestivum L. |
| [138] | Triticum aestivum L. |
| [139] | Triticum durum L. |
| [140] | Triticum durum L. |
| [141] | Triticum aestivum L. |
| [55] | Triticum durum L. |
| [62] | Triticum aestivum L. |
| [80] | Triticum durum L. |
| [60] | Oryza sativa L. |
| [61] | Triticum aestivum L. |
| [129] | Triticum durum L. |
| [126] | Triticum aestivum L. |
| [56] | Triticum aestivum L. |
| [142] | Triticum aestivum L. |
| [37] | Triticumaestivum L., Triticumdurum L. |
| [143] | Triticum aestivum L. |
| [144] | Triticum aestivum L. |
| [145] | Triticum aestivum L. |
| [51] | Triticum aestivum L. |
| Heat stress | |
| [36] | Triticum durum L. |
| [51] | Triticum aestivum L. |
| [32] | Triticum aestivum L. |
| [146] | Triticumaestivum L., Triticumdurum L. |
| [59,147] | Triticum aestivum L. |
| [45] | Triticum aestivum L. |
| [148] | Triticumaestivum L., Triticumdurum L. |
| [31] | Triticum durum L. |
| [145] | Triticum durum L. |
| [39] | Triticum aestivum L. |
| [74] | Triticum aestivum L. |
| [149] | Triticum aestivum L. |
| [46] | Triticum aestivum L. |
| [150] | Triticumaestivum L., Triticumdurum L. |
| [151] | Triticum aestivum L. |
| [37] | Triticumaestivum L., Triticumdurum L. |
| [152] | Triticum aestivum L. |
| [153] | Triticum aestivum L. |
| [41] | Triticum aestivum L. |
| [154] | Triticum durum L. |
References
- Food and Agriculture Organization of the United Nations (FAO). The Future of Food and Agriculture—Alternative Pathways to 2050. Summary Version; FAO: Rome, Italy, 2018; 60p. [Google Scholar]
- United Nations. United Nations Population Division (UNPD). Available online: http://www.un.org/en/development/desa/population/ (accessed on 12 March 2019).
- Ortiz-Bobea, A.; Ault, T.R.; Carillo, C.M.; Chambers, R.G.; Lobell, D.B. Anthropogenic climate change has slowed global agricultural productivity growth. Nat. Clim. Chang. 2021, 11, 306–312. [Google Scholar] [CrossRef]
- Nuttall, J.G.; O’Leary, G.J.; Panozzo, J.F.; Walker, C.K.; Barlow, K.M.; Fitzgerald, G.J. Models of grain quality in wheat—A review. Field Crop. Res. 2017, 202, 136–145. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). World Food and Agriculture. Statistical Pocketbook; FAO: Rome, Italy, 2018; 254p. [Google Scholar]
- Foreign Agriculture Service/United States Department of Agriculture. World Agricultural Production; Circular Series WAP; USDA: Washington, DC, USA, 2019. [Google Scholar]
- Singh, S.; Gupta, A.K.; Kaur, N. Influence of drought and sowing time on protein composition, antinutrients, and mineral contents of wheat. Sci. World J. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef]
- Loladze, I. Hidden shift of the ionome of plants exposed to elevated CO2 depletes minerals at the base of human nutrition. eLife 2014, 3, e02245. [Google Scholar] [CrossRef]
- Zhang, G.; Sakai, H.; Tokida, T.; Usui, Y.; Zhu, C.; Nakamura, H.; Yoshimoto, M.; Fukuoka, M.; Kobayashi, K.; Hasegawa, T. The effects of free-air CO2 enrichment (FACE) on carbon and nitrogen accumulation in grains of rice. J. Exp. Bot. 2013, 64, 3179–3188. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, J.; Wollenweber, B.; Liu, F.; Dai, T.; Cao, W.; Jiang, D. Multiple heat and drought events affect grain yield and accumulations of high molecular weight glutenin subunits and glutenin macropolymers in wheat. J. Cereal Sci. 2013, 57, 134–140. [Google Scholar] [CrossRef]
- Broberg, M.; Högy, P.; Pleijel, H. CO2-Induced Changes in Wheat Grain Composition: Meta-Analysis and Response Functions. Agronomy 2017, 7, 32. [Google Scholar] [CrossRef]
- Ghannoum, O.; Caemmerer, S.V.; Ziska, L.H.; Conroy, J.P. The growth response of C4 plants to rising atmospheric CO2 partial pressure: A reassessment. Plant Cell Environ. 2000, 23, 931–942. [Google Scholar] [CrossRef]
- Loladze, I. Rising atmospheric CO2 and human nutrition: Toward globally imbalanced plant stoichiometry? Trends Ecol. Evol. 2002, 17, 457–461. [Google Scholar] [CrossRef]
- Taub, D.R.; Miller, B.; Allen, H. Effects of elevated CO2 on the protein concentration of food crops: A meta-analysis. Glob. Chang. Biol. 2008, 14, 565–575. [Google Scholar] [CrossRef]
- Medek, D.E.; Schwartz, J.; Myers, S.S. Estimated effects of future atmospheric CO2 concentrations on protein intake and the risk of protein deficiency by country and region. Environ. Health Perspect. 2017, 125, 1–8. [Google Scholar] [CrossRef]
- Högy, P.; Wieser, H.; Köhler, P.; Schwadorf, K.; Breuer, J.; Franzaring, J.; Muntifering, R.; Fangmeier, A. Effects of elevated CO2 on grain yield and quality of wheat: Results from a 3-year free-air CO2 enrichment experiment. Plant Biol. 2009, 11 (Suppl.1), 60–69. [Google Scholar] [CrossRef]
- Fernando, N.; Panozzo, J.; Tausz, M.; Norton, R.M.; Fitzgerald, G.J.; Seneweera, S. Rising atmospheric CO2 concentration affects mineral nutrient and protein concentration of wheat grain. Food Chem. 2012, 133, 1307–1311. [Google Scholar] [CrossRef]
- Fernando, N.; Panozzo, J.; Tausz, M.; Norton, R.M.; Fitzgerald, G.J.; Myers, S.; Nicolas, M.E.; Seneweera, S. Intraspecific variation of wheat grain quality in response to elevated [CO2] at two sowing times under rain-fed and irrigation treatments. J. Cereal Sci. 2014, 59, 137–144. [Google Scholar] [CrossRef]
- Fernando, N.; Panozzo, J.; Tausz, M.; Norton, R.M.; Neumann, N.; Fitzgerald, G.J.; Seneweera, S. Elevated CO2 alters grain quality of two bread wheat cultivars grown under different environmental conditions. Agric. Ecosyst. Environ. 2014, 185, 24–33. [Google Scholar] [CrossRef]
- Fernando, N.; Panozzo, J.; Tausz, M.; Norton, R.M.; Fitzgerald, G.J.; Myers, S.; Walter, C.; Stangoules, J.; Seneweera, S. Wheat grain quality under increasing atmospheric CO2 concentrations in a semi-arid cropping system. J. Cereal Sci. 2012, 56, 684–690. [Google Scholar] [CrossRef]
- Dietterich, L.H.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.B.; Bloom, A.J.; Carlisle, E.; Fernando, N.; Fitzgerald, G.; Hasegawa, T.; et al. Impacts of elevated atmospheric CO2 on nutrient content of important food crops. Sci. Data 2015, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; et al. Rising CO2 threatens human nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef]
- Weyant, C.; Brandeau, M.L.; Burke, M.; Lobell, D.B.; Bendavid, E.; Basu, S. Anticipated burden and mitigation of carbon-dioxide-induced nutritional deficiencies and related diseases: A simulation modeling study. PLoS Med. 2018, 15, e1002586. [Google Scholar] [CrossRef]
- Beach, R.H.; Sulser, T.B.; Crimmins, A.; Cenacchi, N.; Cole, J.; Fukagawa, N.K.; Mason-D’Croz, D.; Myers, S.; Sarofin, M.C.; Smith, M.; et al. Combining the effects of increased atmospheric carbon dioxide on protein, iron, zinc availability and projected climate change on global diets: A modelling study. Lancet Planet. Health 2019, 3, e307–e317. [Google Scholar] [CrossRef]
- Zhu, C.; Kobayashi, K.; Loladze, I.; Zhu, J.; Jiang, Q.; Xu, X.; Liu, G.; Seneweera, S.; Ebi, K.L.; Drewnowski, A.; et al. Carbon dioxide (CO2) levels this century will alter the protein, micronutrients and vitamin content of rice grains with potential health consequences for the poorest rice-dependent countries. Sci. Adv. 2018, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Loladze, I.; Nolan, J.M.; Ziska, L.H.; Knobbe, A.R. Rising atmospheric CO2 lowers concentrations of plant carotenoids essential to human health: A meta-analysis. Mol. Nutr. Food Res. 2019, 63, 1–9. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2014: Synthesis Report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; 151p. [Google Scholar]
- Lopez-Bustins, J.A.; Pla, E.; Nadal, M.; De Herralde, F.; Savé, R. Global change and viticulture in the Mediterranean region: A case of study in north-eastern Spain. Span. J. Agric. Res. 2014, 12, 78–88. [Google Scholar] [CrossRef]
- Meehl, G.A.; Stocker, T.F.; Collins, W.D.; Friedlingstein, P.; Gaye, A.T.; Gregory, J.M.; Kitoh, A.; Knutti, R.; Murphy, J.M.; Noda, A.; et al. Global Climate Projections. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 747–845. [Google Scholar]
- Lizana, X.C.; Calderini, D.F. Yield and grain quality of wheat in response to increased temperatures at key periods for grain number and grain weight determination: Considerations for the climatic change scenarios of Chile. J. Agric. Sci. 2013, 151, 209–221. [Google Scholar] [CrossRef]
- Castro, M.; Peterson, G.J.; Rizza, M.D.; Dellavalle, P.D.; Vázquez, D.; Ibáñez, V.; Ross, A. Influence of heat stress on wheat grain characteristics and protein molecular weight distribution. Wheat Prod. Stress Environ. 2007, 12, 365–371. [Google Scholar] [CrossRef]
- Usui, Y.; Sakai, H.; Tokida, T.; Nakamura, H.; Nakagawa, H.; Hasegawa, T. Heat-tolerant rice cultivars retain grain appearance quality under free-air CO2 enrichment. Rice 2014, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bahuguna, R.N.; Jha, J.; Pal, M.; Shah, D.; Lawas, L.M.; Khetarpal, S.; Jagadish, S.V.K. Physiological and biochemical characterization of NERICA-L-44: A novel source of heat tolerance at the vegetative and reproductive stages in rice. Physiol. Plant. 2015, 154, 543–559. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Bahuguna, R.N.; Pal, M.; Shah, D.; Maurya, S.; Jagadish, K.S.V. Elevated CO2 and heat stress interactions affect grain yield, quality and mineral nutrient composition in rice under field conditions. Field Crop. Res. 2017, 206, 149–157. [Google Scholar] [CrossRef]
- Ferreira, M.S.L.; Martre, P.; Mangavel, C.; Girousse, C.; Rosa, N.N.; Samson, M.F.; Morel, M.H. Physicochemical control of durum wheat grain filling and glutenin polymer assembly under different temperature regimes. J. Cereal Sci. 2012, 56, 58–66. [Google Scholar] [CrossRef]
- Guzmán, C.; Autrique, J.E.; Mondal, S.; Singh, R.P.; Govindan, V.; Morales-Dorantes, A.; Posadas-Romano, G.; Crossa, J.; Ammar, K.; Peña, R.J. Response to drought and heat stress on wheat quality, with special emphasis on bread-making quality, in durum wheat. Field Crop. Res. 2016, 186, 157–165. [Google Scholar] [CrossRef]
- Jing, L.; Wang, J.; Shen, S.; Wang, Y.; Zhu, J.; Wang, Y.; Yang, L. The impact of elevated CO2 and temperature on grain quality of rice grown under open-air field conditions. J. Sci. Food Agric. 2016, 96, 3658–3667. [Google Scholar] [CrossRef] [PubMed]
- Dupont, F.M.; Hurkman, W.J.; Vensel, W.H.; Tanaka, C.; Kothari, K.M.; Chung, O.K.; Altenbach, S.B. Protein accumulation and composition in wheat grains: Effects of mineral nutrients and high temperature. Eur. J. Agron. 2006, 25, 96–107. [Google Scholar] [CrossRef]
- Savill, G.P.; Michalski, A.; Powers, S.J.; Wan, Y.; Tosi, P.; Buchner, P.; Hawkesford, M.J. Temperature and nitrogen supply interact to determine protein distribution gradients in the wheat grain endosperm. J. Exp. Bot. 2018, 69, 3117–3126. [Google Scholar] [CrossRef]
- Wardlaw, I.F. Interaction Between Drought and Chronic High Temperature During Kernel Filling in Wheat in a Controlled Environment. Ann. Bot. 2002, 90, 469–476. [Google Scholar] [CrossRef]
- Yang, H.; Gu, X.; Ding, M.; Lu, W.; Lu, D. Heat stress during grain filling affects activities of enzymes involved in grain protein and starch synthesis in waxy maize. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Monjardino, P.; Smith, A.G.; Jones, R.J. Heat stress effects on protein accumulation of maize endosperm. Crop. Sci. 2005, 45, 1203–1210. [Google Scholar] [CrossRef]
- Ziska, L.H.; Namuco, O.; Moya, T.; Quilang, J. Growth and yield response of field-grown tropical rice to increasing carbon dioxide and air temperature. Agron. J. 1997, 89, 45–53. [Google Scholar] [CrossRef]
- Randall, P.J.; Moss, H.J. Some effects of temperature regime during grain filling on wheat quality. Aust. J. Agric. Res. 1990, 41, 603–617. [Google Scholar] [CrossRef]
- Gooding, M.J.; Ellis, R.H.; Shewry, P.R.; Schofield, J.D. Effects of restricted water availability and increased temperature on the grain filling, drying and quality of winter wheat. J. Cereal Sci. 2003, 37, 295–309. [Google Scholar] [CrossRef]
- Habash, D.Z.; Kehel, Z.; Nachit, M. Genomic approaches for designing durum wheat ready for climate change with a focus on drought. J. Exp. Bot. 2009, 60, 2805–2815. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Summary for Policymakers. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- McKersie, B. Planning for food security in a changing climate. J. Exp. Bot. 2015, 66, 3435–3450. [Google Scholar] [CrossRef]
- Giorgi, F.; Lionello, P. Climate change projections for the Mediterranean region. Glob. Planet. Chang. 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Altenbach, S.B.; DuPont, F.M.; Kothari, K.M.; Chan, R.; Johnson, E.L.; Lieu, D. Temperature, water and fertilizer influence the timing of key events during grain development in a US spring wheat. J. Cereal Sci. 2003, 37, 9–20. [Google Scholar] [CrossRef]
- Russo, A.C.; Gouveia, C.M.; Trigo, R.M.; Liberato, M.L.R.; DaCamara, C. The influence of circulation weather patterns at different spatial scales on drought variability in the Iberian Peninsula. Front. Environ. Sci. 2015, 3, 1–15. [Google Scholar] [CrossRef]
- Araus, J.L.; Slafer, G.A.; Royo, C.; Serret, M.D. Breeding for yield potential and stress adaptation in cereals. Crit. Rev. Plant Sci. 2008, 27, 377–412. [Google Scholar] [CrossRef]
- Rebolledo, M.C.; Luquet, D.; Courtois, B.; Henry, A.; Soulié, J.C.; Rouan, L.; Dingkuhn, M. Can early vigour occur in combination with drought tolerance and efficient water use in rice genotypes? Funct. Plant Biol. 2013, 40, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Kiliç, H.; Yagbasanlar, T. The Effect of drought stress on grain yield, yield components and some quality traits of durum wheat (Triticum turgidum ssp. durum) Cultivars. Not. Bot. Hort. Agrobot. Cluj-Napoca 2010, 38, 164–170. [Google Scholar] [CrossRef]
- Balla, K.; Rakszegi, M.; Li, Z.; Békés, F.; Bencze, S.; Veisz, O. Quality of winter wheat in relation to heat and drought shock after anthesis. Czech J. Food Sci. 2011, 29, 117–128. [Google Scholar] [CrossRef]
- Houshmand, S.; Arzani, A.; Mirmohammadi-Maibody, S.A.M. Effects of salinity and drought stress on grain quality of durum wheat. Commun. Soil Sci. Plant Anal. 2014, 45, 297–308. [Google Scholar] [CrossRef]
- Sharma, S.; Carena, M.J. Grain quality in Maize (Zea mays L.): Breeding implications for short-season drought environments. Euphytica 2016, 212, 247–260. [Google Scholar] [CrossRef]
- Panozzo, J.F.; Eagles, H.A. Cultivar and environmental effects on quality characters in wheat. I. Aust. J. Agric. Res. 1998, 49, 757–766. [Google Scholar] [CrossRef]
- Crusciol, C.A.C.; Arf, O.; Soratto, R.P.; Mateus, G.P. Grain quality of upland rice cultivars in response to cropping systems in the Brazilian tropical savanna. Sci. Agric. 2008, 65, 468–473. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, A.K.; Gupta, S.K.; Kaur, N. Effect of sowing time on protein quality and starch pasting characteristics in wheat (Triticum aestivum L.) genotypes grown under irrigated and rain-fed conditions. Food Chem. 2010, 122, 559–565. [Google Scholar] [CrossRef]
- Abd El-Kareem, T.H.A.; El-Saidy, A.E.A. Evaluation of yield and grain quality of some bread wheat genotypes under normal irrigation and drought stress conditions in calcareous soils. J. Biol. Sci. 2011, 11, 156–164. [Google Scholar] [CrossRef][Green Version]
- Flagella, Z.; Giuliani, M.M.; Giuzio, L.; Volpi, Z.; Masci, S. Influence of water deficit on durum wheat storage protein composition and technological quality. Eur. J. Agron. 2010, 33, 197–207. [Google Scholar] [CrossRef]
- Peleg, Z.; Saranga, Y.; Yazici, A.; Fahima, T.; Ozturk, L.; Cakmak, I. Grain zinc, iron and protein concentrations and zinc-efficiency in wild emmer wheat under contrasting irrigation regimes. Plant Soil 2008, 306, 57–67. [Google Scholar] [CrossRef]
- Wilcox, J.; Makowski, D. A meta-analysis of the predicted effects of climate change on wheat yields using simulation studies. Field Crop. Res. 2014, 156, 180–190. [Google Scholar] [CrossRef]
- Knox, J.; Daccache, A.; Hess, T.; Haro, D. Meta-analysis of climateimpacts and uncertainty on crop yields in Europe. Environ. Res. Lett. 2016, 11, 1–10. [Google Scholar] [CrossRef]
- Dixit, P.N.; Telleria, R.; Al Khatib, A.N.; Allouzi, S.F. Decadal analysis of impact of future climate on wheat production in dry Mediterranean environment: A case of Jordan. Sci. Total. Environ. 2018, 610, 219–233. [Google Scholar] [CrossRef]
- Kimball, B.A.; Morris, C.F.; Pinter, P.J.; Wall, G.W.; Hunsaker, D.J.; Adamsen, F.J.; Lamorte, R.L.; Leavitt, S.W.; Thompson, T.L.; Matthias, A.D.; et al. Elevated CO2, drought, and soil nitrogen effects on wheat grain quality. New Phytol. 2001, 150, 295–303. [Google Scholar] [CrossRef]
- Aranjuelo, I.; Cabrera-Bosquet, L.; Morcuende, R.; Avice, J.-C.; Nogués, S.; Araus, J.L.; Martínez-Carrasco, R.; Pérez, P. Does ear C sink strength contribute to overcoming photosynthetic acclimation of wheat plants exposed to elevated CO2? J. Exp. Bot. 2011, 62, 3957–3969. [Google Scholar] [CrossRef] [PubMed]
- Aranjuelo, I.; Sanz-Sáez, A.; Jauregui, I.; Irigoyen, J.J.; Araus, J.L.; Sánchez-Díaz, M.; Erice, G. Harvest index, a parameter conditioning responsiveness of wheat plants to elevated CO2. J. Exp. Bot. 2013, 64, 1879–1892. [Google Scholar] [CrossRef] [PubMed]
- Lobell, D.B.; Schlenker, W.; Costa-Roberts, J. Climate trends and global crop production since 1980. Science 2011, 333, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Bourgault, M.; Dreccer, M.F.; James, A.T.; Chapman, S.C. Genotypic variability in the response to elevated CO2 of wheat lines differing in adaptive traits. Funct. Plant Biol. 2013, 40, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Pleijel, H.; Högy, P. CO2 dose-response functions for wheat grain, protein and mineral yield based on FACE and open-top chamber experiments. Environ. Pollut. 2015, 198, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.A.C.; Lawlor, D.W.; Mitchell, V.J.; Gibbard, C.L.; White, E.M.; Porter, J.R. Effects of elevated CO2 concentration and increased temperature on winter wheat: Test of ARCWHEAT1 simulation model. Plant Cell Environ. 1995, 18, 736–748. [Google Scholar] [CrossRef]
- Talukder, S.K.; Babar, M.A.; Vijaylakshmi, K.; Poland, J.; Prasad, P.V.V.; Bowden, R.; Fritz, A. Mapping QTL for the traits associated with heat tolerance in wheat (Triticum aestivum L.). BMC Genet. 2014, 15, 97. [Google Scholar] [CrossRef]
- Akter, N.; Islam, M.R. Heat stress effects and management in wheat. A review. Agron. Sustain. Dev. 2017, 37, 1–17. [Google Scholar] [CrossRef]
- Xiong, D.; Ling, X.; Huang, J.; Peng, S. Meta-analysis and dose-response analysis of high temperature effects on rice yield and quality. Environ. Exp. Bot. 2017, 141, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Hernandez-Espinosa, N.; Peña, R.J. The influence of drought and heat stress on the expression of end-use quality parameters of common wheat. J. Cereal Sci. 2013, 57, 73–78. [Google Scholar] [CrossRef]
- Ziska, L.H.; Bunce, J.A.; Shimono, H.; Gealy, D.R.; Baker, J.T.; Newton, P.C.D.; Matthew, P.R.; Jagadish, K.S.V.; Zhu, C.; Howden, M.; et al. Security and climate change: On the potential to adapt global crop production by active selection to rising atmospheric carbon dioxide. Proc. R. Soc. B Biol. Sci. 2012, 279, 4097–4105. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, N.; Bettoni, M.M.; Fuertes-Mendizábal, T.; González-Murua, C.; Aranjuelo, I. Durum wheat quality traits affected by mycorrhizal inoculation, water availability and atmospheric CO2 concentration. Crop Pasture Sci. 2016, 67, 147–155. [Google Scholar] [CrossRef]
- Jung, K.H.; An, G.; Ronald, P.C. Towards a better bowl of rice: Assigning function to tens of thousands of rice genes. Nature 2008, 9, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Thitisaksakul, M.; Jiménez, R.C.; Arias, M.C.; Beckles, D.M. Effects of environmental factors on cereal starch biosynthesis and composition. J. Cereal Sci. 2012, 56, 67–80. [Google Scholar] [CrossRef]
- Högy, P.; Fangmeier, A. Effects of elevated atmospheric CO2 on grain quality of wheat. J. Cereal Sci. 2008, 48, 580–591. [Google Scholar] [CrossRef]
- Fangmeier, A.; De Temmerman, L.; Mortensen, L.; Kemp, K.; Burke, J.; Mitchell, R.; Van Oijen, M.; Weigel, H.J. Effects on nutrients and on grain quality in spring wheat crops grown under elevated CO2 concentrations and stress conditions in the European, multiple-site experiment “ESPACE-wheat”. Eur. J. Agron. 1999, 10, 215–229. [Google Scholar] [CrossRef]
- Worch, S.; Rajesh, K.; Harshavardhan, V.T.; Pietsch, C.; Korzun, V.; Kuntze, L.; Börner, A.; Wobus, U.; Röder, M.S.; Sreenivasulu, N. Haplotyping, linkage mapping and expression analysis of barley genes regulated by terminal drought stress influencing seed quality. BMC Plant Biol. 2011, 11, 1–14. [Google Scholar] [CrossRef]
- Avila, R.G.; Da Silva, E.M.; Magalhães, P.C.; De Alvarenga, A.A.; De Oliveira Lavinsky, A. Drought changes yield and organic and mineral composition of grains of four maize genotypes. Acad. J. Agric. Res. 2017, 5, 243–250. [Google Scholar] [CrossRef]
- Hawker, J.S.; Jenner, C.F. High temperature affects the activity of enzymes in the committed pathway of starch synthesis in developing wheat endosperm. Aust. J. Plant Physiol. 1993, 20, 197–209. [Google Scholar] [CrossRef]
- Keeling, P.L.; Bacon, P.J.; Holt, D.C. Elevated temperature reduced starch deposition in wheat endosperm by reducing the activity of soluble starch synthase. Planta 1993, 191, 342–348. [Google Scholar] [CrossRef]
- Goufo, P.; Falco, V.; Brites, C.M.; Wessel, D.F.; Kratz, S.; Rosa, E.A.S.; Carranca, C.; Trindade, H. Effect of Elevated Carbon Dioxide Concentration on Rice Quality: Nutritive Value, Color, Milling and Cooking/Eating Qualities. Cereal Chem. 2014, 91, 513–521. [Google Scholar] [CrossRef]
- Fangmeier, A.; Chrost, B.; Ho, P.; Krupinska, K. CO2 enrichment enhances flag leaf senescence in barley due to greater grain nitrogen sink capacity. Environ. Exp. Bot. 2000, 44, 151–164. [Google Scholar] [CrossRef]
- Bloom, A.J.; Burger, M.; Kimball, B.A.; Pinter, P.J. Nitrate assimilation is inhibited by elevated CO2 in field-grown wheat. Nat. Clim. Chang. 2014, 4, 477–480. [Google Scholar] [CrossRef]
- Wieser, H.; Manderscheid, R.; Erbs, M.; Weigel, H.J. Effects of elevated atmospheric CO2 concentrations on the quantitative protein composition of wheat grain. J. Agric. Food Chem. 2008, 56, 6531–6535. [Google Scholar] [CrossRef] [PubMed]
- Weegels, P.L.; Hamer, R.J.; Schofield, J.D. Critical review: Functional properties of wheat glutenin. J. Cereal Sci. 1996, 23, 1–17. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J. Grain filling of cereals under soil drying. New Phytol. 2006, 169, 223–236. [Google Scholar] [CrossRef]
- Brdar, M.D.; Kraljević-Balalić, M.M.; Kobiljski, B.D. The parameters of grain filling and yield components in common wheat (Triticum aestivum L.) and durum wheat (Triticum turgidum L. var. durum). Cent. Eur. J. Biol. 2008, 3, 75–82. [Google Scholar] [CrossRef]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef]
- Gallé, A.; Csiszár, J.; Secenji, M.; Guóth, A.; Cseuz, L.; Tari, I.; Györgyey, J.; Erdei, L. Drought response strategies during grain filling in wheat. Glutathione transferase activity and expression pattern in flag leaves. J. Plant Physiol. 2009, 170, 1389–1399. [Google Scholar] [CrossRef]
- Bhullar, S.S.; Jenner, C.F. Effects of temperature on conversion of sucrose to starch in the developing wheat endosperm. Aust. J. Plant. Physiol. 1986, 13, 605–615. [Google Scholar] [CrossRef]
- Wang, Y.; Frei, M. Stressed food—The impact of abiotic environmental stresses on crop quality. Agric. Ecosyst. Environ. 2011, 141, 271–286. [Google Scholar] [CrossRef]
- Blum, A. Improving wheat grain filling under stress by stem reserve mobilization. Euphytica 1998, 100, 77–83. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Wang, Z.; Zhu, Q.; Liu, L. Involvement of abscisic acid and cytokinins int the senescence and remobilization of carbon reserves in wheat subjected to water stress during grain filling. Plant Cell. Environ. 2003, 26, 1621–1631. [Google Scholar] [CrossRef]
- Srivastava, A.; Srivastava, P.; Sharma, A.; Sarlach, R.S.; Bains, N.S. Effect of stem reserve mobilization on grain filling under drought stress conditions in recombinant inbred population of wheat. J. Appl. Nat. Sci. 2017, 9, 1–5. [Google Scholar] [CrossRef][Green Version]
- Houshmandfar, A.; Fitzgerald, G.J.; Tausz, M. Elevated CO2 decreases both transpiration flow and concentrations of Ca and Mg in the xylem sap of wheat. J. Plant Physiol. 2015, 174, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Gifford, R.M.; Barrett, D.J.; Lutze, J.L. The effects of elevated [CO2] on the C:N and C:P mass ratios of plant tissues. Plant Soil 2000, 224, 1–14. [Google Scholar] [CrossRef]
- Bloom, A.J.; Lancaster, K.M. Manganese binding to Rubisco could drive a photorespiratory pathway that increases the energy efficiency of photosynthesis. Nat. Plants 2018, 4, 414–422. [Google Scholar] [CrossRef]
- Stone, P.J.; Grast, P.W.; Nicolas, M.E. The influence of recovery temperature on the effects of a brief heat shock on wheat. III. grain protein composition and dough properties. J. Cereal Sci. 1997, 25, 129–141. [Google Scholar] [CrossRef]
- Ge, T.D.; Sui, F.G.; Nie, S.; Sun, N.B.; Xiao, H.; Tong, C.L. Differential responses of yield and selected nutritional compositions to drought stress in summer maize grains. J. Plant. Nut. 2010, 33, 1811–1818. [Google Scholar] [CrossRef]
- Farahani, S.M.; Mazaheri, D.; Chaichi, M.; Afshari, R.T.; Savaghebi, G. Effect of seed vigour on stress tolerance of barley (Hordeum vulgare) seed at germination stage. Seed Sci. Technol. 2010, 38, 494–507. [Google Scholar] [CrossRef]
- Miller, R.O.; Jacobsen, J.S.; Skogley, E.O. Aerial accumulation and partitioning of nutrients by hard red spring wheat. Commun. Soil Sci. Plant Anal. 1994, 24, 2389–2407. [Google Scholar] [CrossRef]
- Velu, G.; Guzman, C.; Mondal, S.; Autrique, J.E.; Huerta, J.; Singh, R.P. Effect of drought and elevated temperature on grain zinc and iron concentrations in CIMMYT spring wheat. J. Cereal Sci. 2016, 69, 182–186. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio Inc.: Boston, MA, USA, 2016. [Google Scholar]
- Hedges, L.V.; Gurevitch, J.; Curtis, P.S. The meta-analysis of response ratios in experimental ecology. Ecology 1999, 80, 1150–1156. [Google Scholar] [CrossRef]
- Högy, P.; Keck, M.; Niehaus, K.; Franzaring, J.; Fangmeier, A. Effects of atmospheric CO2 enrichment on biomass, yield and low molecular weight metabolites in wheat grain. J. Cereal Sci. 2010, 52, 215–220. [Google Scholar] [CrossRef]
- Högy, P.; Brunnbauer, M.; Koehler, P.; Schwadrof, K.; Breuer, J.; Franzaring, J.; Zhunusbayeva, D.; Fangmeier, A. Grain quality characteristics of spring wheat (Triticum aestivum) as affected by free-air CO2 enrichment. Environ. Exp. Bot. 2013, 88, 11–18. [Google Scholar] [CrossRef]
- Erbs, M.; Manderscheid, R.; Jansen, G.; Seddig, S.; Pacholski, A.; Weigel, H.J. Effects of free-air CO2 enrichment and nitrogen supply on grain quality parameters and elemental composition of wheat and barley grown in a crop rotation. Agric. Ecosyst. Environ. 2010, 136, 59–68. [Google Scholar] [CrossRef]
- Usui, Y.; Sakai, H.; Tokida, T.; Nakamura, H.; Nakagawa, H.; Hasegawa, T. Rice grain yield and quality responses to free-air CO2 enrichment combined with soil and water warming. Glob. Chang. Biol. 2016, 22, 1256–1270. [Google Scholar] [CrossRef]
- Panozzo, J.F.; Walker, C.K.; Partington, D.L.; Neumann, N.C.; Tausz, M.; Seneweera, S.; Fitzgerald, G.J. Elevated carbon dioxide changes grain protein concentration and composition and compromises baking quality. A FACE study. J. Cereal Sci. 2014, 60, 461–470. [Google Scholar] [CrossRef]
- Bencze, S.; Veisz, O.; Bedõ, Z. Effects of high atmospheric CO2 on the morphological and heading characteristics of winter wheat. Cereal Res. Comm. 2004, 32, 233–240. [Google Scholar] [CrossRef]
- Blumenthal, C.; Rawson, H.M.; McKenzie, E.; Gras, P.W.; Barlow, E.W.R.; Wrigley, C.W. Changes in wheat grain quality due to doubling the level of atmospheric CO2. Cereal Chem. 1996, 73, 762–766. [Google Scholar]
- Conroy, J.; Seneweera, S.; Basra, A.; Rogers, G.; Nissen-Wooller, B. Influence of rising atmospheric CO2 concentrations and temperature on growth, yield and grain quality of cereal crops. Aust. J. Plant. Physiol. 1994, 21, 741–758. [Google Scholar] [CrossRef]
- De la Puente, L.S.; Perez, P.P.; Carrasco, R.M.; Morcuende, R.M.; Del Molino, L.M.M. Action of elevated CO2 and high temperature on the mineral chemical composition of two varieties of wheat. Agrochimica 2000, 44, 221–230. [Google Scholar]
- Fangmeier, A.; Grüters, U.; Högy, P.; Vermehren, B.; Jäger, H.J. Effects of elevated CO2, nitrogen supply and tropospheric ozone on spring wheat—II. Nutrients (N, P, K, S, Ca, Mg, Fe, Mn, Zn). Environ. Pollut. 1997, 96, 43–59. [Google Scholar] [CrossRef]
- Fangmeier, A.; Grüters, U.; Vermehren, B.; Jager, H.J. Responses of some cereal cultivars to CO2 enrichment and tropospheric ozone at different levels of nitrogen supply. J. Appl. Bot. Food Qual. 1996, 70, 12–18. [Google Scholar]
- Wroblewitz, S.; Hüther, L.; Manderscheid, R.; Weigel, H.J.; Wätzig, H.; Dänicke, S. Effect of Rising atmospheric carbon dioxide concentration on the protein composition of cereal grain. J. Agric. Food Chem. 2014, 62, 6616–6625. [Google Scholar] [CrossRef]
- Weigel, H.J.; Manderscheid, R. CO2 enrichment effects on forage and grain nitrogen content of pasture and cereal plants. J. Crop Improv. 2005, 13, 73–89. [Google Scholar] [CrossRef]
- Wu, D.X.; Wang, G.X.; Bai, Y.F.; Liao, J.X. Effects of elevated CO2 concentration on growth, water use, yield and grain quality of wheat under two soil water levels. Agric. Ecosyst. Environ. 2004, 104, 493–507. [Google Scholar] [CrossRef]
- Rogers, G.S.; Gras, P.W.; Batey, I.L.; Milham, P.J.; Payne, L.; Conroy, J.P. The influence of atmospheric CO2 concentration on the protein, starch and mixing properties of wheat flour. Aust. J. Plant Physiol. 1998, 25, 387–393. [Google Scholar] [CrossRef]
- Manderscheid, R.; Bender, J.; Jäger, H.J.; Weigel, H.J. Effects of season long CO2 enrichment on cereals. II. Nutrient concentrations and grain quality. Agric. Ecosyst. Environ. 1995, 54, 175–185. [Google Scholar] [CrossRef]
- Erice, G.; Sanz-Sáez, A.; González-Torralba, J.; Mendez-Espinoza, A.M.; Urretavizcaya, I.; Nieto, M.T.; Serret, M.D.; Araus, J.L.; Irigoyen, J.J.; Aranjuelo, I. Impact of elevated CO2 and drought on yield and quality traits of a historical (Blanqueta) and a modern (Sula) durum wheat. J. Cereal Sci. 2019, 87, 194–201. [Google Scholar] [CrossRef]
- Carlisle, E.; Myers, S.; Raboy, V.; Bloom, A. The Effects of Inorganic Nitrogen form and CO2 Concentration on Wheat Yield and Nutrient Accumulation and Distribution. Front. Plant Sci. 2012, 3, 1–13. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, J.; Zhou, H.; Sun, Y.; Yin, Y.; Pei, D.; Ji, R.; Wu, J.; Wang, X. Elevated CO2 levels affects the concentrations of copper and cadmium in crops grown in soil contaminated with heavy metals under fully open-air field conditions. Environ. Sci. Technol. 2011, 45, 6997–7003. [Google Scholar] [CrossRef]
- Ma, H.; Zhu, J.; Xie, Z.; Liu, G.; Zeng, Q.; Han, Y. Responses of rice and winter wheat to free-air CO2 enrichment (China FACE) at rice/wheat rotation system. Plant Soil 2007, 294, 137–146. [Google Scholar] [CrossRef]
- Pleijel, H.; Danielsson, H. Yield dilution of grain Zn in wheat grown in open-top chamber experiments with elevated CO2 and O3 exposure. J. Cereal Sci. 2009, 50, 278–282. [Google Scholar] [CrossRef]
- Beleggia, R.; Fragasso, M.; Miglietta, F.; Cattivelli, L.; Menga, V.; Nigro, F.; Pecchioni, N.; Fares, C. Mineral composition of durum wheat grain and pasta under increasing atmospheric CO2 concentrations. Food Chem. 2018, 242, 53–61. [Google Scholar] [CrossRef]
- Sakai, H.; Tokida, T.; Usui, Y.; Nakamura, H.; Hasegawa, T. Yield responses to elevated CO2 concentration among Japanese rice cultivars released since 1882. Plant Prod. Sci. 2019, 22, 352–366. [Google Scholar] [CrossRef]
- Chang-Xing, Z.; Ming-Rong, H.; Zhen-Lin, W.; Yue-Fu, W.; Qi, L. Effects of different water availability at post-anthesis stage on grain nutrition and quality in strong-gluten winter wheat. Comptes Rendus Biol. 2009, 332, 759–764. [Google Scholar] [CrossRef]
- Eivazi, A.; Habibi, F. Sensitivity wheat genotypes for grain yield and quality traits to drought stress. Int. J. Agron. Plant Prod. 2012, 3, 738–747. [Google Scholar]
- Baric, M.; Keresa, S.; Sarcevic, H.; HabusJercic, I.; Horvat, D.; Drezner, G. Influence of drought during the grain filling period to the yield and quality of winter wheat (T. aestivum L.). In Proceedings of the 3rd International Congress “Flour-Bread 05” and 5th Croatian Congress of Cereal Technologists, Opatija, Croatia, 26–29 October 2005; pp. 19–24. [Google Scholar]
- Houshmand, S.; Arzani, A.; Maibody, S.A.M. Influences of drought and salt stress on grain quality of durum wheat. Genetic Variation for Plant Breeding. In Proceedings of the 17th Eucarpia General Congress, Tulln, Austria, 8–11 September 2004; pp. 383–386. [Google Scholar]
- Arzani, A. Grain quality of durum wheat germplasm as affected by heat and drought stress at grain filling period. Wheat Inf. Serv. 2002, 94, 9–13. [Google Scholar]
- Saint Pierre, C.; Peterson, C.J.; Ross, A.S.; Ohm, J.B.; Verhoeven, M.C.; Larson, M.; Hoefer, B. White wheat grain quality changes with genotype, nitrogen fertilization, and water stress. Agron. J. 2008, 100, 414–420. [Google Scholar] [CrossRef]
- Souza, E.J.; Martin, J.M.; Guttieri, M.J.; O’Brien, K.M.; Habernicht, D.K.; Lanning, S.P.; McLean, R.; Carlson, G.R.; Talbert, L.E. Influence of genotype, environment, and nitrogen management on spring wheat quality. Crop Sci. 2004, 44, 425–432. [Google Scholar] [CrossRef]
- Mkhabela, M.; Bullock, P.; Gervais, M.; Finlay, G.; Sapirstein, H. Assessing indicators of agricultural drought impacts on spring wheat yield and quality on the Canadian prairies. Agric. For. Meteorol. 2010, 150, 399–410. [Google Scholar] [CrossRef]
- Guttieri, M.J.; Stark, J.C.; O’Brien, K.; Souza, E. Relative sensitivity of spring wheat grain yield and quality parameters to moisture deficit. Crop. Sci. 2001, 41, 327–335. [Google Scholar] [CrossRef]
- Li, Y.F.; Wu, Y.; Hernandez-Espinosa, N.; Peña, R.J. Heat and drought stress on durum wheat: Responses of genotypes, yield, and quality parameters. J. Cereal Sci. 2013, 57, 398–404. [Google Scholar] [CrossRef]
- Dias, A.S.; Bagulho, A.S.; Lidon, F.C. Ultrastructure and biochemical traits of bread and durum wheat grains under heat stress. Braz. J. Plant Physiol. 2008, 20, 323–333. [Google Scholar] [CrossRef]
- Panozzo, J.F.; Eagles, H.A. Cultivar and environmental effects on quality characters in wheat. II. Protein. Aust. J. Agric. Res. 2000, 51, 629–636. [Google Scholar] [CrossRef]
- Corbellini, M.; Canevar, M.G.; Mazza, L.; Ciaffi, M.; Lafiandra, D.; Borghi, B. Effect of the Duration and Intensity of Heat Shock During Grain Filling on Dry Matter and Protein Accumulation, Technological Quality and Protein Composition in Bread and Durum Wheat. Aust. J. Plant Physiol. 1997, 24, 245–260. [Google Scholar] [CrossRef]
- Daniel, C.; Triboi, E. Effects of temperature and nitrogen nutrition on the grain composition of winter wheat: Effects on gliadin content and composition. J. Cereal Sci. 2000, 32, 45–56. [Google Scholar] [CrossRef]
- Labuschagne, M.T.; Elago, O.; Koen, E. The influence of temperature extremes on some quality and starch characteristics in bread, biscuit and durum wheat. J. Cereal Sci. 2009, 49, 184–189. [Google Scholar] [CrossRef]
- Spiertz, J.H.J.; Hamer, R.J.; Xu, H.; Primo-Martin, C.; Don, C.; van der Putten, P.E.L. Heat stress in wheat (Triticum aestivum L.): Effects on grain growth and quality traits. Eur. J. Agron. 2006, 25, 89–95. [Google Scholar] [CrossRef]
- Viswanathan, C.; Khanna-Chopra, R. Effect of heat stress on grain growth, starch synthesis and protein synthesis in grains of wheat (Triticum aestivum L.) varieties differing in grain weight stability. J. Agron. Crop. Sci. 2001, 186, 1–7. [Google Scholar] [CrossRef]
- Wrigley, C.; Blumenthal, C.; Gras, P.; Barlow, E. Temperature variation during grain filling and changes in wheat-grain quality. Aust. J. Plant Physiol. 1994, 21, 875–885. [Google Scholar] [CrossRef]
- De Leonardis, A.M.; Fragasso, M.; Beleggia, R.; Ficco, D.B.M.; De Vita, P.; Mastrangelo, A.M. Effects of heat stress on metabolite accumulation and composition, and nutritional properties of durum wheat grain. Int. J. Mol. Sci. 2015, 16, 30382–30404. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Mariem, S.; Soba, D.; Zhou, B.; Loladze, I.; Morales, F.; Aranjuelo, I. Climate Change, Crop Yields, and Grain Quality of C3 Cereals: A Meta-Analysis of [CO2], Temperature, and Drought Effects. Plants 2021, 10, 1052. https://doi.org/10.3390/plants10061052
Ben Mariem S, Soba D, Zhou B, Loladze I, Morales F, Aranjuelo I. Climate Change, Crop Yields, and Grain Quality of C3 Cereals: A Meta-Analysis of [CO2], Temperature, and Drought Effects. Plants. 2021; 10(6):1052. https://doi.org/10.3390/plants10061052
Chicago/Turabian StyleBen Mariem, Sinda, David Soba, Bangwei Zhou, Irakli Loladze, Fermín Morales, and Iker Aranjuelo. 2021. "Climate Change, Crop Yields, and Grain Quality of C3 Cereals: A Meta-Analysis of [CO2], Temperature, and Drought Effects" Plants 10, no. 6: 1052. https://doi.org/10.3390/plants10061052
APA StyleBen Mariem, S., Soba, D., Zhou, B., Loladze, I., Morales, F., & Aranjuelo, I. (2021). Climate Change, Crop Yields, and Grain Quality of C3 Cereals: A Meta-Analysis of [CO2], Temperature, and Drought Effects. Plants, 10(6), 1052. https://doi.org/10.3390/plants10061052










