Silicon in the Soil–Plant Continuum: Intricate Feedback Mechanisms within Ecosystems
Abstract
1. Introduction
2. Historical Overview
3. Soluble and Particulate Silicon in the Soil
3.1. Silicic Acid Effects on Nutrient and Toxicant Availability in Soils
3.2. Amorphous Silica as Control for Water Availability in Soils
4. Silicon Uptake by Plants
4.1. Active Uptake by Intrinsic Transporters
4.2. External Factors Affecting Silicon Uptake
5. The Variability of Silicon in Plants
5.1. Methods for Extracting Si from Plant Material
5.2. Types of Variability
5.3. Form, Location and Function
6. Implications for Ecosystem Structure, Functioning and Services
6.1. Effects on Soil
6.1.1. Si Cycling in Undisturbed and Disturbed Plant–Soil Systems
6.1.2. Concluding Remarks
6.2. Effects on Species Interactions, Community Structure and Net Primary Productivity
6.3. Effects on Biogeochemical Cycles
6.3.1. The Ecosystem Scale
6.3.2. The Global Scale
6.4. Silicon and Ecosystem Services
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Katz, O. Silicon content is a plant functional trait: Implications in a changing world. Flora 2019, 254, 88–94. [Google Scholar] [CrossRef]
- Cooke, J.; Leishman, M.R. Is plant ecology more siliceous than we realise? Trends Plant Sci. 2011, 16, 61–68. [Google Scholar] [CrossRef]
- He, H.; Veneklaas, E.J.; Kuo, J.; Lambers, H. Physiological and ecological significance of biomineralization in plants. Trends Plant Sci. 2014, 19, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E. The anomaly of silicon in plant biology. Proc. Natl. Acad. Sci. USA 1994, 91, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Putra, R.; Powell, J.R.; Hartley, S.E.; Johnson, S.N. Is it time to include legumes in plant silicon research? Funct. Ecol. 2020, 34, 1142–1157. [Google Scholar] [CrossRef]
- Epstein, E. Silicon: Its manifold roles in plants. Ann. Appl. Biol. 2009, 155, 155–160. [Google Scholar] [CrossRef]
- Frew, A.; Weston, L.A.; Reynolds, O.L.; Gurr, G.M. The role of silicon in plant biology: A paradigm shift in research approach. Ann. Bot. 2018, 121, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Richmond, K.E.; Sussman, M. Got silicon? The non-essential beneficial plant nutrient. Curr. Opin. Plant Biol. 2003, 6, 268–272. [Google Scholar] [CrossRef]
- Farooq, M.A.; Dietz, K.J. Silicon as versatile player in plant and human biology: Overlooked and poorly understood. Front. Plant Sci. 2015, 6, 994. [Google Scholar] [CrossRef]
- Meharg, C.; Meharg, A.A. Silicon, the silver bullet for mitigating biotic and abiotic stress, and improving grain quality, in rice? Environ. Exp. Bot. 2015, 120, 8–17. [Google Scholar] [CrossRef]
- Raven, J.A. The transport and function of silicon in plants. Biol. Rev. 1983, 58, 179–207. [Google Scholar] [CrossRef]
- Schoelynck, J.; Struyf, E. Silicon in aquatic vegetation. Funct. Ecol. 2016, 30, 1323–1330. [Google Scholar] [CrossRef]
- Cooke, J.; Leishman, M.R. Consistent alleviation of abiotic stress with silicon addition: A meta-analysis. Funct. Ecol. 2016, 30, 1340–1357. [Google Scholar] [CrossRef]
- Guntzer, F.; Keller, C.; Meunier, J.D. Benefits of plant silicon for crops: A review. Agron. Sustain. Dev. 2012, 32, 201–213. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Functions and transport of silicon in plants. Cell. Mol. Life Sci. 2008, 65, 3049–3057. [Google Scholar] [CrossRef]
- Sahebi, M.; Hanafi, M.M.; Siti Nor Akmar, A.; Rafii, M.Y.; Azizi, P.; Tengoua, F.F.; Nurul Mayzaitul Azwa, J.; Shabanimofrad, M. Importance of silicon and mechanisms of biosilica formation in plants. BioMed Res. Int. 2015, 2015, 396010. [Google Scholar] [CrossRef]
- Katz, O. Beyond grasses: The potential benefits of studying silicon accumulation in non-grass species. Front. Plant Sci. 2014, 5, 376. [Google Scholar] [CrossRef]
- Frings, P.J.; Clymans, W.; Jeppesen, E.; Lauridsen, T.L.; Struyf, E.; Conley, D.J. Lack of steady-state in the global biogeochemical Si cycle: Emerging evidence from lake Si sequestration. Biogeochemistry 2014, 117, 255–277. [Google Scholar] [CrossRef]
- Keller, C.; Guntzer, F.; Barboni, D.; Labreuche, J.; Meunier, J.D. Impact of agriculture on the Si biogeochemical cycle: Input from phytolith studies. C. R. Geosci. 2012, 344, 739–746. [Google Scholar] [CrossRef]
- Carey, J.C.; Fulweiler, R.W. The terrestrial silica pump. PLoS ONE 2012, 7, e52932. [Google Scholar] [CrossRef]
- Cornelis, J.T.; Delvaux, B. Soil processes drive the biological silicon feedback loop. Funct. Ecol. 2016, 30, 1298–1310. [Google Scholar] [CrossRef]
- Katz, O. Silica phytoliths in angiosperms: Phylogeny and early evolutionary history. New Phytol. 2015, 208, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.; Degabriel, J.L. Plant silicon interactions between organisms and the implications for ecosystems. Front. Plant Sci. 2016, 7, 1001. [Google Scholar] [CrossRef]
- Schoelynck, J.; Müller, F.; Vandevenne, F.; Bal, K.; Barão, L.; Smis, A.; Opdekamp, W.; Meire, P.; Struyf, E. Silicon-Vegetation interaction in multiple ecosystems: A review. J. Veg. Sci. 2014, 25, 301–313. [Google Scholar] [CrossRef]
- Schaller, J.; Puppe, D.; Kaczorek, D.; Ellerbrock, R.; Sommer, M. Silicon cycling in soils revisited. Plants 2021, 10, 295. [Google Scholar] [CrossRef]
- Ehrenberg, C.G. Mikrogeologie: Das Erden und Felsen Schaffende Wirken des Unsichtbar Kleinen Selbständigen Lebens auf der Erde; L. Voss: Leipzig, Germany, 1854. [Google Scholar]
- Powers, A.P. Historical review of European phytolith systematics. In Phytolith Systematics; Rapp, G.J., Mulholland, S.C., Eds.; Springer International Publishing: New York, NY, USA, 1992; pp. 15–35. [Google Scholar]
- Darwin, C. An account of the fine dust which often falls on vessels in the Atlantic Ocean. Q. J. Geol. Soc. Lond. 1846, 2, 26–30. [Google Scholar] [CrossRef]
- Struve, G.A. De Silicia in Plantis Nonnullis; University of Berlin: Berlin, Germany, 1835. [Google Scholar]
- Davy, H. Elements of Agricultural Chemistry; John J. Griffin and Company: Glasgow, UK, 1846. [Google Scholar]
- Sachs, J. Ergebnisse einiger neuerer untersuchungen uber die in pflanzen enthaltene Kieselsaure. Flora 1862, 33, 65–71. [Google Scholar]
- Miliarakis, S. Die Verkieselung lebender Elementarorgane bei den Pflanzen; University Würzburg: Würzburg, Germany, 1884. [Google Scholar]
- Kreuzhage, C.; Wolff, E. Bedeutung der kieselsäure für die entwicklung der haferpflanze. Landwirtsch. Versuchs-Stationen 1884, 30, 161–197. [Google Scholar]
- von Marilaun, A.K. Pflanzenleben: Bd. Gestalt und Legen der Pflanze; Verlag des Bibliographischen Institut: Leipzig, Germany, 1887. [Google Scholar]
- Stahl, E. Pflanzen und Schnecken: Eine Biologische Studie Über Die Schutzmittel der Pflanzen Gegen Schneckenfrass; G. Fischer: Jena, Germany, 1888. [Google Scholar]
- Lemmermann, O.; Wießmann, H. Die ertragssteigernde wirkung der kieselsäure bei unzureichender phosphorsäureernährung der pflanzen. Zeitschrift für Pflanzenernährung und Düngung A Wissenschaftlicher Teil 1922, 1, 185–246. [Google Scholar] [CrossRef]
- Lemmermann, O.; Wießmann, H.; Lemmermann, O. Weitere versuche über die ertragssteigernde wirkung der kieselsäure bei unzureichender phosphorsäuredüngung. Zeitschrift für Pflanzenernährung und Düngung A Wissenschaftlicher Teil 1924, 3, 185–197. [Google Scholar] [CrossRef]
- Wießmann, O.L.U.; Sammet, K.; Lemmermann, O. Untersuchungen über die ursache der ertragssteigernden wirkung der kieselsäure. Zeitschrift für Pflanzenernährung und Düngung A Wissenschaftlicher Teil 1925, 4, 265–315. [Google Scholar] [CrossRef]
- Sommer, A.L. Studies Concerning the Essential Nature of Aluminum and Silicon for Plant Growth; University of California Press: Berkeley, CA, USA, 1926. [Google Scholar]
- Ma, J.F.; Takahashi, E. Soil, Fertilizer, and Plant Silicon Research in Japan; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Onodera, I. Chemical studies on rice blast (1). J. Sci. Agric. Soc. 1917, 180, 606–617. [Google Scholar]
- Miyake, K.; Adachi, M. Chmische Untersucungen über die widerstandsfähigkeit der reisarten gegen die “imochi-krankheit”: Zweiter bericht. der einflusz der wasserstoffionenkonzentration auf das wachstum des pilzes. J. Biochem. 1922. [Google Scholar] [CrossRef]
- Kawashima, R. Influence of silica on rice blast disease. Jpn. J. Soil Sci. Plant Nutr. 1927, 1, 86–91. [Google Scholar]
- Miyake, K.; Ikeda, M. Influence of silica application on rice blast. Jpn. J. Soil Sci. Plant Nutr. 1932, 6, 53–76. [Google Scholar]
- Ishibashi, H. Influence of silica on the growth of rice plant. Jpn. J. Soil Sci. Plant Nutr. 1936, 10, 244–256. [Google Scholar]
- Ishibashi, H. The effect of silica on the growth of cultivated plants. V. The effect of silica on the growth of rice plants growing on soils of various depth. J. Sci. Soil Manure 1937, 11, 535–549. [Google Scholar]
- Ishibashi, H. The eefct of silicic acid on the growth of rice plants. J. Sci. Soil Manure 1936, 10, 224–256. [Google Scholar]
- Raleigh, G.J. Evidence for the essentiality of silicon for the beet plant. Plant Physiol. 1939. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F. The importance of silicic acid for the growth of some cultivated plants, their metabolism, and their susceptibility to true mildew. Phytopathol. Zeitschrift 1940, 12, 427–479. [Google Scholar]
- Engel, W. Untersuchungen über die Kieselsäureverbindungen im Roggenhalm. Planta 1953, 41, 358–390. [Google Scholar] [CrossRef]
- Holzapfel, L.; Engel, W. Beeinflussung der Kieselsäure-Aufnahme und-Abgabe bei Weizenpflanzen. Arch. Biochem. Biophys. 1959, 83, 268–274. [Google Scholar] [CrossRef]
- Yoshida, S.; Ohnishi, Y.; Kitagishi, K. Histochemistry of silicon in rice plant: III. The presence of cuticle-silica double layer in the epidermal tissue. Soil Sci. Plant Nutr. 1962, 8, 1–5. [Google Scholar] [CrossRef]
- Okuda, A.; Takahashi, E. Effects of silicon supply on the injuries due to excessive amount of Fe, Mn, Cu, As, Al, Co of barley and rice plants. Jpn. J. Soil Sci. Plant Nutr. 1962, 33, 1–8. [Google Scholar]
- Okuda, A.; Takahashi, E. Studies on the physiological role of silicon in crop plant. Part 3. Effect of various amount of silicon supply on the growth of rice plant and its nutrients uptake. J. Sci. Soil Manure 1961, 32, 533–537. [Google Scholar]
- Lewin, J.; Reimann, B.E.F. Silicon and plant growth. Annu. Rev. Plant Physiol. 1969, 20, 289–304. [Google Scholar] [CrossRef]
- Jones, L.H.P.; Handreck, K.A. Studies of silica in the oat plant—III. Uptake of silica from soils by the plant. Plant Soil 1965, 23, 79–96. [Google Scholar] [CrossRef]
- Jones, L.H.P.; Handreck, K.A. Silica in soils, plants, and animals. Adv. Agron. 1967, 19, 107–149. [Google Scholar] [CrossRef]
- Handreck, K.A.; Jones, L.H.P. Studies of silica in the oat plant—IV. Silica content of plant parts in relation to stage of growth, supply of silica, and transpiration. Plant Soil 1968, 29, 449–459. [Google Scholar] [CrossRef]
- Jones, L.H.P.P.; Milne, A.A.; Wadham, S.M. Studies of silica in the oat plant. Plant Soil 1963, 18, 358–371. [Google Scholar] [CrossRef]
- Jones, L.H.P.; Handreck, K.A. Uptake of silica by Trifolium incarnatum in relation to the concentration in the external solution and to transpiration. Plant Soil 1969, 30, 71–80. [Google Scholar] [CrossRef]
- Sangster, A.G. Characteristics of silica deposition in Digitaria sanguinalis (L.) scop. (Crabgrass). Ann. Bot. 1977, 41, 341–350. [Google Scholar] [CrossRef]
- Sangster, A.G. Intracellular silica deposition in immature leaves in three species of the Gramineae. Ann. Bot. 1970, 34, 245–257. [Google Scholar] [CrossRef]
- Parry, D.W.; Smithson, F. Silicification of bulliform cells in grasses. Nature 1958, 181, 1549–1550. [Google Scholar] [CrossRef]
- Sangster, A.G.; Parry, D.W. Ultrastructure of silica deposits in higher plants. In Silicon and Siliceous Structures in Biological Systems; Simpson, T.L., Volcani, B.E., Eds.; Springer: New York, NY, USA, 1981; pp. 383–407. [Google Scholar]
- Parry, D.W.; Kelso, M. The distribution of silicon deposits in the roots of Molinia caerulea (L.) Moench. and Sorghum bicolor (L.) Moench. Ann. Bot. 1975, 39, 995–1001. [Google Scholar] [CrossRef]
- Sangster, A.G.; Parry, D.W. Endodermal silicon deposits and their linear distribution in developing roots of Sorghum bicolor (L.) Moench. Ann. Bot. 1976, 40, 361–371. [Google Scholar] [CrossRef]
- Blackman, E.; Parry, D.W. Opaline silica deposition in rye (Secale cereale L.). Ann. Bot. 1968, 32, 199–206. [Google Scholar] [CrossRef]
- Blackman, E. Observations on the development of the silica cells of the leaf sheath of wheat (Triticum aestivum). Can. J. Bot. 1969. [Google Scholar] [CrossRef]
- Blackman, E. The pattern and sequence of opaline silica deposition in rye (Secale cereale L.). Ann. Bot. 1968, 32, 207–218. [Google Scholar] [CrossRef]
- Blackman, E. Opaline silica bodies in the range grasses of southern Alberta. Can. J. Bot. 1971, 49, 769–781. [Google Scholar] [CrossRef]
- Sauer, D.; Saccone, L.; Conley, D.J.; Herrmann, L.; Sommer, M. Review of methodologies for extracting plant-available and amorphous Si from soils and aquatic sediments. Biogeochemistry 2006, 80, 89–108. [Google Scholar] [CrossRef]
- Matichenkov, V.V.; Bocharnikova, E.A. The relationship between silicon and soil physical and chemical properties. In Silicon in Agriculture; Datnoff, L.E., Snyder, G.H., Korndörfer, G.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 209–219. [Google Scholar]
- Cornelis, J.T.; Delvaux, B.; Cardinal, D.; André, L.; Ranger, J.; Opfergelt, S. Tracing mechanisms controlling the release of dissolved silicon in forest soil solutions using Si isotopes and Ge/Si ratios. Geochim. Cosmochim. Acta 2010, 74, 3913–3924. [Google Scholar] [CrossRef]
- Opfergelt, S.; de Bournonville, G.; Cardinal, D.; André, L.; Delstanche, S.; Delvaux, B. Impact of soil weathering degree on silicon isotopic fractionation during adsorption onto iron oxides in basaltic ash soils, Cameroon. Geochim. Cosmochim. Acta 2009, 73, 7226–7240. [Google Scholar] [CrossRef]
- Dietzel, M. Dissolution of silicates and the stability of polysilicic acid. Geochim. Cosmochim. Acta 2000, 64, 3275–3281. [Google Scholar] [CrossRef]
- Belton, D.J.; Deschaume, O.; Perry, C.C. An overview of the fundamentals of the chemistry of silica with relevance to biosilicification and technological advances. FEBS J. 2012, 279, 1710–1720. [Google Scholar] [CrossRef]
- Dietzel, M. Interaction of polysilicic and monosilicic acid with mineral surfaces. In Water-Rock Interaction; Stober, I., Bucher, K., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 207–235. [Google Scholar]
- Pallavi, T.; Prakash, N.B. Pools of silicon in soils and their contribution to rice. J. Indian Soc. Soil Sci. 2019, 67, 211–220. [Google Scholar] [CrossRef]
- Majumdar, S.; Prakash, N.B. Quantification of amorphous silicon by optimizing the 1% Na2CO3 method from intensively cultivated rice and sugarcane soils in a tropical climate. Silicon 2020, 12, 2989–3003. [Google Scholar] [CrossRef]
- Reithmaier, G.M.S.; Knorr, K.H.; Arnhold, S.; Planer-Friedrich, B.; Schaller, J. Enhanced silicon availability leads to increased methane production, nutrient and toxicant mobility in peatlands. Sci. Rep. 2017, 7, 8728. [Google Scholar] [CrossRef] [PubMed]
- Sigg, L.; Stumm, W. The interaction of anions and weak acids with the hydrous goethite (α-FeOOH) surface. Colloids Surf. 1981, 2, 101–117. [Google Scholar] [CrossRef]
- Schaller, J.; Faucherre, S.; Joss, H.; Obst, M.; Goeckede, M.; Planer-Friedrich, B.; Peiffer, S.; Gilfedder, B.; Elberling, B. Silicon increases the phosphorus availability of Arctic soils. Sci. Rep. 2019, 9, 449. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Takahashi, E. Effect of silicate on phosphate availability for rice in a P-deficient soil. Plant Soil 1991, 133, 151–155. [Google Scholar] [CrossRef]
- Neu, S.; Schaller, J.; Dudel, E.G. Silicon availability modifies nutrient use efficiency and content, C:N:P stoichiometry, and productivity of winter wheat (Triticum aestivum L.). Sci. Rep. 2017, 7, 40829. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P. Interactions of Silica with Iron Oxides: Effects on Oxide Transformations and Sorption Properties; Whiteshell Laboratories: Pinawa, MB, Canada, 1995. [Google Scholar]
- Meunier, J.D.; Sandhya, K.; Prakash, N.B.; Borschneck, D.; Dussouillez, P. pH as a proxy for estimating plant-available Si? A case study in rice fields in Karnataka (South India). Plant Soil 2018, 432, 143–155. [Google Scholar] [CrossRef]
- Schaller, J.; Frei, S.; Rohn, L.; Gilfedder, B.S. Amorphous silica controls water storage capacity and phosphorus mobility in soils. Front. Environ. Sci. 2020, 8, 94. [Google Scholar] [CrossRef]
- Dol Hamid, R.; Swedlund, P.J.; Song, Y.; Miskelly, G.M. Ionic strength effects on silicic acid (H4SiO4) sorption and oligomerization on an iron oxide surface: An interesting interplay between electrostatic and chemical forces. Langmuir 2011, 27, 12930–12937. [Google Scholar] [CrossRef] [PubMed]
- Pokrovski, G.S.; Schott, J.; Farges, F.; Hazemann, J.L. Iron (III)-silica interactions in aqueous solution: Insights from X-ray absorption fine structure spectroscopy. Geochim. Cosmochim. Acta 2003, 67, 3559–3573. [Google Scholar] [CrossRef]
- Xu, D.; Gao, T.; Fang, X.; Bu, H.; Li, Q.; Wang, X.; Zhang, R. Silicon addition improves plant productivity and soil nutrient availability without changing the grass:legume ratio response to N fertilization. Sci. Rep. 2020, 10, 10295. [Google Scholar] [CrossRef] [PubMed]
- Bhat, J.A.; Shivaraj, S.M.; Singh, P.; Navadagi, D.B.; Tripathi, D.K.; Dash, P.K.; Solanke, A.U.; Sonah, H.; Deshmukh, R. Role of silicon in mitigation of heavy metal stresses in crop plants. Plants 2019, 8, 71. [Google Scholar] [CrossRef]
- Li, L.; Zheng, C.; Fu, Y.; Wu, D.; Yang, X.; Shen, H. Silicate-Mediated alleviation of Pb toxicity in banana grown in Pb-contaminated soil. Biol. Trace Elem. Res. 2012, 145, 101–108. [Google Scholar] [CrossRef]
- Exley, C.; Guerriero, G.; Lopez, X. Silicic acid: The omniscient molecule. Sci. Total Environ. 2019, 665, 432–437. [Google Scholar] [CrossRef]
- Pačes, T. Reversible control of aqueous aluminum and silica during the irreversible evolution of natural waters. Geochim. Cosmochim. Acta 1978, 42, 1487–1493. [Google Scholar] [CrossRef]
- Beardmore, J.; Lopez, X.; Mujika, J.I.; Exley, C. What is the mechanism of formation of hydroxyaluminosilicates? Sci. Rep. 2016, 6, 30913. [Google Scholar] [CrossRef]
- Zhang, S.; Li, S.; Ding, X.; Li, F.; Liu, C.; Liao, X.; Wang, R. Silicon mediated the detoxification of Cr on pakchoi (Brassica chinensis L.) in Cr-contaminated soil. J. Food Agric. Environ. 2013, 11, 814–819. [Google Scholar] [CrossRef]
- Shim, J.; Shea, P.J.; Oh, B.T. Stabilization of heavy metals in mining site soil with silica extracted from corn rob. Water. Air. Soil Pollut. 2014, 225, 2152. [Google Scholar] [CrossRef]
- Da Cunha, K.P.V.; Do Nascimento, C.W.A.; Da Silva, A.J. Silicon alleviates the toxicity of cadmium and zinc for maize (Zea mays L.) grown on a contaminated soil. J. Plant Nutr. Soil Sci. 2008, 171, 849–853. [Google Scholar] [CrossRef]
- Zachara, J.M.; Girvin, D.C.; Schmidt, R.L.; Resch, C.T. Chromate adsorption on amorphous iron oxyhydroxide in the presence of major groundwater ions. Environ. Sci. Technol. 1987, 21, 589–594. [Google Scholar] [CrossRef]
- Gutiérrez-Castorena, M.D.C.; Stoops, G.; Ortiz Solorio, C.A.; López Avila, G. Amorphous silica materials in soils and sediments of the Ex-Lago de Texcoco, Mexico: An explanation for its subsidence. Catena 2005, 60, 205–226. [Google Scholar] [CrossRef]
- Iler, R.K. Surface and Colloid Science; John Wiley & Sons: Toronto, ON, Canada, 1973. [Google Scholar]
- Schaller, J.; Cramer, A.; Carminati, A.; Zarebanadkouki, M. Biogenic amorphous silica as main driver for plant available water in soils. Sci. Rep. 2020, 10, 2424. [Google Scholar] [CrossRef]
- Liang, Y.; Si, J.; Römheld, V. Silicon uptake and transport is an active process in Cucumis sativus. New Phytol. 2005, 167, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Henriet, C.; Draye, X.; Oppitz, I.; Swennen, R.; Delvaux, B. Effects, distribution and uptake of silicon in banana (Musa spp.) under controlled conditions. Plant Soil 2006, 287, 359–374. [Google Scholar] [CrossRef]
- Faisal, S.; Callis, K.L.; Slot, M.; Kitajima, K. Transpiration-dependent passive silica accumulation in cucumber (Cucumis sativus) under varying soil silicon availability. Botany 2012, 90, 1058–1064. [Google Scholar] [CrossRef]
- Mitani, N.; Ma, J.F. Uptake system of silicon in different plant species. J. Exp. Bot. 2005, 56, 1255–1261. [Google Scholar] [CrossRef]
- Piperno, D.R.; Holst, I.; Wessel-Beaver, L.; Andres, T.C. Evidence for the control of phytolith formation in Cucurbita fruits by the hard rind (Hr) genetic locus: Archaeological and ecological implications. Proc. Natl. Acad. Sci. USA 2002, 99, 10923–10928. [Google Scholar] [CrossRef]
- Ma, J.F.; Tamai, K.; Yamaji, N.; Mitani, N.; Konishi, S.; Katsuhara, M.; Ishiguro, M.; Murata, Y.; Yano, M. A silicon transporter in rice. Nature 2006, 440, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Tamai, K.; Konishi, S.; Fujiwara, T.; Katsuhara, M.; Yano, M. An efflux transporter of silicon in rice. Nature 2007, 448, 209–212. [Google Scholar] [CrossRef]
- Yamaji, N.; Mitani, N.; Ma, J.F. A transporter regulating silicon distribution in rice shoots. Plant Cell 2008, 20, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N. A cooperative system of silicon transport in plants. Trends Plant Sci. 2015, 20, 435–442. [Google Scholar] [CrossRef]
- Yamaji, N.; Ma, J.F. The node, a hub for mineral nutrient distribution in graminaceous plants. Trends Plant Sci. 2014, 19, 556–563. [Google Scholar] [CrossRef]
- Yamaji, N.; Sakurai, G.; Mitani-Ueno, N.; Ma, J.F. Orchestration of three transporters and distinct vascular structures in node for intervascular transfer of silicon in rice. Proc. Natl. Acad. Sci. USA 2015, 112, 11401–11406. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Fan, X.; Tan, L.; Yin, C.; Li, T.; Liang, Y. Root silicon deposition and its resultant reduction of sodium bypass flow is modulated by OsLsi1 and OsLsi2 in rice. Plant Physiol. Biochem. 2021, 158, 219–227. [Google Scholar] [CrossRef]
- Yan, G.C.; Nikolic, M.; Ye, M.J.; Xiao, Z.X.; Liang, Y.C. Silicon acquisition and accumulation in plant and its significance for agriculture. J. Integr. Agric. 2018, 17, 2138–2150. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Vivancos, J.; Guérin, V.; Sonah, H.; Labbé, C.; Belzile, F.; Bélanger, R.R. Identification and functional characterization of silicon transporters in soybean using comparative genomics of major intrinsic proteins in Arabidopsis and rice. Plant Mol. Biol. 2013, 83, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Mitani, N.; Yamaji, N.; Ago, Y.; Iwasaki, K.; Ma, J.F. Isolation and functional characterization of an influx silicon transporter in two pumpkin cultivars contrasting in silicon accumulation. Plant J. 2011, 66, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.S.; Yu, C.; Fan, P.P.; Bao, B.F.; Li, T.; Zhu, Z.J. Identification of two cucumber putative silicon transporter genes in cucumis sativus. J. Plant Growth Regul. 2015, 34, 332–338. [Google Scholar] [CrossRef]
- Sahebi, M.; Hanafi, M.M.; Abdullah, S.N.A.; Rafii, M.Y.; Azizi, P.; Nejat, N.; Idris, A.S. Isolation and expression analysis of novel silicon absorption gene from roots of mangrove (Rhizophora apiculata) via suppression subtractive hybridization. BioMed Res. Int. 2014, 2014, 971985. [Google Scholar] [CrossRef] [PubMed]
- Grégoire, C.; Rémus-Borel, W.; Vivancos, J.; Labbé, C.; Belzile, F.; Bélanger, R.R. Discovery of a multigene family of aquaporin silicon transporters in the primitive plant Equisetum arvense. Plant J. 2012, 72, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Trembath-Reichert, E.; Wilson, J.P.; McGlynn, S.E.; Fischer, W.W. Four hundred million years of silica biomineralization in land plants. Proc. Natl. Acad. Sci. USA 2015, 112, 5449–5454. [Google Scholar] [CrossRef]
- Yamaji, N.; Ma, J.F. Spatial distribution and temporal variation of the rice silicon transporter Lsi1. Plant Physiol. 2007, 143, 1306–1313. [Google Scholar] [CrossRef]
- Kumar, N.; Dubey, A.K.; Upadhyay, A.K.; Gautam, A.; Ranjan, R.; Srikishna, S.; Sahu, N.; Behera, S.K.; Mallick, S. GABA accretion reduces Lsi-1 and Lsi-2 gene expressions and modulates physiological responses in Oryza sativa to provide tolerance towards arsenic. Sci. Rep. 2017, 7, 8786. [Google Scholar] [CrossRef]
- Chaiwong, N.; Bouain, N.; Prom-u-thai, C.; Rouached, H. Interplay between silicon and iron signaling pathways to regulate silicon transporter Lsi1 expression in rice. Front. Plant Sci. 2020, 11, 1065. [Google Scholar] [CrossRef]
- Bokor, B.; Bokorová, S.; Ondoš, S.; Švubová, R.; Lukačová, Z.; Hýblová, M.; Szemes, T.; Lux, A. Ionome and expression level of Si transporter genes (Lsi1, Lsi2, and Lsi6) affected by Zn and Si interaction in maize. Environ. Sci. Pollut. Res. 2015, 22, 6800–6811. [Google Scholar] [CrossRef]
- Lux, A.; Luxová, M.; Abe, J.; Tanimoto, E.; Hattori, T.; Inanaga, S. The dynamics of silicon deposition in the sorghum root endodermis. New Phytol. 2003, 158, 437–441. [Google Scholar] [CrossRef]
- Hattori, T.; Inanaga, S.; Araki, H.; An, P.; Morita, S.; Luxová, M.; Lux, A. Application of silicon enhanced drought tolerance in Sorghum bicolor. Physiol. Plant. 2005, 123, 459–466. [Google Scholar] [CrossRef]
- de Melo, S.P.; Korndörfer, G.H.; Korndörfer, C.M.; Lana, R.M.Q.; de Santana, D.G. Silicon accumulation and water deficit tolerance in Brachiaria grasses. Sci. Agric. 2003, 60, 755–759. [Google Scholar] [CrossRef]
- Mayland, H.F.; Wright, J.L.; Sojka, R.E. Silicon accumulation and water uptake by wheat. Plant Soil 1991, 137, 191–199. [Google Scholar] [CrossRef]
- Jenkins, E.; Jamjoum, K.; Nuimat, S.; Stafford, R.; Nortcliff, S.; Mithen, S. Identifying ancient water availability through phytolith analysis: An experimental approach. J. Archaeol. Sci. 2016, 73, 82–93. [Google Scholar] [CrossRef]
- Jenkins, E.; Jamjoum, K.; Al Nuimat, S. Irrigation and phytolith formation: An experimental study. Water Life Civilis. 2011, 347–372. [Google Scholar] [CrossRef][Green Version]
- Rosen, A.M.; Weiner, S. Identifying ancient irrigation: A new method using opaline phytoliths from emmer wheat. J. Archaeol. Sci. 1994, 21, 125–132. [Google Scholar] [CrossRef]
- Katz, O.; Lev-Yadun, S.; Bar (Kutiel), P. Plant silicon and phytolith contents as affected by water availability and herbivory: Integrating laboratory experimentation and natural habitat studies. Silicon 2018, 10, 2387–2389. [Google Scholar] [CrossRef]
- Euliss, K.W.; Dorsey, B.L.; Benke, K.C.; Banks, M.K.; Schwab, A.P. The use of plant tissue silica content for estimating transpiration. Ecol. Eng. 2005, 25, 343–348. [Google Scholar] [CrossRef]
- Katz, O.; Lev-Yadun, S.; Pua Bar, K. Plasticity and variability in the patterns of phytolith formation in Asteraceae species along a large rainfall gradient in Israel. Flora 2013, 208, 438–444. [Google Scholar] [CrossRef]
- Katz, O.; Lev-Yadun, S.; Bar, P. Do phytoliths play an antiherbivory role in southwest Asian Asteraceae species and to what extent? Flora 2014, 209, 349–358. [Google Scholar] [CrossRef]
- Johnston, A.; Bezeau, L.M.; Smoliak, S. Variation in silica content of range grasses. Can. J. Plant Sci. 1967, 47, 65–71. [Google Scholar] [CrossRef]
- Webb, E.A.; Longstaffe, F.J. The relationship between phytolith- and plant-water δ 18O values in grasses. Geochim. Cosmochim. Acta 2003, 67, 1437–1449. [Google Scholar] [CrossRef]
- Frew, A.; Allsopp, P.G.; Gherlenda, A.N.; Johnson, S.N. Increased root herbivory under elevated atmospheric carbon dioxide concentrations is reversed by silicon-based plant defences. J. Appl. Ecol. 2017, 54, 1310–1319. [Google Scholar] [CrossRef]
- Takahashi, N.; Isogai, A.; Ling, P.P.; Kato, Y.; Kurata, K. Effects of elevated atmospheric carbon dioxide concentration on silica deposition in rice (Oryza sativa L.) panicle. Plant Prod. Sci. 2008, 11, 307–315. [Google Scholar] [CrossRef][Green Version]
- Li, N.N.; Jie, D.M.; Ge, Y.; Guo, J.X.; Liu, H.Y.; Liu, L.D.; Qiao, Z.H. Response of phytoliths in Phragmites communis to elevated CO2 concentration in Songnen Grassland, China. Quat. Int. 2014, 321, 97–104. [Google Scholar] [CrossRef]
- Hartley, S.E.; DeGabriel, J.L. The ecology of herbivore-induced silicon defences in grasses. Funct. Ecol. 2016, 30, 1311–1322. [Google Scholar] [CrossRef]
- Massey, F.P.; Roland Ennos, A.; Hartley, S.E. Herbivore specific induction of silica-based plant defences. Oecologia 2007, 152, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Kvedaras, O.L.; An, M.; Choi, Y.S.; Gurr, G.M. Silicon enhances natural enemy attraction and biological control through induced plant defences. Bull. Entomol. Res. 2010, 100, 367–371. [Google Scholar] [CrossRef]
- Islam, T.; Moore, B.D.; Johnson, S.N. Novel evidence for systemic induction of silicon defences in cucumber following attack by a global insect herbivore. Ecol. Entomol. 2020, 45, 1373–1381. [Google Scholar] [CrossRef]
- Johnson, S.N.; Reynolds, O.L.; Gurr, G.M.; Esveld, J.L.; Moore, B.D.; Tory, G.J.; Gherlenda, A.N. When resistance is futile, tolerate instead: Silicon promotes plant compensatory growth when attacked by above- and belowground herbivores. Biol. Lett. 2019, 15, 20190361. [Google Scholar] [CrossRef] [PubMed]
- Soininen, E.M.; Bråthen, K.A.; Jusdado, J.G.H.; Reidinger, S.; Hartley, S.E. More than herbivory: Levels of silica-based defences in grasses vary with plant species, genotype and location. Oikos 2013, 122, 30–41. [Google Scholar] [CrossRef]
- Massey, F.P.; Ennos, A.R.; Hartley, S.E. Grasses and the resource availability hypothesis: The importance of silica-based defences. J. Ecol. 2007, 95, 414–424. [Google Scholar] [CrossRef]
- McNaughton, S.J.; Tarrants, J.L. Grass leaf silicification: Natural selection for an inducible defense against herbivores. Proc. Natl. Acad. Sci. USA 1983, 80, 790–791. [Google Scholar] [CrossRef]
- Bañuelos, M.J.; Obeso, J.R. Effect of grazing history, experimental defoliation, and genotype on patterns of silicification in Agrostis tenuis Sibth. Ecoscience 2000, 7, 45–50. [Google Scholar] [CrossRef]
- Garbuzov, M.; Reidinger, S.; Hartley, S.E. Interactive effects of plant-available soil silicon and herbivory on competition between two grass species. Ann. Bot. 2011, 108, 1355–1363. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Quigley, K.M.; Anderson, T.M. Leaf silica concentration in Serengeti grasses increases with watering but not clipping: Insights from a common garden study and literature review. Front. Plant Sci. 2014, 5, 568. [Google Scholar] [CrossRef]
- Cid, M.S.; Detling, J.K.; Brizuela, M.A.; Whicker, A.D. Patterns in grass silicification: Response to grazing history and defoliation. Oecologia 1989, 80, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Kindomihou, V.M.; Dagbénonbakin, G.D.; Bognonkpe, J.P.; Sinsin, B.A.; Meerts, P.J. Silica concentration is related to leaf traits but not to a specific anatomical tissue in tropical fodder grass species. Eur. J. Sci. Res. 2011, 62, 559–570. [Google Scholar]
- Brizuela, M.A.; Detling, J.K.; Cid, M.S. Silicon concentration of grasses growing in sites with different grazing histories. Ecology 1986, 67, 1098–1101. [Google Scholar] [CrossRef]
- Massey, F.P.; Smith, M.J.; Lambin, X.; Hartley, S.E. Are silica defences in grasses driving vole population cycles? Biol. Lett. 2008, 4, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Huitu, O.; Forbes, K.M.; Helander, M.; Julkunen-Tiitto, R.; Lambin, X.; Saikkonen, K.; Stuart, P.; Sulkama, S.; Hartley, S. Silicon, endophytes and secondary metabolites as grass defenses against mammalian herbivores. Front. Plant Sci. 2014, 5, 478. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.J.H.; Lambin, X.; Massey, F.P.; Reidinger, S.; Sherratt, J.A.; Smith, M.J.; White, A.; Hartley, S.E. Delayed induced silica defences in grasses and their potential for destabilising herbivore population dynamics. Oecologia 2012, 170, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Melzer, S.E.; Knapp, A.K.; Kirkman, K.P.; Smith, M.D.; Blair, J.M.; Kelly, E.F. Fire and grazing impacts on silica production and storage in grass dominated ecosystems. Biogeochemistry 2010, 97, 263–278. [Google Scholar] [CrossRef]
- Hartley, S.E. Round and round in cycles? Silicon-based plant defences and vole population dynamics. Funct. Ecol. 2015, 29, 151–153. [Google Scholar] [CrossRef]
- Clymans, W.; Conley, D.J.; Battles, J.J.; Frings, P.J.; Koppers, M.M.; Likens, G.E.; Johnson, C.E. Silica uptake and release in live and decaying biomass in a northern hardwood forest. Ecology 2016, 97, 3044–3057. [Google Scholar] [CrossRef]
- Narayanaswamy, C.; Prakash, N.B. Evaluation of selected extractants for plant-available silicon in rice soils of Southern India. Commun. Soil Sci. Plant Anal. 2010, 41, 977–989. [Google Scholar] [CrossRef]
- Narayanaswamy, C.; Prakash, N.B. Calibration and categorization of plant available silicon in rice soils of South India. J. Plant Nutr. 2009, 32, 1237–1254. [Google Scholar] [CrossRef]
- DeMaster, D.J. The supply and accumulation of silica in the marine environment. Geochim. Cosmochim. Acta 1981, 45, 1715–1732. [Google Scholar] [CrossRef]
- Puppe, D.; Höhn, A.; Kaczorek, D.; Wanner, M.; Wehrhan, M.; Sommer, M. How big is the influence of biogenic silicon pools on short-term changes in water-soluble silicon in soils? Implications from a study of a 10-year-old soil-plant system. Biogeosciences 2017, 14, 5239–5252. [Google Scholar] [CrossRef]
- Chao, T.T.; Sanzolone, R.F. Decomposition techniques. J. Geochem. Explor. 1992, 44, 65–106. [Google Scholar] [CrossRef]
- Nakamura, R.; Cornelis, J.T.; de Tombeur, F.; Nakagawa, M.; Kitajima, K. Comparative analysis of borate fusion versus sodium carbonate extraction for quantification of silicon contents in plants. J. Plant Res. 2020, 133, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Guntzer, F.; Keller, C.; Meunier, J.D. Determination of the silicon concentration in plant material using Tiron extraction. New Phytol. 2010, 188, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Reidinger, S.; Ramsey, M.H.; Hartley, S.E. Rapid and accurate analyses of silicon and phosphorus in plants using a portable X-ray fluorescence spectrometer. New Phytol. 2012, 195, 699–706. [Google Scholar] [CrossRef]
- Alexandre, A.; Meunier, J.-D.; Colin, F.; Koud, J.-M. Plant impact on the biogeochemical cycle of silicon and related weathering processes. Geochim. Cosmochim. Acta 1997, 61, 677–682. [Google Scholar] [CrossRef]
- Hodson, M.J.; White, P.J.; Mead, A.; Broadley, M.R. Phylogenetic variation in the silicon composition of plants. Ann. Bot. 2005, 96, 1027–1046. [Google Scholar] [CrossRef] [PubMed]
- Prychid, C.J.; Rudall, P.J.; Gregory, M. Systematics and biology of silica bodies in monocotyledons. Bot. Rev. 2003, 69, 377–440. [Google Scholar] [CrossRef]
- Piperno, D.R. The production, deposition, and dissolution of phytoliths. Phytolith Anal. 1988, 11–49. [Google Scholar] [CrossRef]
- Zotz, G. The systematic distribution of vascular epiphytes-a critical update. Bot. J. Linn. Soc. 2013, 171, 453–481. [Google Scholar] [CrossRef]
- Thummel, R.V.; Brightly, W.H.; Strömberg, C.A.E. Evolution of phytolith deposition in modern bryophytes, and implications for the fossil record and influence on silica cycle in early land plant evolution. New Phytol. 2019, 221, 2273–2285. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, J. Phytoliths of pteridophytes. S. Afr. J. Bot. 2011, 77, 10–19. [Google Scholar] [CrossRef]
- Nguyen, M.N.; Meharg, A.A.; Carey, M.; Dultz, S.; Marone, F.; Cichy, S.B.; Tran, C.T.; Le, G.H.; Mai, N.T.; Nguyen, T.T.H. Fern, Dicranopteris linearis, derived phytoliths in soil: Morphotypes, solubility and content in relation to soil properties. Eur. J. Soil Sci. 2019, 70, 507–517. [Google Scholar] [CrossRef]
- Golokhvast, K.S.; Seryodkin, I.V.; Bulakh, E.M.; Chaika, V.V.; Zakharenko, A.M.; Kholodov, A.S.; Pamirsky, I.E.; Chung, G. Mycolith (fungal phytolith) morphotypes and biosilification of proteins in wood-destroying and pileate fungi. Bot. Pacifica 2018, 7, 63–70. [Google Scholar] [CrossRef]
- Mizutani, T.; Nagase, H.; Fujiwara, N.; Ogoshi, H. Silicic acid polymerization catalyzed by amines and polyamines. Bull. Chem. Soc. Jpn. 1998, 71, 2017–2022. [Google Scholar] [CrossRef]
- Coradin, T.; Livage, J. Effect of some amino acids and peptides on silicic acid polymerization. Colloids Surf. B Biointerfaces 2001, 21, 329–336. [Google Scholar] [CrossRef]
- Kauss, H.; Seehaus, K.; Franke, R.; Gilbert, S.; Dietrich, R.A.; Kröger, N. Silica deposition by a strongly cationic proline-rich protein from systemically resistant cucumber plants. Plant J. 2003, 33, 87–95. [Google Scholar] [CrossRef]
- Mann, S.; Perry, C.C. Structural aspects of biogenic silica. Ciba Found. Symp. 1986, 121, 40–58. [Google Scholar] [CrossRef]
- Harrison, C.C. Evidence for intramineral macromolecules containing protein from plant silicas. Phytochemistry 1996, 41, 37–42. [Google Scholar] [CrossRef]
- Ma, J.; Cai, H.; He, C.; Zhang, W.; Wang, L. A hemicellulose-bound form of silicon inhibits cadmium ion uptake in rice (Oryza sativa) cells. New Phytol. 2015, 206, 1063–1074. [Google Scholar] [CrossRef]
- Perry, C.C.; Keeling-Tucker, T. Biosilicification: The role of the organic matrix in structure control. J. Biol. Inorg. Chem. 2000, 5, 537–550. [Google Scholar] [CrossRef]
- Currie, H.A.; Perry, C.C. Silica in plants: Biological, biochemical and chemical studies. Ann. Bot. 2007, 100, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Peleg, Z.; Saranga, Y.; Fahima, T.; Aharoni, A.; Elbaum, R. Genetic control over silica deposition in wheat awns. Physiol. Plant. 2010, 140, 10–20. [Google Scholar] [CrossRef]
- Hodson, M.J. The development of phytoliths in plants and its influence on their chemistry and isotopic composition. Implications for palaeoecology and archaeology. J. Archaeol. Sci. 2016, 68, 62–69. [Google Scholar] [CrossRef]
- Sakai, W.S.; Thom, M. Localization of silicon in specific cell wall layers of the stomatal apparatus of sugar cane by use of energy dispersive x-ray analysis. Ann. Bot. 1979, 44, 245–248. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, L.; Zhang, W.; Zhang, F. Do lignification and silicification of the cell wall precede silicon deposition in the silica cell of the rice (Oryza sativa L.) leaf epidermis? Plant Soil 2013, 372, 137–149. [Google Scholar] [CrossRef]
- He, C.; Ma, J.; Wang, L. A hemicellulose-bound form of silicon with potential to improve the mechanical properties and regeneration of the cell wall of rice. New Phytol. 2015, 206, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Ueno, O.; Agarie, S. Silica deposition in cell walls of the stomatal apparatus of rice leaves. Plant Prod. Sci. 2005, 8, 71–73. [Google Scholar] [CrossRef]
- He, C.; Wang, L.; Liu, J.; Liu, X.; Li, X.; Ma, J.; Lin, Y.; Xu, F. Evidence for “silicon” within the cell walls of suspension-cultured rice cells. New Phytol. 2013, 200, 700–709. [Google Scholar] [CrossRef]
- Rudall, P.J.; Prychid, C.J.; Gregory, T. Epidermal patterning and silica phytoliths in grasses: An evolutionary history. Bot. Rev. 2014, 80, 59–71. [Google Scholar] [CrossRef]
- Schaller, J.; Brackhage, C.; Paasch, S.; Brunner, E.; Bäucker, E.; Dudel, E.G. Silica uptake from nanoparticles and silica condensation state in different tissues of Phragmites australis. Sci. Total Environ. 2013, 442, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Neumann, K.; Strömberg, C.A.E.; Ball, T.; Albert, R.M.; Vrydaghs, L.; Cummings, L.S. International Code for Phytolith Nomenclature (ICPN) 2.0. Ann. Bot. 2019, 124, 189–199. [Google Scholar] [CrossRef]
- Yoshida, S.; Ohnishi, Y.; Kitagishi, K. Chemical forms, mobility and deposition of silicon in rice plant. Soil Sci. Plant Nutr. 1962, 8, 15–21. [Google Scholar] [CrossRef]
- Matichenkov, V.V.; Bocharnikova, E.A.; Kosobryukhov, A.A.; Biel, K.Y. Mobile forms of silicon in plants. Dokl. Biol. Sci. 2008, 418, 39–40. [Google Scholar] [CrossRef]
- Motomura, H.; Fujii, T.; Suzuki, M. Silica deposition in relation to ageing of leaf tissues in Sasa veitchii (Carrière) Rehder (Poaceae: Bambusoideae). Ann. Bot. 2004, 93, 235–248. [Google Scholar] [CrossRef]
- Fernández Honaine, M.; Osterrieth, M.L. Silicification of the adaxial epidermis of leaves of a panicoid grass in relation to leaf position and section and environmental conditions. Plant Biol. 2012, 14, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Hodson, M.J.; Sangster, A.G.; Parry, D.W. An ultrastructural study on the developmental phases and silicification of the glumes of Phalaris canariensis L. Ann. Bot. 1985, 55, 649–665. [Google Scholar] [CrossRef]
- Kaufman, P.B.; Petering, L.B.; Smith, J.G. Ultrastructural development of cork-silica cell pairs in Avena internodal epidermis. Bot. Gaz. 1970, 131, 173–185. [Google Scholar] [CrossRef]
- Hodson, M.J.; Bell, A. The mineral relations of the lemma of Phalaris canariensis L., with particular reference to its silicified macrohairs. Isr. J. Bot. 1986, 35, 241–253. [Google Scholar] [CrossRef]
- Lanning, F.C.; Eleuterius, L.N. Silica and ash in tissues of some coastal plants. Ann. Bot. 1983, 51, 835–850. [Google Scholar] [CrossRef]
- Fernández Honaine, M.; Borrelli, N.L.; Osterrieth, M.; Del Rio, L. Leaf and culm silicification of Pampas grass (Cortaderia selloana) developed on different soils from Pampean region, Argentina. Aust. J. Bot. 2017, 65, 1–10. [Google Scholar] [CrossRef]
- Schaller, J.; Brackhage, C.; Gessner, M.O.; Bäuker, E.; Gert Dudel, E. Silicon supply modifies C:N:P stoichiometry and growth of Phragmites australis. Plant Biol. 2012, 14, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Albert, R.M.; Shahack-Gross, R.; Cabanes, D.; Gilboa, A.; Lev-Yadun, S.; Portillo, M.; Sharon, I.; Boaretto, E.; Weiner, S. Phytolith-rich layers from the Late Bronze and Iron Ages at Tel Dor (Israel): Mode of formation and archaeological significance. J. Archaeol. Sci. 2008, 35, 57–75. [Google Scholar] [CrossRef]
- Tsartsidou, G.; Lev-Yadun, S.; Albert, R.M.; Miller-Rosen, A.; Efstratiou, N.; Weiner, S. The phytolith archaeological record: Strengths and weaknesses evaluated based on a quantitative modern reference collection from Greece. J. Archaeol. Sci. 2007, 34, 1262–1275. [Google Scholar] [CrossRef]
- Portillo, M.; Kadowaki, S.; Nishiaki, Y.; Albert, R.M. Early Neolithic household behavior at Tell Seker al-Aheimar (Upper Khabur, Syria): A comparison to ethnoarchaeological study of phytoliths and dung spherulites. J. Archaeol. Sci. 2014, 42, 107–118. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, R.; Paruya, D.K.; Yao, Y.F.; Li, C.S.; Bera, S. Phytolith spectra in respiratory aerial roots of some mangrove plants of the Indian Sunderbans and its efficacy in ancient deltaic environment reconstruction. Quat. Int. 2014, 325, 179–196. [Google Scholar] [CrossRef]
- Schoelynck, J.; Bal, K.; Puijalon, S.; Meire, P.; Struyf, E. Hydrodynamically mediated macrophyte silica dynamics. Plant Biol. 2012, 14, 997–1005. [Google Scholar] [CrossRef]
- Lanning, F.C.; Eleuterius, L.N. Silica deposition in some C3 and C4 species of grasses, sedges and composites in the USA. Ann. Bot. 1989, 64, 395–410. [Google Scholar] [CrossRef]
- Sangster, A.G.; Hodson, M.J. Silica deposition in subterranean organs. In Phytolith Systematics: Emerging Issues; Rapp, G., Mulholland, S.C., Eds.; Springer: Boston, MA, USA, 1992; pp. 239–251. [Google Scholar]
- Gallego, L.; Distel, R.A. Phytolith assemblages in grasses native to central Argentina. Ann. Bot. 2004, 94, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Novello, A.; Barboni, D. Grass inflorescence phytoliths of useful species and wild cereals from sub-Saharan Africa. J. Archaeol. Sci. 2015, 59, 10–22. [Google Scholar] [CrossRef]
- Mercader, J.; Astudillo, F.; Barkworth, M.; Bennett, T.; Esselmont, C.; Kinyanjui, R.; Grossman, D.L.; Simpson, S.; Walde, D. Poaceae phytoliths from the Niassa Rift, Mozambique. J. Archaeol. Sci. 2010, 37, 1953–1967. [Google Scholar] [CrossRef]
- Fahmy, A.G. Diversity of lobate phytoliths in grass leaves from the Sahel region, West Tropical Africa: Tribe Paniceae. Plant Syst. Evol. 2008, 270, 1–23. [Google Scholar] [CrossRef]
- Golokhvast, K.S.; Seryodkin, I.V.; Chaika, V.V.; Zakharenko, A.M.; Pamirsky, I.E. Phytoliths in taxonomy of phylogenetic domains of plants. BioMed Res. Int. 2014, 2014, 648326. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, O.; Greenbaum, N.; Ayalon, A.; Bar-Matthews, M.; Boaretto, E.; Bruins, H.J.; Cabanes, D.; Horwitz, L.K.; Neumann, F.H.; Porat, N.; et al. Using palaeo-environmental proxies to reconstruct natural and anthropogenic controls on sedimentation rates, Tell es-Safi/Gath, eastern Mediterranean. Anthropocene 2014, 8, 70–82. [Google Scholar] [CrossRef]
- Alexandre, A.; Meunier, J.D.; Lézine, A.M.; Vincens, A.; Schwartz, D. Phytoliths: Indicators of grassland dynamics during the late Holocene in intertropical Africa. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1997, 136, 213–229. [Google Scholar] [CrossRef]
- Morgan-Edel, K.D.; Boston, P.J.; Spilde, M.N.; Reynolds, R.E. Phytoliths (plant-derived mineral bodies) as geobiological and climatic indicators in arid environments. New Mex. Geol. 2015, 37, 3–20. [Google Scholar]
- Piperno, D.R.; Sues, H.D. Dinosaurs dined on grass. Science 2005, 310, 1126–1128. [Google Scholar] [CrossRef] [PubMed]
- Strömberg, C.A.E. Using phytolith assemblages to reconstruct the origin and spread of grass-dominated habitats in the great plains of North America during the late Eocene to early Miocene. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004, 207, 239–275. [Google Scholar] [CrossRef]
- Bremond, L.; Alexandre, A.; Véla, E.; Guiot, J. Advantages and disadvantages of phytolith analysis for the reconstruction of Mediterranean vegetation: An assessment based on modern phytolith, pollen and botanical data (Luberon, France). Rev. Palaeobot. Palynol. 2004, 129, 213–228. [Google Scholar] [CrossRef]
- Bremond, L.; Alexandre, A.; Wooller, M.J.; Hély, C.; Williamson, D.; Schäfer, P.A.; Majule, A.; Guiot, J. Phytolith indices as proxies of grass subfamilies on East African tropical mountains. Glob. Planet. Chang. 2008, 61, 209–224. [Google Scholar] [CrossRef]
- Zurro, D.; García-Granero, J.J.; Lancelotti, C.; Madella, M. Directions in current and future phytolith research. J. Archaeol. Sci. 2016, 68, 112–117. [Google Scholar] [CrossRef]
- Morris, L.R.; West, N.E.; Baker, F.A.; Van Miegroet, H.; Ryel, R.J. Developing an approach for using the soil phytolith record to infer vegetation and disturbance regime changes over the past 200 years. Quat. Int. 2009, 193, 90–98. [Google Scholar] [CrossRef]
- Hart, T.C. Issues and directions in phytolith analysis. J. Archaeol. Sci. 2016, 68, 24–31. [Google Scholar] [CrossRef]
- Shillito, L.M. Grains of truth or transparent blindfolds? A review of current debates in archaeological phytolith analysis. Veg. Hist. Archaeobot. 2013, 22, 71–82. [Google Scholar] [CrossRef]
- Prasad, V.; Strömberg, C.A.E.; Alimohammadian, H.; Sahni, A. Dinosaur coprolites and the early evolution of grasses and grazers. Science 2005, 310, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Strömberg, C.A.E.; Leaché, A.D.; Samant, B.; Patnaik, R.; Tang, L.; Mohabey, D.M.; Ge, S.; Sahni, A. Late Cretaceous origin of the rice tribe provides evidence for early diversification in Poaceae. Nat. Commun. 2011, 2, 480. [Google Scholar] [CrossRef]
- Katz, O. Extending the scope of Darwin’s “abominable mystery”: Integrative approaches to understanding angiosperm origins and species richness. Ann. Bot. 2018, 121, 1–8. [Google Scholar] [CrossRef]
- Schaller, J.; Turner, B.L.; Weissflog, A.; Pino, D.; Bielnicka, A.W.; Engelbrecht, B.M.J. Silicon in tropical forests: Large variation across soils and leaves suggests ecological significance. Biogeochemistry 2018, 140, 161–174. [Google Scholar] [CrossRef]
- Katz, O. Silicon and plant–animal interactions: Towards an evolutionary framework. Plants 2020, 9, 430. [Google Scholar] [CrossRef]
- Zhu, Y.; Gong, H. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 2014, 34, 455–472. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Huynh, W.Q.; Kronzucker, H.J. The role of silicon in higher plants under salinity and drought stress. Front. Plant Sci. 2016, 7, 1072. [Google Scholar] [CrossRef]
- Wilkinson, R. Plant-Environment Interactions; Marcel Dekker: New York, NY, USA, 2000; ISBN 0824703774. [Google Scholar]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Dakora, F.D.; Nelwamondo, A. Silicon nutrition promotes root growth and tissue mechanical strength in symbiotic cowpea. Funct. Plant Biol. 2003, 30, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Steudle, E.; Peterson, C.A. How does water get through roots? J. Exp. Bot. 1998, 49, 775–788. [Google Scholar] [CrossRef]
- Gao, X.; Zou, C.; Wang, L.; Zhang, F. Silicon decreases transpiration rate and conductance from stomata of maize plants. J. Plant Nutr. 2006, 29, 1637–1647. [Google Scholar] [CrossRef]
- Gao, X.; Zou, C.; Wang, L.; Zhang, F. Silicon improves water use efficiency in maize plants. J. Plant Nutr. 2004, 27, 1457–1470. [Google Scholar] [CrossRef]
- Agarie, S.; Uchida, H.; Agata, W.; Kaufman, P.B. Effects of silicon on stomatal blue-light response in rice (Oryza sativa L.). Plant Prod. Sci. 1999, 2, 232–234. [Google Scholar] [CrossRef]
- Shakoor, S.A. Silicon biomineralisation in plants: A tool to adapt global climate change. J. Res. Biol. Sci. 2014, 1, 1–3. [Google Scholar]
- Vandegeer, R.K.; Zhao, C.; Cibils-Stewart, X.; Wuhrer, R.; Hall, C.R.; Hartley, S.E.; Tissue, D.T.; Johnson, S.N. Silicon deposition on guard cells increases stomatal sensitivity as mediated by K+ efflux and consequently reduces stomatal conductance. Physiol. Plant. 2020. [Google Scholar] [CrossRef]
- Aston, M.J.; Jones, M.M. A study of the transpiration surfaces of Avena sterilis L. var. Algerian leaves using monosilicic acid as a tracer for water movement. Planta 1976, 130, 121–129. [Google Scholar] [CrossRef]
- Harizanova, A.; Zlatev, Z.; Koleva, L. Effect of silicon on activity of antioxidant enzymes and photosynthesis in leaves of cucumber plants (Cucumis sativus L.). Türk Tarım ve Doğa Bilim. Derg. 2014, 1, 1812–1817. [Google Scholar]
- Shen, X.; Zhou, Y.; Duan, L.; Li, Z.; Eneji, A.E.; Li, J. Silicon effects on photosynthesis and antioxidant parameters of soybean seedlings under drought and ultraviolet-B radiation. J. Plant Physiol. 2010, 167, 1248–1252. [Google Scholar] [CrossRef]
- Thorne, S.J.; Hartley, S.E.; Maathuis, F.J.M. Is silicon a panacea for alleviating drought and salt stress in crops? Front. Plant Sci. 2020, 11, 1221. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, B.; Jiang, D.; Chen, G. Silicon improves photosynthetic performance by optimizing thylakoid membrane protein components in rice under drought stress. Environ. Exp. Bot. 2019, 158, 117–124. [Google Scholar] [CrossRef]
- Gong, H.; Zhu, X.; Chen, K.; Wang, S.; Zhang, C. Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci. 2005, 169, 313–321. [Google Scholar] [CrossRef]
- Detmann, K.C.; Araújo, W.L.; Martins, S.C.V.; Sanglard, L.M.V.P.; Reis, J.V.; Detmann, E.; Rodrigues, F.Á.; Nunes-Nesi, A.; Fernie, A.R.; Damatta, F.M. Silicon nutrition increases grain yield, which, in turn, exerts a feed-forward stimulation of photosynthetic rates via enhanced mesophyll conductance and alters primary metabolism in rice. New Phytol. 2012, 196, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Agarie, S.; Agata, W.; Uchida, H.; Kubota, F.; Kaufman, P.B. Function of silica bodies in the epidermal system of rice (Oryza sativa L.): Testing the window hypothesis. J. Exp. Bot. 1996, 47, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Vandevenne, F.I.; Barão, A.L.; Schoelynck, J.; Smis, A.; Ryken, N.; Van Damme, S.; Meire, P.; Struyf, E. Grazers: Biocatalysts of terrestrial silica cycling. Proc. R. Soc. B Biol. Sci. 2013, 280. [Google Scholar] [CrossRef]
- Carey, J.C.; Fulweiler, R.W. Human appropriation of biogenic silicon—the increasing role of agriculture. Funct. Ecol. 2016, 30, 1331–1339. [Google Scholar] [CrossRef]
- Müller, F.; Struyf, E.; Hartmann, J.; Weiss, A.; Jensen, K. Impact of grazing management on silica export dynamics of wadden sea saltmarshes. Estuar. Coast. Shelf Sci. 2013, 127, 1–11. [Google Scholar] [CrossRef]
- Struyf, E.; Smis, A.; van Damme, S.; Meire, P.; Conley, D.J. The global biogeochemical silicon cycle. Silicon 2009, 1, 207–213. [Google Scholar] [CrossRef]
- Struyf, E.; Conley, D.J. Emerging understanding of the ecosystem silica filter. Biogeochemistry 2012, 107, 9–18. [Google Scholar] [CrossRef]
- Alexandre, A.; Bouvet, M.; Abbadie, L. The role of savannas in the terrestrial Si cycle: A case-study from Lamto, Ivory Coast. Glob. Planet. Chang. 2011, 78, 162–169. [Google Scholar] [CrossRef]
- Viaroli, P.; Nizzoli, D.; Pinardi, M.; Rossetti, G.; Bartoli, M. Factors affecting dissolved silica concentrations, and DSi and DIN stoichiometry in a human impacted watershed (Po River, Italy). Silicon 2013, 5, 101–114. [Google Scholar] [CrossRef]
- Jacobs, S.; Müller, F.; Teuchies, J.; Oosterlee, L.; Struyf, E.; Meire, P. The vegetation silica pool in a developing tidal freshwater marsh. Silicon 2013, 5, 91–100. [Google Scholar] [CrossRef]
- Farmer, V.C.; Delbos, E.; Miller, J.D. The role of phytolith formation and dissolution in controlling concentrations of silica in soil solutions and streams. Geoderma 2005, 127, 71–79. [Google Scholar] [CrossRef]
- Raven, J.A. Cycling silicon—The role of accumulation in plants. New Phytol. 2003, 158, 419–421. [Google Scholar] [CrossRef]
- Klotzbücher, T.; Leuther, F.; Marxen, A.; Vetterlein, D.; Horgan, F.G.; Jahn, R. Forms and fluxes of potential plant-available silicon in irrigated lowland rice production (Laguna, the Philippines). Plant Soil 2015, 393, 177–191. [Google Scholar] [CrossRef]
- Johnson, S.N.; Hartley, S.E.; Moore, B.D. Silicon defence in plants: Does herbivore identity matter? Trends Plant Sci. 2021. [Google Scholar] [CrossRef]
- Massey, F.P.; Ennos, A.R.; Hartley, S.E. Silica in grasses as a defence against insect herbivores: Contrasting effects on folivores and a phloem feeder. J. Anim. Ecol. 2006, 75, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Shewmaker, G.E.; Mayland, H.F.; Rosenau, R.C.; Asay, K.H. Silicon in C-3 Grasses: Effects on forage quality and sheep preference. J. Range Manag. 1989, 42, 122–127. [Google Scholar] [CrossRef]
- Gali-Muhtasib, H.U.; Smith, C.C.; Higgins, J.J. The effect of silica in grasses on the feeding behavior of the prairie vole, Microtus Ochrogaster. Ecology 1992, 73, 1724–1729. [Google Scholar] [CrossRef]
- Chanas, B.; Pawlik, J.R. Does the skeleton of a sponge provide a defense against predatory reef fish? Oecologia 1996, 107, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Hartley, S.E.; Fitt, R.N.; McLarnon, E.L.; Wade, R.N. Defending the leaf surface: Intra- and inter-specific differences in silicon deposition in grasses in response to damage and silicon supply. Front. Plant Sci. 2015, 6, 35. [Google Scholar] [CrossRef]
- Kvedaras, O.L.; Keeping, M.G.; Goebel, F.R.; Byrne, M.J. Larval performance of the pyralid borer Eldana saccharina Walker and stalk damage in sugarcane: Influence of plant silicon, cultivar and feeding site. Int. J. Pest Manag. 2007, 53, 183–194. [Google Scholar] [CrossRef]
- Grime, J.P.; MacPherson-Stewart, S.F.; Dearman, R.S. An investigation of leaf palatability using the snail Cepaea nemoralis L. J. Ecol. 1968, 56, 405–420. [Google Scholar] [CrossRef]
- Samuels, A.L.; Glass, A.D.M.; Ehret, D.L.; Menzies, J.G. Distribution of silicon in cucumber leaves during infection by powdery mildew fungus (Sphaerotheca fuliginea). Can. J. Bot. 1991, 69, 140–146. [Google Scholar] [CrossRef]
- Samuels, A.L.; Glass, A.D.M.; Menzies, J.G.; Ehret, D.L. Silicon in cell walls and papillae of Cucumis sativus during infection by Sphaerotheca fuliginea. Physiol. Mol. Plant Pathol. 1994, 44, 237–242. [Google Scholar] [CrossRef]
- Kim, S.G.; Kim, K.W.; Park, E.W.; Choi, D. Silicon-induced cell wall fortification of rice leaves: A possible cellular mechanism of enhanced host resistance to blast. Phytopathology 2002, 92, 10959–11103. [Google Scholar] [CrossRef]
- Hall, C.R.; Dagg, V.; Waterman, J.M.; Johnson, S.N. Silicon alters leaf surface morphology and suppresses insect herbivory in a model grass species. Plants 2020, 9, 643. [Google Scholar] [CrossRef]
- Li, Z.; Song, Z.; Yan, Z.; Hao, Q.; Song, A.; Liu, L.; Yang, X.; Xia, S.; Liang, Y. Silicon enhancement of estimated plant biomass carbon accumulation under abiotic and biotic stresses. A meta-analysis. Agron. Sustain. Dev. 2018, 38, 26. [Google Scholar] [CrossRef]
- Ishizuka, Y. Physiology of the rice plant. Adv. Agron. 1971, 23, 241–315. [Google Scholar] [CrossRef]
- Massey, F.P.; Hartley, S.E. Physical defences wear you down: Progressive and. J. Anim. Ecol. 2009, 78, 281–291. [Google Scholar] [CrossRef]
- Mir, S.H.; Rashid, I.; Hussain, B.; Reshi, Z.A.; Assad, R.; Sofi, I.A.; Hodson, M.J.; Johnson, S.N.; Juma, G.; Mir, S.H.; et al. Silicon supplementation of rescuegrass reduces herbivory by a grasshopper. Front. Plant Sci. 2019, 671. [Google Scholar] [CrossRef]
- Massey, F.P.; Hartley, S.E. Experimental demonstration of the antiherbivore effects of silica in grasses: Impacts on foliage digestibility and vole growth rates. Proc. R. Soc. B Biol. Sci. 2006, 273, 2299–2304. [Google Scholar] [CrossRef] [PubMed]
- Rivals, F.; Takatsuki, S.; Albert, R.M.; Macià, L. Bamboo feeding and tooth wear of three sika deer (Cervus nippon) populations from northern Japan. J. Mammal. 2014, 95, 1043–1053. [Google Scholar] [CrossRef]
- Sanson, G.D.; Kerr, S.A.; Gross, K.A. Do silica phytoliths really wear mammalian teeth? J. Archaeol. Sci. 2007, 34, 526–531. [Google Scholar] [CrossRef]
- Kaiser, T.M.; Braune, C.; Kalinka, G.; Schulz-Kornas, E. Nano-indentation of native phytoliths and dental tissues: Implications for herbivore-plant combat and dental wear proxies. Evol. Syst. 2018, 2, 55–63. [Google Scholar] [CrossRef]
- Lev-Yadun, S.; Halpern, M. External and internal spines in plants insert pathogenic microorganisms into herbivore’s tissues for defense. Microb. Ecol. Res. Trends 2008, 155–168. [Google Scholar]
- Song, Z.; Liu, H.; Zhao, F.; Xu, C. Ecological stoichiometry of N:P:Si in China’s grasslands. Plant Soil 2014, 380, 165–179. [Google Scholar] [CrossRef]
- Frew, A.; Weston, L.A.; Gurr, G.M. Silicon reduces herbivore performance via different mechanisms, depending on host–plant species. Austral. Ecol. 2019, 44, 1092–1097. [Google Scholar] [CrossRef]
- Schaller, J.; Schoelynck, J.; Struyf, E.; Meire, P. Silicon affects nutrient content and ratios of wetland plants. Silicon 2016, 8, 479–485. [Google Scholar] [CrossRef]
- Hunt, J.W.; Dean, A.P.; Webster, R.E.; Johnson, G.N.; Ennos, A.R. A novel mechanism by which silica defends grasses against herbivory. Ann. Bot. 2008, 102, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, C.; Corbera, J.A.; Doreste, F.; Padrón, T.R.; Morales, M. Silica urolithiasis in the dromedary camel in a subtropical climate. Vet. Res. Commun. 2002, 26, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.B. Silica metabolism and silica urolithiasis in ruminants: A review. Can. J. Anim. Sci. 1981, 61, 219–235. [Google Scholar] [CrossRef]
- Vicari, M.; Bazely, D.R. Do grasses fight back? The case for antiherbivore defences. Trends Ecol. Evol. 1993, 8, 137–141. [Google Scholar] [CrossRef]
- Bhatt, T.; Coombs, M.; O’Neill, C. Biogenic silica fibre promotes carcinogenesis in mouse skin. Int. J. Cancer 1984, 34, 519–528. [Google Scholar] [CrossRef]
- Cherif, M.; Asselin, A.; Belanger, R.R. Defence responses induced by soluble silicon in cucumber roots infected by Phytium spp. Phytopathology 1994, 84, 236–242. [Google Scholar] [CrossRef]
- Cai, K.; Gao, D.; Luo, S.; Zeng, R.; Yang, J.; Zhu, X. Physiological and cytological mechanisms of silicon-induced resistance in rice against blast disease. Physiol. Plant. 2008, 134, 324–333. [Google Scholar] [CrossRef]
- Fauteux, F.; Rémus-Borel, W.; Menzies, J.G.; Bélanger, R.R. Silicon and plant disease resistance against pathogenic fungi. FEMS Microbiol. Lett. 2005, 249, 1–6. [Google Scholar] [CrossRef]
- Wang, M.; Gao, L.; Dong, S.; Sun, Y.; Shen, Q.; Guo, S. Role of silicon on plant–pathogen interactions. Front. Plant Sci. 2017, 8, 701. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Wallis, C.M.; Uddin, W. Silicon-Induced systemic defense responses in perennial ryegrass against infection by Magnaporthe oryzae. Phytopathology 2015, 105, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Leroy, N.; De Tombeur, F.; Walgraffe, Y.; Cornélis, J.T.; Verheggen, F.J. Silicon and plant natural defenses against insect pests: Impact on plant volatile organic compounds and cascade effects on multitrophic interactions. Plants 2019, 8, 444. [Google Scholar] [CrossRef] [PubMed]
- Fauteux, F.; Chain, F.; Belzile, F.; Menzies, J.G.; Bélanger, R.R. The protective role of silicon in the Arabidopsis-powdery mildew pathosystem. Proc. Natl. Acad. Sci. USA 2006, 103, 17554–17559. [Google Scholar] [CrossRef] [PubMed]
- Schoelynck, J.; Bal, K.; Backx, H.; Okruszko, T.; Meire, P.; Struyf, E. Silica uptake in aquatic and wetland macrophytes: A strategic choice between silica, lignin and cellulose? New Phytol. 2010, 186, 385–391. [Google Scholar] [CrossRef]
- Klotzbücher, T.; Klotzbücher, A.; Kaiser, K.; Vetterlein, D.; Jahn, R.; Mikutta, R. Variable silicon accumulation in plants affects terrestrial carbon cycling by controlling lignin synthesis. Glob. Chang. Biol. 2018, 24, e183–e189. [Google Scholar] [CrossRef]
- Cooke, J.; Leishman, M.R. Tradeoffs between foliar silicon and carbon-based defences: Evidence from vegetation communities of contrasting soil types. Oikos 2012, 121, 2052–2060. [Google Scholar] [CrossRef]
- Frew, A.; Powell, J.R.; Sallam, N.; Allsopp, P.G.; Johnson, S.N. Trade-Offs between silicon and phenolic defenses may explain enhanced performance of root herbivores on phenolic-rich plants. J. Chem. Ecol. 2016, 42, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.N.; Hartley, S.E. Elevated carbon dioxide and warming impact silicon and phenolic-based defences differently in native and exotic grasses. Glob. Chang. Biol. 2018, 24, 3886–3896. [Google Scholar] [CrossRef]
- Schaller, J.; Brackhage, C.; Dudel, E.G. Silicon availability changes structural carbon ratio and phenol content of grasses. Environ. Exp. Bot. 2012, 77, 283–287. [Google Scholar] [CrossRef]
- Biru, F.N.; Cazzonelli, C.I.; Elbaum, R.; Johnson, S.N. Contrasting effects of Miocene and Anthropocene levels of atmospheric CO2 on silicon accumulation in a model grass. Biol. Lett. 2020, 16, 20200608. [Google Scholar] [CrossRef]
- Fulweiler, R.W.; Maguire, T.J.; Carey, J.C.; Finzi, A.C. Does elevated CO2 alter silica uptake in trees? Front. Plant Sci. 2015, 5, 793. [Google Scholar] [CrossRef]
- Brightly, W.H.; Hartley, S.E.; Osborne, C.P.; Simpson, K.J.; Strömberg, C.A.E. High silicon concentrations in grasses are linked to environmental conditions and not associated with C4 photosynthesis. Glob. Chang. Biol. 2020, 26, 7128–7143. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.; DeGabriel, J.L.; Hartley, S.E. The functional ecology of plant silicon: Geoscience to genes. Funct. Ecol. 2016, 30, 1270–1276. [Google Scholar] [CrossRef]
- Sommer, M.; Kaczorek, D.; Kuzyakov, Y.; Breuer, J. Silicon pools and fluxes in soils and landscapes—A review. J. Plant Nutr. Soil Sci. 2006, 169, 310–329. [Google Scholar] [CrossRef]
- Street-Perrott, F.A.; Barker, P.A. Biogenic silica: A neglected component of the coupled global continental biogeochemical cycles of carbon and silicon. Earth Surf. Process. Landforms 2008, 33, 1436–1457. [Google Scholar] [CrossRef]
- Cornelis, J.T.; Delvaux, B.; Georg, R.B.; Lucas, Y.; Ranger, J.; Opfergelt, S. Tracing the origin of dissolved silicon transferred from various soil-plant systems towards rivers: A review. Biogeosciences 2011, 8, 89–112. [Google Scholar] [CrossRef]
- Fraysse, F.; Cantais, F.; Pokrovsky, O.S.; Schott, J.; Meunier, J.D. Aqueous reactivity of phytoliths and plant litter: Physico-chemical constraints on terrestrial biogeochemical cycle of silicon. J. Geochem. Explor. 2006, 88, 202–205. [Google Scholar] [CrossRef]
- Fraysse, F.; Pokrovsky, O.S.; Schott, J.; Meunier, J.D. Surface chemistry and reactivity of plant phytoliths in aqueous solutions. Chem. Geol. 2009, 258, 197–206. [Google Scholar] [CrossRef]
- Dürr, H.H.; Meybeck, M.; Hartmann, J.; Laruelle, G.G.; Roubeix, V. Global spatial distribution of natural riverine silica inputs to the coastal zone. Biogeosciences 2011, 8, 597–620. [Google Scholar] [CrossRef]
- Struyf, E.; Smis, A.; Van Damme, S.; Garnier, J.; Govers, G.; Van Wesemael, B.; Conley, D.J.; Batelaan, O.; Frot, E.; Clymans, W.; et al. Historical land use change has lowered terrestrial silica mobilization. Nat. Commun. 2010, 1, 129. [Google Scholar] [CrossRef]
- Vandevenne, F.I.; Delvaux, C.; Hughes, H.J.; André, L.; Ronchi, B.; Clymans, W.; Baraõ, L.; Govers, G.; Meire, P.; Struyf, E. Landscape cultivation alters δ30Si signature in terrestrial ecosystems. Sci. Rep. 2015, 5, 7732. [Google Scholar] [CrossRef]
- Vandevenne, F.I.; Barão, L.; Ronchi, B.; Govers, G.; Meire, P.; Kelly, E.F.; Struyf, E. Silicon pools in human impacted soils of temperate zones. Glob. Biogeochem. Cycles 2015, 9, 1439–1450. [Google Scholar] [CrossRef]
- Clymans, W.; Struyf, E.; Govers, G.; Vandevenne, F.; Conley, D.J. Anthropogenic impact on amorphous silica pools in temperate soils. Biogeosciences 2011, 8, 2281–2293. [Google Scholar] [CrossRef]
- Sommer, M.; Jochheim, H.; Höhn, A.; Breuer, J.; Zagorski, Z.; Busse, J.; Barkusky, D.; Meier, K.; Puppe, D.; Wanner, M.; et al. Si cycling in a forest biogeosystem-the importance of transient state biogenic Si pools. Biogeosciences 2013, 10, 4991–5007. [Google Scholar] [CrossRef]
- Desplanques, V.; Cary, L.; Mouret, J.C.; Trolard, F.; Bourrié, G.; Grauby, O.; Meunier, J.D. Silicon transfers in a rice field in Camargue (France). J. Geochemical Explor. 2006, 88, 190–193. [Google Scholar] [CrossRef]
- Guntzer, F.; Keller, C.; Poulton, P.R.; McGrath, S.P.; Meunier, J.D. Long-Term removal of wheat straw decreases soil amorphous silica at Broadbalk, Rothamsted. Plant Soil 2012, 352, 173–184. [Google Scholar] [CrossRef]
- Meunier, J.D.; Guntzer, F.; Kirman, S.; Keller, C. Terrestrial plant-Si and environmental changes. Mineral. Mag. 2008, 72, 263–267. [Google Scholar] [CrossRef]
- Vandevenne, F.; Struyf, E.; Clymans, W.; Meire, P. Agricultural silica harvest: Have humans created a new loop in the global silica cycle? Front. Ecol. Environ. 2012, 10, 243–248. [Google Scholar] [CrossRef]
- Schaller, J.; Puppe, D. Heat improves silicon availability in mineral soils. Geoderma 2021, 386, 114909. [Google Scholar] [CrossRef]
- Haynes, R.J. What effect does liming have on silicon availability in agricultural soils? Geoderma 2019, 337, 375–383. [Google Scholar] [CrossRef]
- Savant, N.K.; Snyder, G.H.; Datnoff, L.E. Silicon management and sustainable rice production. Adv. Agron. 1996, 58, 151–199. [Google Scholar] [CrossRef]
- Savant, N.K.; Korndörfer, G.H.; Datnoff, L.E.; Snyder, G.H. Silicon nutrition and sugarcane production: A review. J. Plant Nutr. 1999, 22, 1853–1903. [Google Scholar] [CrossRef]
- Berhane, M.; Xu, M.; Liang, Z.; Shi, J.; Wei, G.; Tian, X. Effects of long-term straw return on soil organic carbon storage and sequestration rate in North China upland crops: A meta-analysis. Glob. Chang. Biol. 2020, 26, 2686–2701. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dai, M.; Dai, S.; Dong, X. Current status and environment impact of direct straw return in China’s cropland—A review. Ecotoxicol. Environ. Saf. 2018, 159, 293–300. [Google Scholar] [CrossRef]
- Song, Z.; Müller, K.; Wang, H. Biogeochemical silicon cycle and carbon sequestration in agricultural ecosystems. Earth-Sci. Rev. 2014, 139, 268–278. [Google Scholar] [CrossRef]
- Hodson, M.J. The relative importance of cell wall and lumen phytoliths in carbon sequestration in soil: A hypothesis. Front. Earth Sci. 2019, 7, 167. [Google Scholar] [CrossRef]
- Caubet, M.; Cornu, S.; Saby, N.P.A.; Meunier, J.D. Agriculture increases the bioavailability of silicon, a beneficial element for crop, in temperate soils. Sci. Rep. 2020, 10, 19999. [Google Scholar] [CrossRef] [PubMed]
- Puppe, D.; Kaczorek, D.; Schaller, J.; Barkusky, D.; Sommer, M. Crop straw recycling prevents anthropogenic desilication of agricultural soil-plant sys-tems in the temperate zone—Results from a long-term field experiment in NE Germany. Geoderma. in revision.
- Kaczorek, D.; Puppe, D.; Busse, J.; Sommer, M. Effects of phytolith distribution and characteristics on extractable silicon fractions in soils under different vegetation—An exploratory study on loess. Geoderma 2019, 356, 113917. [Google Scholar] [CrossRef]
- Cornelis, J.T.; Titeux, H.; Ranger, J.; Delvaux, B. Identification and distribution of the readily soluble silicon pool in a temperate forest soil below three distinct tree species. Plant Soil 2011, 342, 369–378. [Google Scholar] [CrossRef]
- Puppe, D.; Ehrmann, O.; Kaczorek, D.; Wanner, M.; Sommer, M. The protozoic Si pool in temperate forest ecosystems—Quantification, abiotic controls and interactions with earthworms. Geoderma 2015, 243–244, 196–204. [Google Scholar] [CrossRef]
- Haynes, R.J. Significance and role of Si in crop production. Adv. Agron. 2017, 146, 83–166. [Google Scholar] [CrossRef]
- Puppe, D.; Sommer, M. Experiments, uptake mechanisms, and functioning of silicon foliar fertilization—A review focusing on maize, rice, and wheat. Adv. Agron. 2018, 152, 1–49. [Google Scholar] [CrossRef]
- Klotzbücher, T.; Treptow, C.; Kaiser, K.; Klotzbücher, A.; Mikutta, R. Sorption competition with natural organic matter as mechanism controlling silicon mobility in soil. Sci. Rep. 2020, 10, 11225. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, F.; Cornelis, J.T.; Song, Z.; Wang, X.; Delvaux, B. Combined silicon-phosphorus fertilization affects the biomass and phytolith stock of rice plants. Front. Plant Sci. 2020, 11, 67. [Google Scholar] [CrossRef]
- Miles, N.; Manson, A.D.; Rhodes, R.; van Antwerpen, R.; Weigel, A. Extractable silicon in soils of the South African sugar industry and relationships with crop uptake. Commun. Soil Sci. Plant Anal. 2014, 45, 2949–2958. [Google Scholar] [CrossRef]
- Korndörfer, G.H.; Snyder, G.H.; Ulloa, M.; Powell, G.; Datnoff, L.E. Calibration of soil and plant silicon analysis for rice production. J. Plant Nutr. 2001, 24, 1071–1084. [Google Scholar] [CrossRef]
- de Tombeur, F.; Vander Linden, C.; Cornélis, J.T.; Godin, B.; Compère, P.; Delvaux, B. Soil and climate affect foliar silicification patterns and silica-cellulose balance in sugarcane (Saccharum officinarum). Plant Soil 2020, 452, 529–546. [Google Scholar] [CrossRef]
- Gocke, M.; Liang, W.; Sommer, M.; Kuzyakov, Y. Silicon uptake by wheat: Effects of Si pools and pH. J. Plant Nutr. Soil Sci. 2013, 176, 551–560. [Google Scholar] [CrossRef]
- Marxen, A.; Klotzbücher, T.; Jahn, R.; Kaiser, K.; Nguyen, V.S.; Schmidt, A.; Schädler, M.; Vetterlein, D. Interaction between silicon cycling and straw decomposition in a silicon deficient rice production system. Plant Soil 2016, 398, 153–163. [Google Scholar] [CrossRef]
- Keeping, M.G. Uptake of silicon by sugarcane from applied sources may not reflect plant-available soil silicon and total silicon content of sources. Front. Plant Sci. 2017, 8, 760. [Google Scholar] [CrossRef] [PubMed]
- Saccone, L.; Conley, D.J.; Likens, G.E.; Bailey, S.W.; Buso, D.C.; Johnson, C.E. Factors that control the range and variability of amorphous silica in soils in the Hubbard Brook Experimental Forest. Soil Sci. Soc. Am. J. 2008, 72, 1637–1644. [Google Scholar] [CrossRef]
- Fishkis, O.; Ingwersen, J.; Streck, T. Phytolith transport in sandy sediment: Experiments and modeling. Geoderma 2009, 151, 168–178. [Google Scholar] [CrossRef]
- Fishkis, O.; Ingwersen, J.; Lamers, M.; Denysenko, D.; Streck, T. Phytolith transport in soil: A field study using fluorescent labelling. Geoderma 2010, 157, 27–36. [Google Scholar] [CrossRef]
- Song, Z.; McGrouther, K.; Wang, H. Occurrence, turnover and carbon sequestration potential of phytoliths in terrestrial ecosystems. Earth-Sci. Rev. 2016, 158, 19–30. [Google Scholar] [CrossRef]
- Maguire, T.J.; Templer, P.H.; Battles, J.J.; Fulweiler, R.W. Winter climate change and fine root biogenic silica in sugar maple trees (Acer saccharum): Implications for silica in the Anthropocene. J. Geophys. Res. Biogeosci. 2017, 122, 708–715. [Google Scholar] [CrossRef]
- Turpault, M.P.; Calvaruso, C.; Kirchen, G.; Redon, P.O.; Cochet, C. Contribution of fine tree roots to the silicon cycle in a temperate forest ecosystem developed on three soil types. Biogeosciences 2018, 15, 2231–2249. [Google Scholar] [CrossRef]
- Puppe, D.; Leue, M. Physicochemical surface properties of different biogenic silicon structures: Results from spectroscopic and microscopic analyses of protistic and phytogenic silica. Geoderma 2018, 330, 212–220. [Google Scholar] [CrossRef]
- de Tombeur, F.; Turner, B.L.; Laliberté, E.; Lambers, H.; Mahy, G.; Faucon, M.P.; Zemunik, G.; Cornelis, J.T. Plants sustain the terrestrial silicon cycle during ecosystem retrogression. Science 2020, 369, 1245–1248. [Google Scholar] [CrossRef] [PubMed]
- Hinsinger, P.; Plassard, C.; Tang, C.; Jaillard, B. Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: A review. Plant Soil 2003, 248, 43–59. [Google Scholar] [CrossRef]
- Hinsinger, P.; Fernandes Barros, O.N.; Benedetti, M.F.; Noack, Y.; Callot, G. Plant-Induced weathering of a basaltic rock: Experimental evidence. Geochim. Cosmochim. Acta 2001, 65, 137–152. [Google Scholar] [CrossRef]
- Gattullo, C.E.; Allegretta, I.; Medici, L.; Fijan, R.; Pii, Y.; Cesco, S.; Mimmo, T.; Terzano, R. Silicon dynamics in the rhizosphere: Connections with iron mobilization. J. Plant Nutr. Soil Sci. 2016, 179, 409–417. [Google Scholar] [CrossRef]
- Puppe, D. Review on protozoic silica and its role in silicon cycling. Geoderma 2020, 365, 114224. [Google Scholar] [CrossRef]
- Qin, Y.; Puppe, D.; Zhang, L.; Sun, R.; Li, P.; Xie, S. How does Sphagnum growing affect testate Amoeba communities and corresponding protozoic Si pools? Results from field analyses in SW China. Microb. Ecol. 2021. [Google Scholar] [CrossRef]
- Qin, Y.; Puppe, D.; Payne, R.; Li, L.; Li, J.; Zhang, Z.; Xie, S. Land-Use change effects on protozoic silicon pools in the Dajiuhu National Wetland Park, China. Geoderma 2020, 368, 114305. [Google Scholar] [CrossRef]
- Ehrlich, H.; Demadis, K.D.; Pokrovsky, O.S.; Koutsoukos, P.G. Modern views on desilicification: Biosilica and abiotic silica dissolution in natural and artificial environments. Chem. Rev. 2010, 110, 4656–4689. [Google Scholar] [CrossRef]
- Haynes, R.J. A contemporary overview of silicon availability in agricultural soils. J. Plant Nutr. Soil Sci. 2014, 177, 831–844. [Google Scholar] [CrossRef]
- Crusciol, C.A.C.; De Arruda, D.P.; Fernandes, A.M.; Antonangelo, J.A.; Alleoni, L.R.F.; Do Nascimento, C.A.C.; Rossato, O.B.; McCray, J.M. Methods and extractants to evaluate silicon availability for sugarcane. Sci. Rep. 2018, 8, 916. [Google Scholar] [CrossRef]
- Mithen, S.; Jenkins, E.; Jamjoum, K.; Nuimat, S.; Nortcliff, S.; Finlayson, B. Experimental crop growing in Jordan to develop methodology for the identification of ancient crop irrigation. World Archaeol. 2008, 40, 7–25. [Google Scholar] [CrossRef]
- Ma, J.; Nishimura, K.; Takahashi, E. Effect of silicon on the growth of rice plant at different growth stages. Soil Sci. Plant Nutr. 1989, 35, 347–356. [Google Scholar] [CrossRef]
- Pati, S.; Pal, B.; Badole, S.; Hazra, G.C.; Mandal, B. Effect of silicon fertilization on growth, yield, and nutrient uptake of rice. Commun. Soil Sci. Plant Anal. 2016, 47, 284–290. [Google Scholar] [CrossRef]
- Cuong, T.X.; Ullah, H.; Datta, A.; Hanh, T.C. Effects of silicon-based Fertilizer on growth, yield and nutrient uptake of rice in tropical zone of Vietnam. Rice Sci. 2017, 24, 283–290. [Google Scholar] [CrossRef]
- Agostinho, F.B.; Tubana, B.S.; Martins, M.S.; Datnoff, L.E. Effect of different silicon sources on yield and silicon uptake of rice grown under varying phosphorus rates. Plants 2017, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Seebold, K.W.; Datnoff, L.E.; Correa-Victoria, F.J.; Kucharek, T.A.; Snyder, G.H. Effect of silicon rate and host resistance on blast, scald, and yield of upland price. Plant Dis. 2000, 84, 871–876. [Google Scholar] [CrossRef]
- Detling, J.K.; Painter, E.L. Defoliation responses of western wheatgrass populations with diverse histories of prairie dog grazing. Oecologia 1983, 57, 65–71. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, S.J.; Tarrants, J.L.; McNaughton, M.M.; Davis, R.D. Silica as a defense against herbivory and a growth promotor in African grasses. Ecology 1985, 66, 528–535. [Google Scholar] [CrossRef]
- Strömberg, C.A.E. Decoupled taxonomic radiation and ecological expansion of open-habitat grasses in the Cenozoic of North America. Proc. Natl. Acad. Sci. USA 2005, 102, 11980–11984. [Google Scholar] [CrossRef] [PubMed]
- Strömberg, C.A.E.; Di Stilio, V.S.; Song, Z. Functions of phytoliths in vascular plants: An evolutionary perspective. Funct. Ecol. 2016, 30, 1286–1297. [Google Scholar] [CrossRef]
- Kistler, L.; Haney, J.M.; Newsom, L.A. Experimental investigation of pathogenic stress on phytolith formation in Cucurbita pepo var. texana (wild gourd). Veg. Hist. Archaeobot. 2013, 22, 165–170. [Google Scholar] [CrossRef]
- Wieczorek, M.; Zub, K.; Szafrańska, P.A.; Ksiazek, A.; Konarzewski, M. Plant-Herbivore interactions: Silicon concentration in tussock sedges and population dynamics of root voles. Funct. Ecol. 2015, 29, 187–194. [Google Scholar] [CrossRef]
- Schuldt, A.; Ebeling, A.; Kunz, M.; Staab, M.; Guimarães-Steinicke, C.; Bachmann, D.; Buchmann, N.; Durka, W.; Fichtner, A.; Fornoff, F.; et al. Multiple plant diversity components drive consumer communities across ecosystems. Nat. Commun. 2019, 10, 1460. [Google Scholar] [CrossRef]
- Schaller, J.; Roscher, C.; Hillebrand, H.; Weigelt, A.; Oelmann, Y.; Wilcke, W.; Ebeling, A.; Weisser, W.W. Plant diversity and functional groups affect Si and Ca pools in aboveground biomass of grassland systems. Oecologia 2016. [Google Scholar] [CrossRef]
- Schaller, J.; Hodson, M.J.; Struyf, E. Is relative Si/Ca availability crucial to the performance of grassland ecosystems? Ecosphere 2017, 8, e01726. [Google Scholar] [CrossRef]
- Conley, D.J.; Carey, J.C. Biogeochemistry: Silica cycling over geologic time. Nat. Geosci. 2015, 8, 431–432. [Google Scholar] [CrossRef]
- Cornelis, J.T.; Ranger, J.; Iserentant, A.; Delvaux, B. Tree species impact the terrestrial cycle of silicon through various uptakes. Biogeochemistry 2010, 97, 231–245. [Google Scholar] [CrossRef]
- Derry, L.A.; Kurtz, A.C.; Ziegler, K.; Chadwick, O.A. Biological control of terrestrial silica cycling and export fluxes to watersheds. Nature 2005, 433, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.; Struyf, E.; Hartmann, J.; Wanner, A.; Jensen, K. A comprehensive study of silica pools and fluxes in Wadden Sea salt marshes. Estuaries Coasts 2013, 36, 1150–1164. [Google Scholar] [CrossRef]
- Brackhage, C.; Schaller, J.; Bäucker, E.; Dudel, E.G. Silicon availability affects the stoichiometry and content of calcium and micro nutrients in the leaves of common reed. Silicon 2013, 5, 199–204. [Google Scholar] [CrossRef]
- Hu, A.Y.; Xu, S.N.; Qin, D.N.; Li, W.; Zhao, X.Q. Role of silicon in mediating phosphorus imbalance in plants. Plants 2021, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Minden, V.; Schaller, J.; Olde Venterink, H. Plants increase silicon content as a response to nitrogen or phosphorus limitation: A case study with Holcus lanatus. Plant Soil 2020. [Google Scholar] [CrossRef]
- Nelwamondo, A.; Dakora, F.D. Silicon promotes nodule formation and nodule function in symbiotic cowpea (Vigna unguiculata). New Phytol. 1999, 142, 463–467. [Google Scholar] [CrossRef]
- Dakora, F.D.; Le Roux, R. Phosphorus nutrition alters root flavonoid content, nitrogen fixation, and phosphorus partitioning in cowpea. In Nitrogen Fixation: Fundememntals and Applications; Tikhonovich, I.A., Provorov, N.A., Romanov, V.I., Newton, W.E., Eds.; Springer: Dordrecht, The Netherlands, 1995; p. 324. [Google Scholar]
- Teixeira, G.C.M.; de Mello Prado, R.; Rocha, A.M.S.; de Cássia Piccolo, M. Root- and foliar-applied silicon modifies C: N: P ratio and increases the nutritional efficiency of pre-sprouted sugarcane seedlings under water deficit. PLoS ONE 2020, 15, e0240847. [Google Scholar] [CrossRef]
- Frazão, J.J.; de Mello Prado, R.; de Souza Júnior, J.P.; Rossatto, D.R. Silicon changes C:N:P stoichiometry of sugarcane and its consequences for photosynthesis, biomass partitioning and plant growth. Sci. Rep. 2020, 10, 12492. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Yang, S.; Song, Z.; Li, Z.; Ding, F.; Yu, C.; Hu, G.; Liu, H. Silicon affects plant stoichiometry and accumulation of C, N, and P in grasslands. Front. Plant Sci. 2020, 11, 1304. [Google Scholar] [CrossRef] [PubMed]
- Schaller, J.; Struyf, E. Silicon controls microbial decay and nutrient release of grass litter during aquatic decomposition. Hydrobiologia 2013, 709, 201–212. [Google Scholar] [CrossRef]
- Schaller, J.; Hines, J.; Brackhage, C.; Bäucker, E.; Gessner, M.O. Silica decouples fungal growth and litter decomposition without changing responses to climate warming and N enrichment. Ecology 2014, 95, 3181–3189. [Google Scholar] [CrossRef]
- Das, S.; Lee, J.G.; Cho, S.R.; Song, H.J.; Kim, P.J. Silicate fertilizer amendment alters fungal communities and accelerates soil organic matter decomposition. Front. Microbiol. 2019, 10, 2950. [Google Scholar] [CrossRef] [PubMed]
- Emsens, W.J.; Schoelynck, J.; Grootjans, A.P.; Struyf, E.; van Diggelen, R. Eutrophication alters Si cycling and litter decomposition in wetlands. Biogeochemistry 2016, 130, 289–299. [Google Scholar] [CrossRef]
- Hömberg, A.; Broder, T.; Knorr, K.H.; Schaller, J. Divergent effect of silicon on greenhouse gas production from reduced and oxidized peat organic matter. Geoderma 2021, 386, 114916. [Google Scholar] [CrossRef]
- Hömberg, A.; Obst, M.; Knorr, K.H.; Kalbitz, K.; Schaller, J. Increased silicon concentration in fen peat leads to a release of iron and phosphate and changes in the composition of dissolved organic matter. Geoderma 2020, 374, 114422. [Google Scholar] [CrossRef]
- Song, Z.; Liu, H.; Strömberg, C.A.E.; Yang, X.; Zhang, X. Phytolith carbon sequestration in global terrestrial biomes. Sci. Total Environ. 2017, 603, 502–509. [Google Scholar] [CrossRef]
- Ji, Z.; Yang, X.; Song, Z.; Liu, H.; Liu, X.; Qiu, S.; Li, J.; Guo, F.; Wu, Y.; Zhang, X. Silicon distribution in meadow steppe and typical steppe of northern China and its implications for phytolith carbon sequestration. Grass Forage Sci. 2018, 73, 482–492. [Google Scholar] [CrossRef]
- Li, Z.; Song, Z.; Cornelis, J.T. Impact of rice cultivar and organ on elemental composition of phytoliths and the release of bio-available silicon. Front. Plant Sci. 2014, 5, 529. [Google Scholar] [CrossRef] [PubMed]
- Parr, J.; Sullivan, L.; Chen, B.; Ye, G.; Zheng, W. Carbon bio-sequestration within the phytoliths of economic bamboo species. Glob. Chang. Biol. 2010, 16, 2661–2667. [Google Scholar] [CrossRef]
- Schaller, J.; Heimes, R.; Ma, J.F.; Meunier, J.D.; Shao, J.F.; Fujii-Kashino, M.; Knorr, K.H. Silicon accumulation in rice plant aboveground biomass affects leaf carbon quality. Plant Soil 2019, 444, 399–407. [Google Scholar] [CrossRef]
- Song, Z.; Wang, H.; Strong, P.J.; Li, Z.; Jiang, P. Plant impact on the coupled terrestrial biogeochemical cycles of silicon and carbon: Implications for biogeochemical carbon sequestration. Earth-Sci. Rev. 2012, 115, 319–331. [Google Scholar] [CrossRef]
- Carter, J.A. Atmospheric carbon isotope signatures in phytolith-occluded carbon. Quat. Int. 2009, 193, 20–29. [Google Scholar] [CrossRef]
- Asscher, Y.; Weiner, S.; Boaretto, E. A new method for extracting the insoluble occluded carbon in archaeological and modern phytoliths: Detection of 14C depleted carbon fraction and implications for radiocarbon dating. J. Archaeol. Sci. 2017, 78, 57–65. [Google Scholar] [CrossRef]
- Causey, G.W. Cytological investigations with the electron microscope. Ann. R. Coll. Surg. Engl. 1958, 22, 43–53. [Google Scholar]
- Alexandre, A.; Balesdent, J.; Cazevieille, P.; Chevassus-Rosset, C.; Signoret, P.; Mazur, J.C.; Harutyunyan, A.; Doelsch, E.; Basile-Doelsch, I.; Miche, H.; et al. Direct uptake of organically derived carbon by grass roots and allocation in leaves and phytoliths: 13C labeling evidence. Biogeosciences 2016, 13, 1693–1703. [Google Scholar] [CrossRef]
- Alexandre, A.; Basile-Doelsch, I.; Delhaye, T.; Borshneck, D.; Mazur, J.C.; Reyerson, P.; Santos, G.M. New highlights of phytolith structure and occluded carbon location: 3-D X-ray microscopy and NanoSIMS results. Biogeosciences 2015, 12, 863–873. [Google Scholar] [CrossRef]
- dos Santos, G.M. Comment on Atmospheric carbon isotope signatures in phytolith occluded carbon, Carter, J.A., Quaternary International, this volume. Quat. Int. 2009, 193, 30–31. [Google Scholar] [CrossRef]
- Santos, G.M.; Alexandre, A.; Southon, J.R.; Treseder, K.K.; Corbineau, R.; Reyerson, P.E. Possible source of ancient carbon in phytolith concentrates from harvested grasses. Biogeosciences 2012, 9, 1873–1884. [Google Scholar] [CrossRef]
- Santos, G.M.; Alexandre, A.; Coe, H.H.G.; Reyerson, P.E.; Southon, J.R.; De Carvalho, C.N. The phytolith 14c puzzle: A tale of background determinations and accuracy tests. Radiocarbon 2010, 52, 113–128. [Google Scholar] [CrossRef]
- Santos, G.M.; Alexandre, A. The phytolith carbon sequestration concept: Fact or fiction? A comment on “Occurrence, turnover and carbon sequestration potential of phytoliths in terrestrial ecosystems by Song et al. doi:10.1016/j.earscirev.2016.04.007”. Earth-Sci. Rev. 2017, 164, 251–255. [Google Scholar] [CrossRef]
- Reyerson, P.E.; Alexandre, A.; Harutyunyan, A.; Corbineau, R.; De La Torre, H.A.M.; Badeck, F.; Cattivelli, L.; Santos, G.M. Unambiguous evidence of old soil carbon in grass biosilica particles. Biogeosciences 2016, 13, 1269–1286. [Google Scholar] [CrossRef]
- Elbaum, R.; Melamed-Bessudo, C.; Tuross, N.; Levy, A.A.; Weiner, S. New methods to isolate organic materials from silicified phytoliths reveal fragmented glycoproteins but no DNA. Quat. Int. 2009, 193, 11–19. [Google Scholar] [CrossRef]
- Song, Z.; Liu, C.; Müller, K.; Yang, X.; Wu, Y.; Wang, H. Silicon regulation of soil organic carbon stabilization and its potential to mitigate climate change. Earth-Sci. Rev. 2018, 185, 463–475. [Google Scholar] [CrossRef]
- Song, Z.; Liu, H.; Li, B.; Yang, X. The production of phytolith-occluded carbon in China’s forests: Implications to biogeochemical carbon sequestration. Glob. Chang. Biol. 2013, 19, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Liu, H.; Si, Y.; Yin, Y. The production of phytoliths in China’s grasslands: Implications to the biogeochemical sequestration of atmospheric CO 2. Glob. Chang. Biol. 2012, 18, 3647–3653. [Google Scholar] [CrossRef]
- Friedlingstein, P.; Jones, M.W.; O’Sullivan, M.; Andrew, R.M.; Hauck, J.; Peters, G.P.; Peters, W.; Pongratz, J.; Sitch, S.; Le Quéré, C.; et al. Global carbon budget 2019. Earth Syst. Sci. Data 2019, 11, 1783–1838. [Google Scholar] [CrossRef]
- Fraysse, F.; Pokrovsky, O.S.; Meunier, J.D. Experimental study of terrestrial plant litter interaction with aqueous solutions. Geochim. Cosmochim. Acta 2010, 74, 70–84. [Google Scholar] [CrossRef]
- Exley, C. Silicon in life:A bioinorganic solution to bioorganic essentiality1JD Birchall memorial lecture.1. J. Inorg. Biochem. 1998, 69, 139–144. [Google Scholar] [CrossRef]
- Ikegami, N.; Satake, T.; Nagayama, Y.; Inubushi, K. Changes in silica in litterfall and available silica in the soil of forests invaded by bamboo species (Phyllostachys pubescens and P. bambusoides) in western Japan. Soil Sci. Plant Nutr. 2014, 60, 731–739. [Google Scholar] [CrossRef]
- Borrelli, N.; Alvarez, M.F.; Osterrieth, M.L.; Marcovecchio, J.E. Silica content in soil solution and its relation with phytolith weathering and silica biogeochemical cycle in Typical Argiudolls of the Pampean Plain, Argentina-a preliminary study. J. Soils Sediments 2010, 10, 983–994. [Google Scholar] [CrossRef]
- Cabanes, D.; Shahack-Gross, R. Understanding fossil phytolith preservation: The role of partial dissolution in paleoecology and archaeology. PLoS ONE 2015, 10, e0125532. [Google Scholar] [CrossRef] [PubMed]
- Cabanes, D.; Weiner, S.; Shahack-Gross, R. Stability of phytoliths in the archaeological record: A dissolution study of modern and fossil phytoliths. J. Archaeol. Sci. 2011, 38, 2480–2490. [Google Scholar] [CrossRef]
- Butterfield, N.J. Animals and the invention of the Phanerozoic Earth system. Trends Ecol. Evol. 2011, 26, 81–87. [Google Scholar] [CrossRef]
- Pausas, J.G.; Bond, W.J. On the three major recycling pathways in terrestrial ecosystems. Trends Ecol. Evol. 2020, 35, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Callaway, R.M.; Kikodze, D.; Chiboshvili, M.; Khetsuriani, L. Unpalatable plants protect neighbors from grazing and increase plant community diversity. Ecology 2005, 86, 1856–1862. [Google Scholar] [CrossRef]
- Gabay, O.; Perevolotsky, A.; Bar Massada, A.; Carmel, Y.; Shachak, M. Differential effects of goat browsing on herbaceous plant community in a two-phase mosaic. Plant Ecol. 2011, 212, 1643–1653. [Google Scholar] [CrossRef]
- Hadar, L.; Noy-Meir, I.; Perevolotsky, A. The effect of shrub clearing and grazing on the composition of a Mediterranean plant community: Functional groups versus species. J. Veg. Sci. 1999, 10, 673–682. [Google Scholar] [CrossRef]
- Díaz, S.; Lavorel, S.; McIntyre, S.; Falczuk, V.; Casanoves, F.; Milchunas, D.G.; Skarpe, C.; Rusch, G.; Sternberg, M.; Noy-Meir, I.; et al. Plant trait responses to grazing—A global synthesis. Glob. Chang. Biol. 2007, 13, 313–341. [Google Scholar] [CrossRef]
- Fernández Honaine, M.; Osterrieth, M.L.; Zucol, A.F. Plant communities and soil phytolith assemblages relationship in native grasslands from southeastern Buenos Aires province, Argentina. Catena 2009, 76, 89–96. [Google Scholar] [CrossRef]
- Shahack-Gross, R. Herbivorous livestock dung: Formation, taphonomy, methods for identification, and archaeological significance. J. Archaeol. Sci. 2011, 38, 205–218. [Google Scholar] [CrossRef]
- Shahack-Gross, R.; Finkelstein, I. Subsistence practices in an arid environment: A geoarchaeological investigation in an Iron Age site, the Negev Highlands, Israel. J. Archaeol. Sci. 2008, 35, 965–982. [Google Scholar] [CrossRef]
- Shahack-Gross, R.; Boaretto, E.; Cabanes, D.; Katz, O.; Finkelstein, I. Subsistence economy in the Negev Highlands: The iron age and the byzantine/ early islamic period. Levant 2014, 46, 98–117. [Google Scholar] [CrossRef]
- Shahack-Gross, R.; Marshall, F.; Ryan, K.; Weiner, S. Reconstruction of spatial organization in abandoned Maasai settlements: Implications for site structure in the Pastoral Neolithic of East Africa. J. Archaeol. Sci. 2004, 31, 1395–1411. [Google Scholar] [CrossRef]
- Delhon, C.; Martin, L.; Argant, J.; Thiébault, S. Shepherds and plants in the Alps: Multi-proxy archaeobotanical analysis of neolithic dung from “La Grande Rivoire” (Isère, France). J. Archaeol. Sci. 2008, 35, 2937–2952. [Google Scholar] [CrossRef]
- Johansen, P.G. Landscape, monumental architecture, and ritual: A reconsideration of the South Indian ashmounds. J. Anthropol. Archaeol. 2004, 23, 309–330. [Google Scholar] [CrossRef]
- Tsartsidou, G.; Lev-Yadun, S.; Efstratiou, N.; Weiner, S. Ethnoarchaeological study of phytolith assemblages from an agro-pastoral village in Northern Greece (Sarakini): Development and application of a Phytolith Difference Index. J. Archaeol. Sci. 2008, 35, 600–613. [Google Scholar] [CrossRef]
- Shahack-Gross, R.; Albert, R.M.; Gilboa, A.; Nagar-Hilman, O.; Sharon, I.; Weiner, S. Geoarchaeology in an urban context: The uses of space in a Phoenician monumental building at Tel Dor (Israel). J. Archaeol. Sci. 2005, 32, 1417–1431. [Google Scholar] [CrossRef]
- Katz, O. Plant silicon and phytolith research and the earth-life superdiscipline. Front. Plant Sci. 2018, 9, 1281. [Google Scholar] [CrossRef] [PubMed]
- Schuiling, R.D.; Krijgsman, P. Enhanced weathering: An effective and cheap tool to sequester CO2. Clim. Chang. 2006, 74, 349–354. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Katz, M.E.; Knoll, A.H.; Quigg, A.; Raven, J.A.; Schofield, O.; Taylor, F.J.R. The evolution of modern eukaryotic phytoplankton. Science 2004, 305, 354–360. [Google Scholar] [CrossRef]
- Bergvall, U.A.; Rautio, P.; Kesti, K.; Tuomi, J.; Leimar, O. Associational effects of plant defences in relation to within- and between-patch food choice by a mammalian herbivore: Neighbour contrast susceptibility and defence. Oecologia 2006, 147, 253–260. [Google Scholar] [CrossRef]
- Wheeler, J.A.; Schnider, F.; Sedlacek, J.; Cortés, A.J.; Wipf, S.; Hoch, G.; Rixen, C. With a little help from my friends: Community facilitation increases performance in the dwarf shrub Salix herbacea. Basic Appl. Ecol. 2015, 16, 202–209. [Google Scholar] [CrossRef]
- Berendse, F.; Scheffer, M. The angiosperm radiation revisited, an ecological explanation for Darwin’s “abominable mystery”. Ecol. Lett. 2009, 12, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Augusto, L.; Davies, T.J.; Delzon, S.; de Schrijver, A. The enigma of the rise of angiosperms: Can we untie the knot? Ecol. Lett. 2014, 17, 1326–1338. [Google Scholar] [CrossRef] [PubMed]
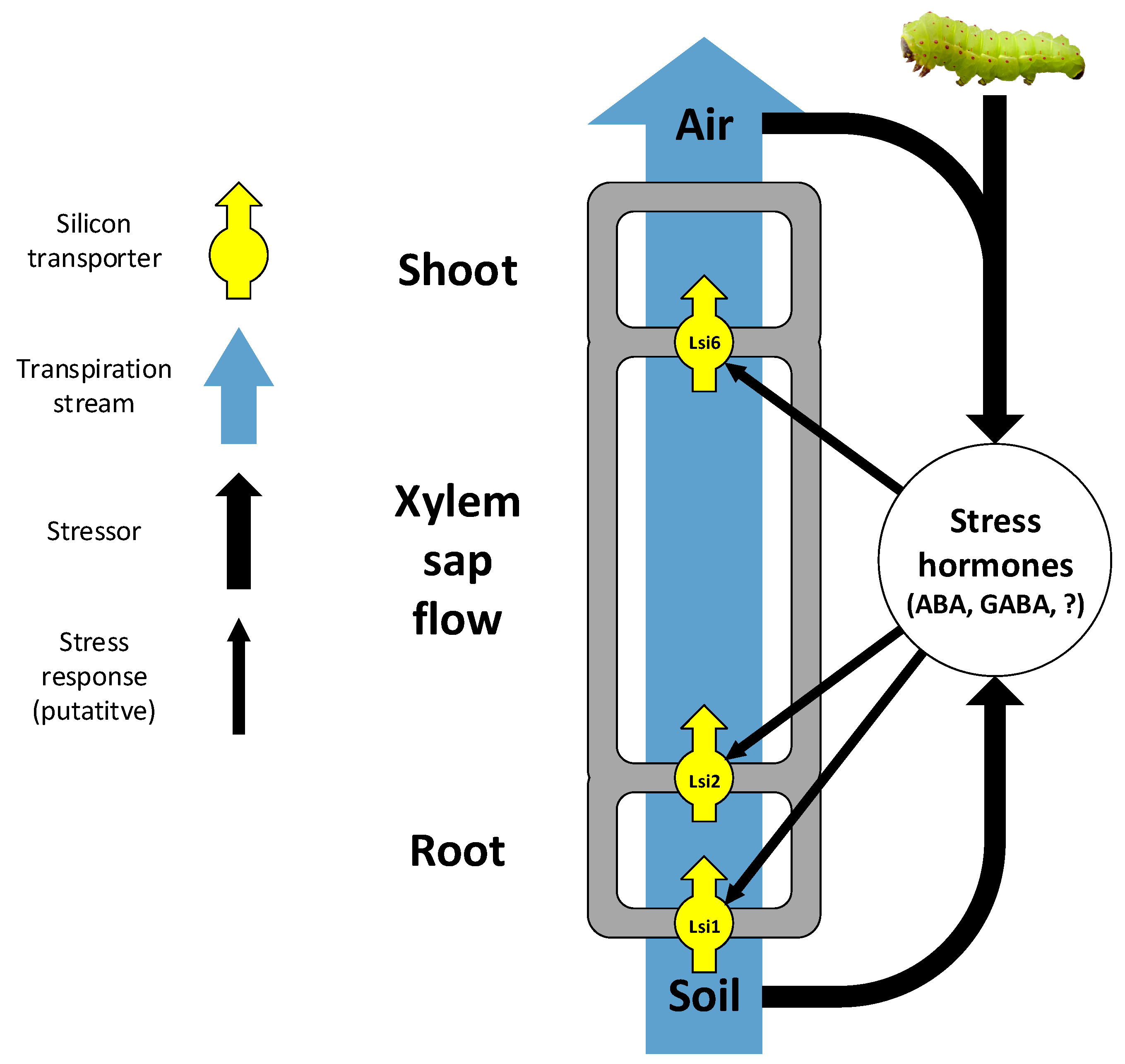
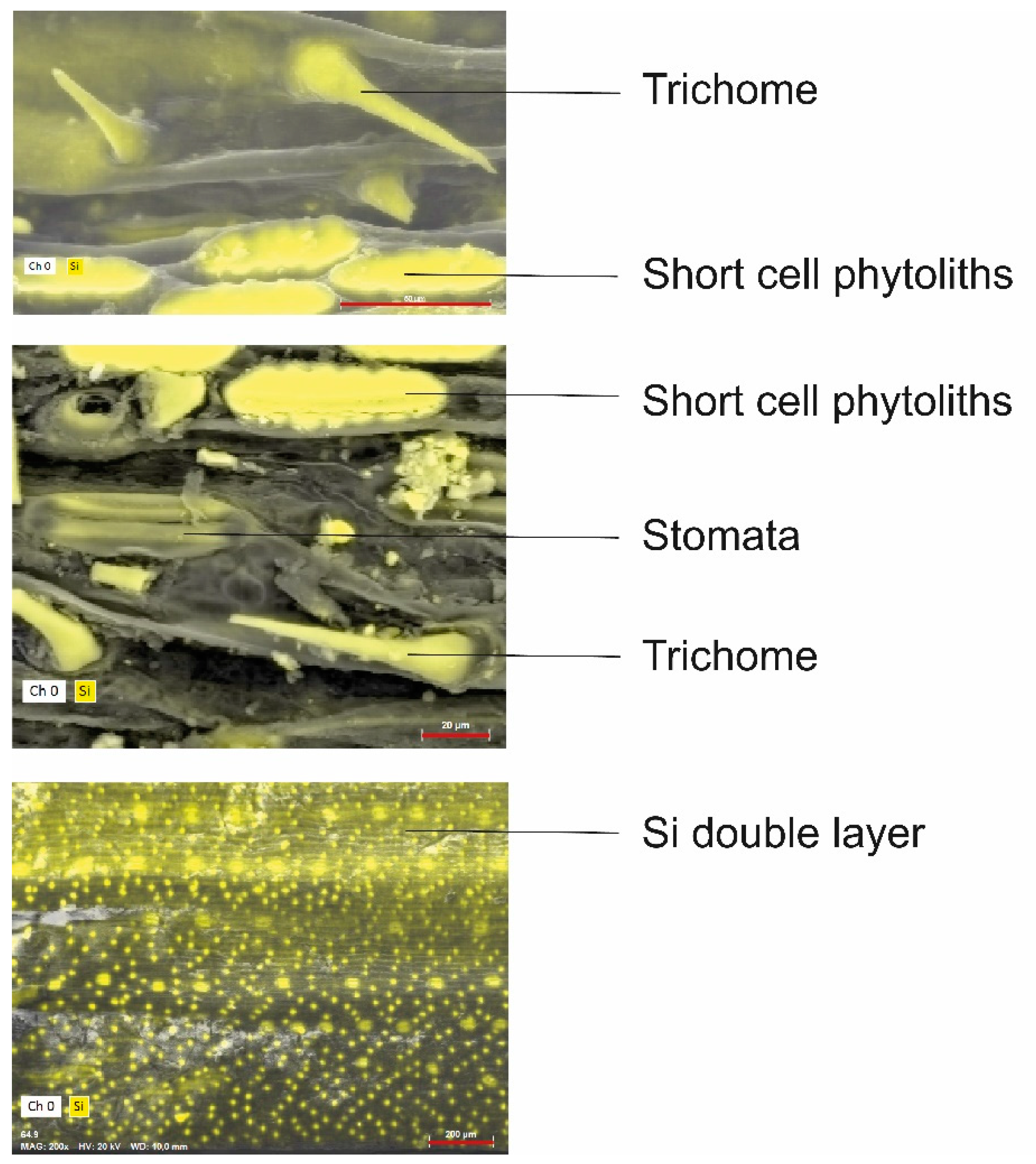
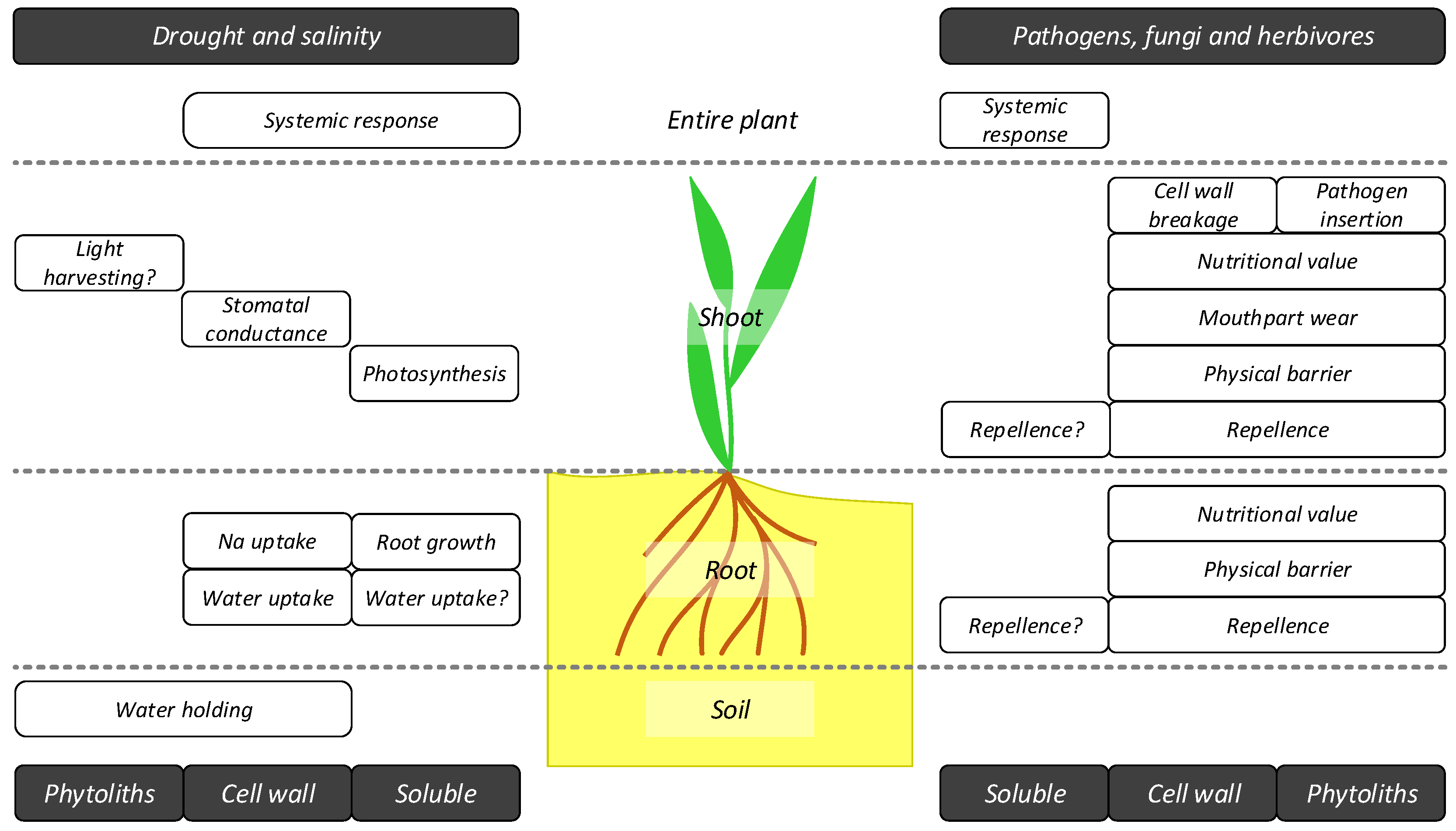
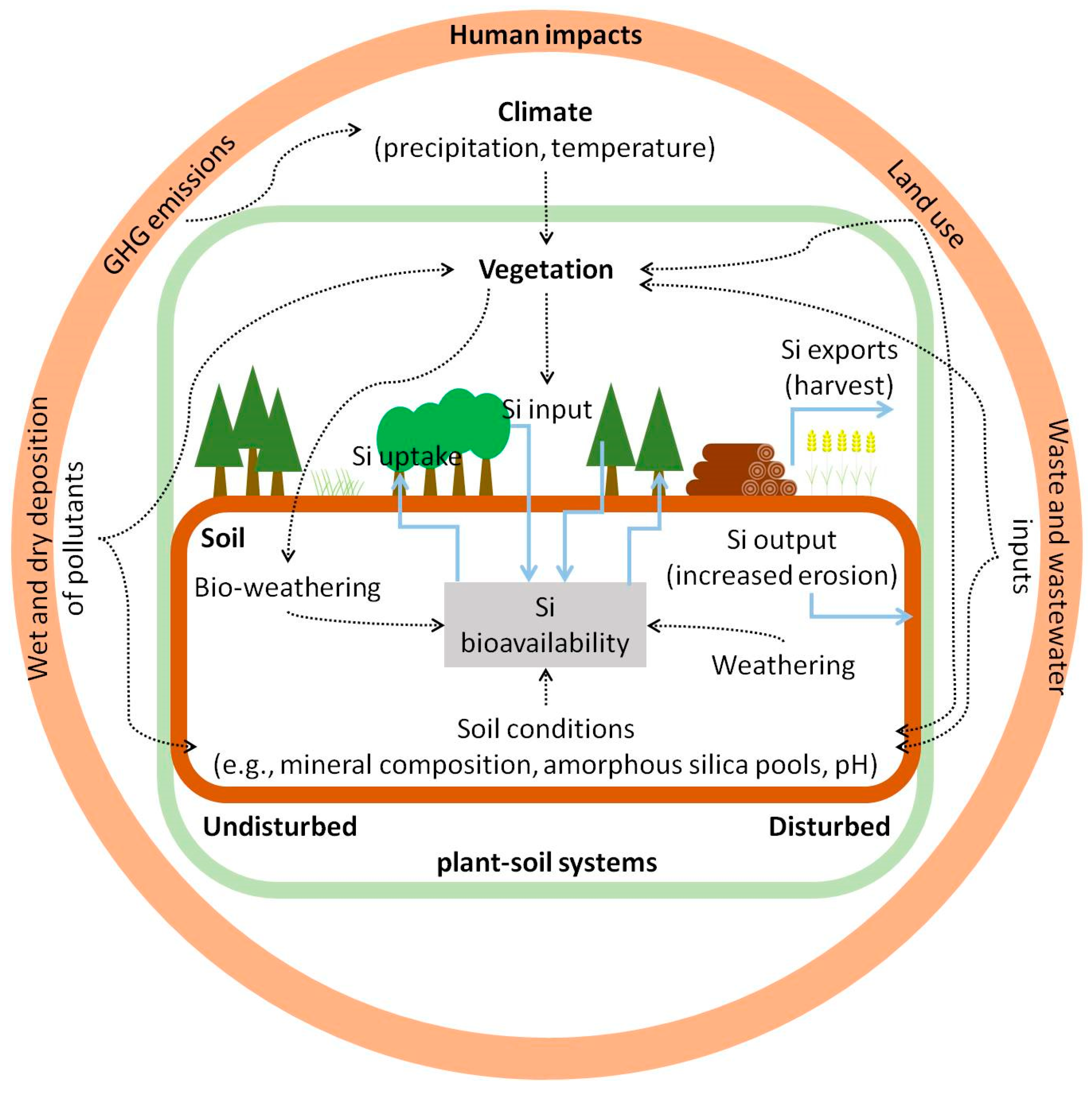
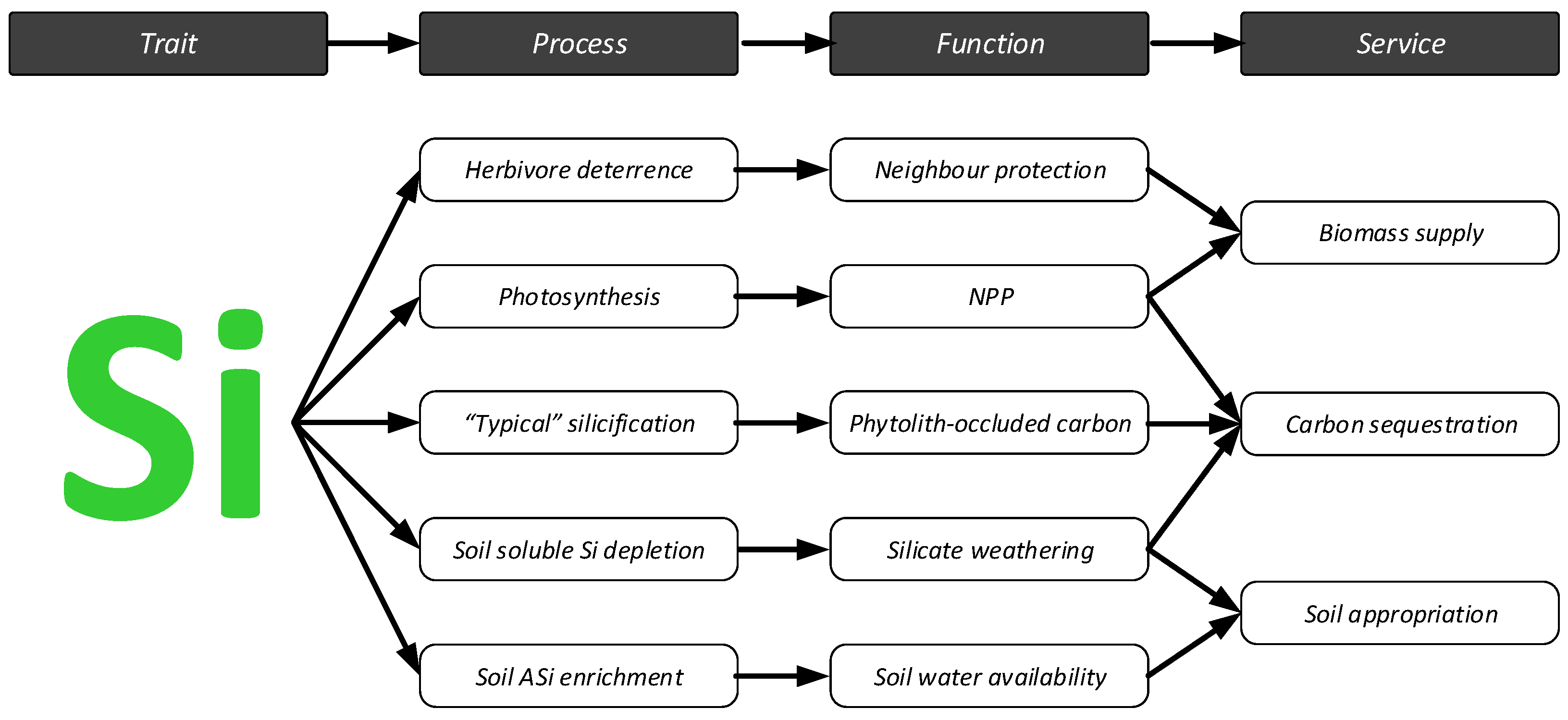
| Extractant | Procedure | Reference |
|---|---|---|
| 1% Na2CO3 solution | ~30 mg plant material extracted in 1% Na2CO3 solution at 85 °C | [162,165] |
| 0.5 M NaOH solution | ~100 mg plant material extracted in 0.5 M NaOH solution at 85 °C | [162] |
| 2-step HF | Step 1: ~100 mg plant material digested in a mixture of distilled water, nitric acid and hydrofluoric acid (40%) at 190 °C Step 2: hydrofluoric acid is neutralized by 10 mL a 4% boric acid solution at 150 °C | [166] |
| Lithium metaborate fusion | Plant material ashed at 500 °C. The ash is mixed with lithium meta-tetraborate at 1000 °C. The obtained bead is transferred into nitric acid. | [167,168,171] |
| Tiron (C6H4Na2O8S2) | Plant material added to tiron solution buffered at pH 10.5 at 85 °C. | [169] |
| No extractant, but XRF | ~100 mg plant material homogenized to a powder, but calibration is required. | [170] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katz, O.; Puppe, D.; Kaczorek, D.; Prakash, N.B.; Schaller, J. Silicon in the Soil–Plant Continuum: Intricate Feedback Mechanisms within Ecosystems. Plants 2021, 10, 652. https://doi.org/10.3390/plants10040652
Katz O, Puppe D, Kaczorek D, Prakash NB, Schaller J. Silicon in the Soil–Plant Continuum: Intricate Feedback Mechanisms within Ecosystems. Plants. 2021; 10(4):652. https://doi.org/10.3390/plants10040652
Chicago/Turabian StyleKatz, Ofir, Daniel Puppe, Danuta Kaczorek, Nagabovanalli B. Prakash, and Jörg Schaller. 2021. "Silicon in the Soil–Plant Continuum: Intricate Feedback Mechanisms within Ecosystems" Plants 10, no. 4: 652. https://doi.org/10.3390/plants10040652
APA StyleKatz, O., Puppe, D., Kaczorek, D., Prakash, N. B., & Schaller, J. (2021). Silicon in the Soil–Plant Continuum: Intricate Feedback Mechanisms within Ecosystems. Plants, 10(4), 652. https://doi.org/10.3390/plants10040652









