Abstract
Plants belonging to the subfamily Bombacoideae (family Malvaceae) consist of about 304 species, many of them having high economical and medicinal properties. In the past, this plant group was put under Bombacaceae; however, modern molecular and phytochemical findings supported the group as a subfamily of Malvaceae. A detailed search on the number of publications related to the Bombacoideae subfamily was carried out in databases like PubMed and Science Direct using various keywords. Most of the plants in the group are perennial tall trees usually with swollen tree trunks, brightly colored flowers, and large branches. Various plant parts ranging from leaves to seeds to stems of several species are also used as food and fibers in many countries. Members of Bombacoides are used as ornamentals and economic utilities, various plants are used in traditional medication systems for their anti-inflammatory, astringent, stimulant, antipyretic, microbial, analgesic, and diuretic effects. Several phytochemicals, both polar and non-polar compounds, have been detected in this plant group supporting evidence of their medicinal and nutritional uses. The present review provides comprehensive taxonomic, ethno-pharmacological, economic, food and phytochemical properties of the subfamily Bombacoideae.
1. Introduction
The plant group Bombacoideae is a subfamily of Malvaceae (kapok, cotton family). The subfamily contains about 304 species, most of them with high economical and medicinal values. Considering their importance, some of the plants are given special cultural status. For instance, the Ceiba pentandra tree is the national tree of Guatemala. Among the Mayan and Aztec civilizations in the Meso-America, the Ceiba species is considered as a sacred “World Tree”. The Indian kapok tree, Bombax ceiba, is worshipped by the Hindu community in North India as a nakshatra tree and home of the female spirits Yakshi [1]. There is West African belief that the first human was born from the trunk of a baobab tree (Adansonia spp.) and these plants are regarded as the “Tree of Life”. Many plants of the Bombacoideae are valued as ornamentals in various parts of the world because of their large branches and brightly colored flowers [2]. Moreover, many genera of this subfamily are known for producing fibers, timber, fruits, and vegetables, thereby, regarded as one of the important economic and commercial plant groups.
This group was previously recognized as a distinct family, Bombacaceae, based on the type genus Bombax by some traditional taxonomists. From the days of the natural system to the present days of phyletic classification, the status of this plant group is continuously debated. Apart from that, the number of genera under this family varied from one classification system to another. There are various arguments in favor of a distinct family or whether to subsume under a subfamily or tribe. The study of palyno-morphological characteristics supported the justification of separating Bombacaceae from Sterculiaceae, Malvaceae, and Tiliaceae [3]. Most of the traditional methodical educations related to the subfamily Bombacoideae are on the basis of the characteristics of the flower, especially the androecium [4]. Recently, morphological, anatomical, palynological, phytochemical, and molecular phylogenetic analyses have shown that separation of Bombacaceae from its related groups viz. Malvaceae, Tiliaceae, and Sterculiaceae is inconsistent [5]. This plant group includes several plants, which are used for medicinal and economic utilities. A detailed search on the number of publications related to the Bombacoideae subfamily was carried out in databases like PubMed and Science Direct and it was found that, as per the PubMed database, around 20 articles have been published during the years 1999–2020 and among them, 12 articles are full texts (https://pubmed.ncbi.nlm.nih.gov/?term=Bombacoideae; accessed on 12 October 2020). Interestingly, from a total of 20 articles, 16 were published during the last 10 years (2010–2020). Similarly, the Science Direct databases show a total of 53 articles were published during the years 1999–2020, of which, 42 are research articles, 2 are review articles, 4 book chapters, 1 short communication, 2 encyclopedia, and 2 others (https://www.sciencedirect.com/search?qs=Bombacoideae&show=100; accessed on 12 October 2020). Considering the importance, the authors attempted to extensively review the taxonomic, phytochemical, and medicinal utilities of the members of the subfamily Bombacoideae.
2. Taxonomy of the Subfamily Bombacoideae
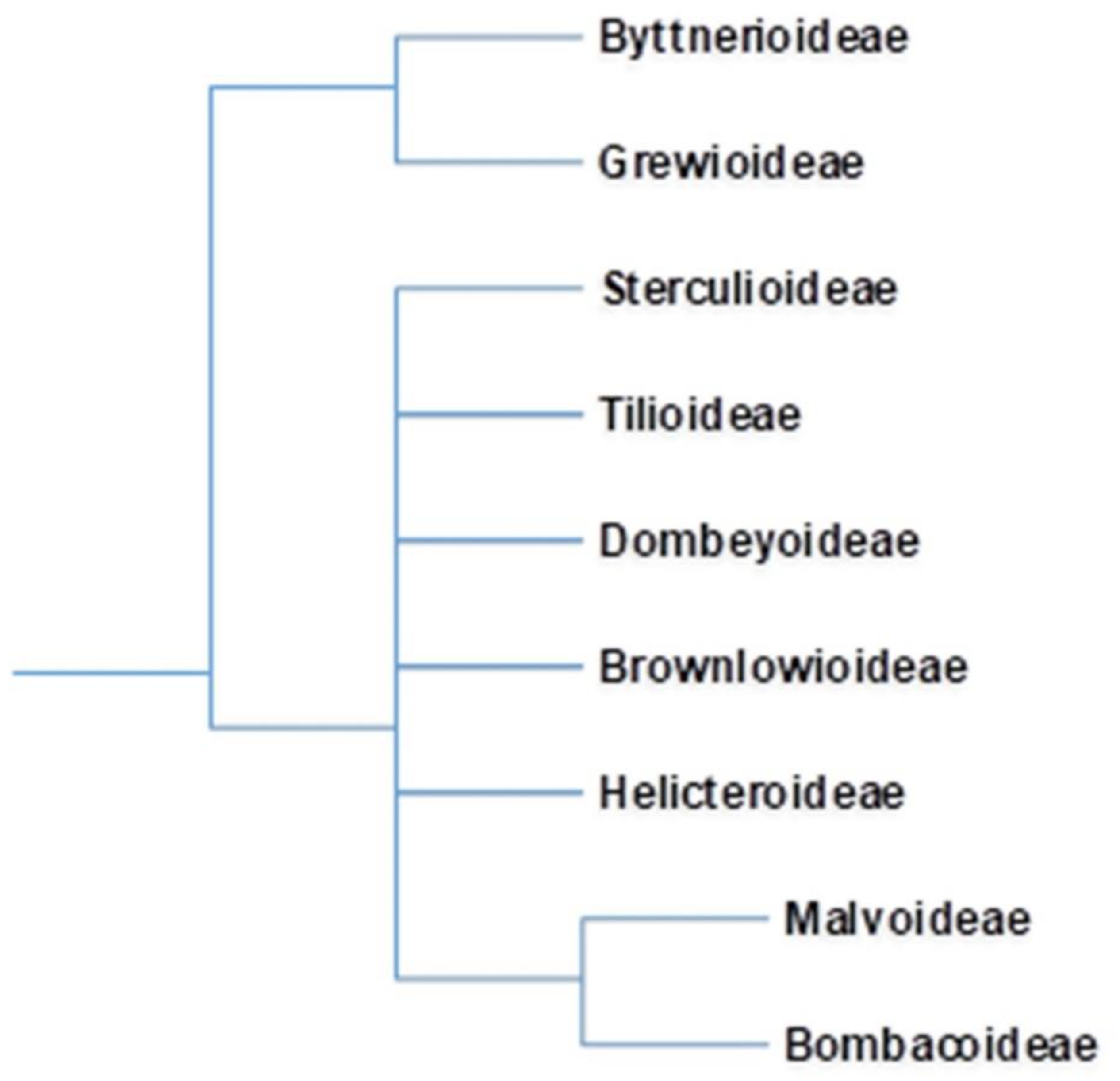
The advent of new taxonomical tools has revolutionized taxonomical circumscriptions. Morphological and molecular analyses revealed that Bombacaceae is not a monophyletic group. Furthermore, families such as Tiliaceae, Sterculiaceae, and Malvaceae are largely nonmonophyletic. Singh [6] considered that traditional distinctions amongst these four families are random and unpredictable and fusion of four would form a monophyletic group. Bayer et al. [7], grouped these four families together into Malvaceae considering their common characteristics and assumed them to be monophyletic. The Malvaceae sensu lato is characterized by apomorphic inflorescence, presence of bicolor unit, 3-bracted cyme, and trimerous epicalyx. Bombacaceae was distributed into two subfamilies, Bombacoideae and Helicteroideae, within the family Malvaceae. The confinement of Bombacoideae and Malvoideae is still under controversy, as the former appears to be paraphyletic without the latter [8]. Most of the plants are included in the subfamily Bombacoideae. At present, Bombacoideae is one of the clades in the family Malvaceae (Figure 1). The taxonomic location of Bombacoideae as per different systems of classification is shown in Figure 2 [4].
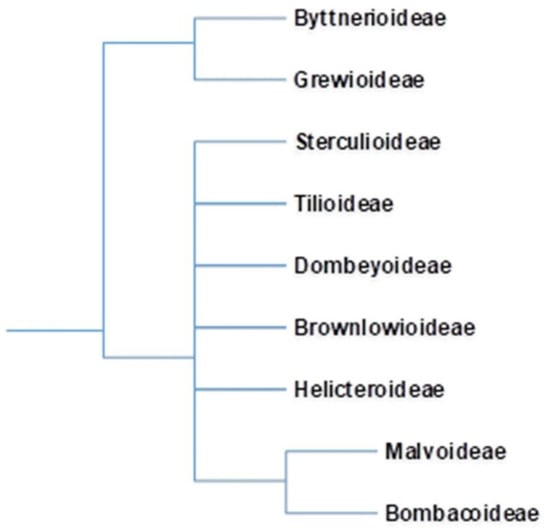
Figure 1.
In the Angiosperm Phylogeny Group (APG) classification, erstwhile family Bombacaceae is allocated as subfamily Bombacoideae of the family Malvaceae. Cladogram of the Malvaceae is after Bayer et.al. 1999 and online version of APG (http://www.mobot.org/MOBOT/research/APweb; accessed on 10 January 2021).
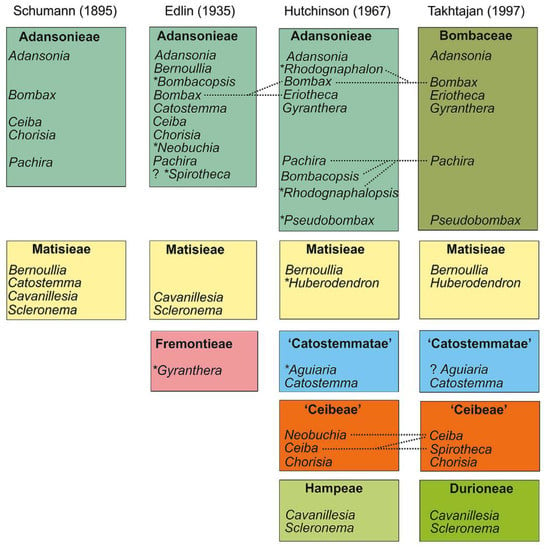
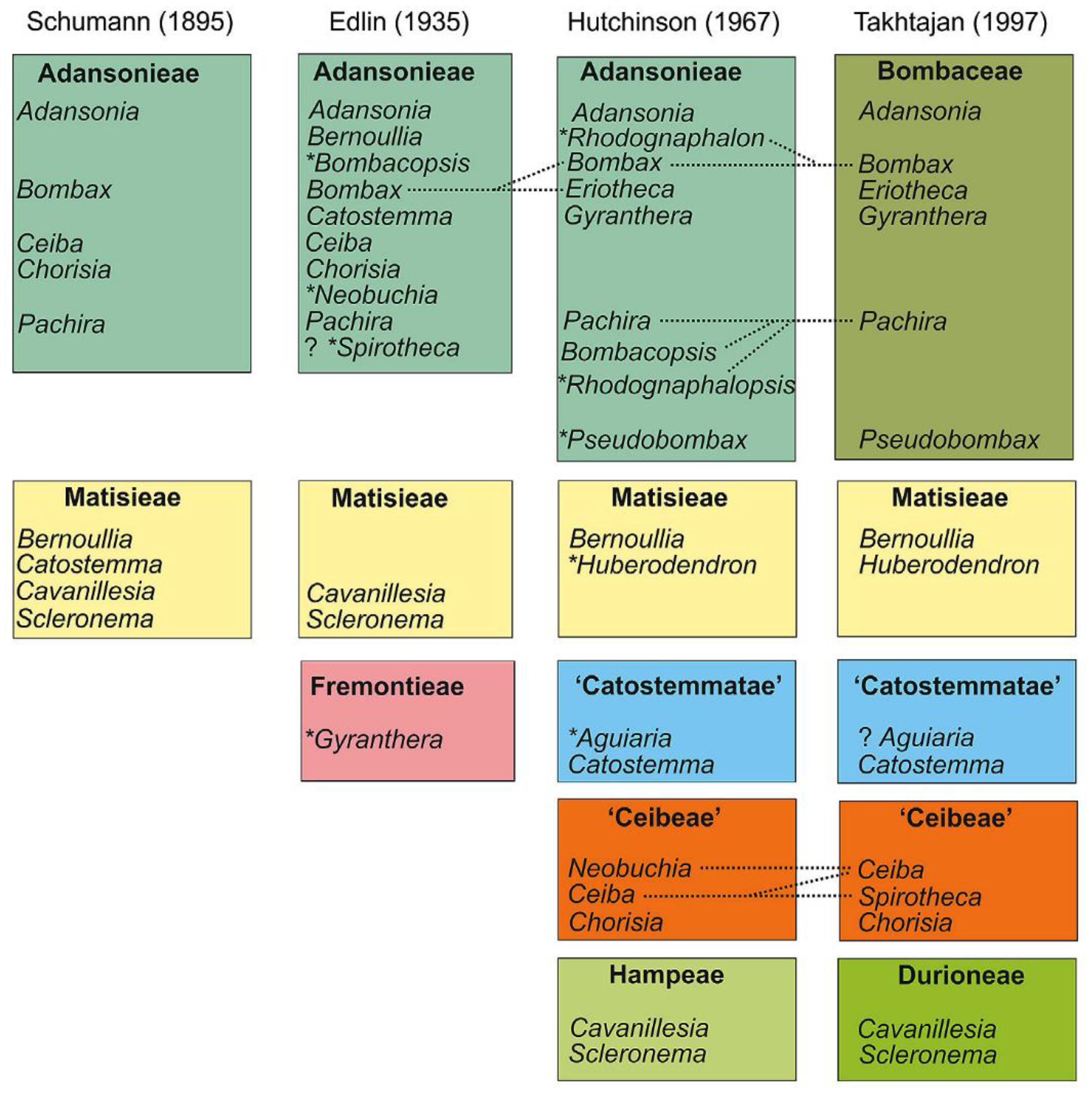
Figure 2.
Taxonomic location of Bombacoideae as per different systems of classification. Dotted lines specify the changes in the genus limitation and the genera, which is described after the previous action, and are specified by a symbol (*), while the citation marks represent the tribes which are not validly published. Reproduced with permission from Carvalho-Sobrinho et al. [4] (originally Figure 1).
As a consequence of changes in circumscription and status of Bombacoideae, has led to the inclusion of 22 genera comprised of 120 species under this subfamily mainly distributed in the tropical regions. The Angiosperm Phylogeny Group (APG) IV Classification listed 24 genera in this subfamily. However, the classification by Maarten et.al. listed 27 genera under Bombacoideae [9]. This classification includes genera like Camptostemon, Lagunaria, and Uladendron in the Bombacoideae sub family. Molecular phylogenetic analysis established on nuclear (ETS, ITS) and plastid genes (matK, trnL-trnF, trnS-trnG) revealed that there are three key lineages noticeable by the kapok clade, seed, or fruit traits—the winged seed clade, and the spongy endocarp clade [4]. Such studies established the monophyly of the core Bombacoideae subfamily and the entire genera without Pachira. The monospecific Septotheca falls outside the core Bombacoideae in many studies [4,10].
3. Habitat, Distribution, and Characteristics of the Subfamily Bombacoideae
Bombacoideae occupies different habitats in various parts of the world (Figure 3) [11]. Adansonia digitata is confined to semi-arid, stony, hot, dry, and woodland areas, with low rainfall. This plant favors well-drained soils ranging from clays to sandy soils [12]. However, some other plants favor wet and humid habitats. For instance, Bombax ceiba favors humid lowland deciduous forest and is sometimes found near stream banks [13,14]. Species belonging to Spirotheca are epiphytic stranglers. Some species are part of mangrove vegetation in the tropical regions, for example, Pachira aquatica, Camptostemon philippinense, et cetera. The majority of the species in Bombacoideae prefer rain forest biome and seasonally dry biomes [11]. Several representative plant species belonging to the subfamily Bombacoideae grow in different habitats. Adansonia digitata L. grows in the hot, semi-arid region with poorly drained soil [15]. Plants such as Bombax ceiba L., Ceiba pentandra; and Gyranthera caribensis Pittier grow in wet habitats [16]. Pachira aquatica Aubl. and Camptostemon philippinense (S.Vidal) Becc. grow in mangrove habitats [17,18]. Spirotheca rivieri (Decne.) Ulbr. grows in the epiphyte environment [19] and Ceiba pentandra grows in the savannah habitat [15].
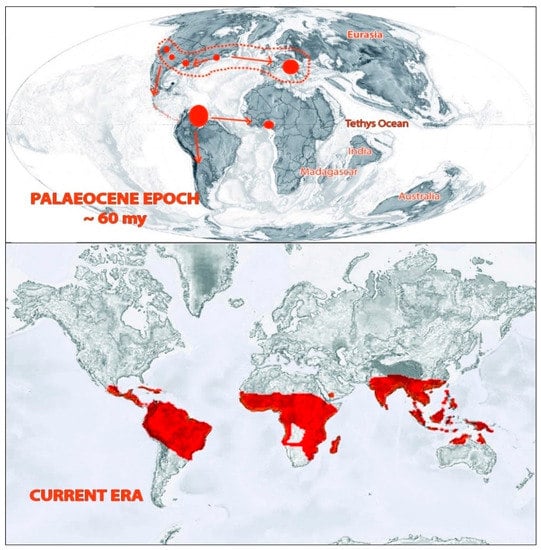
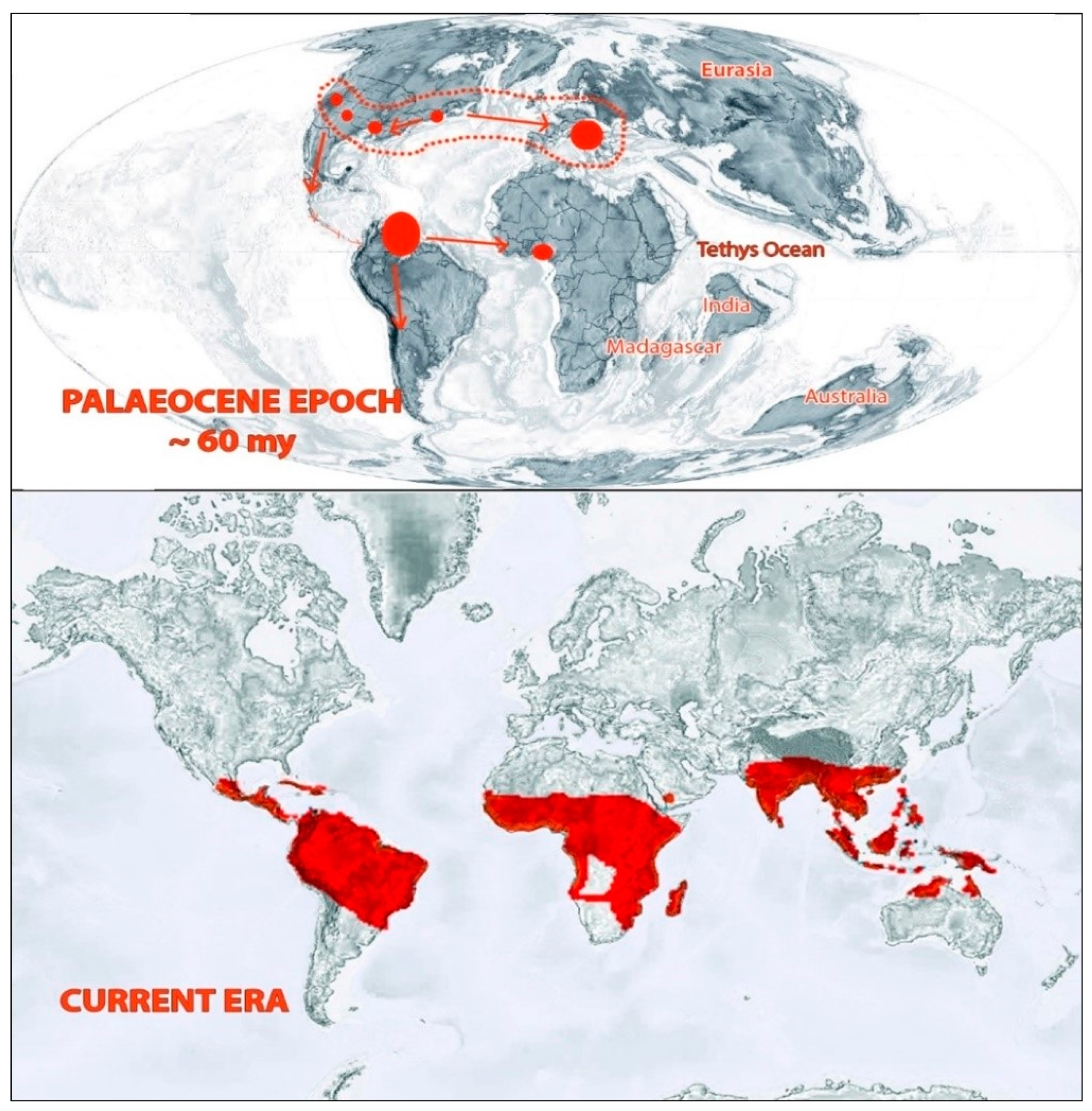
Figure 3.
Distribution of Bombacoideae in past and present eras. Adapted from Krutzsch, [20], Zizka et.al. [11], and Angiosperm Phylogeny website version 14 (www.mobot.org/MOBOT/research/APweb; accessed on 10 January 2021).
The early distribution of this plant group can be ascertained from fossil records. There are various arguments for the distribution of Bombacoideae. Croizat (1952) favored the knowledge of an African entrance with Bombacoideae transferring northwards from Antarctica by Madagascar, across into Africa and through the East Indies to Australia [21]. However, the concept cannot be supported by floral evolution and geological shreds of evidence [22]. According to another view based on palyno-morphological characteristics exhibited by the members of this plant group, the subfamily is assumed to have a triphyletic origin—with southern Central America, East Africa, Madagascar, and Southeast Asia as centers of origin [3]. Fossil records of this subfamily mainly belonged to microfossils (classified as belonging to the pollen genus Bombacacidites) and some macrofossils [23]. This plant group occurred in the North Tethyan flora and reached tropical regions of South America through Central America during the transition phase between the Cretaceous and Tertiary periods. Then they moved to Central Africa in the Paleocene epoch. During the Pliocene and Pleistocene periods, this group extended its distribution to the Caribbean and Central America. When the tropical flora reduced along with the North Tethys during the Upper Paleogene, the Bombacoideae retreated to North India and reached South East Asia during the Miocene epoch. From there, they expanded to New Guinea and North Australia [20,23]. In the present era, the distribution of the extant species mainly falls in the tropical regions, particularly in Africa, America, and Australia. More than 80% of the species’ richness of this subfamily lies in the Neotropical region [8,11].
There are many reports of native species in Asian countries. Various native species are introduced to other parts of the world through human activities and other influences. The center of origin of the species of this plant group differs according to the genus. Species of this plant group can be categorized into two groups—plants endemic to a certain area and plants widely distributed through introduction. Of the endemic species, Adansonia suarezensis H.Perrier and Adansonia gregorii F.Muell. are restricted to Madagascar and NW Australia, respectively [24]. Madagascar has many endemic species of Adansonia such as Adansonia fony Baill., Adansonia madagascariensis Baill., Adansonia za Baill., and Adansonia perrieri Capuron [25,26,27]. Wild regions of the endemic species are as follows: Adansonia suarezensis, Adansonia fony Baill., Adansonia madagascariensis Baill., Adansonia za Baill., Adansonia perrieri Capuron, and Adansonia grandidieri Baill. are the endemic species in the Madagascar region [24]. Similarly, Adansonia gregorii F.Muell., Aguiaria excelsa Ducke, Uladendron codesuri Marc.-Berti, Gyranthera darienensis Pittier, Cavanillesia chicamochae Fern. Alonso, Gyranthera caribensis Pittier, and Neobuchia paulinae Urb. are endemic to Australia, Brazil, Venezuela, Panama, Colombia, Venezuela, and Haiti, respectively [4,8,24,28,29,30].
Native regions of various species in this group fall within the tropical region of Africa, America, and Asia. From their native regions, many species have been introduced to other parts of the world. Adansonia digitata is amongst the most widely distributed ones covering Asia, Australia, Northern America, and some oceanic islands. Distribution of this plant in the Caribbean and parts of America is through human agencies, where people from West Africa were transported between the sixteenth and nineteenth centuries for sugarcane plantations in the New World countries. In the Indian subcontinent, Arab traders or medieval Muslim rulers who maintained African slave armies mainly introduced this species. However, genetic analyses conducted in Indian populations revealed that the introduction occurred through multiple phases [31]. Most of the species have neotropical distribution, with some species having native ranges in Asia. Bombax ceiba has wild distribution in South East Asia and India. The place of origin of some plants is uncertain. The origins of wild areas of Ceiba pentandra (L.) Gaertn. are uncertain but now it is distributed throughout tropical regions including Asia [32].
4. Characteristics
Plants of Bombacoideae are usually perennial tall trees usually with swollen tree trunks. Trees of wet forests are usually evergreen while those of dry forests are deciduous [33]. Tree trunks may contain parenchymatous water storage tissue or mucilage cells. Pneumatophores are present in the Camptostemon, a mangrove genus [8]. Barks are usually thin, often green. Most of the plants of Bombacoideae are characterized by their large size gigantic flowers with brush types [8]. Plants in these groups have a terminal flower and three bracts that exhibit a “bicolor unit”. The first, lowermost bracts remain sterile, however, other bracts subtend cymose partial inflorescences. Flowers are usually subtended by an involucre of bracts. Sepals are usually large and fused and petals are usually fused to the stamen tube [34]. The fruit capsule has a hairy endocarp. Leaves are usually peltately-palmate. Petioles are pulvinate, k connate with or without lobes. Monothecal anthers are present. These characteristics are assumed to have resulted from the splitting of whole stamens. Transitional forms are observed in some plants [8]. Anther walls have 5–7 cells across. Staminodes are usually absent. Pollen may be flattened, triangular in polar view. Seeds are usually large and usually more than two cm long.
Most of the members of this subfamily are trees, especially shrubs, with characteristic two to five carpels, fruit capsules, rarely indehiscent, endocarp usually pubescent, pollen usually without spines, seeds usually glabrous, and exceptionally spinulose [8]. Some plants have a ploidy level other than diploidy. The lowermost chromosome numbers in this group were witnessed in Bombax insigne (2n = 18) from India and Pachira macrocarpa (2x = 26) from China, while uppermost numbers were documented in Eriotheca species (6x = 276) in Brazil [35]. Distinguishing characteristucs of the genera in this subfamily is provided in Table 1.

Table 1.
General synopsis of the genera under Bombacoideae.
The status of genera under Bombacoideae might be subjected to change in future revisions. The single species Chiranthodendron pentadactylon can be crossed with Fremontodendron sp. [8] exhibiting compatible genotypes. Neobuchia paulinae is an imperfectly known species that may be included in Ceiba [8].
5. Phytochemical Configuration of Bombacoideae Subfamily
Phytochemical investigations of Bombacoideae plant species resulted in the extraction and isolation of several classes of secondary metabolites. Among the most studied genera, there are Adansonia, Bombax, and Chorisia [2,37,38]. Bombax ceiba (syn. Bombax malabaricum, Bombax malabarica, Salmalia malabaricum, Gossampinus malabarica), Adansonia digitata, and Chorisia speciosa are the most chemically and biologically investigated species.
A wide spectrum of phytochemicals has been identified and has confirmed that this family is a rich source of phytochemicals. Table 2 lists the main alkaloids, anthocyanins, coumarins, flavonoids, lignans and neolignans, sesquiterpenes and sesquiterpene lactones, sterols, tannins, and triterpenes isolated from the Bombacoideae subfamily. Volatiles and fatty acids were also reported (Table 2).

Table 2.
The main phytochemicals identified in plant species from the Bombacoideae subfamily.
The fruit pulp of A. digitata from Mali is characterized by flavonol glycosides and procyanidins as dominant classes of compounds [50]. Tiliroside was identified as a major constituent. A. digitata fruits from Nigeria showed hydroxycinnamic acid glycosides, iridoid glycosides, and phenylethanoid glycosides, secondary metabolites not detected in the fruits from Mali [88]. More recently, procyanidins, phenolic acids, and flavonol glycosides were identified in A. digitata fruits from Cameroon [89]. In particular, fruit pulp was characterized by the presence of non-flavonoid compounds such as hydroxycinnamic derivatives and flavonoids, mainly flavones, flavanols, proanthocyanidins, and flavonols.
Furthermore, polar compounds identified in leaf extracts consisted of several classes of flavonoids and hydroxycinnamic acids. Leaves from Cameroon [89] exhibited a very similar profile compared to the leaves from Mali [50].
Previously, Tembo et al. [90], quantifying several compounds in fresh A. digitata pulp and investigating quantitatively variations of some of these molecules induced by pasteurization and thermal preservation, described a high content of epicatechin, gallic acid, and procyanidin B2 in Malawi A. digitata fruits. Nasr et al. [65] isolated two flavonoid glycosides, namely, rhoifolin and tiliroside, in the alcoholic extract of C. speciosa leaves from Egypt, together with some sterols and triterpenes. The sesquiterpenes, bombamalin and isohemigossypol-1-methyl ether, and the phenols, 4-hydroxy-3,5-dimethoxybenzoic acid, 3,4,5-trimethoxyphenol-1-(β-xylopyranosyl-(1→2))-β-glucopyranoside, shorealactone, (−)-epicatechin 5-O-β-D-xylopyranoside, and 2-C-(β-D-apiofuranosyl-(1→6))-β-D-glucopyranosyl-1,3,6-trihydroxy-7-methoxyxanthone have been isolated from the ethanol extract of B. malabarica root bark [73].
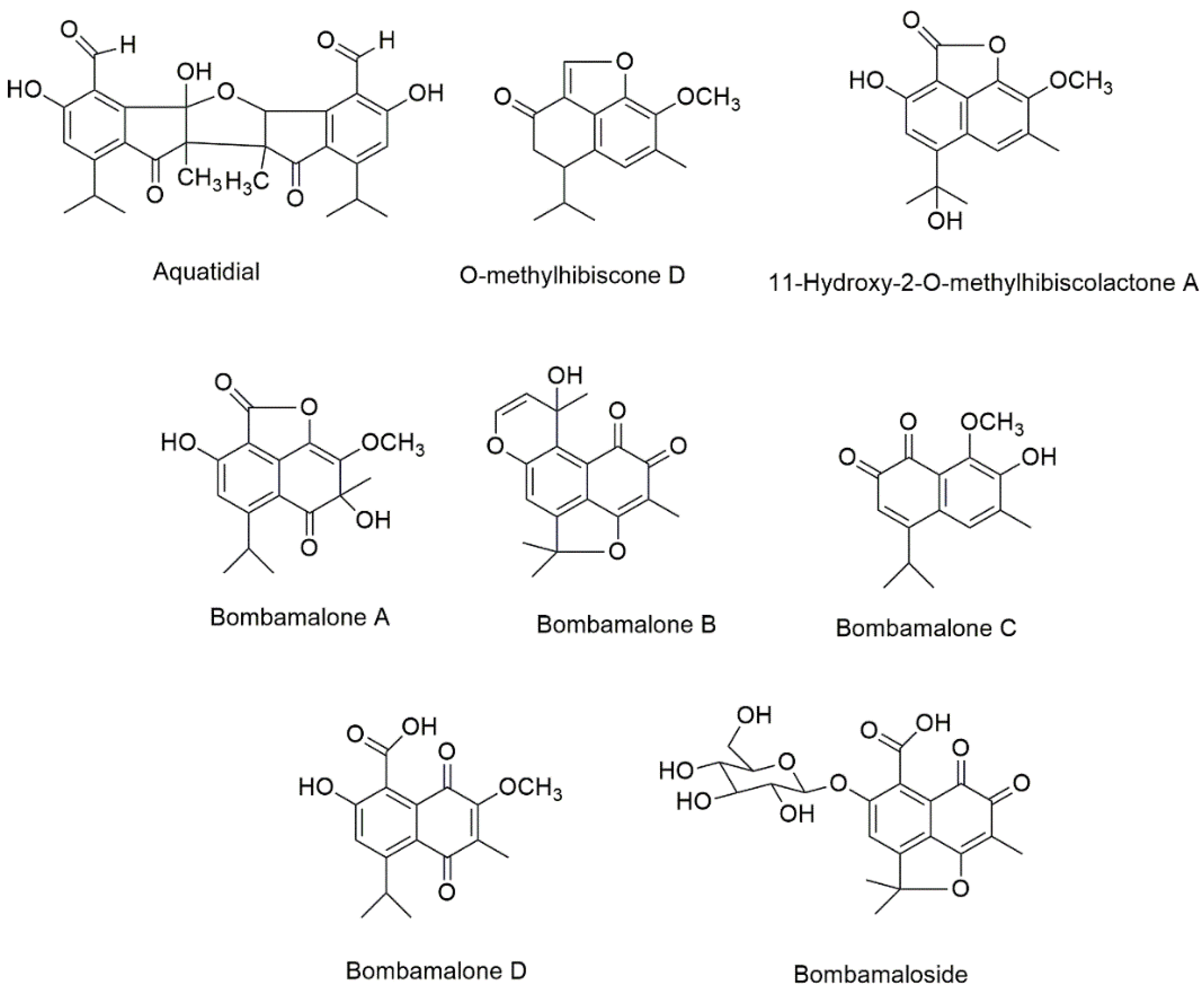
Five new compounds, namely, bombamaloside and bombamalones A–D (Figure 4), were obtained by Zhang et al. [74] from the H2O/acetone (3:7) extract of B. malabaricum roots, along with other known constituents such as bombaxquinone B, lacinilene C, isohemigossypol-1-methyl ester, and 2-O-methylisohemigossylic acid lactone.
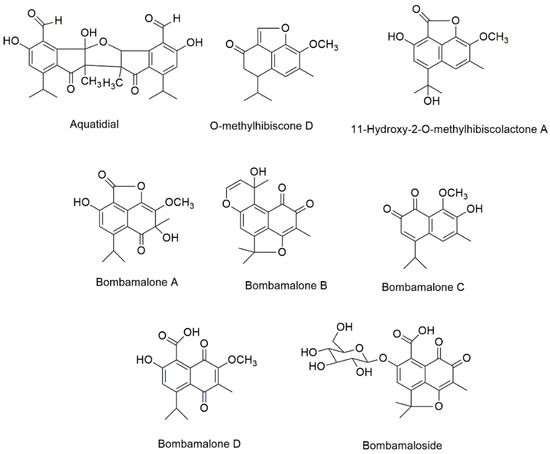
Figure 4.
The chemical structures of new isolated compounds from Bombacoideae species.
Aquatidial (Figure 4) was previously isolated from a chloroform extracts of P. aquatica roots together with the known compounds lupeol, triacontyl p-coumarate, and isohemigossypolone [72]. Aquatidial is a new bis-norsesquiterpene with an uncommon skeleton, putatively derived from isohemigossypolone. Two new naphthofuranones, 11-hydroxy-2-O-methylhibiscolactone A and O-methylhibiscone D (Figure 4), have been extracted from the P. aquatica stems [48].
Several volatiles have also been described from some Bombacoideae species. Sulfur compounds (15.3%), benzenoids (7.8%), monoterpene hydrocarbons (0.6%), and oxygenated monoterpenes (0.2%) were identified in the flowers of A. digitata [91]. The oil obtained from the flowers of C. pentandra showed monoterpene hydrocarbons (34%), sesquiterpene hydrocarbons (26.9%), oxygenated monoterpenes (8.4%), benzenoids (7.8%), and miscellaneous compounds (2%) [91].
The most common fatty acids in the Bombacoideae subfamily are oleic, linoleic, linolenic, stearic, and palmitic acids. The cyclopropenoid fatty acids, malvalic acid and sterculic acid, have been identified in A. digitata [92,93,94], A. fony [94], and Bombax oleagineum, C. acuminata, and C. pentandra [61]. Recently, the seeds’ n-hexane extract of C. speciosa from Italy showed linoleic acid (28.22%) and palmitic acid (19.56%) as the most abundant fatty acids [95]. Percentages of 16.15 and 11.11% were found for malvalic acid and sterculic acid, respectively.
Linoleic acid (38.8%), palmitic acid (24.3%), and oleic acid (21.9%) were identified as the dominant fatty acids of C. pentandra seed oil from Malaysia [96]. Malvalic and sterculic acids were also identified. A lower percentage of linoleic acid was found in the seed oil of C. pentandra from India [97]. Saturated fatty acids and monounsaturated fatty acids were obtained from the seeds of P. aquatica by using the Soxhlet apparatus and n-hexane as solvent. Palmitic acid and oleic acid were the most abundant with percentages of 49.0 and 18.2%, respectively [98]. Linoleic acid (11.2%) is the only polyunsaturated fatty acid identified.
6. Details of the Extraction and Isolation Procedure of Major Compounds from Bombacoideae for Industrial Applications
Different bioactive constituents, mainly terpenes, flavonoids, alkaloids, steroids, and fatty acids, have been isolated from the Bombacoideae subfamily. The extraction technique is the first pivotal step to obtaining active phytochemicals from plants. The choice of extraction procedure would depend mainly on the advantages and disadvantages of the process, including yield, biological activity, environmental friendliness, and safety. The fruit pulp of A. digitata revealed the presence of iridoids and phenols by using 70% ethanol as solvent [88]. Proanthocyanidins were obtained as major constituents from the pericarp of A. digitata fruits [54] by using a hydroalcoholic solution (methanol/H2O 80:20 v/v). Maceration with 95% ethanol of B. malabarica root bark led to the isolation of several sesquiterpenes, triterpenes, phenols, and sterols [73]. Conversely, cadinene sesquiterpenes were extracted from the roots of B. malabaricum by using H2O/acetone (3:7 v/v) [74]. B. malabaricum flowers extracted by 70% (v/v) aq. ethanol is characterized by different lignans.
Until now, the most applied extraction technique to isolate phytochemicals from the Bombacoideae subfamily is maceration. Researchers are exploring other extraction procedures using less energy and less solvent while producing higher yields and that are more environmentally friendly. Some advanced methods (i.e., pressurized and accelerated fluid extraction, supercritical extraction) have demonstrated to be useful in mediating related extraction difficulties along with increased extraction yields. Two of the most commonly employed extraction techniques of flavonoids are microwave- (MAE) and ultrasound-assisted extraction (UAE). High extraction efficiency and less destruction of the active constituents are the many advantages of UAE [99,100,101]. Nevertheless, MAE is preferred over UAE because MAE has been shown to increase the mass transfusion through the solid matrix, faster mixing of the extraction solvent thus preserving the highest possible driving forces, and ensuring the highest quality and quantity of the extracted constituents. Indeed, several works have proven that MAE allows for great extraction yields, a reduction of the volumes of solvents used, and a reduction of the extraction times [99,100]. MAE has been applied to extract flavonoids, tanshinones, coumarins, and terpenes [101]. These characteristics along with the simplicity of operation would position MAE as a valuable and suitable technology for industries with the growing demand for increased productivity and efficiency. However, until now little progress has been described for the MAE application to Bombacoideae species. Surely, taking into account all the MAE features, in the future, it will be possible to optimize the process by exploiting the opportunity to apply this innovative extraction method to the study of species belonging to the Bombacoideae family.
7. Application in Food/Use as Food
From ancient periods until today, many plants of the Bombacoideae have been used as food in various corners of the world. Parts used may range from leaves, seeds, tuberous roots to stem, flowers, et cetera. There are various variations in the use of food according to genera and cultures associated. Native African populations commonly use fruits of Adansonia digitata as famine food to make sauces, decoctions, and refreshing beverages [102]. The leaves, seeds, and pulp of the fruit of this plant are all edible. Lim (2012) reported the use of young leaves, seeds, fruit pulp, and tuberous roots of Adansonia gregorii F. Muell as food. Along with Adansonia spp, Ceiba pentandra is another one of the plant foods common to West Africa. Leaves of this plant are cooked in the form of slurry sauce [103]. The utilization as food for this plant group is not restricted to Africa but observed in other parts of tropical countries. In Central and South America, flowers and tender leaves of Pachira aquatica, a wetland tree, are cooked and used as vegetables [15]. Young roots of Bombax ceiba are eaten raw or roasted in Cambodia. The cuipo tree (Cavanillesia platanifolia), growing in Central America, is used by the natives for getting water. To collect water, a piece of the root is cut and the bark is removed on one end after keeping the root horizontal. When the clean end of the root is lowered, the water drains out through the cut end [104].
The use and preparation of food from the members of Bombacoideae dates back to time immemorial. For instance, in South America, from the ancient pre-Colombian period [105], flowers of Quararibea funebris were used as an additive to chocolate drinks. Ancient Mayans used the sap from Pseudobombax ellipticum to make an intoxicating drink by fermentation. This drink was likely used in religious ceremonies such as sacrifice and self-mutilation [33]. The use of various members of Bombacoides as fruits, vegetables, and other forms are highlighted in Table 3.

Table 3.
Plant and parts used as a food.
8. Traditional and Economic Uses
Various members of Bombacoideae are used as fiber and other utilities and some are also used as ornamental plants. Adansonia digitata is a multipurpose plant with various economic and social values [106]. In African countries, Adansonia digitata is very popular and reported to have more than three hundred traditional uses [102]. Ceiba Mill. is now popular throughout the tropical regions for ornamental landscaping [114]. Many species of the genus Ceiba were sacred to the Mayan civilization as depicted in ancient ceramics because of their cultural importance [33].
Many Bombacoideae species are economically important. Some species are collected for their wood that is soft and can easily be carved into canoes and other useful products. One popular wood is balsa wood obtained from the Ochroma pyramidale [16] and other species were widely used for making dugout canoes in ancient South America. Ancient Peruvians are believed to have used legendary Kon-Tiki rafts made from balsa wood to navigate across the Pacific Ocean and settle in the Polynesian islands [115]. The silky cotton-like fluff (kapok) present in the seed pods of Ceiba pentandra is used for stuffing pillows, bedding, and soft toys in various parts [116]. Silk hair present in seeds of Bombax ceiba are used in India from time immemorial for stuffing cushions, mattresses, pillows, and making clothes [117]. Various traditional and economic uses of the members of this subfamily are summarized in Table 4.

Table 4.
Economic and traditional uses of Bombacoideae members.
9. Ethnopharmacology
In various tropical countries, plants of Bombacoideae are used in traditional medicine mainly for pharmacological properties like anti-inflammatory, astringent, antimicrobial, stimulant, antipyretic, analgesic, and diuretic [2]. For instance, various parts of Bombax ceiba such as the stem bark, flowers, fruits, seeds, leaves, and root of young plants, are traditionally used as remedy in South India [108]. Its main therapeutic applications include diabetes, urinogenital disorders, gastrointestinal and skin diseases, gynecological, and general debility [129]. Another important plant from this subfamily in the Indian ayurvedic system is Ceiba pentandra known as Sweta Salmali for its acrid, bitter, thermogenic, diuretic, and purgative properties. The known pharmacological activities of Ceiba pentandra include hepatoprotective, antidiabetic, antipyretic, laxative, and anti-inflammatory [130]. Adansonia digitata is one of the most studied species for its therapeutic properties against antipyretic, diarrhea, dysentery, and as a substitute for cinchona in traditional medicinal preparations [105]. Different species under Bombacoideae having reported ethnopharmacological uses are summarized in Table 5.

Table 5.
Plants belonging to Bombacoideae with ethnopharmacological uses.
10. Pharmacological Potential of Bombacoideae
The different species, viz. Adansonia digitata, Bombax ceiba, B. malabaricum, and Ceiba pentandra of the Bombacoideae family [136,146], were reported for their various pharmacological potentials, which are summarized in the following section (Table 6; Figure 5).

Table 6.
Pharmacological studies on some of the plant species of Bombacoideae subfamily.

Figure 5.
Photo of representative plant species from the Bombacoideae subfamily. Reproduced under Creative Commons Attribution-Non-Commercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/; accessed on 27 February 2021) from Rameshwar et al. [136] (originally Figure 1).
10.1. Antioxidant Properties
Adansonia digitata L.
The methanolic fruit pulp and leaf extracts of A. digitata exhibited in vitro antioxidant activities as studied by 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH), 2,2-azinobis—(3-ethylbenzothiazoline-6-sulfonate) (ABTS), ferric reducing antioxidant power (FRAP), β-carotene bleaching test, superoxide-scavenging assays [50,150]. The methanol extracts of leaf, seed, bark, fruit wall, and floral extracts of A. digitata were reported for their DPPH scavenging potential [147]. The DPPH scavenging activity was highest in seed extract (27.69%) and lowest in fruit wall (20.69%) extract. The methanolic leaf extract of A. digitata could maintain the antioxidant status of the streptozotocin (STZ) induced diabetic rats by normalizing the elevated levels of reduced glutathione (GSH) superoxide dismutase (SOD), and catalase (CAT) [148]. The ethanolic leaf, bark, and fruit extracts of A. digitata could scavenge the DPPH free radicals with percentages of inhibition of 13.4, 29.23, and 39.21%, respectively [149].
Bombax ceiba L.
The methanolic root extract of Bombax ceiba could scavenge DPPH radicals, lipid peroxidation, and ascorbyl radicals with an EC50 value of 87 µg/mL. The extract also inhibited lipid peroxidation in rat-liver microsome induced by ascorbyl and peroxynitrite radicals with IC50 values of 141 µg/mL and 115 µg/mL, respectively [192]. In another study, the methanol root extract of B. ceiba scavenged DPPH radical with an EC50 value of 15.07 µg. The extract also could reduce the Fe3+ to Fe2+ in a dose-dependent manner with the maximum activity at 500 µg. The study also demonstrated that the administration of 3 g root powder could raise the antioxidant status in the human volunteer. The antioxidant activity properties of the root extract are attributed to their high phenolic and tannin contents [152]. The aqueous soluble partition (AQSF) of the methanolic root extracts of B. ceiba scavenged DPPH radical with an IC50 value of 3.33 μg/mL [153]. Further, the methanol and petroleum ether root extract of B. ceiba was reported to scavenge DPPH radical with IC50 values of 144.77 and 214.83 μg/mL [155]. The methanolic stem bark extract of B. ceiba exhibited antiradical activity with EC50 values of 18.78, 23.62, and 139.4 μg/mL for nitric oxide, DPPH, and reducing power activity assay, respectively [193]. Similarly, Hossain et al. [154] reported the antioxidant activity of methanolic root extract of B. ceiba by DPPH scavenging assay (IC50 value of 58.6 μg/mL). Gandhare et al. [156] reported that aqueous and ethanolic extracts of the B. ceiba bark exhibited DPPH, ABTS, nitric oxide, and superoxide radical scavenging activity along with total antioxidant activity. Besides the extract also inhibited lipid peroxidation and reduced ferric ions. The IC50 values of aqueous extracts of B. ceiba varied between 85.71 and 102.45 µg/mL, and for ethanolic extract, it varied between 85.48 and 103.4 µg/mL. Komati et al. [163] reported that aqueous methanol extract of B. ceiba calyx reduced the level of reactive oxygen species (ROS), NADPH oxidase (NOX), and thereby lowered the mitochondrial dysfunction in methylglyoxal induced protein glycation. Further, in HEK-293 cells, Mn and Cu/Zn-superoxide dismutase and glutathione reductase antioxidant enzymes levels were improved. The whole plant methanolic extract of B. ceiba scavenged DPPH radical with an IC50 value of 68 µg/mL [158]. The petroleum ether (PE) of B. ceiba flowers exhibited DPPH and Fe-chelating activities with IC50 values of 37.6 and 33.5 μg/mL and diethyl ether extracts (DE) exhibited beta-carotene bleaching test with an IC50 value of 58.3 μg/mL. The antioxidant properties of B. ceiba flower extracts are attributed to the presence of beta-sitosterol and some fatty acids [80]. Similarly, another study reported that aqueous flower extracts of B. ceiba could scavenge DPPH radicals with an IC50 value of 50.21 μg/mL [159]. The aqueous flower extracts of B. ceiba exhibited antioxidant activities against DPPH, hydroxyl, hydrogen peroxide, and ferric ion reducing antioxidant power (FRAP) activity with IC50 values of 1.70 mg/mL, 4.20 mg/mL, 3.51 mg/mL, and 2.15 mg/mL, respectively [160]. The hexane, benzene, chloroform, ethyl acetate, acetone, methanol, and ethanol extracts prepared from methanolic flower extract of B. ceiba exhibited DPPH scavenging activity [161]. The hexane, chloroform, and methanolic extracts prepared from dried powder extracts of B. ceiba flower exhibited antioxidant activity in terms of FRAP, DPPH, and reducing power assay [162].
Bombax malabaricum DC.
The n-hexane and methanol flower extracts of B. malabaricum scavenged DPPH radicals over a concentration range of 0.55–0.0343 mg/mL and 0.5–0.0312 mg/mL, respectively. The maximum DPPH scavenging was observed in the range of 0.55–0.5 mg/mL for both extracts [49]. The antioxidant potential of flower extract was attributed to the presence of bioactive constituent, viz. apigenin, cosmetin, xanthomicrol, saponarin, vicenin 2, isovitexin, and linarin. Similarly, in another study, the aqueous, acetone, and ethanol flower extracts of B. malabaricum flowers showed DPPH radical-scavenging properties along with Oxygen radical absorbance capacity (ORAC), reducing power, and liposome peroxidation inhibition activities [151].
Ceiba pentandra L.
The different stem bark extracts of C. pentandra such as decoction, maceration, and methanol scavenged DPPH radical with IC50 values of 87.84, 54.77, and 6.15 µg/mL, respectively. The extracts also restrained the H2O2-induced hemolysis and lipid peroxidation [165]. The Soxhlet seed oil extracts at 100 mg/mL concentration of C. pentandra exhibited DPPH, and OH radical scavenging along with FRAP, reducing power activities by 47.65%, 39.69%, and 309 FRAP units, and 20.52 μg of ascorbic acid equivalent, respectively [193]. The in vitro antioxidant evaluation of C. pentandra ethanol leaf extract demonstrated that the extract could scavenge DPPH, nitric oxide, and hydroxyl radicals with IC50 values of 27.4, 24.45, and 51.65 µg/mL, respectively. The Gas chromatography-mass spectrometry (GC-MS) study revealed the presence of 9 compounds, amongst which, hexadecanoic acid was found to be the most prominent compound [167]. In another study, Fitria et al. [166] demonstrated that a compound vavain or 5, 3′-dihydroxy-7, 4 ′, 5′- trimethoxyisoflavone isolated from the ethyl acetate fraction of stem bark of C. pentandra could scavenge DPPH radical with IC50 value of 81.66 µg/mL. However, the ethyl acetate extract of the aerial part of C. pentandra scavenged the DPPH radicals with an IC50 value of 0.0716 mg/mL [184]. The aqueous and methanol stem bark extracts of C. pentandra inhibited superoxide (O2•−) (IC50 values of 51.81 and 34.26 μg/mL), hydrogen peroxide (44.84 and 1.78 μg/mL), and protein oxidation induced by H2O2 (120.60 and 140.40 μg/mL) [168].
10.2. Anti-Inflammatory Activity
Adansonia digitata L.
The methanol leaf extracts of A. digitata reduced iNOS and NF-kB expression in LPS-stimulated RAW264.7, thereby showing its anti-inflammatory potential [181]. The extract could inhibit NO production with an IC50 value of 28.6 µg/mL. Similarly, the Dimethyl sulfoxide (DMSO) fruit pulp and aqueous leaf extract of A. digitata inhibited expressions of proinflammatory cytokine IL-8 [182]. The leaf extract (70 µg/mL) exhibited better anti-inflammatory activity compared to pulp extract (247 µg/mL).
Bombax ceiba L.
The petroleum ether, ethanol, and aqueous bark extracts of B. ceiba at 1000 µg/mL concentration exhibited anti-inflammatory potential by stabilizing the Human red blood cell (HRBC) membrane. Amongst the different solvent extracts, better anti-inflammatory activity is shown by ethanol extract followed by aqueous and petroleum ether extract [183].
Ceiba pentandra (L.) Gaertn
The ethyl acetate extracts of aerial parts of C. pentandra upon oral administration at 400 mg/kg dose could inhibit methotrexate (MTX)-initiated apoptotic and inflammatory cascades. The extract could improve the architecture of histopathological changes observed in the renal tissue of MTX-induced nephrotoxic rats [184].
10.3. Antimicrobial Activity
Adansonia digitata L.
The methanolic and ethanolic leaf and stem bark extracts of A. digitata inhibited the growth of S. aureus and E. coli at different concentrations, viz, 100, 200, 500, and 1000 mg/mL with a minimum bactericidal concentration (MIC) at 100 mg/mL [169].
Bombax ceiba L.
The methanolic stem bark extract of B. ceiba could inhibit the growth of both Gram-negative (Escherichia coli, Pseudomonas aeruginosa, and Salmonella typhi) and Gram-positive bacteria (Bacillus subtilis, Staphylococcus aureus) dose-dependently. The order of sensitivity from highest to lowest was S. aureus > E. coli > P. aeruginosa > B. subtilis > S. typhi [157]. The methanolic flower extract of B. ceiba exhibited antibacterial activity against Klebsiella pneumonia, E. coli, P. aeruginosa (Gram-negative), and S. aureus, B. subtilis (Gram-positive) bacteria with the MIC value ranging between 3.125 and 12.500 μg/mL [161]. The methanol, dichloromethane, and PE extracts of B. ceiba roots exhibited mild to moderate antibacterial activity against different bacterial strains including Sarcina lutea, Bacillus megaterium, B. subtilis, S. aureus, B. cereus, P. aeruginosa, Salmonella typhi, E. coli, Vibrio mimicus, Shigella boydii, and S. dysenteriae with a 7–13 mm zone of inhibition [155].
Bombax malabaricum DC.
The n-hexane and methanol extracts (at 100 µg/mL) of B. malabaricum demonstrated antimicrobial activities against Gram-positive (E. coli, Neisseria gonorrhoeae, P. aeruginosa), Gram-negative (S. aureus, B. subtilis, Streptococcus faecalis) bacteria and fungi (Aspergillus niger, A. flavus Candida albicans). Of the two extracts, the methanol extract showed better activity against all the studied bacterial strains and C. albicans. Further, only the methanol extract exhibited moderate activities against A. niger and A. flavus [49].
Ceiba pentandra (L.) Gaertn
The ethyl acetate fraction of leaf and bark of C. pentandra showed antimicrobial activity against E. coli, Salmonella typhi, B. subtilis, Kleibsiella pneumonia, and S. aureus [170]. Similarly, aqueous, methanol, ethanol, and acetone seed extracts of C. pentandra exhibited antimicrobial activity against E. coli, S. aureus, K. pneumonia, Enterobacter aerogenes, P. aeruginosa, Salmonella typhimurium, S.typhi, Staphylococcus epidermidis, and Proteus vulgaris [171]. Another study revealed that ethanol leaf extract of C. pentandra dose-dependently inhibits antibacterial activity against E. coli and S. aureus [167].
10.4. Anticancer and Cytotoxicity Activity
Adansonia digitata L.
The seed and pulp extracts of A. digitata (at 10, 100, and 500 µg/mL) exhibited anticancer activity against MCF- 7 (breast cancer cell), Hep-G2 (liver cancer cell), and COLO-205 (colon cancer cell) in a dose dependent manner [172]. The results of the MTT study revealed that the inhibition ranges between 22.57 and 29.96% for MCF-7 cell line; 25.85 and 37.81% for Hep-G2 cell line, and 20.75 and 27.34% for COLO-205 cell line. The dichloromethane and methanolic leaf extracts of A. digitata demonstrated cytotoxic activity against human breast development cell lines BT474 evaluated by MTT assay. The methanol leaves of the plant exhibited moderate cytotoxic activity (56%) against the BT474 cell line with IC50 values of 15.3 ± 0.4 µg/mL [185].
Bombax ceiba L.
The diethyl ether and light petroleum ether extracts of B. ceiba flowers exhibited antiproliferative activity against human renal adenocarcinoma cell (ACHN) with respective IC50 values of 53.2 and 45.5 μg/mL. The antiproliferative properties were attributed to the presence of beta-sitosterol and some fatty acids in B. ceiba flowers [80]. The brine shrimp lethality bioassay revealed that the petroleum ether, dichloromethane, and methanol extracts of B. ceiba roots exhibited cytotoxic effect with LC50 values of 22.58, 37.72, and 70.72 μg/mL, respectively [155].
Ceiba pentandra (L.) Gaertn
The petroleum and acetone stem bark extracts of C. pentandra at 15 and 30 mg/kg doses could reduce tumor weight by >50% and tumor volume on the 30th day in Dalton’s lymphoma ascites (DLA) model [173]. Similarly, both these extracts of C. pentandra exhibited cytotoxic effects against Ehrlich ascites carcinoma (EAC) cells as evaluated by trypan blue assay [173]. At 15 mg/kg doses, both the extracts showed improvement in mean survival time and decline in tumor induced increase in body weight. Further, the petroleum ether, benzene, chloroform, acetone, and ethanolic extract of this plant demonstrated cytotoxicity in a concentration dependent manner after 3 h of incubation with EAC cells with EC50 values of 53.30, 70.58, 250.48, 67.30, and 56.11 µg/mL, respectively.
10.5. Hepatoprotective Activity
Adansonia digitata L.
The aqueous fruit pulp extract of A. digitata showed hepatoprotective potential in carbon-tetrachloride (CCL4) -induced hepatotoxic rat models as significant reductions in serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatize (ALP), and bilirubin levels were observed in extract-treated hepatotoxic rats [186,187]. The liver protection potential could be attributed to the presence of triterpenoids, β-sitosterol, β-amyrin palmitate, and α-amyrin or without, and ursolic acid in the fruit pulp [188]. The methanolic fruit pulp extract of A. digitata exhibited hepatoprotective potential in paracetamol-induced hepatotoxic rat models. The disturbances in the liver function such as ALT, AST, ALP, total bilirubin, and total protein measurements of the hepatotoxic rats were normalized due to the administration of paracetamol [189].
Bombax ceiba L.
The hepatoprotective property of aqueous [159] and methanolic [190] flower extracts of B. ceiba was studied in CCl4-induced hepatotoxic rats. Treatment with extracts decreased elevated levels of glutamate oxaloacetate transaminase (SGOT), glutamic pyruvic transaminase (SGPT), alkaline phosphatize (ALP), bilirubin, triglycerides, and total protein. Treatment with the extract further attenuated the damage caused to the liver as seen by histological studies. The young roots of B. ceiba exhibited hepatoprotective activities in alloxan induced diabetic mice. Administration of ethanolic root extracts at 400 mg/kg decreased the hepatotoxicity in diabetic mice by reducing the elevated levels of SGOT and SGPT [176].
Ceiba pentandra (L.) Gaertn
The ethyl acetate fraction of methanolic stem bark extract of C. pentandra exhibited a hepatoprotective effect against paracetamol-induced hepatotoxic rats by reducing the serum enzyme levels of SGOT, SGPT, ALP, and total bilirubin content [191].
10.6. Antidiabetic Activity
Adansonia digitata L.
The methanolic fruit pulp and leaf extracts of A. digitata exhibited in vitro antidiabetic activities by inhibiting the digestive enzyme α-glucosidase dose-dependently [50]. The IC50 values of the fruit extracts ranged between 1.71 ± 0.23 and 2.39 ± 0.22 µg/mL while the leaf extract had an IC50 value of 1.71 ± 0.23 µg/mL. Similarly, the methanolic leaf extract of this plant inhibited α-amylase, α-glucosidase, and aldolase reductase [150]. The antidiabetic potency of the extracts may be attributed to the presence of catechin, epicatechin, rutin, quercitrin, quercetin, kaempferol, luteolin (flavonoids), gallic, chlorogenic, caffeic, and ellagic acids (phenolic acids). The methanolic leaf extract of A. digitata reduced the elevated blood glucose, glycosylated hemoglobin levels in streptozotocin (STZ) induced diabetic rats [148].
Bombax ceiba L.
The dichloromethane, ethanol, and aqueous thalamus and flower extracts of B. ceiba were reported for their antidiabetic properties in terms of their capacity to inhibit alpha-amylase and alpha-glucosidase enzymes under in vitro condition. The corresponding IC50 values for alpha-amylase inhibition activities for thalamus were 36.22 µg/mL (dichloromethane extract), 35.32 µg/mL (ethanolic extract), and 31.31 µg/mL (aqueous extract) and for flowers, 38.13 µg/mL (dichloromethane extract), 35.23 µg/mL (ethanolic extract), and 33.00 µg/mL (aqueous extract) [174]. The n-hexane fraction of sepals [175] and ethanolic leaf extracts [177] of B. ceiba exhibited antidiabetic activities in STZ-induced diabetic rats. The n-hexane fraction at 0.1 gm/kg bw, b.d. dose reduced the fasting blood sugar level and restored the levels of serum insulin, Hb, and glycated hemoglobin in diabetic rats. Histological studies of also showed marked improvement in diminution in the area of the islets of Langerhans of pancreases in diabetic rats treated with the plant extracts [175]. Similarly, the leaf extract of B. ceiba (at 70, 140, and 280 mg/kg doses) decreased the fasting blood glucose, glycosylated hemoglobin, and increased the oral glucose tolerance in the STZ-induced diabetic rats. The antidiabetic property may be attributed to the antioxidant activity and protecting pancreatic β-cells of the extract [177]. The young roots of B. ceiba exhibited antidiabetic activities in alloxan-induced diabetic mice. Administration of ethanolic root extracts at 400 mg/kg decreased blood glucose levels in diabetic mice as compared to untreated diabetic mice at different time points (0–24 h). [176]. However, at 600 mg/kg dose the extract could significantly decrease elevated levels of blood glucose in diabetic rats [178].
Ceiba pentandra (L.) Gaertn
The aqueous stem bark extracts of C. pentandra exhibited antihyperglycemic, insulin-sensitizing potential, and cardioprotective effects in dexamethasone-induced insulin-resistant rats. Extracts of both 75 or 150 mg/kg doses could decrease the level of glycemia [179]. The decoction extracts of stem bark of C. pentandra decreased glucose level by increasing glucose uptake in the liver and skeletal muscle cells by 56.57% and 94.19%, respectively. The extract also reduced the glucose release in liver cells by 33.94% in a hypoglycemic milieu [165]. The ethanolic bark extract of C. pentandra at 200 mg/kg dose exhibited antihyperglycemic activity in STZ-induced diabetic rats by decreasing the levels of blood glucose, total cholesterol, and triglycerides, preventing degeneration of liver and pancreas, and increasing serum insulin and liver glycogen content [180]. The aqueous stem bark extracts of C. pentandra inhibited alpha-amylase and glucosidase with IC50 values of 6.15 and 76.61 μg/mL, respectively, whereas the methanol extract inhibited alpha-amylase and glucosidase with IC50 values of 54.52 and 86.49 μg/mL, respectively [168].
10.7. Miscellaneous Activities
The petroleum ether and methanol extract from B. ceiba stem bark displayed increased osteogenic activity as demonstrated by Chauhan et al. [37] in UMR-106 cells and surgical ovariectomy models in female Wistar albino rats. It has been reported that the administration of the extracts for 28 days ameliorated the consequences of ovariectomy-induced bone porosity, restoring the normal architecture of bone in experimented rats. The in vitro osteogenic activity of the extracts could be attributed to the presence of lupeol, gallic acid, and β-sitosterol in B. ceiba.
Komati et al. [163] reported the antiglycation properties of aqueous methanolic calyx extract of B. ceiba in methylglyoxal-induced protein glycation and oxidative stress in HEK-293 cells. The extract could inhibit advanced glycation end products (AGEs) formation and restrained Receptor for advanced glycation end products (RAGE) up-regulation in HEK-293 cells.
The aqueous and crude ethanol fruit extracts of B. ceiba exhibited diuretic effects in rats. Both aqueous and ethanol extracts could increase the urine output in the rats. The aqueous extract increased the urinary Na+ and K+ levels demonstrating the diuretic effect of the extracts [194]. The ethanolic leaf, bark, and fruit extracts of A. digitata exhibited antipyretic activity in albino rats at 400 and 800 mg/kg doses [149].
11. Mechanism of Action of Extracts and Bioactive Compounds of the Plants’ Species with Pharmacological Properties
The different plant species of Bombacoideae are well known for their medicinal properties and can act as a useful bio-resource for medicines, nutraceuticals, pharmaceuticals, and chemical analogs for synthetic drugs. Bombacoideae plant species contain several bioactive phytocompounds such as alkaloids, anthocyanins, coumarins, flavonoids, lignans and neolignans, sesquiterpenes, sesquiterpene lactones, sterols, tannins, triterpenes, et cetera, which may be responsible for their antimicrobial properties. The antimicrobial action of phytocompounds might be due to their capacity to disintegrate cytoplasmic membrane, destabilize proton motive force, electron flow, active transport, and coagulation of the cell content in microbes [195]. Silva and Fernandes [196] also reviewed the antimicrobial properties of plants and concluded that different chemical classes of phytochemicals including alkaloids, flavonoids, terpenoids, phenols, tannins, et cetera may be responsible for their antimicrobial potential.
Several phytochemicals of the different classes of compounds such as alkaloids, flavonoids, saponins, terpenoids, vitamins, glycosides, phenols, et cetera play significant roles in inhibiting or arresting cancer cell progression by different mechanisms such as (a) by inhibiting cancer cell-activating signaling pathways such as Cdc2, CDK2, and CDK4 kinases, topoisomerase enzyme, cyclooxygenase, and COX-2, Bcl-2, cytokines, PI3K, Akt, MAPK/ERK, MMP, and TNK; (b) activating mechanisms of DNA repairing, viz. p21, p27, p51, and p53 genes, and Bax, Bid, and Bak proteins; or (c) by stimulating the formation of protective enzymes, viz. Caspase-3, 7, 8, 9, 10, and 12 [197].
Plants enriched with phenolic acids, flavonoids, coumarins, lignans, terpenoids, et cetera can exert antioxidant action by scavenging radicals and chelating metal ions by acting as reducing agents, hydrogen donors, singlet oxygen quenchers, metal chelators, or reductants of ferryl hemoglobin [198]. Therefore, the antioxidant potential of different species of Bombacoideae may be due to the presence of several classes of phytoconstituents including vicenin 2, linarin, saponarin, cosmetin, isovitexin, xanthomicrol, vavain, apigenin, beta-sitosterol, et cetera [151,184].
The different bioactive phytocomponents could exhibit anti-inflammatory activities by down regulating of signaling pathways like NF- κB pathway. This is done by different mechanisms such as (a) inhibiting common mediators of inflammation like NO, iNOS, and pro-inflammatory cytokines like TNF-α, IL-1β, IL-6, and IL-12p40; (b) inhibition of chemokines such as RANTES and MCP-1; (c) downregulating mediators of inflammation such as cycloxygenase-2 (COX-2), prostaglandins, and leukotrienes; (d) reducing the production of ROS and lipid peroxidation; and (e) upregulating enzymatic (superoxide dismutase, catalase, etc.) and non-enzymatic (glutathione, etc.) defense systems [199]. The different species of Bombacoideae such as A. digitata and C. pentandra could inhibit inhibition against proinflammatory cytokine IL-8 expression or by reducing iNOS and NF-kB expression [181,182], and the activity is attributed to the presence of different phytoconstituents, viz. quercitrin, cinchonains 1a and 1b, cis-clovamide, trans-clovamide, and glochidioboside [184]. The phytoconstituents of different plants could show antidiabetic activities by inhibiting carbohydrate metabolizing enzymes like amylase and glucosidase enzymes, or by stimulating insulin release or by increasing glucose uptake by cells or by decreasing insulin resistance [200]. Several studies have rightly pointed out that different Bombacoideae plants could exhibit antidiabetic activities by inhibiting α-amylase and α-glucosidase enzymes [148].
12. Conclusions
Plants are considered important natural resources as food supplements and in traditional and modern medicine in different regions of the world. Bioactive phytochemicals are valued candidates for the discovery of new drugs. Detailed reporting of plants with food value, and therapeutic and economic importance, of subfamily Bombacoideae, was undertaken in this review. Isolated phytochemicals of diversified classes of secondary metabolites are reported to possess numerous therapeutic properties against different ailments. The bioactive phytochemicals from plants of this subfamily will play important roles in the development of new drug leads with less toxicity and side effects.
Author Contributions
G.D., H.-S.S., S.S.N., A.D.T., H.U., R.T., S.K.D. and J.K.P., writing—original draft preparation, investigation, resources, data curation; G.D., A.D.T., S.K.D., R.T. and J.K.P., writing—review and editing, methodology, formal analysis; J.K.P., conceptualization, supervision, project administration, funding acquisition, visualization. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1G1A1004667), Republic of Korea for support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data presented in the manuscript are available in the form of tables and figures in the manuscript.
Acknowledgments
All authors are grateful to their respective institutions for support. J.K.P. acknowledges the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1G1A1004667), Republic of Korea for support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jain, V.; Verma, S.; Katewa, S. Myths, traditions and fate of multipurpose Bombax ceiba L.—An appraisal. Indian J. Tradit. Knowl. 2009, 8, 638–644. [Google Scholar]
- Refaat, J.; Desoky, S.Y.; Ramadan, M.A.; Kamel, M.S. Bombacaceae: A phytochemical review. Pharm. Biol. 2013, 51, 100–130. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, H.P. Pollen morphology of the family Bombacaceae. Rev. Palaeobot. Palynol. 1967, 3, 119–132. [Google Scholar] [CrossRef]
- Carvalho-Sobrinho, J.G.; Alverson, W.S.; Alcantara, S.; Queiroz, L.P.; Mota, A.C.; Baum, D.A. Revisiting the phylogeny of Bombacoideae (Malvaceae): Novel relationships, morphologically cohesive clades, and a new tribal classification based on multilocus phylogenetic analyses. Mol. Phylogenetics Evol. 2016, 101, 56–74. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.B.; Bovini, M.G.; da Souza Conceição, A. Bombacoideae, Byttnerioideae, Grewioideae and Helicterioideae (Malvaceae s.l.) in the Raso da Catarina Ecoregion, Bahia, Brazil. Biota Neotrop. 2019, 19, 1–21. [Google Scholar] [CrossRef]
- Singh, G. Plant Systematics: An Integrated Approach, 4th ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Bayer, C.; Fay, M.; Bruijn, A.; Savolainen, V.; Morton, C.; Kubitzki, K.; Alverson, W.; Chase, M. Support for an expanded family concept of Malvaceae within a recircumscribed order Malvales: A combined analysis of plastid atpB and rbcL DNA sequences. Bot. J. Linn. Soc. 1999, 129, 267–303. [Google Scholar] [CrossRef]
- Kubitzki, K.; Bayer, C. The Families and Genera of Vascular Plants; Springer: Berlin/Heidelberg, Germany, 2003; Volume 5. [Google Scholar]
- Christenhusz, M.J.M.; Fay, M.F.; Chase, M.W. Plants of the World: An Illustrated Encyclopedia of Vascular Plants; University of Chicago Press: Chicago, IL, USA, 2017. [Google Scholar]
- Duarte, M. Phylogenetic Analyses of Eriotheca and Related Genera (Bombacoideae, Malvaceae). Syst. Bot. 2011, 36, 690–701. [Google Scholar] [CrossRef]
- Zizka, A.; Carvalho-Sobrinho, J.G.; Pennington, R.T.; Queiroz, L.P.; Alcantara, S.; Baum, D.A.; Bacon, C.D.; Antonelli, A. Transitions between biomes are common and directional in Bombacoideae (Malvaceae). J. Biogeogr. 2020, 47, 1–12. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Vermaak, I.; Viljoen, A.M. An updated review of Adansonia digitata: A commercially important African tree. S. Afr. J. Bot. 2011, 77, 908–919. [Google Scholar] [CrossRef]
- Barwick, M. Tropical & Subtropical Trees: A Worldwide Encyclopaedic Guide; Thames & Hudson: London, UK, 2004. [Google Scholar]
- Wickens, G.E. Edible Nuts; FAO: Rome, Italy, 1995; Volume 5. [Google Scholar]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2012; Volume 1. [Google Scholar]
- Roth, I.; Lindorf, H. South American Medicinal Plants: Botany, Remedial Properties and General Use; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Middeljans, M. The Species Composition of the Mangrove Forest along the Abatan River in Lincod, Maribojoc, Bohol, Philippines and the Mangrove Forest Structure and its Regeneration Status between Managed and Unmanaged Nipa palm (Nypa fruticans Wurmb); Wageningen University: Wageningen, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Infante-Mata, D.; Moreno-Casasola, P.; Madero-Vega, C. Pachira aquatica, un indicador del límite del manglar? Rev. Mex. Biodivers. 2014, 85, 143–160. [Google Scholar] [CrossRef]
- Gibbs, P.E.; Alverson, W.S. How many species of Spirotheca (Malvaceae sl, Bombacoideae)? Brittonia 2006, 58, 245–258. [Google Scholar] [CrossRef]
- Krutzsch, W. Paleogeography and historical phytogeography (paleochorology) in the Neophyticum. Plant Syst. Evol. 1989, 162, 5–61. [Google Scholar] [CrossRef]
- Croizat, L. Manual of Phytogeography: An Account of Plant-Dispersal Throughout the World; Springer Science + Business: Dordrecht, The Netherlands, 1952. [Google Scholar]
- Wickens, G.E.; Lowe, P. The Baobabs: Pachycauls of Africa, Madagascar and Australia; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Ehrendorfer, F. Woody Plants—Evolution and Distribution Since the Tertiary: Proceedings of a Symposium Organized by Deutsche Akademie Der Naturforscher LEOPOLDINA in Halle/Saale, German Democratic Republic, 9–11 October 1986; Springer: Vienna, Austria, 1989. [Google Scholar]
- Odetokun, S. The nutritive value of Baobab fruit (Adansonia digitata). Riv. Ital. Sostanze Grasse 1996, 73, 371–373. [Google Scholar]
- Chevalier, M.A. Les Baobabs (Adansonia) de lʹAfrique continentale. Bull. Société Bot. Fr. 1906, 53, 480–496. [Google Scholar] [CrossRef][Green Version]
- Hostettmann, K.; Schaller, F. Antimicrobial Diterpenes. U.S. Patent US5929124A, 27 July 1999. [Google Scholar]
- Inngjerdingen, K.; Nergård, C.; Diallo, D.; Mounkoro, P.; Paulsen, B. An Ethnopharmacological survey of plants used for wound healing in Dogonland, Mali, West Africa. J. Ethnopharmacol. 2004, 92, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, D.; Carvalho-Sobrinho, J.; Zartman, C.; Komura, D.; Queiroz, L. Unexplored Amazonian diversity: Rare and phylogenetically enigmatic tree species are newly collected. Neodiversity 2015, 8, 55–73. [Google Scholar] [CrossRef]
- Mitré, M. Gyranthera darienensis. IUCN Red List Threat. Species 1998 1998, e.T30572A9554110. [Google Scholar] [CrossRef]
- Díaz-Pérez, C.N.; Puerto-Hurtado, M.A.; Fernández-Alonso, J.L. Evaluación Del Hábitat, Las Poblaciones Y El Estatus De Conservación Del Barrigón (Cavanillesia chicamochae, Malvaceae–Bombacoideae). Caldasia 2011, 33, 105–119. [Google Scholar]
- Bell, K.L.; Rangan, H.; Kull, C.A.; Murphy, D.J. The history of introduction of the African baobab (Adansonia digitata, Malvaceae: Bombacoideae) in the Indian subcontinent. R. Soc. Open Sci. 2015, 2, 150370. [Google Scholar] [CrossRef] [PubMed]
- Mansfeld, R.; Hanelt, P. Mansfeld’s Encyclopedia of Agricultural and Horticultural Crops: (Except Ornamentals); Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Zidar, C.; Elisens, W. Sacred Giants: Depiction of Bombacoideae on Maya Ceramics in Mexico, Guatemala, and Belize. Econ. Bot. 2009, 63, 119–129. [Google Scholar] [CrossRef]
- Byng, J.W. The Flowering Plants Handbook: A Practical Guide to Families and Genera of the World; Plant Gateway Ltd.: Hertford, UK, 2014. [Google Scholar]
- Marinho, R.C.; Mendes-Rodrigues, C.; Balao, F.; Ortiz, P.L.; Yamagishi-Costa, J.; Bonetti, A.M.; Oliveira, P.E. Do chromosome numbers reflect phylogeny? New counts for Bombacoideae and a review of Malvaceae s.l. Am. J. Bot. 2014, 101, 1456–1465. [Google Scholar] [CrossRef]
- Fay, M. Index to Plant Chromosome Numbers 2004–2006. Regnum Vegetabile 152; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Chauhan, S.; Sharma, A.; Upadhyay, N.K.; Singh, G.; Lal, U.R.; Goyal, R. In-vitro osteoblast proliferation and in-vivo anti-osteoporotic activity of Bombax ceiba with quantification of Lupeol, gallic acid and β-sitosterol by HPTLC and HPLC. BMC Complement. Altern. Med. 2018, 18, 233. [Google Scholar] [CrossRef]
- Deepshikha, R.; Geetanjali; Ram, S. Phytochemistry and Pharmacology of Genus Bombax. Nat. Prod. J. 2019, 9, 184–196. [Google Scholar] [CrossRef]
- Osman, M.A. Chemical and nutrient analysis of baobab (Adansonia digitata) fruit and seed protein solubility. Plant Foods Hum. Nutr. 2004, 59, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Zennie, T.M.; Cassady, J.M.; Raffauf, R.F. Funebral, a new pyrrole lactone alkaloid from Quararibea funebris. J. Nat. Prod. 1986, 49, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Zennie, T.M.; Cassady, J.M. Funebradiol, a New Pyrrole Lactone Alkaloid from Quararibea funebris Flowers. J. Nat. Prod. 1990, 53, 1611–1614. [Google Scholar] [CrossRef] [PubMed]
- Raffauf, R.F.; Zennie, T.M.; Onan, K.D.; Le Quesne, P.W. Funebrine, a structurally novel pyrrole alkaloid, and other. gamma.-hydroxyisoleucine-related metabolites of Quararibea funebris (Llave) Vischer (Bombacaceae). J. Org. Chem. 1984, 49, 2714–2718. [Google Scholar] [CrossRef]
- Scogin, R. Reproductive phytochemistry of Bombacaceae: Floral anthocyanins and nectar constituents. Aliso: A J. Syst. Evol. Bot. 1986, 11, 377–385. [Google Scholar] [CrossRef]
- Niranjan, G.; Gupta, P. Anthocyanins from the flowers of Bombax malabaricum. Planta Med. 1973, 24, 196–199. [Google Scholar] [CrossRef]
- Rizk, A.; Al-Nowaihi, A. The Phytochemistry of the Horticultural Plants of Qatar; Alden Press: Oxford, UK, 1989. [Google Scholar]
- Paula, V.F.; Barbosa, L.C.; Demuner, A.J.; Howarth, O.W.; Veloso, D.P. Chemical constituents of Ochroma lagopus Swartz. Quim. Nova 1996, 19, 225–229. [Google Scholar]
- Joshi, K.R.; Devkota, H.P.; Yahara, S. Chemical analysis of flowers of Bombax ceiba from Nepal. Nat. Prod. Commun. 2013, 8, 1934578X1300800508. [Google Scholar] [CrossRef]
- Cheng, L.-Y.; Liao, H.-R.; Chen, L.-C.; Wang, S.-W.; Kuo, Y.-H.; Chung, M.-I.; Chen, J.-J. Naphthofuranone derivatives and other constituents from Pachira aquatica with inhibitory activity on superoxide anion generation by neutrophils. Fitoterapia 2017, 117, 16–21. [Google Scholar] [CrossRef] [PubMed]
- El-Hagrassi, A.M.; Ali, M.M.; Osman, A.F.; Shaaban, M. Phytochemical investigation and biological studies of Bombax malabaricum flowers. Nat. Prod. Res. 2011, 25, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Braca, A.; Sinisgalli, C.; De Leo, M.; Muscatello, B.; Cioni, P.L.; Milella, L.; Ostuni, A.; Giani, S.; Sanogo, R. Phytochemical Profile, Antioxidant and Antidiabetic Activities of Adansonia digitata L. (Baobab) from Mali, as a Source of Health-Promoting Compounds. Molecules 2018, 23, 3104. [Google Scholar] [CrossRef] [PubMed]
- El-Alfy, T.; El-Sawi, S.; Sleem, A.; Moawad, D. Investigation of flavonoidal content and biological activities of Chorisia insignis Hbk. leaves. Aust. J. Basic Appl. Sci. 2010, 4, 1334–1348. [Google Scholar]
- Noreen, Y.; El-Seedi, H.; Perera, P.; Bohlin, L. Two new isoflavones from Ceiba pentandra and their effect on cyclooxygenase-catalyzed prostaglandin biosynthesis. J. Nat. Prod. 1998, 61, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, E.; Martínez, E.M.; Cogordán, J.A.; Delgado, G. Triterpenes, Phenols, and Other Constituents from the leaves of Ochroma pyramidale (Balsa Wood, Bombacaceae): Preferred Conformations of 8-C-β-D-Glucopyranosyl-apigenin (vitexin). Rev. Soc. Química México 2002, 46, 254–258. [Google Scholar]
- Shahat, A.A. Procyanidins from Adansonia digitata. Pharm. Biol. 2006, 44, 445–450. [Google Scholar] [CrossRef]
- Qi, Y.; Guo, S.; Xia, Z.; Xie, D. Chemical constituents of Gossampinus malabarica (L.) Merr.(II). Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Med. 1996, 21, 234. [Google Scholar]
- Ueda, H.; Kaneda, N.; Kawanishi, K.; Alves, S.M.; Moriyasu, M. A new isoflavone glycoside from Ceiba pentandra (L.) Gaertner. Chem. Pharm. Bull. 2002, 50, 403–404. [Google Scholar] [CrossRef] [PubMed]
- Sichaem, J.; Siripong, P.; Khumkratok, S.; Tip-Pyang, S. Chemical constituents from the roots of Bombax anceps. J. Chil. Chem. Soc. 2010, 55, 325–327. [Google Scholar] [CrossRef]
- Paula, V.F.; Cruz, M.P.; Barbosa, L.C.d.A. Chemical constituents of Bombacopsis glabra (Bombacaceae). Química Nova 2006, 29, 213–215. [Google Scholar] [CrossRef]
- Chauhan, J.; Kumar, S.; Chaturvedi, R. A new flavanonol glycoside from Adansonia digitata roots. Planta Med. 1984, 50, 113. [Google Scholar] [CrossRef]
- Gopal, H.; Gupta, R. Chemical constituents of Salmalia malabarica Schott and Endl. flowers. J. Pharm. Sci. 1972, 61, 807–808. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Lavaud, C.; Bourdy, G.; Giménez, A.; Sauvainc, M. First bioguided phytochemical approach to Cavanillesia Aff. hylogeiton. Rev. Boliv. Química 2002, 19, 18–24. [Google Scholar]
- Ngounou, F.; Meli, A.; Lontsi, D.; Sondengam, B.; Choudhary, M.I.; Malik, S.; Akhtar, F. New isoflavones from Ceiba pentandra. Phytochemistry 2000, 54, 107–110. [Google Scholar] [CrossRef]
- Chauhan, J.; Chaturvedi, R.; Kumar, S. A new flavonol glycoside from the stem of Adansonia-Digitata. Indian J. Chem. B Org. Incl. Med. Chem. 1982, 21, 254–255. [Google Scholar]
- Coussio, J. Isolation of rhoifolin from Chorisia species (Bombacaceae). Experientia 1964, 20, 562. [Google Scholar] [CrossRef] [PubMed]
- Nasr, E.M.; Assaf, M.H.; Darwish, F.M.; Ramadan, M.A. Phytochemical and biological study of Chorisia speciosa A. St. Hil. cultivated in Egypt. J. Pharmacogn. 2018, 7, 649–656. [Google Scholar]
- Faizi, S.; Ali, M. Shamimin: A new flavonol C-glycoside from leaves of Bombax ceiba. Planta Med. 1999, 65, 383–385. [Google Scholar] [CrossRef]
- Saleem, R.; Ahmad, S.I.; Ahmed, M.; Faizi, Z.; Zikr-ur-Rehman, S.; Ali, M.; Faizi, S. Hypotensive activity and toxicology of constituents from Bombax ceiba stem bark. Biol. Pharm. Bull. 2003, 26, 41–46. [Google Scholar] [CrossRef]
- Chauhan, J.; Kumar, S.; Chaturvedi, R. A new flavanone glycoside from the roots of Adansonia digitata. Natl. Acad. Sci. Lett. India 1987, 10, 177–179. [Google Scholar]
- Paula, V.F.; Barbosa, L.C.; Howarth, O.W.; Demuner, A.J.; Cass, Q.B.; Vieira, I.J. Lignans from Ochroma lagopus Swartz. Tetrahedron 1995, 51, 12453–12462. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, X.-H.; Zhang, S.-W.; Xuan, L.-J. Three Novel Compounds from the Flowers of Bombax malabaricum. Helv. Chim. Acta 2008, 91, 136–143. [Google Scholar] [CrossRef]
- Wang, G.K.; Lin, B.B.; Rao, R.; Zhu, K.; Qin, X.Y.; Xie, G.Y.; Qin, M.J. A new lignan with anti-HBV activity from the roots of Bombax ceiba. Nat. Prod. Res. 2013, 27, 1348–1352. [Google Scholar] [CrossRef] [PubMed]
- Paula, V.F.; Rocha, M.E.; Barbosa, L.C.d.A.; Howarth, O.W. Aquatidial, a new bis-Norsesquiterpenoid from Pachira aquatica Aubl. J. Braz. Chem. Soc. 2006, 17, 1443–1446. [Google Scholar] [CrossRef]
- Lam, S.-H.; Chen, J.-M.; Tsai, S.-F.; Lee, S.-S. Chemical investigation on the root bark of Bombax malabarica. Fitoterapia 2019, 139, 104376. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, H.; Zhang, S.; Yu, Q.; Xuan, L. Sesquiterpenoids from Bombax malabaricum. J. Nat. Prod. 2007, 70, 1526–1528. [Google Scholar] [CrossRef]
- Sankaram, A.V.B.; Reddy, N.S.; Shoolery, J.N. New sesquiterpenoids of Bombax malabaricum. Phytochemistry 1981, 20, 1877–1881. [Google Scholar] [CrossRef]
- Puckhaber, L.; Stipanovic, R. Revised structure for a sesquiterpene lactone from Bombax malbaricum. J. Nat. Prod. 2001, 64, 260–261. [Google Scholar] [CrossRef]
- Rao, K.V.; Sreeramulu, K.; Gunasekar, D.; Ramesh, D. Two new sesquiterpene lactones from Ceiba pentandra. J. Nat. Prod. 1993, 56, 2041–2045. [Google Scholar] [CrossRef]
- Sood, R.P.; Suri, K.A.; Suri, O.P.; Dhar, K.L.; Atal, C.K. Sesquiterpene lactone from Salmalia malbarica. Phytochemistry 1982, 21, 2125–2126. [Google Scholar] [CrossRef]
- Bianchini, J.-P.; Ralaimanarivo, A.; Gaydou, E.M.; Waegell, B. Hydrocarbons, sterols and tocopherols in the seeds of six Adansonia species. Phytochemistry 1982, 21, 1981–1987. [Google Scholar] [CrossRef]
- Tundis, R.; Rashed, K.; Said, A.; Menichini, F.; Loizzo, M.R. In vitro cancer cell growth inhibition and antioxidant activity of Bombax ceiba (Bombacaceae) flower extracts. Nat. Prod. Commun. 2014, 9, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Dhar, D.; Munjal, R. Chemical examination of the seeds of Bombax malabaricum. Planta Med. 1976, 29, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Sipra, D.; Dan, S.S. Phytochemical study of Adansonia digitata, Coccoloba excoriata, Psychotria adenophylla and Schleichera oleosa. Fitoterapia 1986, 57, 445–446. [Google Scholar]
- Matsuda, M.; Endo, Y.; Fushiya, S.; Endo, T.; Nozoe, S. Cytotoxic 6-substituted 5, 6-dihydro-2H-pyran-2-ones from a Brazilian medicinal plant, Chorisia crispiflora. Heterocycles 1994, 38, 1229–1232. [Google Scholar] [CrossRef]
- Vijaya Bhaskar Reddy, M.; Kesava Reddy, M.; Gunasekar, D.; Marthanda Murthy, M.; Caux, C.; Bodo, B. A new sesquiterpene lactone from Bombax malabaricum. Chem. Pharm. Bull. 2003, 51, 458–459. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bell, A.A.; Stipanovic, R.D.; O’Brien, D.H.; Fryxell, P.A. Sesquiterpenoid aldehyde quinones and derivatives in pigment glands of Gossypium. Phytochemistry 1978, 17, 1297–1305. [Google Scholar] [CrossRef]
- Kishore, P.H.; Reddy, M.V.B.; Gunasekar, D.; Caux, C.; Bodo, B. A new naphthoquinone from Ceiba pentandra. J. Asian Nat. Prod. Res. 2003, 5, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Shibatani, M.; Hashidoko, Y.; Tahara, S. A Major fungitoxin from Pachira aquatica and its accumulation in outer bark. J. Chem. Ecol. 1999, 25, 347–353. [Google Scholar] [CrossRef]
- Li, X.-N.; Sun, J.; Shi, H.; Yu, L.L.; Ridge, C.D.; Mazzola, E.P.; Okunji, C.; Iwu, M.M.; Michel, T.K.; Chen, P. Profiling hydroxycinnamic acid glycosides, iridoid glycosides, and phenylethanoid glycosides in baobab fruit pulp (Adansonia digitata). Food Res. Int. 2017, 99, 755–761. [Google Scholar] [CrossRef]
- Tsetegho Sokeng, A.J.; Sobolev, A.P.; Di Lorenzo, A.; Xiao, J.; Mannina, L.; Capitani, D.; Daglia, M. Metabolite characterization of powdered fruits and leaves from Adansonia digitata L. (baobab): A multi-methodological approach. Food Chem. 2019, 272, 93–108. [Google Scholar] [CrossRef]
- Tembo, D.T.; Holmes, M.J.; Marshall, L.J. Effect of thermal treatment and storage on bioactive compounds, organic acids and antioxidant activity of baobab fruit (Adansonia digitata) pulp from Malawi. J. Food Compos. Anal. 2017, 58, 40–51. [Google Scholar] [CrossRef]
- Pettersson, S.; Ervik, F.; Knudsen, J.T. Floral scent of bat-pollinated species: West Africa vs. the New World. Biol. J. Linn. Soc. 2004, 82, 161–168. [Google Scholar] [CrossRef]
- Gaydou, E.; Bianchini, D.J.P.; Ralaimanarivo, A. Cyclopropenoid fatty acids in Malagasy baobab: Adansonia grandidieri (Bombacaceae) seed oil. Fette Seifen Anstrichm. 1982, 84, 468–472. [Google Scholar] [CrossRef]
- Ralaimanarivo, A.; Gaydou, E.M.; Bianchini, J.P. Fatty acid composition of seed oils from six Adansonia species with particular reference to cyclopropane and cyclopropene acids. Lipids 1982, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, D.; Dennis, T.; Shingare, M. Constituents of Adansonia digitata root bark. Fitoterapia 1992, 63, 278–279. [Google Scholar]
- Rosselli, S.; Tundis, R.; Bruno, M.; Leporini, M.; Falco, T.; Gagliano Candela, R.; Badalamenti, N.; Loizzo, M.R. Ceiba speciosa (A. St.-Hil.) Seeds Oil: Fatty Acids Profiling by GC-MS and NMR and Bioactivity. Molecules 2020, 25, 1037. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.K. The characteristics of the kapok (Ceiba pentadra, Gaertn.) seed oil. Pertanika 1979, 2, 1–4. [Google Scholar]
- Ravi Kiran, C.; Raghava Rao, T. Lipid Profiling by GC-MS and Anti-inflammatory Activities of Ceiba pentandra Seed Oil. J. Biol. Act. Prod. Nat. 2014, 4, 62–70. [Google Scholar]
- Sunday, A.S.; Gillian, I.O.; John, I.O. Fatty acid composition of seed oil from Pachira aquatica Grown in Nigeria. J. Agric. Ecol. Res. Int. 2019, 18, 1–9. [Google Scholar] [CrossRef]
- Krishnan, R.Y.; Chandran, M.N.; Vadivel, V.; Rajan, K. Insights on the influence of microwave irradiation on the extraction of flavonoids from Terminalia chebula. Sep. Purif. Technol. 2016, 170, 224–233. [Google Scholar] [CrossRef]
- Wang, X.-H.; Wang, J.-P. Effective extraction with deep eutectic solvents and enrichment by macroporous adsorption resin of flavonoids from Carthamus tinctorius L. J. Pharm. Biomed. Anal. 2019, 176, 112804. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Serradilla, J.; Japon-Lujan, R.; de Castro, M.L. Simultaneous microwave-assisted solid–liquid extraction of polar and nonpolar compounds from Alperujo. Anal. Chim. Acta 2007, 602, 82–88. [Google Scholar] [CrossRef]
- Buchmann, C.; Prehsler, S.; Hartl, A.; Vogl, C.R. The Importance of Baobab (Adansonia digitata L.) in Rural West African Subsistence—Suggestion of a Cautionary Approach to International Market Export of Baobab Fruits. Ecol. Food Nutr. 2010, 49, 145–172. [Google Scholar] [CrossRef]
- Friday, E.T.; James, O.; Olusegun, O.; Gabriel, A. Investigations on the nutritional and medicinal potentials of Ceiba pentandra leaf: A common vegetable in Nigeria. Int. J. Plant Physiol. Biochem. 2011, 3, 95–101. [Google Scholar]
- Brown, T. Tom Brown’s Guide to Wild Edible and Medicinal Plants; Berkley Books: New York, NY, USA, 1986. [Google Scholar]
- Paula, V.F.; Barbosa, L.C.A.; Demuner, A.J.; Piló-Veloso, D. A química da família bombacaceae. Química Nova 1997, 20, 627–630. [Google Scholar] [CrossRef][Green Version]
- Chadare, F.J.; Linnemann, A.; Hounhouigan, D.; Nout, M.J.; Boekel, M. Baobab Food Products: A Review on their Composition and Nutritional Value. Crit. Rev. Food Sci. Nutr. 2009, 49, 254–274. [Google Scholar] [CrossRef] [PubMed]
- Divakar, T.; Rao, Y.H. Biological synthesis of silver nano particles by using Bombax ceiba plant. J. Chem. Pharm. Sci. 2017, 10, 574–576. [Google Scholar]
- R Nayagam, D.J. IBA Induced Rooting Characteristics in Marathi Moggu Stem Cuttings: Evaluation Using SVI Concept. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 734–739. [Google Scholar] [CrossRef][Green Version]
- Assogba, G.A.; Fandohan, A.B.; Gandji, K.; Salako, V.K.; Assogbadjo, A.E. Bombax costatum (Malvaceae): State of knowns, unknowns and prospects in West Africa. BASE 2018, 22, 267–275. [Google Scholar]
- Van Roosmalen, M.G. Fruits of the Guianan Flora; Institute of Systematic Botany, Utrecht University: Utrecht, The Netherlands, 1985. [Google Scholar]
- Macbride, J.F. Flora of Peru; Field Museum of Natural History: Chicago, IL, USA, 1936; Volume 13. [Google Scholar]
- Rosengarten, F. An unusual spice from Oaxaca: The flowers of Quararibea funebris. Bot. Mus. Leafl. Harv. Univ. 1977, 25, 183–202. [Google Scholar]
- Vickers, W.T.; Plowman, T. Useful Plants of the Siona and Secoya Indians of Eastern Ecuador; Field Museum of Natural History: Chicago, IL, USA, 1984. [Google Scholar]
- Tripathi, S.; Farooqui, A.; Singh, V.; Singh, S.; Kumar Roy, R. Morphometrical analysis of Ceiba Mill. (Bombacoideae, Malvaceae) pollen: A sacred plant of the Mayan (Mesoamerican) civilisation. Palynology 2019, 43, 551–573. [Google Scholar] [CrossRef]
- Easterling, K.; Harrysson, R.; Gibson, L.; Ashby, M.F. On the mechanics of balsa and other woods. Proc. R. Soc. Lond. A Math. Phys. Sci. 1982, 383, 31–41. [Google Scholar]
- Zheng, Y.; Wang, A. Kapok Fiber: Applications. In Biomass and Bioenergy: Applications; Hakeem, K.R., Jawaid, M., Rashid, U., Eds.; Springer: Cham, Switzerland, 2014; pp. 251–266. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Rani, S.; Rana, J. Traditionally used common fibre plants in outer siraj area, Himachal Pradesh. Indian J. Nat. Prod. Resour. 2014, 5, 190–194. [Google Scholar]
- Sidibe, M.; Williams, J.T. Baobab: Adansonia digitata L.; International Centre for Underutilised Crops: Southampton, UK, 2002; Volume 4. [Google Scholar]
- Watt, G. A Dictionary of the Economic Products of India; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Sint, K.M.; Adamopoulos, S.; Koch, G.; Hapla, F.; Militz, H. Wood Anatomy and Topochemistry of Bombax ceiba L. and Bombax insigne Wall. BioResources 2013, 8, 530–544. [Google Scholar] [CrossRef]
- Oyen, L.P.A. Bombax costatum Pellegr. & Vuillet. In Record from PROTA4U; PROTA: Wageningen, The Netherlands, 2011. [Google Scholar]
- Quattrocchi, U. CRC World Dictionary of Medicinal and Poisonous Plants: Common Names, Scientific Names, Eponyms, Synonyms, and Etymology; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Günter, S.; Weber, M.; Stimm, B.; Mosandl, R. Silviculture in the Tropics; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Ballesteros, J.L.; Bracco, F.; Cerna, M.; Vita Finzi, P.; Vidari, G. Ethnobotanical Research at the Kutukú Scientific Station, Morona-Santiago, Ecuador. BioMed Res. Int. 2016, 2016, 9105746. [Google Scholar] [CrossRef]
- Weniger, B.; Estrada, A.; Aragon, R.; Deharo, E.; Arango, G.; Lobstein, A.; Anton, R. Ethnopharmacology in the search for new leishmanicidal drugs. Etudes Chim. Pharmacol. 2002, 1, 391–394. [Google Scholar]
- DeWalt, S.J.; Geneviève, B.; Lia, R.C.d.M.; Celin, Q. Ethnobotany of the Tacana: Quantitative Inventories of Two Permanent Plots of Northwestern Bolivia. Econ. Bot. 1999, 53, 237–260. [Google Scholar] [CrossRef]
- Ruffinatto, F.; Crivellaro, A. Atlas of Macroscopic Wood Identification: With a Special Focus on Timbers Used in Europe and CITES-Listed Species; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Lorenzi, H. Instituto Plantarum de Estudos da Flora. Brazilian Trees: A Guide to the Identification and Cultivation of Brazilian Native Trees; Instituto Plantarum de Estudos da Flora: Nova Odessa, Brazil, 2002. [Google Scholar]
- Jain, V.; Verma, S. Assessment of credibility of some folk medicinal claims on Bombax ceiba L. Indian J. Tradit. Knowl. 2014, 13, 87–94. [Google Scholar]
- Elumalai, A.; Mathangi, N.; Didala, A.; Kasarla, R.; Venkatesh, Y. A Review on Ceiba pentandra and its medicinal features. Asian J. Pharma. Technol. 2012, 2, 83–86. [Google Scholar]
- Watt, J.M.; Breyer-Brandwijk, M.G. The Medicinal and Poisonous Plants of Southern and Eastern Africa Being an Account of Their Medicinal and other Uses, Chemical Composition, Pharmacological Effects and Toxicology in Man and Animal; E & S Livingstone Ltd.: Edinburgh, UK, 1962. [Google Scholar]
- Van Wyk, B.E.; Gericke, N. People’s Plants: A Guide to Useful Plants of Southern Africa; Briza Publications: Pretoria, South Africa, 2000. [Google Scholar]
- Adesanya, S.A.; Idowu, T.B.; Elujoba, A.A. Antisickling activity of Adansonia digitata. Planta Med. 1988, 54, 374. [Google Scholar] [CrossRef]
- Tapsoba, H.; Deschamps, J.P. Use of medicinal plants for the treatment of oral diseases in Burkina Faso. J. Ethnopharmacol. 2006, 104, 68–78. [Google Scholar] [CrossRef]
- Rosado, F.L. Manual de los Árboles de Tikal; Agencia Española de Cooperación Internacional, Programa de Preservación del Patrimonio Cultural en Iberoamérica: Barcelona, Spain, 1996. [Google Scholar]
- Rameshwar, V.; Kishor, D.; Tushar, G.; Siddharth, G.; Sudarshan, G. A Pharmacognostic and pharmacological overview on Bombax ceiba. Sch. Acad. J. Pharm. 2014, 3, 100–107. [Google Scholar]
- Chaudhary, P.H.; Khadabadi, S.S. Bombax ceiba Linn.: Pharmacognosy, ethnobotany and phyto-pharmacology. Pharmacogn. Commun. 2012, 2, 2–9. [Google Scholar] [CrossRef]
- DeFilipps, R.; Maina, S.; Crepin, J. Medicinal Plants of the Guianas (Guyana, Surinam, French Guiana); DC Department of Botany: Washington, DC, USA, 2004. [Google Scholar]
- Nsimba, M.M.; Lami, J.N.; Hayakawa, Y.; Yamamoto, T.K. Decreased thrombin activity by a Congolese herbal medicine used in sickle cell anemia. J. Ethnopharmacol. 2013, 148, 895–900. [Google Scholar] [CrossRef]
- Weniger, B.; Robledo, S.; Arango, G.J.; Deharo, E.; Aragón, R.; Muñoz, V.; Callapa, J.; Lobstein, A.; Anton, R. Antiprotozoal activities of Colombian plants. J. Ethnopharmacol. 2001, 78, 193–200. [Google Scholar] [CrossRef]
- Pabon, A.; TV, V.M.; Cardona, F.; Blair, S.; Ramírez, O. Ethnobotany of medicinal plants used to treat malaria by traditional healers from ten indigenous Colombian communities located in Waupes Medio. Biodivers. Int. J. 2017, 1, 151–167. [Google Scholar] [CrossRef][Green Version]
- Mors, W.B.; Rizzini, C.T.; Pereira, N.A. Medicinal Plants of Brazil; Reference Publications Inc.: Algonac, MI, USA, 2000. [Google Scholar]
- Standley, P.C.; Steyermark, J.A. Flora of Guatemala; Natural History Museum: Chicago, IL, USA, 1946. [Google Scholar]
- Ribeiro, R.V.; Bieski, I.G.C.; Balogun, S.O.; Martins, D.T.d.O. Ethnobotanical study of medicinal plants used by Ribeirinhos in the North Araguaia microregion, Mato Grosso, Brazil. J. Ethnopharmacol. 2017, 205, 69–102. [Google Scholar] [CrossRef]
- Moraes, L.L.C.; Freitas, J.L.; Matos Filho, J.R.; Silva, R.B.L.; Borges, C.H.A.; Santos, A.C. Ethno-knowledge of medicinal plants in a community in the eastern Amazon. Revista Ciências Agrárias 2019, 42, 291–300. [Google Scholar]
- Rahul, J.; Jain, M.K.; Singh, S.P.; Kamal, R.K.; Anuradha; Naz, A.; Gupta, A.K.; Mrityunjay, S.K. Adansonia digitata L. (baobab): A review of traditional information and taxonomic description. Asian Pac. J. Trop. Biomed. 2015, 5, 79–84. [Google Scholar] [CrossRef]
- Talari, S.; Gundu, C.; Koila, T.; Nanna, R.S. In vitro free radical scavenging activity of different extracts of Adansonia digitata L. Int. J. Environ. Agric. Biotechnol. 2017, 2, 238781. [Google Scholar] [CrossRef]
- Ebaid, H.; Bashandy, S.A.; Alhazza, I.M.; Hassan, I.; Al-Tamimi, J. Efficacy of a methanolic extract of Adansonia digitata leaf in alleviating hyperglycemia, hyperlipidemia, and oxidative stress of diabetic rats. BioMed Res. Int. 2019, 2019, 2835152. [Google Scholar] [CrossRef]
- Kiyawa, S.A.; Mukhtar, Z.G. Antioxidant and Antipyretic Activities of Adansonia Digitata (African Baobab) Fruit, Leaf And Bark Extracts. Chem. J. 2019, 3, 1–8. [Google Scholar]
- Irondi, E.A.; Akintunde, J.K.; Agboola, S.O.; Boligon, A.A.; Athayde, M.L. Blanching influences the phenolics composition, antioxidant activity, and inhibitory effect of Adansonia digitata leaves extract on α-amylase, α-glucosidase, and aldose reductase. Food Sci. Nutr. 2017, 5, 233–242. [Google Scholar] [CrossRef]
- Yu, Y.-G.; He, Q.-T.; Yuan, K.; Xiao, X.-L.; Li, X.-F.; Liu, D.-M.; Wu, H. In vitro antioxidant activity of Bombax malabaricum flower extracts. Pharm. Biol. 2011, 49, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Verma, S.; Katewa, S.; Anandjiwala, S.; Singh, B. Free radical scavenging property of Bombax ceiba Linn. root. Res. J. Med. Plant 2011, 5, 462–470. [Google Scholar] [CrossRef]
- Rahman, M.H.; Rashid, M.A.; Chowdhury, T.A. Studies of Biological Activities of the Roots of Bombax ceiba L. Bangladesh Pharm. J. 2019, 22, 219–223. [Google Scholar] [CrossRef]
- Hossain, S.; Islam, J.; Ahmed, F.; Hossain, M.; Siddiki, M.; Hossen, S. Free radical scavenging activity of six medicinal plants of Bangladesh: A potential source of natural antioxidant. J. Appl. Pharm. 2015, 7, 96–104. [Google Scholar] [CrossRef]
- Hoque, N.; Rahman, S.; Jahan, I.; Shanta, M.A.; Tithi, N.S.; Nasrin, N. A Comparative Phytochemical and Biological Study between Different Solvent Extracts of Bombax ceiba Roots Available in Bangladesh. Pharmacol. Pharm. 2018, 9, 53–66. [Google Scholar] [CrossRef][Green Version]
- Gandhare, B.; Soni, N.; Dhongade, H.J. In vitro antioxidant activity of Bombax ceiba. Int. J. Biomed. Res 2010, 1, 31–36. [Google Scholar] [CrossRef][Green Version]
- Masood Ur, R.; Akhtar, N.; Mustafa, R. Antibacterial And Antioxidant Potential Of Stem Bark Extract Of Bombax ceiba Collected Locally From South Punjab Area Of Pakistan. Afr. J. Tradit. Complementary Altern. Med. 2017, 14, 9–15. [Google Scholar] [CrossRef]
- Shyur, L.-F.; Tsung, J.-H.; Chen, J.-H.; Chiu, C.-Y.; Lo, C.-P. Antioxidant properties of extracts from medicinal plants popularly used in Taiwan. Int. J. Appl. Sci. Eng. 2005, 3, 195–202. [Google Scholar]
- Wanjari, M.M.; Gangoria, R.; Dey, Y.N.; Gaidhani, S.N.; Pandey, N.K.; Jadhav, A.D. Hepatoprotective and antioxidant activity of Bombax ceiba flowers against carbon tetrachloride-induced hepatotoxicity in rats. Hepatoma Res. 2016, 2, 144–150. [Google Scholar] [CrossRef]
- Sharma, A.; Batish, D.R.; Singh, H.P. In vitro antioxidant potential of the edible flowers of Bombax ceiba—An underutilized tropical tree. In Proceedings of the 6th International Conference on Recent Trends in Engineering, Science & Management, Punjab, India, 8 January 2017; pp. 364–374. [Google Scholar]
- Rathore, D.; Srivastava, R.; Geetanjali, G.; Singh, R. Phytochemical screening, antioxidant and antibacterial activities of Bombax ceiba flower. Alger. J. Nat. Prod. 2019, 7, 651–656. [Google Scholar]
- Donipati, R.S.; Subhasini, P. Determining the Antioxidant Activity of Bombax ceiba Flower Extracts. Int. J. Pharm. Res. Sch. 2016, 5, 146–150. [Google Scholar]
- Komati, A.; Anand, A.; Shaik, H.; Mudiam, M.K.R.; Babu, K.S.; Tiwari, A.K. Bombax ceiba (Linn.) calyxes ameliorate methylglyoxal-induced oxidative stress via modulation of RAGE expression: Identification of active phytometabolites by GC-MS analysis. Food Funct. 2020, 11, 5486–5497. [Google Scholar] [CrossRef]
- Kiran, C.R.; Rao, D.B.; Sirisha, N.; Rao, T.R. Assessment of phytochemicals and antioxidant activities of raw and germinating Ceiba pentandra (kapok) seeds. J. Biomed. Res. 2015, 29, 414. [Google Scholar]
- Fofie, C.K.; Wansi, S.L.; Nguelefack-Mbuyo, E.P.; Atsamo, A.D.; Watcho, P.; Kamanyi, A.; Nole, T.; Nguelefack, T.B. In vitro anti-hyperglycemic and antioxidant properties of extracts from the stem bark of Ceiba pentandra. J. Complementary Integr. Med. 2014, 11, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Fitria, Z.; Efdi, A.; Efdi, M. Isolation and characterization of antioxidative constituent from stem bark extract of Ceiba pentandra L. J. Chem. Pharm. Res. 2015, 7, 257–260. [Google Scholar]
- Bhavani, R.; Bhuvaneswari, E.; Rajeshkumar, S. Antibacterial and Antioxidant activity of Ethanolic extract of Ceiba pentandra leaves and its Phytochemicals Analysis using GC-MS. Res. J. Pharm. Technol. 2016, 9, 1922–1926. [Google Scholar] [CrossRef]
- Nguelefack, T.B.; Fofie, C.K.; Nguelefack-Mbuyo, E.P.; Wuyt, A.K. Multimodal α-Glucosidase and α-Amylase Inhibition and Antioxidant Effect of the Aqueous and Methanol Extracts from the Trunk Bark of Ceiba pentandra. BioMed Res. Int. 2020, 2020, 3063674. [Google Scholar] [CrossRef]
- Datsugwai, M.S.S.; Yusuf, A.S. Phytochemical analysis and antimicrobial activity of baobab (Adansonia digitata) leaves and stem bark extracts on Staphylococcus aureus and Escherichia coli. J. Biosci. Biotechnol. 2017, 6, 9–16. [Google Scholar]
- Osuntokun, O.; AjayiAyodele, A.M. Assessment of Antimicrobial and Phytochemical Properties of Crude Leaf and Bark Extracts of Ceiba Pentandra on Selected Clinical Isolates. JBMOA 2017, 4, 17–23. [Google Scholar]
- Parulekar, G. Antibacterial and phytochemical analysis of Ceiba pentandra (L.) seed extracts. J. Pharmacogn. Phytochem. 2017, 6, 586–589. [Google Scholar]
- Kadam, S.D.; Kondawar, M. Evaluation of In-Vitro Anticancer Activity and Quantitation of Active Ingredient of Adansonia digitata L. Fruit. Int. J. Pharm. Sci. Drug Res. 2019, 11, 358–369. [Google Scholar]
- Kumar, R.; Kumar, N.; Ramalingayya, G.V.; Setty, M.M.; Pai, K.S. Evaluation of Ceiba pentandra (L.) Gaertner bark extracts for in vitro cytotoxicity on cancer cells and in vivo antitumor activity in solid and liquid tumor models. Cytotechnology 2016, 68, 1909–1923. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.A.; Mir, B.A.; Bisht, A.; Rao, Z.; Singh, D. Antidiabetic properties and metal analysis of Bombax ceiba flower extracts. Glob. J. Addict. Rehabil. Med. 2017, 1, 1–7. [Google Scholar] [CrossRef]
- De, D.; Ali, K.M.; Chatterjee, K.; Bera, T.K.; Ghosh, D. Antihyperglycemic and antihyperlipidemic effects of n-hexane fraction from the hydro-methanolic extract of sepals of Salmalia malabarica in streptozotocin-induced diabetic rats. J. Complement. Integr. Med. 2012, 9, 12. [Google Scholar] [CrossRef]
- Sharmin, R.; Joarder, H.; Alamgir, M.; Mostofa, G.; Islam, M. Antidiabetic and hepatoprotective activities of Bombax ceiba young roots in alloxan-induced diabetic mice. J. Nutr. Health Food Sci. 2018, 6, 1–7. [Google Scholar]
- Guang-Kai, X.; Xiao-Ying, Q.; Guo-Kai, W.; Guo-Yong, X.; Xu-Sen, L.; Chen-Yu, S.; Bao-Lin, L.; Min-Jian, Q. Antihyperglycemic, antihyperlipidemic and antioxidant effects of standard ethanol extract of Bombax ceiba leaves in high-fat-diet-and streptozotocin-induced Type 2 diabetic rats. Chin. J. Nat. Med. 2017, 15, 168–177. [Google Scholar]
- Bhavsar, C.; Talele, G.S. Potential anti-diabetic activity of Bombax ceiba. Bangladesh J. Pharmacol. 2013, 8, 102–106. [Google Scholar] [CrossRef]
- Fofié, C.K.; Nguelefack-Mbuyo, E.P.; Tsabang, N.; Kamanyi, A.; Nguelefack, T.B. Hypoglycemic properties of the aqueous extract from the stem bark of Ceiba pentandra in dexamethasone-induced insulin resistant rats. Evid. Based Complement. Altern. Med. 2018, 2018, 4234981. [Google Scholar] [CrossRef]
- Satyaprakash, R.; Rajesh, M.; Bhanumathy, M.; Harish, M.; Shivananda, T.; Shivaprasad, H.; Sushma, G. Hypoglycemic and antihyperglycemic effect of Ceiba pentandra L. gaertn in normal and streptozotocininduced diabetic rats. Ghana Med. J. 2013, 47, 121–127. [Google Scholar]
- Ayele, Y.; Kim, J.-A.; Park, E.; Kim, Y.-J.; Retta, N.; Dessie, G.; Rhee, S.-K.; Koh, K.; Nam, K.-W.; Kim, H.S. A methanol extract of Adansonia digitata L. leaves inhibits pro-inflammatory iNOS possibly via the inhibition of NF-κB activation. Biomol. Ther. 2013, 21, 146. [Google Scholar] [CrossRef]
- Selvarani, V. Multiple inflammatory and antiviral activities in Adansonia digitata (Baobab) leaves, fruits and seeds. J. Med. Plants Res. 2009, 3, 576–582. [Google Scholar]
- Anandarajagopal, K.; Sunilson, J.A.J.; Ajaykumar, T.; Ananth, R.; Kamal, S. In-vitro anti-inflammatory evaluation of crude Bombax ceiba extracts. Eur. J. Med. Plants 2013, 3, 99–104. [Google Scholar] [CrossRef]
- Abouelela, M.E.; Orabi, M.A.; Abdelhamid, R.A.; Abdelkader, M.S.; Madkor, H.R.; Darwish, F.M.; Hatano, T.; Elsadek, B.E. Ethyl acetate extract of Ceiba pentandra (L.) Gaertn. reduces methotrexate-induced renal damage in rats via antioxidant, anti-inflammatory, and antiapoptotic actions. J. Tradit. Complement. Med. 2019, 10, 478–486. [Google Scholar] [CrossRef]
- Basipogu, D.; Basha Syed, N.; Chitta, S.K. Cytotoxic effect of Adansonia digitata L. on breast cancer cell lines. Eur. J. Biomed. Pharm. Sci. 2018, 5, 956–959. [Google Scholar]
- Sa’id, A.; Musa, A.; Mashi, J.; Maigari, F.; Nuhu, M. Phytochemical Screening and Hepatoprotective Potential of Aqueous Fruit Pulp Extract of Adansonia digitata against CCL4 Induced Liver Damage in Rats. Asian J. Biochem. Genet. Mol. Biol. 2020, 12–21. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Mohamed, I.; El-Din, T.T.; Hassan, A.; Hassan, M. Hepatoprotective effect of Adansonia digitata L.(Baobab) fruits pulp extract on CCl4-induced hepatotoxicity in rats. World J. Pharm. Res. 2015, 4, 368–377. [Google Scholar]
- Al-Qarawi, A.; Al-Damegh, M.; El-Mougy, S. Hepatoprotective influence of Adansonia digitata pulp. J. Herbs Spices Med. Plants 2003, 10, 1–6. [Google Scholar] [CrossRef]
- Badr-Eldin, S.M.; Aldawsari, H.M.; Hanafy, A.; Abdlmoneim, M.S.; Abdel-Hady, S.E.-S.; Hasan, A. Enhanced Hepatoprotective Effect of Adansonia Digitata Extract on Paracetamol-Induced Hepatotoxicity. Res. J. Pharm. Biol. Chem. Sci. 2017, 8, 787–794. [Google Scholar]
- Lin, C.-c.; Chen, S.-y.; Lin, J.-m.; Chiu, H.-f. The Pharmacological and Pathological Studies on Taiwan Folk Medicine (Vlll): The Anti-inflammatory and Liver Protective Effects of Mu-mien. Am. J. Chin. Med. 1992, 20, 135–146. [Google Scholar] [CrossRef]
- Bairwa, N.K.; Sethiya, N.K.; Mishra, S. Protective effect of stem bark of Ceiba pentandra linn. against paracetamol-induced hepatotoxicity in rats. Pharmacogn. Res. 2010, 2, 26–30. [Google Scholar] [CrossRef]
- Vieira, T.O.; Said, A.; Aboutabl, E.; Azzam, M.; Creczynski-Pasa, T.B. Antioxidant activity of methanolic extract of Bombax ceiba. Redox Rep. 2009, 14, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Ch, R.K.; Madhavi, Y.; Raghava Rao, T. Evaluation of phytochemicals and antioxidant activities of Ceiba pentandra (kapok) seed oil. J. Bioanal. Biomed. 2012, 4, 068–073. [Google Scholar]
- Jalalpure, S.; Gadge, N. Diuretic effects of young fruit extracts of Bombax ceiba L. in rats. Indian J. Pharm. Sci. 2011, 73, 306. [Google Scholar] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Silva, N.C.C.; Fernandes Júnior, A. Biological properties of medicinal plants: A review of their antimicrobial activity. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 402–413. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Kanwal, S.; Ali, B.; Shah, S.A.; Khalil, A.T. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 2017, 7, 1129–1150. [Google Scholar] [CrossRef]
- Kumaran, A. Antioxidant and free radical scavenging activity of an aqueous extract of Coleus aromaticus. Food Chem. 2006, 97, 109–114. [Google Scholar] [CrossRef]
- Sarkar, D. The anti-inflammatory activity of plant derived ingredients: An analytical review. Int. J. Pharm. Sci. Res. 2020, 11, 496–506. [Google Scholar] [CrossRef]
- Das, S.K.; Samantaray, D.; Patra, J.K.; Samanta, L.; Thatoi, H. Antidiabetic potential of mangrove plants: A review. Front. Life Sci. 2016, 9, 75–88. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).