Dose–Effect Relationship of Water Salinity Levels on Osmotic Regulators, Nutrient Uptake, and Growth of Transplanting Vetiver [Vetiveria zizanioides (L.) Nash]
Abstract
1. Introduction
2. Results
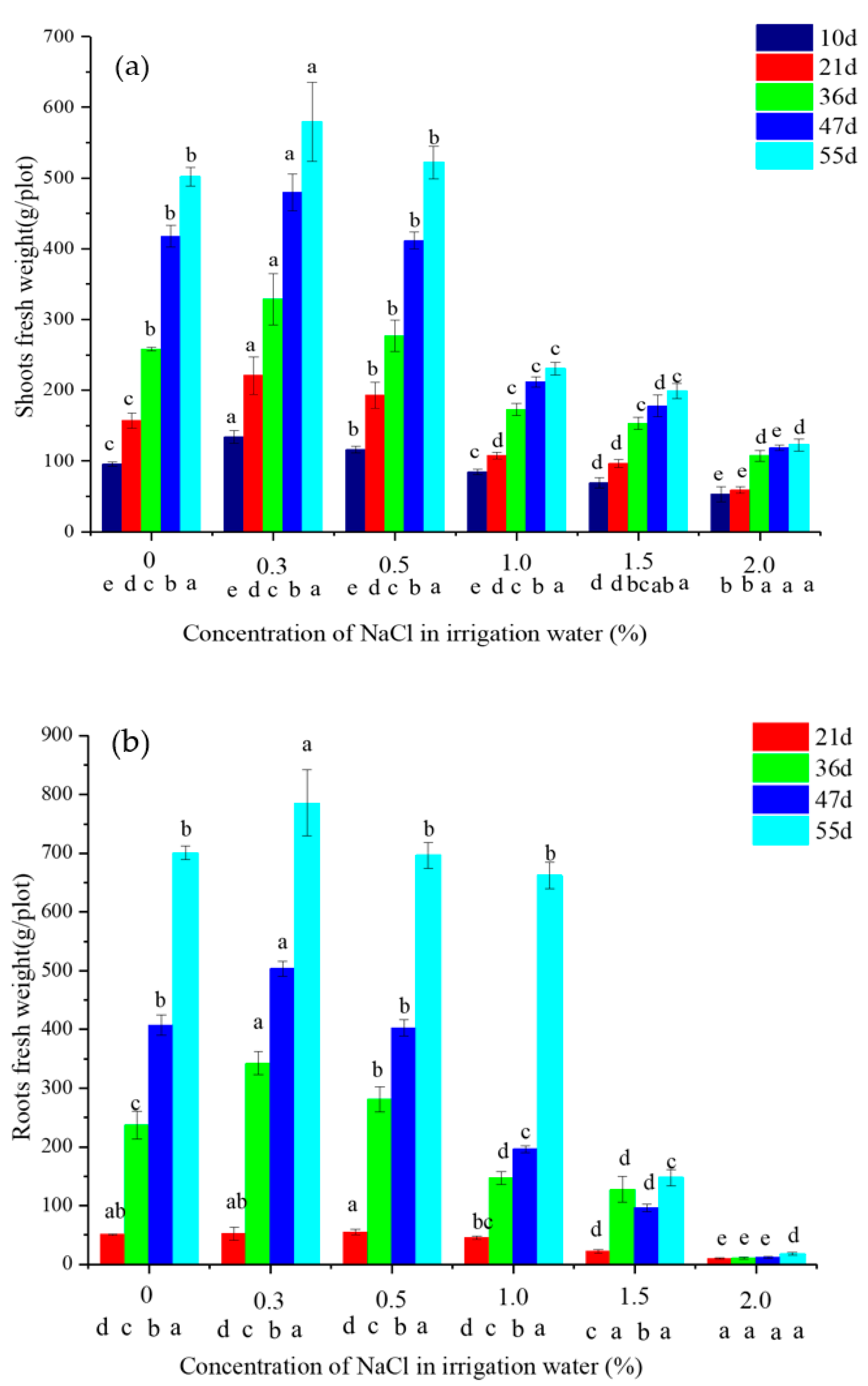
2.1. The Growth of Vetiver
2.2. The Content of Nutrients in Vetiver
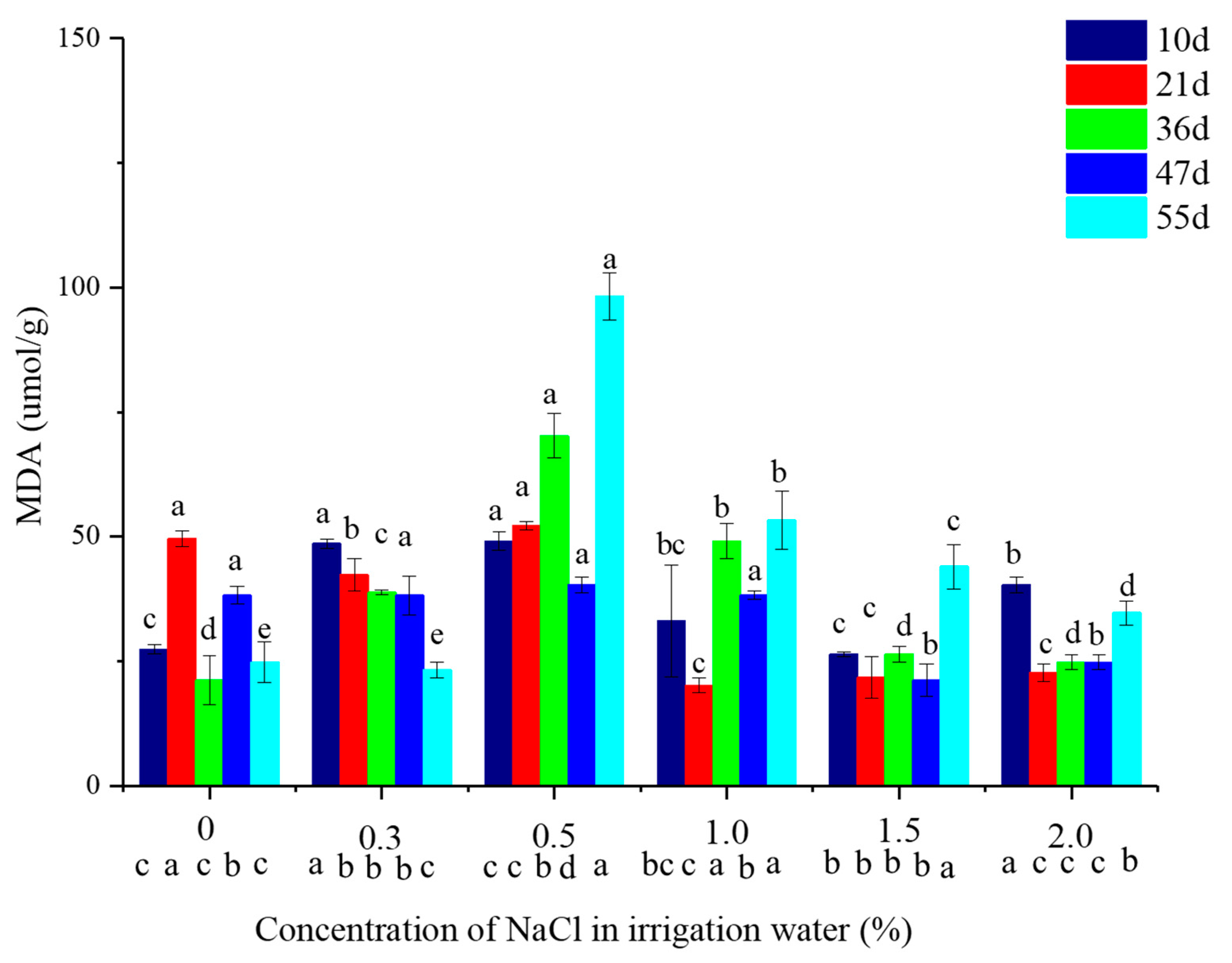
2.3. Malondialdehyde (MDA) Concentation
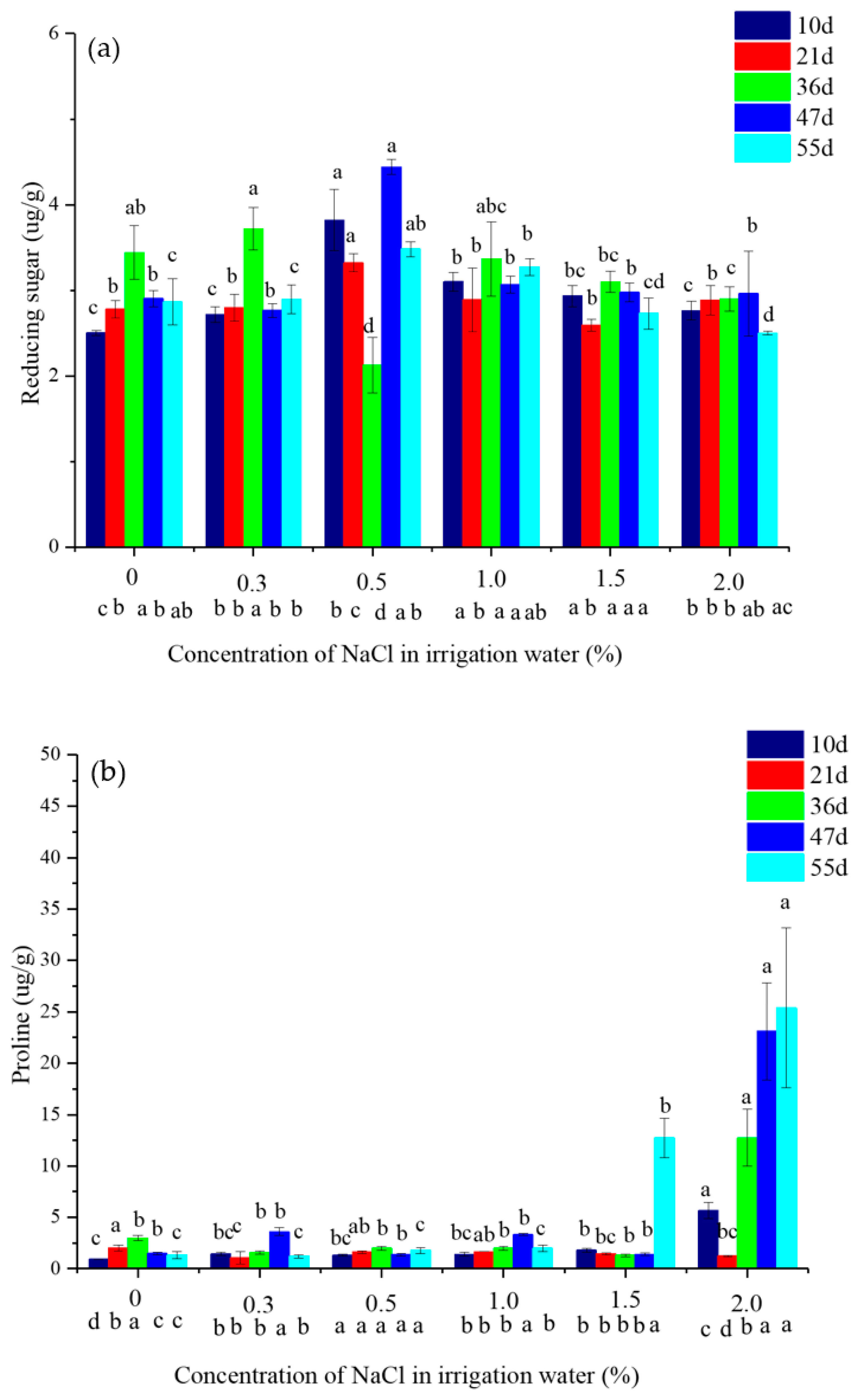
2.4. The Content of Reducing Sugar and Prolin in Vetivere
2.5. Ion Regulation in Vetiver
3. Discussion
3.1. The Effect of Water Salinity Levels on Vetiver
3.2. The Adaptability of Saline Soil to Transplanting and Introduction of Vetiver
4. Materials and Methods
4.1. The Experimental Design
4.2. Determination of Plant Samples
4.3. Data Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, J.; Kang, Y.; Wan, S.; Hu, W.; Jiang, S.; Zhang, T. Soil salinity management with drip irrigation and its effects on soil hydraulic properties in north China coastal saline soils. Agric. Water Manag. 2012, 115, 10–19. [Google Scholar] [CrossRef]
- Guangyi, L.; Guoxiong, G.; Zhongdong, Y. Summary on the Domestic and Foreign Dynamic Research of Protection Forest System Structure Benefit. Res. Soil Water Conserv. 1995, 2, 70–78. (In Chinese) [Google Scholar]
- Cui, B.; Yang, Q.; Yang, Z.; Zhang, K. Evaluating the ecological performance of wetland restoration in the Yellow River Delta, China. Ecol. Eng. 2009, 35, 1090–1103. [Google Scholar] [CrossRef]
- Jing, C.; Xu, Z.; Zou, P.; Tang, Q.; Li, Y.; You, X.; Zhang, C. Coastal halophytes alter properties and microbial community structure of the saline soils in the Yellow River Delta, China. Appl. Soil Ecol. 2019, 134, 1–7. [Google Scholar] [CrossRef]
- Qadir, M.; Oster, J.D. Crop and irrigation management strategies for saline-sodic soils and waters aimed at environmentally sustainable agriculture. Sci. Total Environ. 2004, 323, 1–19. [Google Scholar] [CrossRef]
- Bharti, P.; Singh, B.; Bauddh, K.; Dey, R.K.; Korstad, J. Efficiency of bioenergy plant in phytoremediation of saline and sodic soil. In Phytoremediation Potential of Bioenergy Plants; Bauddh, K., Singh, B., Korstad, J., Eds.; Springer: Singapore, 2017; pp. 353–369. [Google Scholar]
- Zheng, L.X.; Hong, S.J.; Zhai, L.K.; Hang, H.X. Effect of planting Suaeda salsa on Coastal Saline Soil. Adv. Mar. Sci. 2005, 1, 65–69. (In Chinese) [Google Scholar]
- Xia, L.X.; Tao, Z.; Li, T.X.; Ping, M.L.; Li, W.W. Profile analysis of soil ions and soil nutrients in Tamarix Suaeda community in Yellow River Delta Wetland. J. Ludong Univ. Nat. Sci. Ed. 2020, 3, 258–264. (In Chinese) [Google Scholar]
- Liu, W.; Liu, J.; Yao, M.; Ma, Q. Salt tolerance of a wild ecotype of vetiver grass (Vetiveria zizanioides L.) in southern China. Bot. Stud. 2016, 57, 1–8. [Google Scholar]
- Xia, H.; Wang, M.; Xu, L. Is vetiver grass planted in China an invasive alien species and becoming a weed? Chin. J. Ecol. 2015, 2015, 2327–2332. (In Chinese) [Google Scholar]
- Emilie, B.; Jean, J.F.; Hugues, B. Volatile constituents of Vetiver: A review. Flavour Fragr. 2005, 30, 26–82. [Google Scholar]
- Murtaza, G.; Azooz, M.M.; Murtaza, B.; Usman, Y.; Saqib, M. Nitrogen-Use-Efficiency (NUE) in Plants Under NaCl Stress. In Salt Stress in Plants: Signalling, Omics and Adaptations; Ahmad, P., Azooz, M.M., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2013; pp. 415–437. [Google Scholar]
- Ashraf, M. Some important physiological selection criteria for salt tolerance in plants. Flora-Morphol. Distrib. Funct. Ecol. Plants 2004, 199, 361–376. [Google Scholar] [CrossRef]
- Deng, S.; Qiu, Q. Study on the influence of soil salinity for the growth of the veliveria zizanioides introducted into Kuitun. Guangdong Agric. Sci. 2011, 6, 70–73. (In Chinese) [Google Scholar]
- Zhou, Q.; Yu, B.J. Accumulation of inorganic and organic osmolytes and their role in osmotic adjustment in NaCl-stressed vetiver grass seedlings. Russ. J. Plant Physiol. 2009, 56, 678–685. [Google Scholar] [CrossRef]
- Mane, A.V.; Wagh, V.B.; Karadge, B.A.; Samant, J.S.; Lah, M.K.B.C.; Nordin, M.N.B.; Isa, M.B.M.; Khanif, Y.M.; Jahan, M.S. Effect of varying contents of salinity on some biochemical parameters involved in nitrogen metabolism of four grass species. ScienceAsia 2011, 37, 285–290. [Google Scholar] [CrossRef]
- Rozema, J.; Flowers, T. Crops for a salinized world. Science 2008, 322, 1478–1480. [Google Scholar] [CrossRef] [PubMed]
- Greenway, H.; Munns, R. Mechanisms of salt tolerance in nonhalophytes. Annu. Rev. Plant Physiol. 1980, 31, 149–190. [Google Scholar] [CrossRef]
- Bidalia, A.; Vikram, K.; Yamal, G.; Rao, K.S. Effect of Salinity on Soil Nutrients and Plant Health. In Salt Stress, Microbes, and Plant Interactions: Causes and Solution; Akhtar, M.S., Ed.; Springer: Singapore, 2019; pp. 273–297. [Google Scholar]
- Miura, K. Nitrogen and Phosphorus Nutrition under Salinity Stress. In Ecophysiology and Responses of Plants under Salt Stress; Ahmad, P., Azooz, M.M., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2013; pp. 425–441. [Google Scholar]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef]
- SU, J. Study on antioxidative enzymes of vetiver to salt stress. Shandong For. Sci. Technol. 2018, 6, 46–47. (In Chinese) [Google Scholar]
- Ahanger, M.A.; Tyagi, S.R.; Wani, M.R.; Ahmad, P. Drought Tolerance: Role of Organic Osmolytes, Growth Regulators, and Mineral Nutrients. In Physiological Mechanisms and Adaptation Strategies in Plants under Changing Environment; Ahmad, P., Wani, M.R., Eds.; Springer: New York, NY, USA, 2014; pp. 25–55. [Google Scholar]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef]
- Chakdar, H.; Borse, D.N.; Verma, S.; Choudhary, P.; Das, S. Microbial management of crop salinity stress: Mechanisms, applications, and prospects. In Salt Stress, Microbes, and Plant Interactions: Mechanisms and Molecular Approaches; Akhtar, M., Ed.; Springer: Singapore, 2019; pp. 1–25. [Google Scholar]
- Kerepesi, I.; Galiba, G. Osmotic and salt stress-induced alteration in soluble carbohydrate content in wheat seedlings. Crop Sci. 2000, 40, 482–487. [Google Scholar] [CrossRef]
- Gzik, A. Accumulation of proline and pattern of α-amino acids in sugar beet plants in response to osmotic, water and salt stress. Environ. Exp. Bot. 1996, 36, 29–38. [Google Scholar] [CrossRef]
- Jain, S.; Nainawatee, H.S.; Jain, R.K.; Chowdhury, J.B. Proline status of genetically stable salt-tolerant Brassica juncea L. somaclones and their parent cv. Prakash. Plant Cell Rep. 1991, 9, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Petrusa, L.M.; Winicov, I. Proline status in salt-tolerant and salt-sensitive alfalfa cell lines and plants in response to NaCl. Plant Physiol. Biochem. 1997, 35, 303–310. [Google Scholar]
- Koca, H.; Bor, M.; Özdemir, F.; Türkan, İ. The effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environ. Exp. Bot. 2007, 60, 344–351. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.; Thomas, J.C.; Vernon, D.M.; Bohnert, H.J.; Jensen, R.G. Distinct cellular and organismic responses to salt stress. Plant Cell Physiol. 1992, 33, 1215–1223. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef]
- Wu, H.; Li, Z. The Importance of Cl− Exclusion and Vacuolar Cl− Sequestration: Revisiting the Role of Cl− Transport in Plant Salt Tolerance. Front. Plant Sci. 2019, 10, 1418. [Google Scholar] [CrossRef]
- Teakle, N.L.; Tyerman, S.D. Mechanisms of Cl- transport contributing to salt tolerance. Plant Cell Environ. 2010, 33, 566–589. [Google Scholar] [CrossRef]
- Guo, H.H.; Yang, L.P. Theoretical Basis and Practice of Ecological Control of Coastal Saline Soils; China Environmental Science Press: Beijing, China, 2007. (In Chinese) [Google Scholar]
- Van-Du, L.; Truong, P. Vetiver grass for sustainable agriculture on adverse soils and climate in South Vietnam. In Proceedings of the Fourth International Vetiver Conference, Caracas, Venezuela, 22–26 October 2006. [Google Scholar]
- Pongvichian, P.; Uaemkhli, P.; Phruekapong, A.; Phothipan, P. The role of salt on the growth and development of vetiver. Bhumivarin 2005, 19, 22–26. [Google Scholar]
- Cuong, D.C.; Van Minh, V.; Truong, P. Effects of sea water salinity on the growth of vetiver grass (Chrysopogon Zizanioides L.). Mod. Environ. Sci. Eng. 2015, 1, 185–191. [Google Scholar] [CrossRef]
- Truong, P.; Gordon, I.; Armstrong, F.; Shepherdson, J.; Ecotec, W.A.; Kalgoorlie, W.A. Vetiver grass for saline land rehabilitation under tropical and Mediterranean climate. In Proceedings of the Eighth National Conference Productive Use of Saline Lands, Fremantle, Australia, 16–20 September 2002. [Google Scholar]
- Ernster, L.; Nordenbrand, K. Microsomal lipid peroxidation. Methods Enzymol. 1967, 10, 574–580. [Google Scholar]
- Ábrahám, E.; Hourton-Cabassa, C.; Erdei, L.; Szabados, L. Methods for determination of proline in plants. In Plant Stress Tolerance: Methods and Protocols; Sunkar, R., Ed.; Humana Press: Herts, UK, 2010; pp. 317–331. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Faithfull, N.T. Methods in Agricultural Chemical Analysis: A Practical Handbook; Cabi: Aberystwyth, UK, 2002. [Google Scholar]
| 0 (CK) | 0.3 | 0.5 | 1 | 1.5 | 2 | ||
|---|---|---|---|---|---|---|---|
| Shoots | N | 3.7 ± 0.7b | 4.4 ± 0.7b | 4.2 ± 1.3ab | 2.6 ± 0.5b | 6.5 ± 1.2a | 6.0 ± 1.0a |
| P | 1.5 ± 0.2a | 1.5 ± 1.0a | 1.5 ± 0.1a | 1.5 ± 0.3a | 1.8 ± 0.2a | 1.5 ± 0.5a | |
| K | 10.8 ± 1.9b | 14.5 ± 0.8a | 11.0 ± 1.1bc | 12.7 ± 0.5b | 10.5 ± 0.6b | 11.0 ± 0.9b | |
| Na | 1.65 ± 0.26d | 1.05 ± 0.06d | 0.87 ± 0.02e | 2.67 ± 0.29c | 5.41 ± 0.18a | 4.50 ± 0.25b | |
| Cl | 3.34 ± 0.92c | 4.83 ± 0.43b | 3.67 ± 0.049c | 7.34 ± 1.83ab | 5.70 ± 1.20b | 8.57 ± 1.41a | |
| Roots | N | 17.5 ± 1.1c | 21.8 ± 1.0b | 28.5 ± 0.8a | 12.2 ± 1.8e | 13.9 ± 1.5d | 23.6 ± 0.8b |
| P | 0.88 ± 0.17a | 0.87 ± 0.10a | 0.89 ± 0.17a | 0.90 ± 0.26a | 0.64 ± 0.26a | 0.89 ± 0.35a | |
| K | 2.95 ± 0.16c | 5.96 ± 0.15b | 5.84 ± 0.18b | 6.71 ± 0.15a | 5.60 ± 0.20b | 5.65 ± 0.37b | |
| Na | 1.82 ± 0.15e | 5.03 ± 0.59d | 4.85 ± 0.29d | 10.56 ± 1.27c | 18.91 ± 1.01a | 17.40 ± 0.66b | |
| Cl | 3.95 ± 0.15c | 9.81 ± 1.30a | 6.96 ± 1.45b | 3.69 ± 0.65c | 3.78 ± 0.22c | 5.35 ± 0.64b |
| 0 (CK) | 0.3 | 0.5 | 1 | 1.5 | 2 | ||
|---|---|---|---|---|---|---|---|
| K | 3.66 | 2.43 | 1.88 | 1.89 | 1.88 | 1.95 | |
| TCs | Na | 0.91 | 0.21 | 0.18 | 0.25 | 0.29 | 0.26 |
| Cl | 0.85 | 0.49 | 0.53 | 1.99 | 1.51 | 1.6 | |
| Rshoot | K/Na | 6.54 | 13.81 | 12.64 | 4.76 | 1.94 | 2.44 |
| Rroot | K/Na | 1.62 | 1.19 | 1.2 | 0.64 | 0.3 | 0.33 |
| SK/Na | K/Na | 4.04 | 11.61 | 10.53 | 7.44 | 6.47 | 7.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, J.; Qiu, Y.; Yang, X.; Li, S.; Hu, Z. Dose–Effect Relationship of Water Salinity Levels on Osmotic Regulators, Nutrient Uptake, and Growth of Transplanting Vetiver [Vetiveria zizanioides (L.) Nash]. Plants 2021, 10, 562. https://doi.org/10.3390/plants10030562
Su J, Qiu Y, Yang X, Li S, Hu Z. Dose–Effect Relationship of Water Salinity Levels on Osmotic Regulators, Nutrient Uptake, and Growth of Transplanting Vetiver [Vetiveria zizanioides (L.) Nash]. Plants. 2021; 10(3):562. https://doi.org/10.3390/plants10030562
Chicago/Turabian StyleSu, Jing, Yanhua Qiu, Xiaosong Yang, Songyan Li, and Zhengyi Hu. 2021. "Dose–Effect Relationship of Water Salinity Levels on Osmotic Regulators, Nutrient Uptake, and Growth of Transplanting Vetiver [Vetiveria zizanioides (L.) Nash]" Plants 10, no. 3: 562. https://doi.org/10.3390/plants10030562
APA StyleSu, J., Qiu, Y., Yang, X., Li, S., & Hu, Z. (2021). Dose–Effect Relationship of Water Salinity Levels on Osmotic Regulators, Nutrient Uptake, and Growth of Transplanting Vetiver [Vetiveria zizanioides (L.) Nash]. Plants, 10(3), 562. https://doi.org/10.3390/plants10030562







