Comparative Study of Drought Stress Effects on Traditional and Modern Apple Cultivars
Abstract
1. Introduction
2. Results
2.1. Effect of Drought Stress on Chlorophyll Fluorescence Parameters
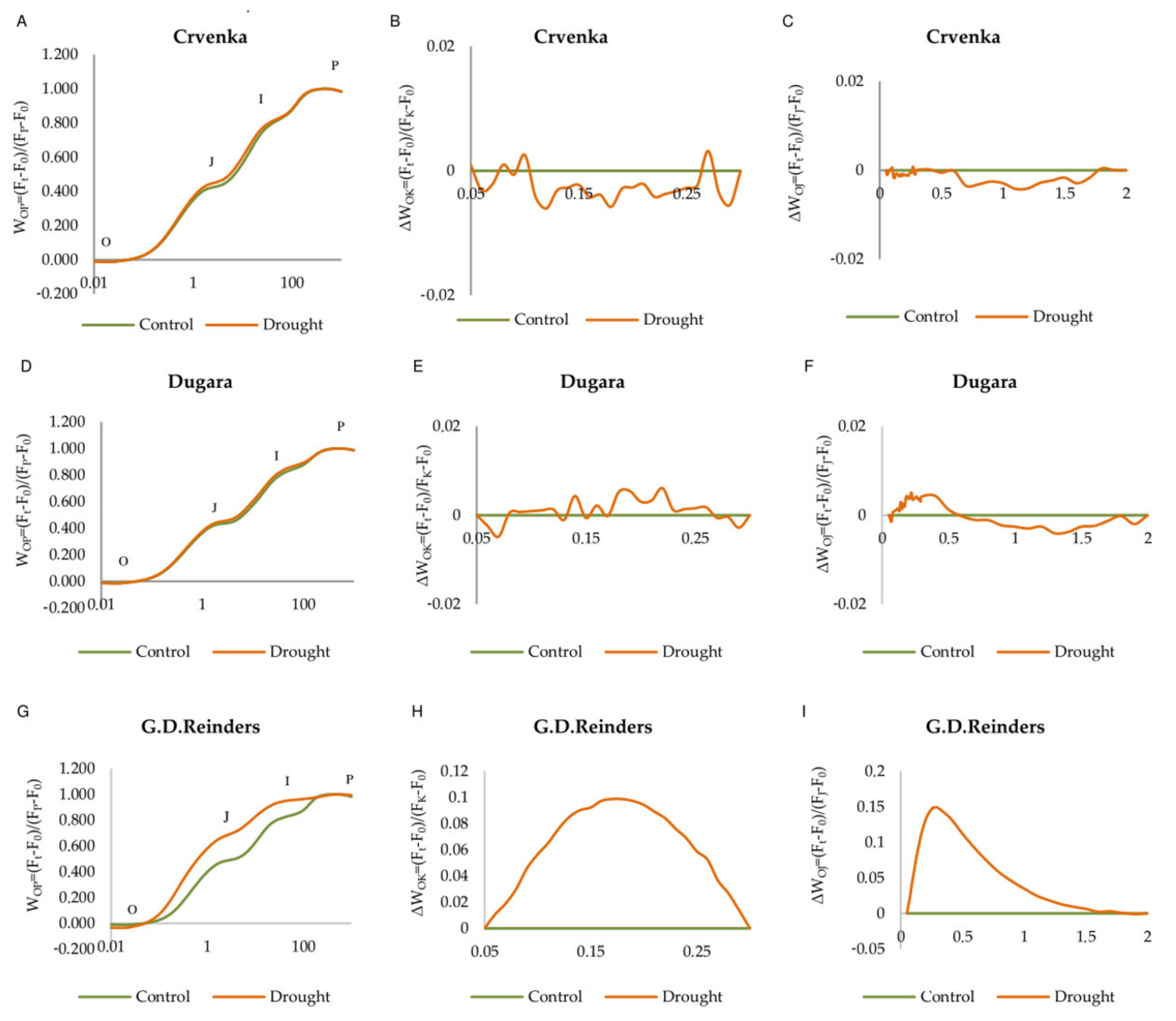
2.2. OJIP Curve
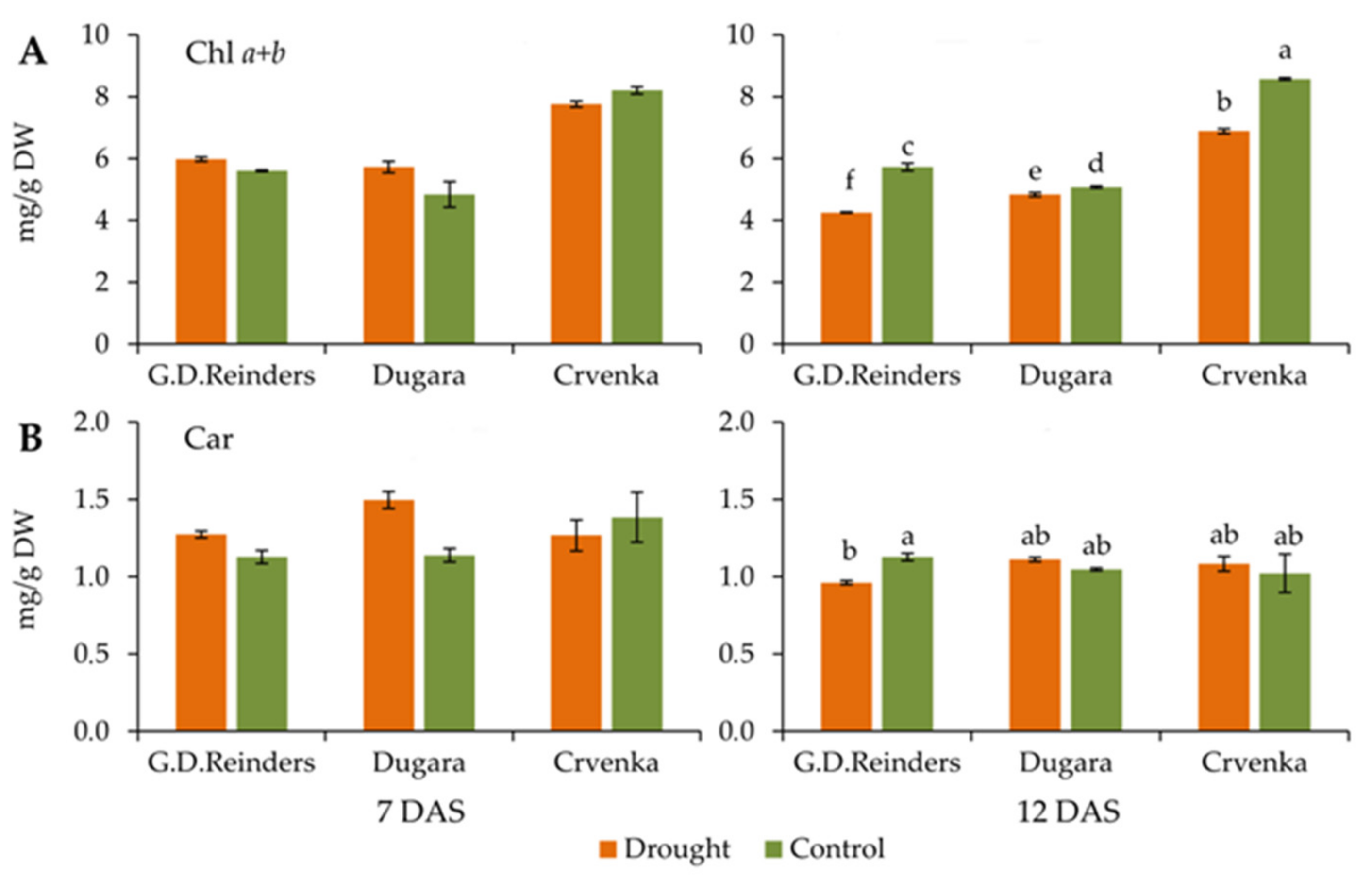
2.3. Chlorophyll and Carotenoid Content
2.4. MDA and H2O2 Content
2.5. Proline and Phenols Content
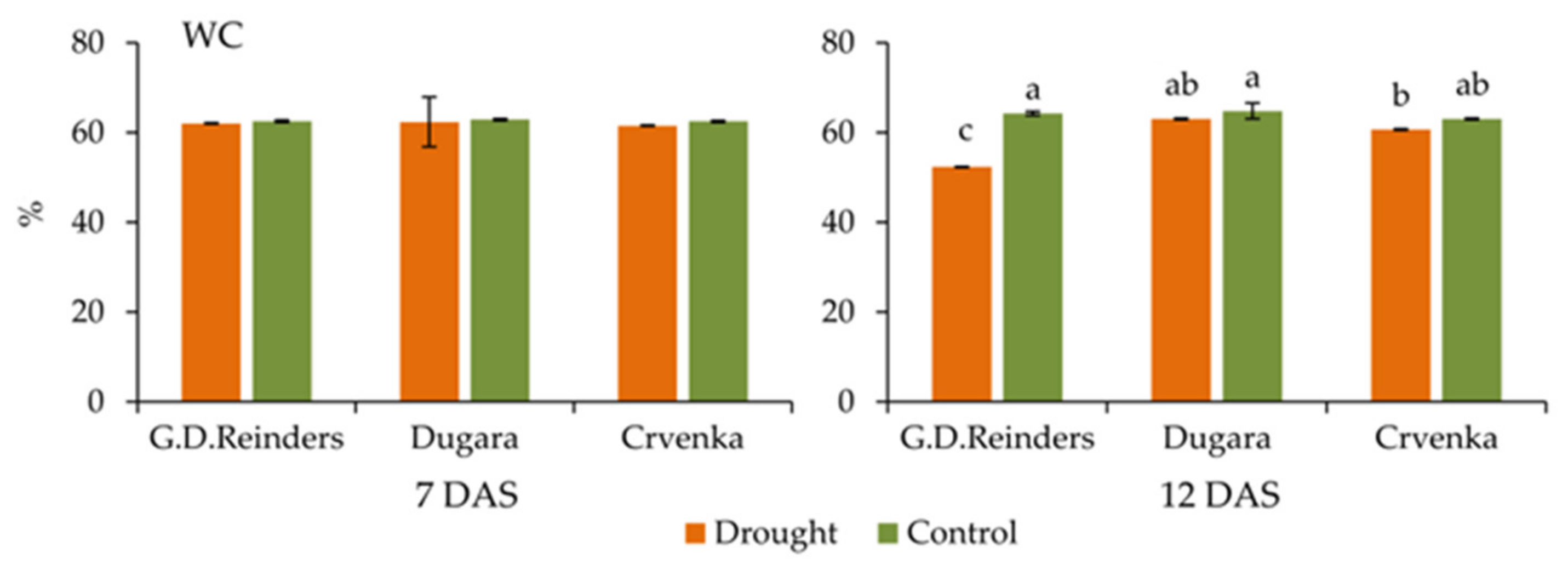
2.6. Water Content in the Leaves
3. Discussion
4. Materials and Methods
4.1. Apple Cultivars, Growth Conditions and Experimental Setup
4.2. Measurement of Chlorophyll a Fluorescence
4.3. Analysis of Total Chlorophyll Content and Carotenoids
4.4. Extraction and Determination of Lipid Peroxidation and Hydrogen Peroxide
4.5. Extraction and Determination of Total Phenolic and Proline Content
4.6. Determination of Water Content
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berger, J.; Palta, J.; Vadez, V. Review: An integrated framework for crop adaptation to dry environments: Responses to transient and terminal drought. Plant Sci. 2016, 252, 58–67. [Google Scholar] [CrossRef]
- Pérez-Pérez, J.G.; Romero, P.; Navarro, J.M.; Botía, P. Response of sweet orange cv “Lane late” to deficit irrigation in two rootstocks. I: Water relations, leaf gas exchange and vegetative growth. Irrig. Sci. 2008, 26, 415–425. [Google Scholar] [CrossRef]
- Zu, X.; Lu, Y.; Wang, Q.; Chu, P.; Miao, W.; Wang, H.; La, H. A new method for evaluating the drought tolerance of upland rice cultivars. Crop. J. 2017, 5, 488–498. [Google Scholar] [CrossRef]
- Massonnet, C.; Costes, E.; Rambal, S.; Dreyer, E.; Regnard, J.L. Stomatal regulation of photosynthesis in apple leaves: Evidence for different water-use strategies between two cultivars. Ann. Bot. 2007, 100, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Yin, C.; Li, C. Differences in some morphological, physiological, and biochemical responses to drought stress in two contrasting populations of Populus przewalskii. Physiol. Plant. 2006, 127, 182–191. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. The roles of reactive oxygen metabolism in drought: Not so cut and dried. Plant Physiol. 2014, 164, 1636–1648. [Google Scholar] [CrossRef] [PubMed]
- Scandalios, J.G. Oxygen stress and superoxide dismutases. Plant Physiol. 1993, 101, 7–12. [Google Scholar] [CrossRef]
- Smirnoff, N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993, 125, 27–58. [Google Scholar] [CrossRef]
- Viljevac, M.; Dugalic, K.; Mihaljević, I.; Šimić, D.; Sudar, R.; Jurković, Z.; Lepeduš, H. Chlorophylls content and photosynthetic efficiency in two sour cherry Prunus cerasus (L.) genotypes under drought stress. Acta Bot. Croat. 2013, 72, 221–235. [Google Scholar] [CrossRef]
- Bhusal, N.; Han, S.G.; Yoon, T.M. Impact of drought stress on photosynthetic response, leaf water potential, and stem sap flow in two cultivars of bi-leader apple trees (Malus × domestica Borkh.). Sci. Hortic. 2019, 246, 535–543. [Google Scholar] [CrossRef]
- Chaves, M.M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.; Ricardo, C.P.P.; Osório, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How plants cope with water stress in the field: Photosynthesis and growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef]
- Asensi-Fabado, M.A.; Munné-Bosch, S. Vitamins in plants: Occurrence, biosynthesis and antioxidant function. Trends Plant Sci. 2010, 15, 582–592. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Chapter 1, Drought Stress in Plants: An Overview. In Plant Responses to Drought Stress; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–33. [Google Scholar]
- Faize, M.; Burgos, L.; Faize, L.; Piqueras, A.; Nicolas, E.; Barba-Espin, G.; Clemente-Moreno, M.J.; Alcobendas, R.; Artlip, T.; Hernandez, J.A. Involvement of cytosolic ascorbate peroxidase and Cu/Zn-superoxide dismutase for improved tolerance against drought stress. J. Exp. Bot. 2011, 62, 2599–2613. [Google Scholar] [CrossRef]
- Shehab, G.G.; Ahmed, O.K.; El-Beltagi, H.S. Effects of various chemical agents for alleviation of drought stress in rice plants Oryza sativa (L.). Not. Bot. Horti Agrobot. 2010, 38, 139–148. [Google Scholar]
- Xu, Z.; Sun, M.; Jiang, X.; Sun, H.; Dang, X.; Cong, H.; Qiao, F. Glycinebetaine biosynthesis in response to osmotic stress depends on jasmonate signaling in watermelon suspension cells. Front. Plant Sci. 2018, 9, 1469. [Google Scholar] [CrossRef] [PubMed]
- Dien, D.C.; Mochizuki, T.; Yamakawa, T. Effect of various drought stresses and subsequent recovery on proline, total soluble sugar and starch metabolisms in Rice Oryza sativa (L.) varieties. Plant Prod. Sci. 2019, 22, 530–545. [Google Scholar] [CrossRef]
- Mahdavi, A.; Moradi, P.; Mastinu, A. Variation in Terpene Profiles of Thymus vulgaris in Water Deficit Stress Response. Molecules 2020, 25, 1091. [Google Scholar] [CrossRef] [PubMed]
- Naservafaei, S.; Sohrabi, Y.; Moradi, P.; Mac Sweeney, E.; Mastinu, A. Biological Response of Lallemantia iberica to Brassinolide Treatment under Different Watering Conditions. Plants 2021, 10, 496. [Google Scholar] [CrossRef]
- Harris, S.A.; Robinson, J.P.; Juniper, B.E. Genetic clues to the origin of the apple. Trends Genet. 2002, 8, 426–430. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Lexer, M.J. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Skendrović Babojelić, M.; Korent, P.; Šindrak, Z.; Jemrić, T. Pomološka svojstva i kakvoća ploda tradicionalnih sorata jabuka. Glas. Zaštite Bilja 2014, 37, 20–27. [Google Scholar]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Torello Marinoni, D.; Cerutti, A.K.; Canterino, S.; Bounous, G. Application of sensory, nutraceutical and genetic techniques to create a quality profile of ancient apple cultivars. J. Food Qual. 2012, 35, 169–181. [Google Scholar] [CrossRef]
- Feliciano, R.P.; Antunes, C.; Ramos, A.; Serra, A.T.; Figueira, M.E.; Duarte, C.M.M.; de Carvalho, A.; Bronze, M.R. Characterization of traditional and exotic apple varieties from Portugal. Part 1—Nutritional, phytochemical and sensory evaluation. J. Funct. Foods. 2010, 2, 35–45. [Google Scholar] [CrossRef]
- Cvetković, M.; Tomić, L.; Botu, M.; Gjamovski, V.; Jemrić, T.; Lazović, B.; Ognjanov, V.; Pintea, M.; Sevo, R.; Achim, G.; et al. SEEDNet’s WG for Fruit and Vitis. In Balkan Pomology, Apples; Exaktaprinting AB: Malmo, Sweden, 2012; pp. 12–13. [Google Scholar]
- Çiçek, N.; Erdal, Ş.Ç.; Arslan, Ö.; Nalçaiyi, S.B.; Çil, A.N.; Şahin, V.; Kaya, Y.; Ekmekçi, Y. Assessing drought tolerance in field-grown sunflower hybrids by chlorophyll fluorescence kinetics. Rev. Bras. Bot. 2019, 42, 249–260. [Google Scholar] [CrossRef]
- Wang, Z.; Li, G.; Sun, H.; Ma, L.; Guo, Y.; Zhao, Z.; Gao, H.; Mei, L. Effects of drought stress on photosynthesis and photosynthetic electron transport chain in young apple tree leaves. Biol. Open 2018, 7, bio035279. [Google Scholar] [CrossRef] [PubMed]
- Roostaei, M.; Mohammadi, S.A.; Amri, A.; Majidi, E.; Nachit, M.; Haghparast, R. Chlorophyll fluorescence parameters and drought tolerance in a mapping population of winter bread wheat in the highlands of Iran. Russ. J. Plant Physiol. 2011, 58, 351–358. [Google Scholar] [CrossRef]
- Milinović, B.; Vujević, P.; Halapija Kazija, D.; Jelačić, T.; Čiček, D.; Biško, A. Productivity and fruit quality of traditional apple cultivars within intensive production systems. Pomol. Croat. 2017, 21, 3–4. [Google Scholar]
- Barać, G.; Milić, B.; Magazin, N.; Ognjanov, V.; Keserović, Z.; Ivanišević, D.; Kalajdžić, M.; Vuković, D.; Mihaljević, I.; Tomaš, V.; et al. Varieties and Selections of Fruit Trees from Eastern Croatia and Northwestern Serbia; Grafika: Osijek, Croatia, 2020. [Google Scholar]
- Gasi, F.; Simon, S.; Pojskic, N.; Kurtovic, M.; Pejic, I.; Mekjell, M.; Kaiser, C. Evaluation of apple (Malus x domestica Borkh) genetic resources in Bosnia and Herzegovina using microsatellite markers. HortScience 2013, 48, 13–21. [Google Scholar] [CrossRef]
- Haldiman, P.; Strasser, R.J. Effects of anaerobiosis as probed by the polyphasic chlorophyll a fluorescence rise kinetic in pea Pisum sativum (L.). Photosynth. Res. 1999, 62, 67–83. [Google Scholar] [CrossRef]
- Yan, K.; Shao, H.; Shao, C.; Zhao, S.; Brestic, M. Dissection of photosynthetic electron transport process in sweet sorghum under heat stress. PLoS ONE 2013, 8, e62100. [Google Scholar] [CrossRef]
- Strasser, R.J.; Stirbet, A.D. Heterogeneity of photosystem II probed by the numerically simulated chlorophyll a fluorescence rise (O-J-I-P). Math Comput. Simul. 1998, 48, 3–9. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration; Papageorgiou, G.G.C., Ed.; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 321–362. [Google Scholar]
- Redillas, M.C.F.R.; Strasser, R.J.; Jin, S.J.; Kim, Y.S.; Kim, J.K. The use of JIP test to evaluate drought-tolerance of transgenic rice overexpressing OsNAC10. Plant Biotechnol. Rep. 2011, 5, 169–175. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samoborska, I.A.; Cetner, M.D.; Lukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Gomes, M.T.G.; da Luz, A.C.; Rossi dos Santos, M.; Do Carmo Pimentel Batitucci, M.; Moura Silva, D.; Falqueto, A.R. Drought tolerance of passion fruit plants assessed by the OJIP chlorophyll a fluorescence transient. Sci. Hortic. 2012, 142, 49–56. [Google Scholar] [CrossRef]
- Arslan, Ö.; Balkan Nalçaiyi, A.S.; Çulha Erdal, Ş.; Pekcan, V.; Kaya, Y.; Çiçek, N.; Ekmekçi, Y. Analysis of drought response of sunflower inbred lines by chlorophyll a fluorescence induction kinetics. Photosynthetica 2020, 58, 348–357. [Google Scholar] [CrossRef]
- Yusuf, M.A.; Kumar, D.; Rajwanshi, R.; Strasser, R.J.; Tsimilli-Michael, M.; Govindjee; Sarin, N.B. Overexpression of gamma-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: Physiological and chlorophyll a fluorescence measurements. Biochim. Biophys. Acta 2010, 1979, 1428–1438. [Google Scholar] [CrossRef]
- Wang, Z.X.; Chen, L.; Ai, J.; Qin, H.Y.; Liu, Y.X.; Xu, P.L.; Jiao, Z.Q.; Zhao, Y.; Zhang, Q.T. Photosynthesis and activity of photosystem II in response to drought stress in Amur Grape (Vitis amurensis Rupr.). Photosynthetica 2012, 50, 189–196. [Google Scholar] [CrossRef]
- Boguszewska-Mańkowska, D.; Pieczyński, M.; Wyrzykowska, A.; Kalaji, H.M.; Sieczko, L.; Szweykowska-Kulińska, Z.; Zagdańska, B. Divergent strategies displayed by potato Solanum tuberosum (L.) cultivars to cope with soil drought. J. Agron. Crop. Sci. 2018, 204, 13–30. [Google Scholar] [CrossRef]
- Schansker, G.; Toth, S.Z.; Strasser, R.J. Methylviologen and dibromothymo-quinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP. Biochim. Biophys. Acta 2005, 1706, 250–261. [Google Scholar] [CrossRef]
- Jia, Y.; Xiao, W.; Ye Xiaolin, Y.; Xiaoli, W.; Guohong, L.; Gang Li, W.; Wang, Y. Response of photosynthetic performance to drought duration and re-watering in maize. Agronomy 2020, 10, 533. [Google Scholar] [CrossRef]
- Krause, G.H.; Weis, E. Chlorophyll fluorescence and photosynthesis: The basics. Plant Mol. Biol. 1991, 42, 313–349. [Google Scholar] [CrossRef]
- Faraloni, C.; Cutino, I.; Petruccelli, R.; Leva, A.R.; Lazzeri, S.; Torzillo, G. Chlorophyll fluorescence technique as a rapid tool for in vitro screening of olive cultivars Olea europaea (L.) tolerant to drought stress. Environ. Exp. Bot. 2011, 73, 49–56. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, J. Effect of water stress on photosystem II photochemistry and its thermostability in wheat plants. Exp. Bot. 1999, 336, 1199–1206. [Google Scholar] [CrossRef]
- Arunyanark, A.; Jogloy, S.; Akkasaeng, C.; Vorasot, N.; Kesmala, T.; Nageswara Rao, R.C.; Wright, G.C.; Patanothai, A. Chlorophyll stability is an indicator of drought tolerance in peanut. J. Agron. Crop. Sci. 2008, 194, 113–125. [Google Scholar] [CrossRef]
- Alizadeh, A.; Alizade, V.; Nassery, L.; Eivazi, A. Effect of drought stress on apple dwarf rootstocks. TJEAS 2011, 1, 86–94. [Google Scholar]
- Bolat, I.; Dikilitas, M.; Ercisli, S.; Ikinci, A.; Tonkaz, T. The effect of water stress on some morphological, physiological, and biochemical characteristics and bud success on apple and quince rootstocks. Sci. World J. 2014, 2014, 769732. [Google Scholar] [CrossRef] [PubMed]
- Cogdell, R.J.; Gardiner, A.T. Functions of carotenoids in photosynthesis. Methods Enzymol. 1993, 214, 185–193. [Google Scholar]
- Khoyerdi, F.; Shamshiri, M.H.; Estaji, A. Changes in some physiological and osmotic parameters of several pistachio genotypes under drought stress. Sci. Hortic. 2016, 198, 44–51. [Google Scholar] [CrossRef]
- Jahns, P.; Holzwarth, A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta 2012, 1817, 182–193. [Google Scholar] [CrossRef]
- Benhassaine-Kesri, G.; Aid, F.; Demandre, C.; Kader, J.C.; Mazliak, P. Drought stress affects chloroplast lipid metabolism in rape (Brassica napus) leaves. Physiol. Plant. 2002, 115, 221–227. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Lee, D.J.; Cheema, S.A.; Aziz, T. Comparative time course action of the foliar applied glycinebetaine, salicylic acid, nitrous oxide, brassinosteroids and spermine in improving drought resistance of rice. J. Agron. Crop. Sci. 2010, 196, 336–345. [Google Scholar] [CrossRef]
- Kocsy, G.; Laurie, R.; Szalai, G.; Szilagyi, V.; Simon-Sarkadi, L.; Galiba, G.; de Ronde, J.A. Genetic manipulation of proline levels affects antioxidants in soybean subjected to simultaneous drought and heat stress. Physiol. Plant. 2005, 124, 227–235. [Google Scholar] [CrossRef]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Petridis, A.; Therios, I.; Samouris, G.; Koundouras, S.; Giannakoula, A. Effect of water deficit on leaf phenolic composition, gas exchange, oxidative damage and antioxidant activity of four Greek olive Olea europaea (L.) cultivars. Plant Physiol. Biochem. 2012, 60, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Miao, L.F. Adaptive responses to progressive drought stress in two poplar species originating from different altitudes. Silva Fenn. 2010, 44, 23–37. [Google Scholar] [CrossRef]
- Umar, M.; Shaheed Siddiqui, Z. Physiological performance of sunflower genotypes under combined salt and drought stress. Acta Bot. Croat. 2018, 77, 36–44. [Google Scholar] [CrossRef]
- Bandurska, H.; Jόźwiak, W. A comparison of the effects of drought on proline accumulation and peroxidases activity in leaves of Festuca rubra (L.) and Lolium perenne (L.). Acta Soc. Bot. Pol. 2010, 79, 111–116. [Google Scholar] [CrossRef]
- Tounekti, T.; Mahdhi, M.; Al-Turki, T.; Khemira, H. Water relations and photo-protection mechanisms during drought stress in four coffee (Coffea arabica) cultivars from southwestern Saudi Arabi. S. Afr. J. Bot. 2018, 117, 17–25. [Google Scholar] [CrossRef]
- Sumera, I.; Asghari, B. Effect of drought and abscisic acidapplication on the osmotic adjustment of four wheat cultivars. J. Chem. Soc. Pak. 2010, 32, 13–19. [Google Scholar]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Ashraf, U.; Hussain, S.; Shahzad, B.; Khan, I.; Wang, L. Effect of progressive drought stress on growth, leaf gas exchange, and antioxidant production in two maize cultivars. Environ. Sci. Pollut. Res. 2016, 23, 17132–17141. [Google Scholar] [CrossRef]
- Man, D.; Bao, Y.X.; Han, L.B. Drought tolerance associated with proline and hormone metabolism in two tall fescue cultivars. HortScience 2011, 1, 1027. [Google Scholar] [CrossRef]
- Rampino, P.; Patale, S.; Gerardi, C.; Mita, G.; Perrotta, C. Drought stress responses in wheat: Physiological and molecular analysis of resistant and sensitive genotypes. Plant Cell Environ. 2006, 29, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress. Ann. Bot. 2003, 9, 179–194. [Google Scholar] [CrossRef]
- Quan, N.T.; Anh, L.H.; Khang, D.T.; Tuyen, P.T.; Toan, N.P.; Minh, T.N.; Bach, D.T.; Ha, P.T.T.; Elzaawely, A.A.; Khanh, T.D.; et al. Involvement of secondary metabolites in response to drought stress of rice Oryza sativa (L.). Agriculture 2016, 6, 23. [Google Scholar] [CrossRef]
- Hura, T.; Hura, K.; Grzesiak, S. Physiological and biochemical parameters for identifications of QTLs controlling the winter triticale drought tolerance at the seedling stage. Plant Physiol. Biochem. 2009, 47, 210–214. [Google Scholar] [CrossRef]
- Puente-Garza, C.A.; Cristina Meza, M.; Desiree Ochoa, M.; Silverio García, L. Effect of in vitro drought stress on phenolic acids, flavonols, saponins, and antioxidant activity in Agave salmiana. Plant Physiol. Biochem. 2017, 115, 400–407. [Google Scholar] [CrossRef]
- Jakobek, L.; Ištuk, J.; Buljeta, I.; Voća, S.; Šic Žlabur, J.; Skendrović Babojelić, M. Traditional, indigenous apple varieties, a fruit with potential for beneficial effects: Their quality traits and bioactive polyphenol contents. Foods 2020, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanism, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor and Francis: London, UK, 2000; pp. 443–480. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Verma, S.; Dubey, R.S. Leads toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2003, 164, 645–655. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Woodrow, P.; Ciarmiello, L.F.; Annunziata, M.G.; Pacifico, S.; Iannuzzi, F.; Mirto, A.; D’Amelia, L.; Dell’Aversana, E.; Piccolella, S.; Fuggi, A.; et al. Durum wheat seedling responses to simultaneous high light and salinity involve a fine reconfiguration of amino acids and carbohydrate metabolism. Physiol. Plant. 2017, 159, 290–312. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Kumar, D.; Al Hassan, M.; Naranjo, M.A.; Agrawal, V.; Boscaiu, M.; Vicente, O. Effects of salinity and drought on growth, ionic relations, compatible solutes and activation of antioxidant systems in oleander Nerium oleander (L.). PLoS ONE 2017, 12, e0185017. [Google Scholar] [CrossRef] [PubMed]
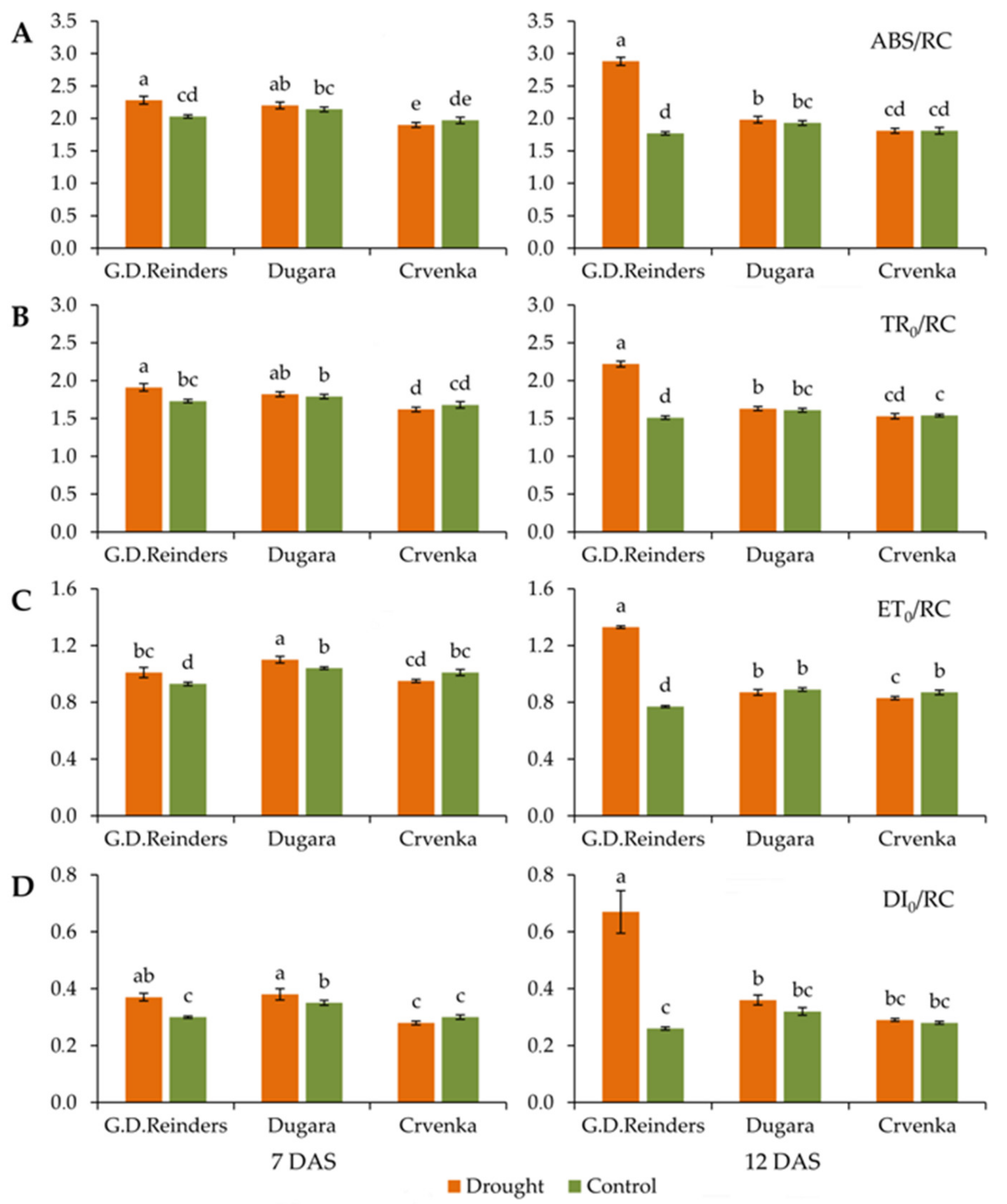
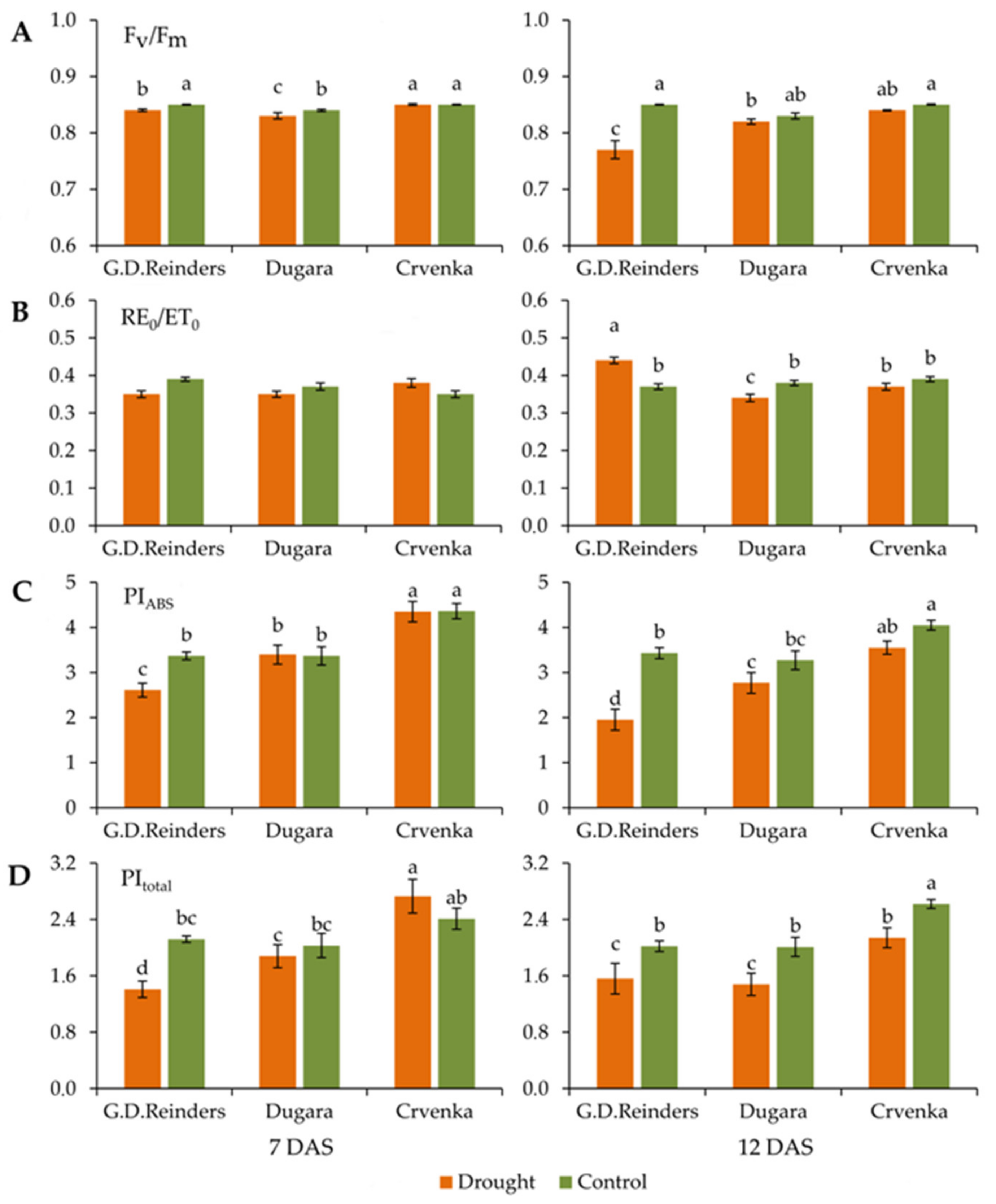

| TR0/ABS: Maximum quantum yield of PSII; TR0/ABS = [1 − (F0/Fm)] |
| ABS/RC: Absorption per active RC; ABS/RC = M0 (1/VJ) [1/(Fv/Fm)] |
| TR0/RC: Trapping per active RC; TR0/RC = M0 (1/VJ) |
| ET0/RC: Electron transport per active RC; ET0/RC = M0 (1/VJ) (1 − VJ) |
| DI0/RC: Dissipation per active RC; DI0/RC = (ABS/RC) − (TR0/RC) |
| RE0/ET0: The efficiency with which an electron can move from the reduced intersystem electron acceptors to the PS I end electron acceptors; (1 − VI)/(1 − VJ) |
| PIABS: Performance index; PI = (RC/ABS) (TR0/DI0) [ET0/(TR0 − ET0)] |
| PItotal: Performance index for energy conservation from exciton to the reduction of PSI end acceptors; |
| PItotal = PIABS × [(RE0/ET0)/(1 − RE0/ET0)] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihaljević, I.; Viljevac Vuletić, M.; Šimić, D.; Tomaš, V.; Horvat, D.; Josipović, M.; Zdunić, Z.; Dugalić, K.; Vuković, D. Comparative Study of Drought Stress Effects on Traditional and Modern Apple Cultivars. Plants 2021, 10, 561. https://doi.org/10.3390/plants10030561
Mihaljević I, Viljevac Vuletić M, Šimić D, Tomaš V, Horvat D, Josipović M, Zdunić Z, Dugalić K, Vuković D. Comparative Study of Drought Stress Effects on Traditional and Modern Apple Cultivars. Plants. 2021; 10(3):561. https://doi.org/10.3390/plants10030561
Chicago/Turabian StyleMihaljević, Ines, Marija Viljevac Vuletić, Domagoj Šimić, Vesna Tomaš, Daniela Horvat, Marko Josipović, Zvonimir Zdunić, Krunoslav Dugalić, and Dominik Vuković. 2021. "Comparative Study of Drought Stress Effects on Traditional and Modern Apple Cultivars" Plants 10, no. 3: 561. https://doi.org/10.3390/plants10030561
APA StyleMihaljević, I., Viljevac Vuletić, M., Šimić, D., Tomaš, V., Horvat, D., Josipović, M., Zdunić, Z., Dugalić, K., & Vuković, D. (2021). Comparative Study of Drought Stress Effects on Traditional and Modern Apple Cultivars. Plants, 10(3), 561. https://doi.org/10.3390/plants10030561







