Using Zinc Oxide Nanoparticles to Improve the Color and Berry Quality of Table Grapes Cv. Crimson Seedless
Abstract
:1. Introduction
2. Results
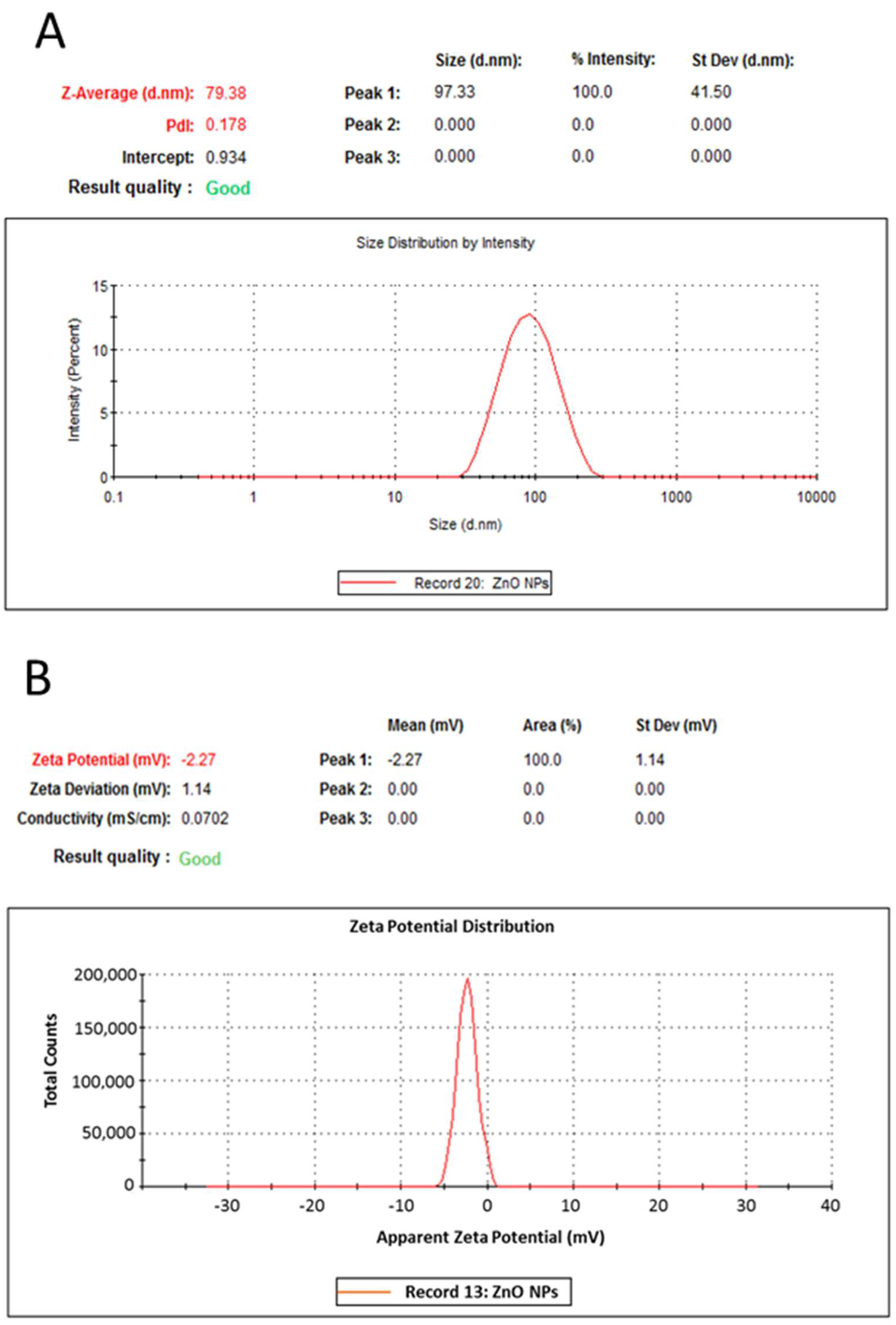
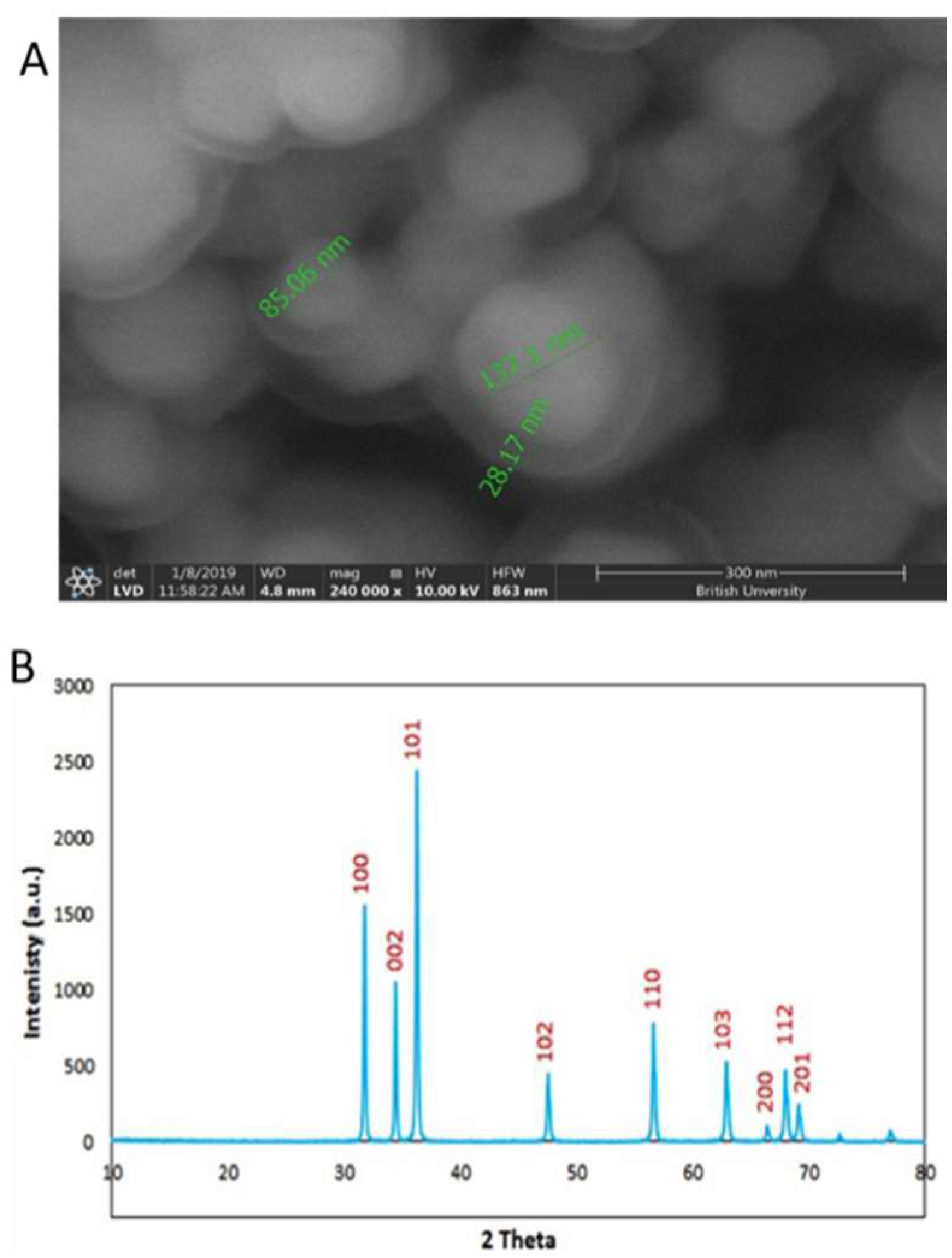
2.1. Characterization of Zinc Oxide Nanoparticles (ZnO NPs)
2.2. Physiochemical Fruit Properties Evaluation
2.3. Chemical Fruit Characteristics
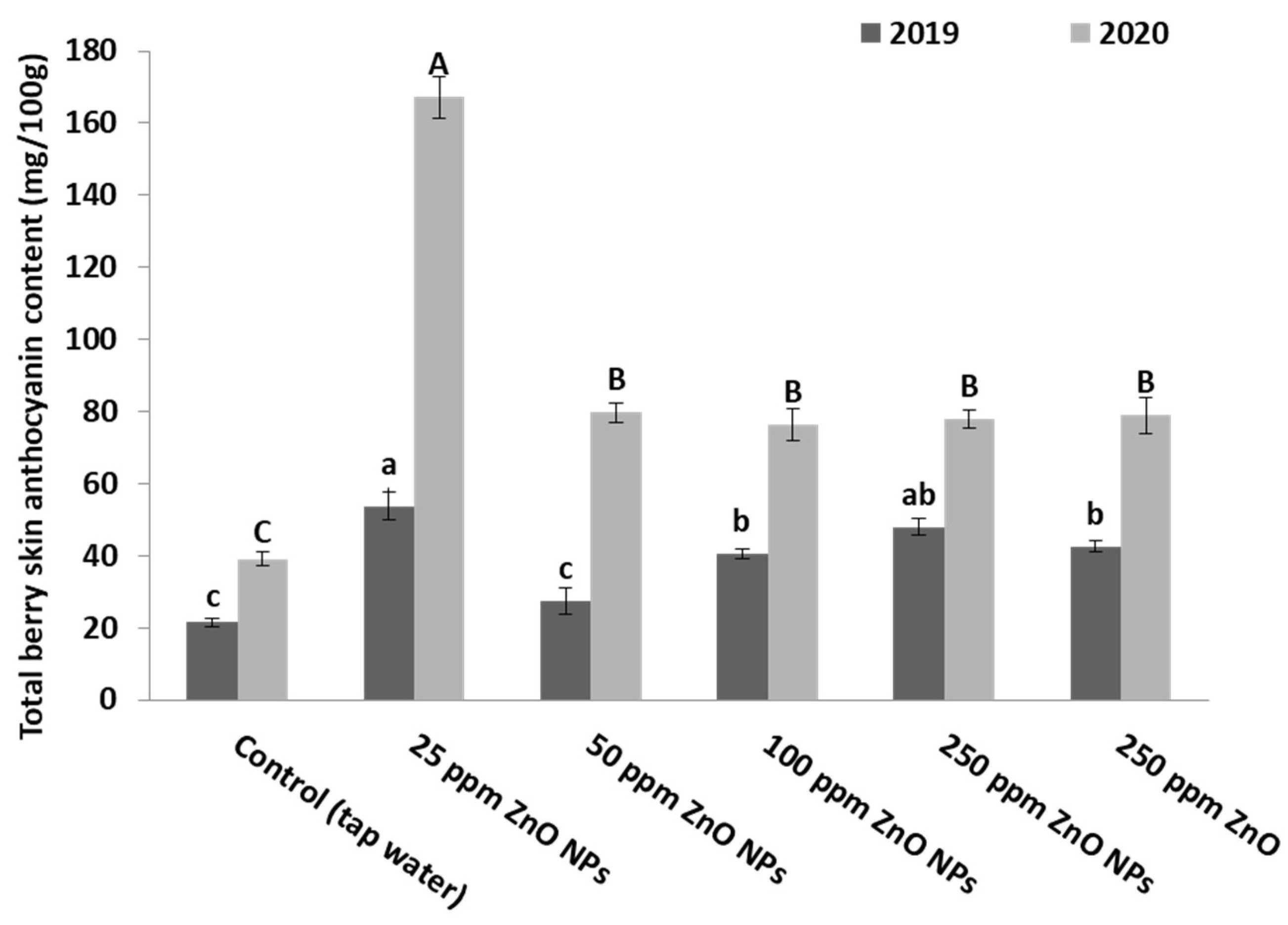
2.3.1. Total Anthocyanin
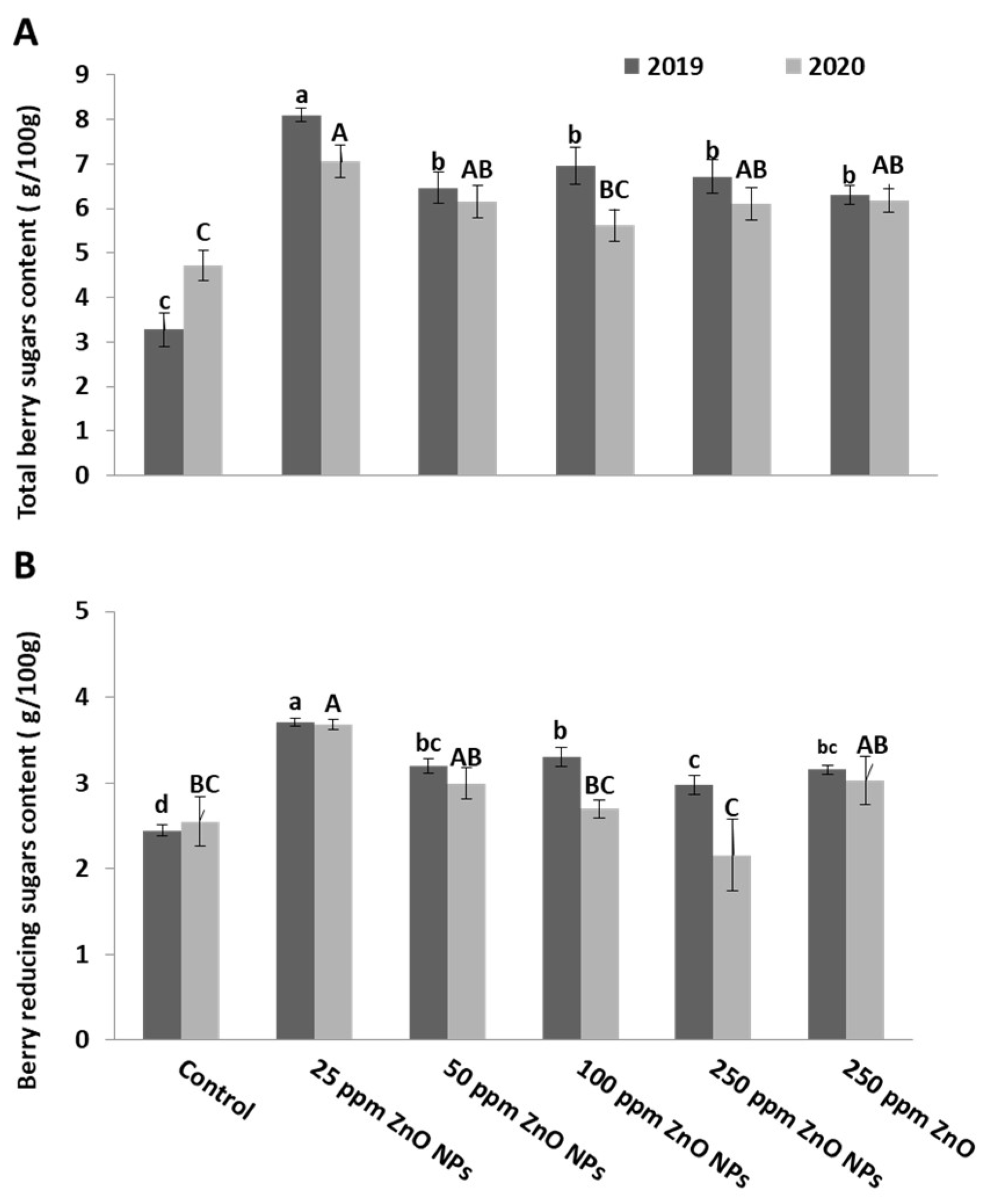
2.3.2. Total and Reducing Sugars
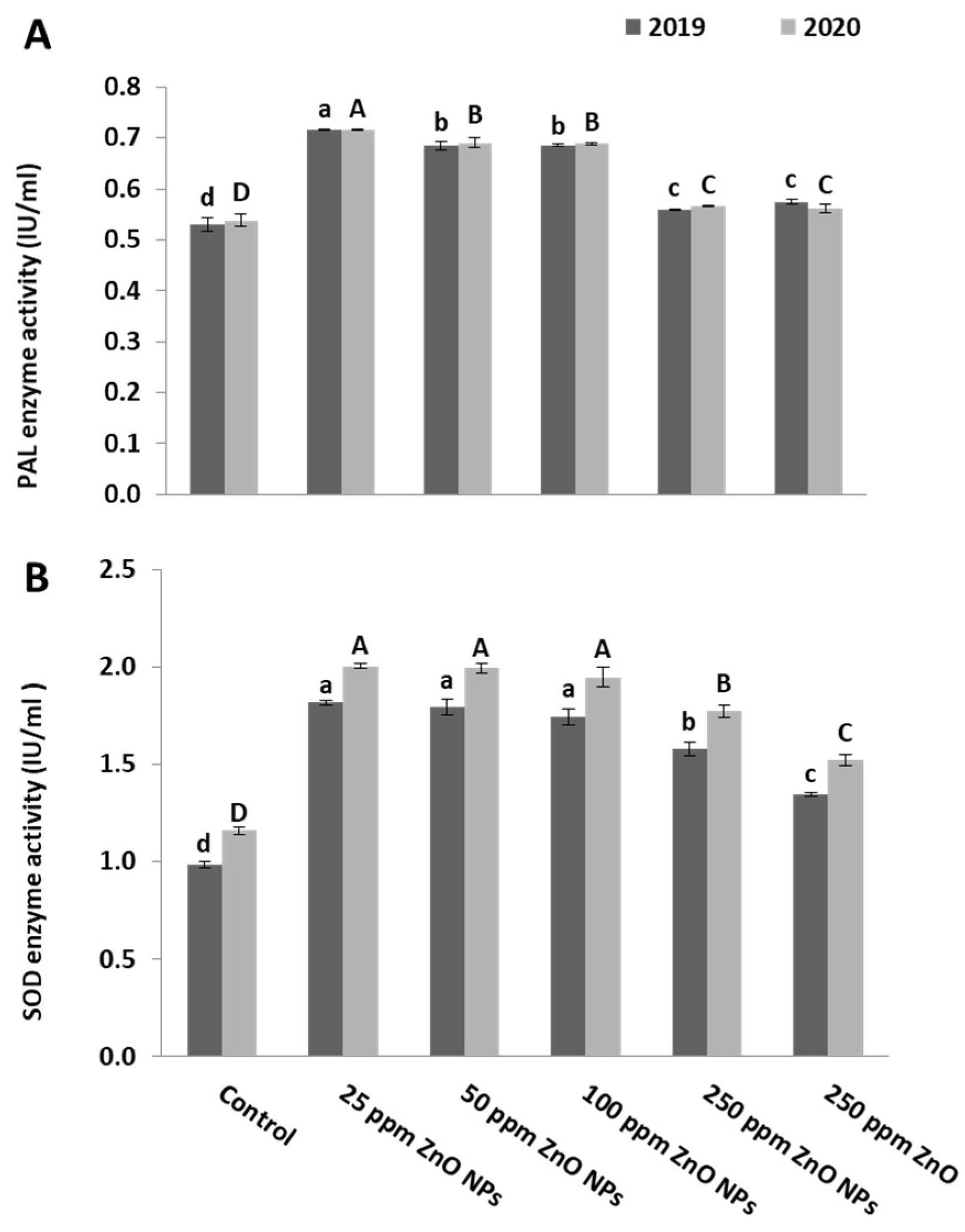
2.4. Enzyme Activity
3. Discussion
4. Materials and Methods
4.1. Preparation and Characterization of Zinc Oxide Nanoparticles (ZnO NPs)
4.2. Plant Material and Treatments
4.3. Leaf Zn Content Analysis
4.4. Physiochemical Fruit Properties Evaluation
4.5. Enzyme Activity
4.6. Fruit Bio-Chemical Components
4.6.1. Total Anthocyanin
4.6.2. Total and Reducing Sugars
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Baghdady, G.A.; Abdrabboh, G.A.; Shahda, M.A. Effect of Some Pre-Harvest Treatments on Yield and Fruit Quality of Crimson Seedless Grapevines. J. Biol. Chem. Environ. Sci. 2020, 15, 1–14. [Google Scholar]
- El-Sayed, M.E.A. Improving Fruit Quality and Marketing of “Crimson Seedless” Grape Using Some Preharvest Treatments. J. Hort. Sci. Ornamen. Plants. 2013, 5, 218–226. [Google Scholar] [CrossRef]
- Işçi, B.; Kacar, E.; Altındişli, A. The Effects of Some Exogenous Applications on Quality in ‘Crimson Seedless’ Grape. Erwerbs-Obstbau 2020, 62 (Suppl. 1), S87–S100. [Google Scholar] [CrossRef]
- Dokoozlian, N.; Peacock, B.; Luvisi, D.; Vasquez, S. Cultural Practices for Crimson Seedless Table Grapes. Calif. Agricult. 1995, 49, 36–40. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Song, C.; Falginella, L.; Castellarin, S.D. Day Temperature has a Stronger Effect than Night Temperature on Anthocyanin and Flavonol Accumulation in “Merlot” (Vitis vinifera L.) Grapes during Ripening. Front. Plant. Sci. 2020, 11, 1095. [Google Scholar] [CrossRef]
- Karagiannis, E.; Tanou, G.; Samiotaki, M.; Michailidis, M.; Diamantidis, G.; Minas, I.S.; Molassiotis, A. Comparative physiological and proteomic analysis reveal distinct regulation of peach skin quality traits by altitude. Front. Plant. Sci. 2016, 7, 1–14. [Google Scholar] [CrossRef]
- Karagiannis, E.; Michailidis, M.; Tanou, G.; Scossa, F.; Sarrou, E.; Stamatakis, G.; Samiotaki, M.; Martens, S.; Fernie, A.R.; Molassiotis, A. Decoding altitude-activated regulatory mechanisms occurring during apple peel ripening. Hortic. Res. 2020, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Sugaya, S.; Gemma, H. Decreased anthocyanin biosynthesis in grape berries grown under elevated night temperature condition. Sci. Hort. 2005, 105, 319–330. [Google Scholar] [CrossRef]
- Yahuaca, B.; Martinez-Peniche, R.; Reyes, J.L.; Madero, E. Effect of ethephon and girdling on berry firmness during storage of “Malaga Roja” grape. Acta Hort. 2006, 727, 459–465. [Google Scholar] [CrossRef]
- Smart, R.E.; Smith, S.M.; Winchester, R.V. Light quality and quantity effects on fruit ripening for Cabernet Sauvignon. Am. J. Enol. Vitic. 1988, 39, 250–258. [Google Scholar]
- Hunter, J.J.; De Villiers, O.T.; Watts, J.E. The effect of partial defoliation on quality characteristics of Vitis vinifera L. cv. Cabernet Sauvignon grapes. II. Skin color, skin sugar, and wine quality. Am. J. Enol. Vitic. 1991, 42, 13–18. [Google Scholar]
- Medda, S.; Dessena, L.; Mulas, M. Monitoring of the PAL Enzymatic Activity and Polyphenolic Compounds in Leaves and Fruits of Two Myrtle Cultivars during Maturation. Agriculture 2020, 10, 389. [Google Scholar] [CrossRef]
- El-Kereamy, A.; Chervin, C.; Roustan, J.-P.; Cheynier, V.; Souquet, J.-M.; Moutounet, M.; Raynal, J.; Ford, C.; Latché, A.; Pech, J.-C.; et al. Exogenous ethylene stimulates the long-term expression of genes related to anthocyanin biosynthesis in grape berries. Physiol. Plant. 2003, 119, 175–182. [Google Scholar] [CrossRef]
- Kataoka, I.; Sugiura, A.; Utsunomiya, N.; Tomana, T. Effect of abscisic acid and defoliation on anthocyanin accumulation in Kyoho grapes (Vitis vinifera L. x V. labruscana Bailey). Vitis 1982, 21, 325–332. [Google Scholar] [CrossRef]
- Samaan, M.S.F.; Nasser, M.A. Effect of spraying Paclobutrazol (PP333) on yield and fruit quality of Crimson seedless grape. J. Plant Prod. Mansoura Univ. 2020, 11, 1031–1034. [Google Scholar] [CrossRef]
- Zhang, W.; Curtin, C.; Kikuchi, M.; Franco, C. Integration of jasmonic acid and light irradiation for enhancement of anthocyanin biosynthesis in Vitis vinifera suspension cultures. Plant. Sci. 2002, 162, 459–468. [Google Scholar] [CrossRef]
- Wen, P.-F.; Chen, J.-Y.; Kong, W.-F.; Pan, Q.-H.; Wan, S.-B.; Huang, W.-D. Salicylic acid induced the expression of phenylalanine ammonia-lyase gene in grape berry. Plant. Sci. 2005, 169, 928–934. [Google Scholar] [CrossRef]
- Ban, T.; Ishimaru, M.; Kobayashi, S.; Shiozaki, S.; Goto-Yamamoto, N.; Horiuchi, S. Abscisic acid and 2,4-dichlorophenoxyacetic acid affect the expression of anthocyanin biosynthetic pathway genes in ‘Kyoho’ grape berries. J. Hortic. Sci. Biotechnol. 2003, 78, 586–589. [Google Scholar] [CrossRef]
- Jeong, S.T.; Goto-Yamamoto, N.; Kobayashi, S.; Esaka, M. Effects of plant hormones and shading on the accumulation of anthocyanins and the expression of anthocyanin biosynthetic genes in grape berry skins. Plant. Sci. 2004, 167, 247–252. [Google Scholar] [CrossRef]
- González-SanJosé, M.L.; Diez, C. Relationship between anthocyanins and sugars during the ripening of grape berries. Food Chem. 1992, 43, 193–197. [Google Scholar] [CrossRef]
- Song, C.-Z.; Liu, M.-Y.; Meng, J.-F.; Chi, M.; Xi, Z.-M.; Zhang, Z.-W. Promoting Effect of Foliage Sprayed Zinc Sulfate on Accumulation of Sugar and Phenolics in Berries of Vitis vinifera cv. Merlot Growing on Zinc Deficient Soil. Molecules 2015, 20, 2536–2554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in Sustainable Agriculture: Recent Developments, Challenges, and Perspectives. Front. Microbiol. 2017, 8, 1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Deng, H.; Hwang, H. The current application of nanotechnology in food and agriculture. J. Food Drug Anal. 2018, 27, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, Y.; Hasan, M.K.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of Nanotechnology in Plant Growth and Crop Protection: A Review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef] [Green Version]
- War, J.M.; Fazili, M.A.; Mushtaq, W.; Wani, A.; Bhat, M.Y. Role of Nanotechnology in Crop Improvement. In Nanobiotechnology in Agriculture, Nanotechnology in the Life Sciences; Hakeem, K.R., Pirzadah, T.B., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 63–97. [Google Scholar] [CrossRef]
- Abdel Wahab, M.M.; Abdelaziz, S.M.; El-Mogy, M.M.; Abdeldaym, E.A. Effect of Foliar ZnO and FeO Nanoparticles Application on Growth and Nutritional Quality of Red Radish and Assessment of Their Accumulation on Human Health. Agriculture 2019, 65, 16–29. [Google Scholar] [CrossRef] [Green Version]
- Prasad, T.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Reddy, K.; Sreeprasad, T.; Sajanlal, P.; Pradeep, T. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant. Nutr. 2012, 35, 905–927. [Google Scholar] [CrossRef]
- Tarafdar, J.; Raliya, R.; Mahawar, H.; Rathore, I. Development of zinc nanofertilizer to enhance crop production in pearl millet (Pennisetum americanum). Agri. Res. 2014, 3, 257–262. [Google Scholar] [CrossRef]
- Venkatachalam, P.; Priyanka, N.; Manikandan, K.; Ganeshbabu, I.; Indiraarulselvi, P.; Geetha, N.; Muralikrishna, K.; Bhattacharya, R.C.; Tiwari, M.; Sharma, N.; et al. Enhanced plant growth promoting role of phycomolecules coated zinc oxide nanoparticles with P supplementation in cotton (Gossypium hirsutum L.). Plant. Physiol Biochem. 2017, 110, 118–127. [Google Scholar] [CrossRef]
- Iziy, E.; Majd, A.; Vaezi-Kakhki, M.R.; Nejadsattari, T.; Noureini, S.K. Effects of zinc oxide nanoparticles on enzymatic and nonenzymatic antioxidant content, germination, and biochemical and ultrastructural cell characteristics of Portulaca oleracea L. Acta Soc. Bot. Pol. 2019, 88, 3639. [Google Scholar] [CrossRef] [Green Version]
- Rossi, L.; Fedenia, L.N.; Sharifan, H.; Ma, X.; Lombardini, L. Effects of foliar application of zinc sulfate and zinc nanoparticles in coffee (Coffea arabica L.) plants. Plant. Physiol. Biochem. 2019, 135, 160–166. [Google Scholar] [CrossRef]
- Narendhran, S.; Rajiv, P.; Sivaraj, R. Influence of zinc oxide nanoparticles on growth of Sesamum indicum L. in zinc deficient soil. Int. J. Pharm. Sci. 2016, 3, 365–371. [Google Scholar]
- García-López, J.I.; Niño-Medina, G.; Olivares-Sáenz, E.; Lira-Saldivar, R.H.; Barriga-Castro, E.D.; Vázquez-Alvarado, R.; Rodríguez-Salinas, P.A.; Zavala-García, F. Foliar Application of Zinc Oxide Nanoparticles and Zinc Sulfate Boosts the Content of Bioactive Compounds in Habanero Peppers. Plants 2019, 8, 254. [Google Scholar] [CrossRef] [Green Version]
- Alharby, H.F.; Metwali, E.M.R.; Fuller, M.P.; Aldhebiani, A.Y. Impact of application of zinc oxide nanoparticles on callus induction, plant regeneration, element content and antioxidant enzyme activity in tomato (Solanum lycopersicum Mill.) under salt stress. Arch. Biol. Sci. 2016, 68, 723–735. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, P.; Alyemeni, M.N.; Al-Huqail, A.A.; Alqahtani, M.A.; Wijaya, L.; Ashraf, M.; Kaya, C.; Bajguz, A. Zinc Oxide Nanoparticles Application Alleviates Arsenic (As) Toxicity in Soybean Plants by Restricting the Uptake of as and Modulating Key Biochemical Attributes, Antioxidant Enzymes, Ascorbate-Glutathione Cycle and Glyoxalase System. Plants 2020, 9, 825. [Google Scholar] [CrossRef]
- Karimzadeh, F.; Haddad, R.; Garoosi, G.; Khademian, R. Effects of Nanoparticles on Activity of Lignan Biosynthesis Enzymes in Cell Suspension Culture of Linum usitatissimum L. Russ. J. Plant. Physiol. 2019, 66, 756–762. [Google Scholar] [CrossRef]
- Farghaly, F.A.; Radi, A.A.; Al-Kahtany, F.A.; Hamada, A.M. Impacts of zinc oxide nano and bulk particles on redox-enzymes of the Punica granatum callus. Sci. Rep. 2020, 10, 1922. [Google Scholar] [CrossRef]
- Fawzi, A.F.A.; Elfouly, M.M.; Moubarak, Z.M. The Need of Grain Legumes for Iron, Manganese, and Zinc Fertilization under Egyptian Soil-Conditions—Effect and Uptake of Metalosates. J. Plant. Nutr. 1993, 16, 813–823. [Google Scholar] [CrossRef]
- Pandey, N.; Gupta, B.; Pathak, G.C. Enhanced yield and nutritional enrichment of seeds of Pisum sativum L. through foliar application of zinc. Sci. Hortic. 2013, 164, 474–483. [Google Scholar] [CrossRef]
- Saadati, S.; Moallemi, N.; Mortazavi, S.M.H.; Seyyednejad, S.M. Effects of zinc and boron foliar application on soluble carbohydrate and oil contents of three olive cultivars during fruit ripening. Sci. Hortic. 2013, 164, 30–34. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, C.-X.; Tan, Q.-L.; Zheng, C.-S.; Gui, H.-P.; Zeng, W.-N.; Sun, X.-C.; Zhao, X.-H. Plant nutrition status yield and quality of satsuma mandarin (Citrus unshiu Marc.) under soil application of Fe-EDDHA and combination with zinc and manganese in calcareous soil. Sci. Hortic. 2014, 174, 46–53. [Google Scholar] [CrossRef]
- Song, C.-Z.; Liu, M.-Y.; Men, J.-F.; Shi, P.-B.; Zhang, Z.-W.; Xi, Z.-M. Influence of foliage-sprayed zinc sulfate on grape quality and wine aroma characteristics of Merlot. Eur. Food Res. Technol. 2015, 242, 609–623. [Google Scholar] [CrossRef]
- Davarpanah, S.; Tehranifar, A.; Davarynejad, G.; Abadía, J.; Khorasani, R. Effects of foliar applications of zinc and boron nano-fertilizers on pomegranate (Punica granatum cv. Ardestani) fruit yield and quality. Sci. Hortic. 2016, 210, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Usha, K.; Singh, B. Effects of macro and micro nutrient spray on fruit yield and quality of grapes (Visit vinifera L.) cv. Perlette. Acta. Hort. 2002, 594, 197–202. [Google Scholar] [CrossRef]
- Abou-Zaid, E.A.A.; Shaaban, M.M. Growth, yield and berries quality in Red Roomy grapevines improved under different foliar application of Spirulina algae, zinc and boron. Mid. East J. Agri. Res. 2019, 8, 654–661. [Google Scholar]
- Hu, C.; Liu, Y.; Li, X.; Li, M. Biochemical responses of duckweed (Spirodela polyrhiza) to zinc oxide nanoparticles. Arch. Environ. Cont. Toxicol. 2013, 64, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Faraz, A.; Yusuf, M.; Khan, S.T.; Hayat, S. Zinc oxide nanoparticle-mediated changes in photosynthetic efficiency and antioxidant system of tomato plants. Photosynthetica 2018, 56, 678–686. [Google Scholar] [CrossRef]
- Faizan, M.; Faraz, A.; Hayat, S. Effective use of zinc oxide nanoparticles through root dipping on the performance of growth, quality, photosynthesis and antioxidant system in tomato. J. Plant. Bioch. Biotech. 2020, 29, 553–567. [Google Scholar] [CrossRef]
- Kouhi, S.M.M.; Lahouti, M.; Ganjeali, A.; Entezari, M.H. Comparative Phytotoxicity of ZnO Nanoparticles, ZnO Microparticles, and Zn2+ on Rapeseed (Brassica napus L.): Investigating a wide Range of Concentrations. Toxicol. Environ. Chem. 2015, 96, 861–868. [Google Scholar] [CrossRef]
- Thomas, B.F.; ElSohly, M.A. Biosynthesis and Pharmacology of Phytocannabinoids and Related Chemical Constituents. Anal. Chem. Cannabis. 2016, 27–41. [Google Scholar] [CrossRef]
- Wadhwa, N.; Joshi, U.N.; Mehta, N. Zinc Induced Enzymatic Defense Mechanisms in Rhizoctonia Root Rot Infected Cluster Bean Seedlings. J. Bot. 2014, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Mori, K.; Goto-Yamamoto, N.; Kitayama, M.; Hashizume, K. Loss of anthocyanins in red-wine grape under high temperature. J. Exp. Bot. 2007, 58, 1935–1945. [Google Scholar] [CrossRef]
- Cohen, S.D.; Tarara, J.M.; Kennedy, J.A. Assessing the impact of temperature on grape phenolic metabolism. Anal. Chim. Acta 2008, 621, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Regules, A.; Romero-Cascales, I.; López-Roca, J.M.; Ros-García, J.M.; Gómez-Plaza, E. Anthocyanin fingerprint of grapes: Environmental and genetic variations. J. Sci. Food Agr. 2006, 86, 1460–1467. [Google Scholar] [CrossRef]
- Biswas, T.; Mathur, A. Plant Anthocyanins: Biosynthesis, Bioactivity and in vitro Production from tissue cultures. Adv. Biotech. Micro. 2017, 5, 555672. [Google Scholar] [CrossRef]
- Hashemi, S.; Asrar, Z.; Pourseyedi, S.; Nadernejad, N. Investigation of ZnO Nanoparticles on Proline, Anthocyanin Contents and Photosynthetic Pigments and Lipid Peroxidation in the Soybean. IET Nanobiotechnol. 2018, 13, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Raigond, P.; Raigond, B.; Kaundal, B.; Singh, B.; Joshi, A.; Dutt, S. Effect of Zinc Nanoparticles on Antioxidative System of Potato Plants. J. Environ. Biol. 2017, 38, 435–439. [Google Scholar] [CrossRef]
- Oloumi, H.; Soltaninejad, R.; Baghizadeh, A. The comparative Effects of Nano and Bulk Size Particles of CuO and ZnO on Glycyrrhiza glabra L. Seedlings. Ind. J. Plant. Physiol. 2015, 20, 157–161. [Google Scholar] [CrossRef]
- Suganya, A.; Saravanan, A.; Manivannan, N. Role of Zinc Nutrition for Increasing Zinc Availability, Uptake, Yield and Quality of Maize (Zea Mays L.) Grains: An Overview. Comm. Soil Sci. Plant. Anal. 2020, 51, 2001–2021. [Google Scholar] [CrossRef]
- Long, L.; Chen, J.; Zhang, Y.; Liang, X.; Ni, H.; Zhang, B.; Yin, Y. Comparison of porous and nano zinc oxide for replacing high-dose dietary regular zinc oxide in weaning piglets. PLoS ONE 2017, 12, e0182550. [Google Scholar] [CrossRef] [Green Version]
- Washington, I.M.; Van Hoosier, G. Clinical Biochemistry and Hematology. In The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents; Academic Press: Cambridge, MA, USA, 2012; pp. 57–116. [Google Scholar] [CrossRef]
- Bashandy, S.A.E.; Alaamer, A.; Moussa, S.A.A.; Omara, E.A. Role of zinc oxide nanoparticles in alleviating hepatic fibrosis and nephrotoxicity induced by thioacetamide in rats. Canadian J. Physi. Pharma. 2018, 96, 337–344. [Google Scholar] [CrossRef] [Green Version]
- Sidra, S.; Arshad, M.; Chaudhari, S.K. Zinc Oxide Nanoparticles for Revolutionizing Agriculture: Synthesis and Applications. Sci. World J. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hassan, N.S.; Salah El Din, T.A.; Hendawey, M.H.; Borai, I.H.; Mahdi, A.A. Magnetite and zinc oxide nanoparticles alleviated heat stress in wheat plants. Curr. Nanomater. 2018, 3, 32–43. [Google Scholar] [CrossRef]
- Wolf, T.K. Effects of rootstock and nitrogen fertilization on the growth yield of grapevines in New York. Am. J. Enol. Vitic. 1988, 39, 29–33. [Google Scholar]
- Carter, G.A. Responses of leaf spectral reflectance to plant stress. Am. J. Bot. 1993, 80, 239–243. [Google Scholar] [CrossRef]
- AOAC-Association of Official Analytical Chemists International. Official Methods of Analysis, 16th ed.; AOAC: Arlington, VA, USA, 1995. [Google Scholar]
- Şirin, S.; Aydaş, S.B.; Aslim, B. Biochemical Evaluation of Phenylalanine Ammonia Lyase (PAL) from Endemic Cyathobasis fruticulosa (Bunge) Aellen for the Dietary Treatment of Phenylketonuria (PKU). Food Technol. Biotechnol. 2016, 54, 296–313. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in condition. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Connor, A.M.; Luby, J.J.; Finn, C.E.; Hancock, J.F. Genotypic and environmental variation in antioxidant activity among blueberry cultivars. Acta Hortic. 2002, 574, 209–213. [Google Scholar] [CrossRef]
- Lima, A.B.; Corrêa, A.D.; Saczk, A.A.; Martins, M.P.; Castilho, R.O. Anthocyanins, pigment stability and antioxidant activity in jabuticaba [Myrciaria cauliflora (Mart.) O. Berg]. Rev. Bras. Frutic. 2011, 33, 877–887. [Google Scholar] [CrossRef] [Green Version]
- Shaffer, P.A.; Hartmann, A.F. The Iodometric Determination of Copper and Its Use in Sugar Analysis. J. Biol. Chem. 1921, 45, 349–364. [Google Scholar] [CrossRef]
- Duncan, D.B. Multiple range and multiple F tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
| Character (Means) | T.S.S (Brix) | Acidity % | T.S.S/Acid Ratio | Firmness (g/mm) | T.S.S (Brix) | Acidity % | T.S.S/Acid Ratio | Firmness (g/mm) |
|---|---|---|---|---|---|---|---|---|
| Treatments | First season (2019) | Second season (2020) | ||||||
| 0 ppm (Control) | 15.0 c | 0.672 a | 22.4 c | 130.6 b | 15.6 b | 0.657 a | 23.7 b | 207.9 d |
| 25 ppm ZnO NPs | 18.1 a | 0.616 ab | 29.5 a | 152.8 a | 19.5 a | 0.613 ab | 32.0 a | 240.0 bc |
| 50 ppm ZnO NPs | 17.7 a | 0.598 b | 29.7 a | 156.9 a | 18.6 a | 0.591 b | 31.7 a | 236.0 bc |
| 100 ppm ZnO NPs | 17.2 a | 0.666 a | 25.9 b | 160.1 a | 18.6 a | 0.627 ab | 29.9 a | 261.1 ab |
| 250 ppm ZnO NPs | 17.5 a | 0.628 ab | 27.9 ab | 165.6 a | 19.3 a | 0.626 ab | 30.9 a | 264.3 a |
| 250 ppm ZnO | 16.3 b | 0.616 ab | 25.8 b | 154.7 a | 16.5 b | 0.648 a | 25.7 b | 218.6 cd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abou El-Nasr, M.K.; El-Hennawy, H.M.; Samaan, M.S.F.; Salaheldin, T.A.; Abou El-Yazied, A.; El-Kereamy, A. Using Zinc Oxide Nanoparticles to Improve the Color and Berry Quality of Table Grapes Cv. Crimson Seedless. Plants 2021, 10, 1285. https://doi.org/10.3390/plants10071285
Abou El-Nasr MK, El-Hennawy HM, Samaan MSF, Salaheldin TA, Abou El-Yazied A, El-Kereamy A. Using Zinc Oxide Nanoparticles to Improve the Color and Berry Quality of Table Grapes Cv. Crimson Seedless. Plants. 2021; 10(7):1285. https://doi.org/10.3390/plants10071285
Chicago/Turabian StyleAbou El-Nasr, Mohamed K., Hussein M. El-Hennawy, Mina S. F. Samaan, Taher A. Salaheldin, Ahmed Abou El-Yazied, and Ashraf El-Kereamy. 2021. "Using Zinc Oxide Nanoparticles to Improve the Color and Berry Quality of Table Grapes Cv. Crimson Seedless" Plants 10, no. 7: 1285. https://doi.org/10.3390/plants10071285
APA StyleAbou El-Nasr, M. K., El-Hennawy, H. M., Samaan, M. S. F., Salaheldin, T. A., Abou El-Yazied, A., & El-Kereamy, A. (2021). Using Zinc Oxide Nanoparticles to Improve the Color and Berry Quality of Table Grapes Cv. Crimson Seedless. Plants, 10(7), 1285. https://doi.org/10.3390/plants10071285









