Chemical Screening of Metabolites Profile from Romanian Tuber spp.
Abstract
1. Introduction
2. Results and Discussion
2.1. Mass Spectrometry Analysis of Tuber magnatum pico and Tuber brumale
2.2. Screening and Classification of Metabolites
3. Materials and Methods
3.1. Reagents
3.2. GC-MS Analysis
3.3. GC-MS Separation Conditions
3.4. Mass Spectrometry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, S. Food, Health and Agricultural Importance of Truffles: A Review of Current Scientific Literature. Importance of Truffles. Curr. Trends Biotechnol. Pharm. 2012, 6, 2230–7303. [Google Scholar]
- Gajos, M.; Hilszczańska, D. Research on truffles: Scientific journals analysis. Sci. Res. Essays 2013, 8, 1837–1847. [Google Scholar]
- Splivallo, R. Biological Significance of Truffle Secondary Metabolites. In Secondary Metabolites in Soil Ecology; Soil Biology 14; Karlovsky, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Trappe, J.M.; Molina, R.; Luoma, D.L.; Cázares, E.; Pilz, D.; Smith, J.E.; Castellano, M.A.; Miller, S.L.; Trappe, M.J. Diversity, Ecology, and Conservation of Truffle Fungi in Forests of the Pacific Northwest; General Technical Report, PNW-GTR-772; Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2009; pp. 158–164.
- Hospodar, M. Aphrodisiac Foods: Bringing Heaven to Earth. Gastron. J. Crit. Food Stud. 2004, 4, 82–93. [Google Scholar] [CrossRef]
- Üstün, N.Ş.; Bulam, S.; Pekşen, A. Biochemical Properties, Biological Activities and Usage of Truffles. In Proceedings of the International Congress on Engineering and Life Science (ICELIS 2018), Kastamonu, Turkey, 26–29 April 2018; Republic of Turkey Kastamonu University: Kastamonu, Turkey, Proceeding Book; 2018; pp. 772–778. ISBN 978-605-4697-20-5. [Google Scholar]
- Li, X.; Zhang, X.; Ye, L.; Kang, Z.; Jia, D.; Yang, L.; Zhang, B. LC-MS-Based Metabolomic Approach Revealed the Significantly Different Metabolic Profiles of Five Commercial Truffle Species. Front. Microbiol. 2019, 10, 2227. [Google Scholar] [CrossRef] [PubMed]
- Khojasteh, S.M.B.; Amiri, L.; Sheikhzadeh, F. Effect of the Alcoholic Extract of Terfezia Boudieri on Reproductive Hormones in Male Rats. Int. J. Pharm. Biol. Sci. 2013, 3, 517–522. [Google Scholar]
- Bone, E. Buried Treasure that Is Filled with Mystery, Dining & Wine. The New York Times, 24 December 2012. [Google Scholar]
- Vita, F.; Taiti, C.; Pompeiano, A.; Bazihizina, N.; Lucarotti, V.; Mancuso, S.; Alpi, A. Volatile organic compounds in truffle (Tuber magnatum Pico): Comparison of samples from different regions of Italy and from different seasons. Sci. Rep. 2015, 5, 12629. [Google Scholar] [CrossRef]
- Al-Ruqaie, I.M. Effect of Treatment Process and Preservation Methods on Shelf Life of Truffles: II. Non-Conventional Methods (Radiation). Int. J. Biol. Chem. 2009, 3, 126–131. [Google Scholar] [CrossRef][Green Version]
- Shavit, E. Medicinal Mushrooms, Truffles Roasting in the Evening Fires. Fungi 2008, 1, 18–23. [Google Scholar]
- Dincă, M.; Dincă, L.C. Truffles and soil. Res. J. Agric. Sci. 2015, 47, 44–50. [Google Scholar]
- Zambonelli, A.; Iotti, M.; Murat, C. True Truffle (Tuber spp.) in the World: Soil Ecology, Systematics, and Biochemistry; Springer—Soil Biology; Springer: Cham, Swizerland, 2016; ISBN 978-2-3-19-31434-1. ISSN 1613-3382. [Google Scholar]
- Al-Laith, A.A.A. Antioxidant components and antioxidant/antiradical activities of desert truffle (Tirmania nivea) from various Middle Eastern origins. J. Food Compos. Anal. 2010, 23, 15–22. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, G.; Song, D.; Liu, J.H.; Zhou, Y.; Ou, J.; Sun, S. Fourier transform infrared spectroscopic study of truffles. In Proceedings of the SPIE, ICO20: Biomedical Optics, Changchun, China, 21–26 August 2006; Volume 6026, p. 60260H. [Google Scholar]
- Bouatia, M.; Touré, H.A.; Cheikh, A.; Eljaoudi, R.; Rahali, Y.; Oulad Bouyahya Idrissi, M.; Khabar, L.; Draoui, M. Analysis of nutrient and antinutrient content of the truffle (Tirmania pinoyi) from Morocco. Int. Food Res. J. 2018, 25, 174–178. [Google Scholar]
- El Enshasy, H.; Elsayed, E.A.; Aziz, R.; Wadaan, M.A. Mushrooms and Truffles: Historical Biofactories for Complementary Medicine in Africa and in the Middle East. Evid.-Based Complement. Altern. Med. 2013, 2013, 620451. [Google Scholar] [CrossRef]
- Patel, S.; Rauf, A.; Khan, H.; Khalid, S.; Mubarak, M.S. Potential health benefits of natural products derived from truffles: A review. Trends Food Sci. Technol. 2017, 70, 1–8. [Google Scholar] [CrossRef]
- Gao, J.M.; Zhanga, A.L.; Chena, H.; Liu, J.K. Molecular species of ceramides from the ascomycete truffle Tuber indicum. Chem. Phys. Lipids 2004, 131, 205–213. [Google Scholar] [CrossRef]
- Hamza, A.; Zouari, N.; Zouari, S.; Jdir, H.; Zaidi, S.; Gtari, M.; Neffati, M. Nutraceutical potential, antioxidant and antibacterial activities of Terfezia boudieri Chatin, a wild edible desert truffle from Tunisia arid zone. Arab. J. Chem. 2016, 9, 383–389. [Google Scholar] [CrossRef]
- Yan, X.; Wang, Y.; Sang, X.; Fan, L. Nutritional value, chemical composition and antioxidant activity of three Tuber species from China. AMB Express 2017, 7, 136. [Google Scholar] [CrossRef]
- Dahham, S.S.; Al-Rawi, S.S.; Ibrahim, A.H.; Majid, A.S.A.; Majid, A.M.S.A. Antioxidant, anticancer, apoptosis properties and chemical composition of black truffle Terfezia claveryi. Saudi J. Biol. Sci. 2018, 25, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.K. Secondary metabolites from higher fungi in China and their biological activity. Drug Discov. Ther. 2007, 1, 94–103. [Google Scholar] [PubMed]
- Kivrak, Ş.; Kivrak, İ. Investigation of Chemical Composition and Nutritional Value of Truffle Mushroom (Tuber nitidum Vittad.). J. Nat. Appl. Sci. 2018, 22, 339–344. [Google Scholar] [CrossRef]
- Nagy, M.; Socaci, S.; Tofană, M.; Biris-Dorhoi, E.S.; Țibulcă, D.; Salanta, L.; Petrut, G. Chemical Composition and Bioactive Compounds of Some Wild Edible Mushrooms. Bull. UASVM Food Sci. Technol. 2017, 74. [Google Scholar] [CrossRef]
- Saddiq, A.A.; Yousef, J.M.; Mohame, A.M. The Potential Antibacterial Role of Terfezia Claveryi Extract Against Immune-Inflammatory Disorder and Oxidative Damage Induced by Pseudomonas Aeruginosa in Rat Corneas. Rom. Biotechnol. Lett. 2016, 21, 11781–11801. [Google Scholar]
- Tejedor-Calvo, E.; Morales, D.; Marco, P.; Sánchez, S.; Garcia-Barreda, S.; Ribeiro, S.; Iacominic, M.; Villalva, M.; Santoyo, S.; Soler-Rivasa, C. Screening of bioactive compounds in truffles and evaluation of pressurized liquid extractions (PLE) to obtain fractions with biological activities. Food Res. Int. 2020, 132, 109054. [Google Scholar] [CrossRef] [PubMed]
- Allen, F.; Greiner, R.; Wishart, D. Competitive fragmentation modelling of ESI-MS/MS spectra for putative metabolite identification. Metabolomics 2015, 11, 98–110. [Google Scholar] [CrossRef]
- Segneanu, A.E.; Sfirloaga, P.; David, I.; Balcu, I.; Grozescu, I. Characterisation of truffles using electrochemical and analytical methods. Dig. J. Nanomater. Biostruct. 2012, 7, 199–205. [Google Scholar]
- Zhang, A.; Sun, H.; Wang, P.; Han, Y.; Wang, X. Modern analytical techniques in metabolomics analysis. Analyst 2012, 137, 293–300. [Google Scholar] [CrossRef]
- Clish, C.B. Metabolomics: An emerging but powerful tool for precision medicine. Cold Spring Harb. Mol. Case Stud. 2015, 1, a000588. [Google Scholar] [CrossRef]
- Stewart, D.; McDougall, G.J.; Sungurtas, J.; Verrall, S.; Graham, J.; Martinussen, I. Metabolomic approach to identifying bioactive compounds in berries: Advances toward fruit nutritional enhancement. Mol. Nutr. Food Res. 2007, 51, 645–651. [Google Scholar] [CrossRef]
- Sinem, N. Metabolomics: Basic Principles and Strategies. In Molecular Medicine; Nalbantoglu, S., Amri, H., Eds.; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Piasecka, A.; Kachlicki, P.; Stobiecki, M. Analytical Methods for Detection of Plant Metabolomes Changes in Response to Biotic and Abiotic Stresses. Int. J. Mol. Sci. 2019, 20, 379. [Google Scholar] [CrossRef]
- Hill, C.B.; Roessner, U. Metabolic Profiling of Plants by GC–MS. In The Handbook of Plant Metabolomics, 1st ed.; Weckwerth, W., Kahl, G., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2013. [Google Scholar]
- Aprea, E.; Biasioli, F.; Carlin, S.; Versini, G.; Märk, T.D.; Gasperi, F. Rapid white truffle headspace analysis by proton transfer reaction mass spectrometry and comparison with solid-phase microextraction coupled with gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 2564–2572. [Google Scholar] [CrossRef]
- Torregiani, E.; Lorier, S.; Sagratini, G.; Maggi, F.; Vittori, S.; Capriol, G. Comparative Analysis of the Volatile Profile of 20 Commercial Samples of Truffles, Truffle Sauces, and Truffle-Flavored Oils by Using HS-SPME-GC-MS. Food Anal. Methods 2017, 10, 1857–1869. [Google Scholar] [CrossRef]
- Sawaya, W.N.; Al-Shalhat, A.; Al-Sogair, A.; AL-Mohammad, M. Chemical Composition and Nutritive Value of Truffles of Saudi Arabia. J. Food Sci. 1985, 50, 450–453. [Google Scholar] [CrossRef]
- Feng, T.; Shui, M.; Song, S.; Zhuang, H.; Sun, M.; Ya, L. Characterization of the Key Aroma Compounds in Three Truffle Varieties from China by Flavoromics Approach. Molecules 2019, 24, 3305. [Google Scholar] [CrossRef]
- Culleré, L.; Ferreira, V.; Venturini, M.E.; Marco, P.; Blanc, D. Chemical and sensory effects of the freezing process on the aroma profile of black truffles (Tuber melanosporum). Food Chem. 2013, 136, 518–525. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.J. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S. Biological Interaction of Sulphur Compounds; CRC Press: Boca Raton, FL, USA, 1996; ISBN 0748402446. [Google Scholar]
- Hackett, M.J.; Zaro, J.L.; Shen, W.C.; Guley, P.C.; Cho, M.J. Fatty Acids as Therapeutic Auxiliaries for Oral and Parenteral Formulations. Adv. Drug Deliv. Rev. 2013, 65, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Rajendrakumar, N.; Vasantha, K.; Mohan, V.R. GC-MS Analysis of Bioactive Components of Tubers of Ruellia tuberosa L. (Acanthaceae). Am. J. Phytomed. Clin. Ther. 2014, 2, 209–216. [Google Scholar]
- Suttiarporn, P.; Chumpolsri, W.; Mahatheeranont, S.; Luangkamin, S.; Teepsawang, S.; Leardkamolkarn, V. Structures of Phytosterols and Triterpenoids with Potential Anti-Cancer Activity in Bran of Black Non-Glutinous Rice. Nutrients 2015, 7, 1672–1687. [Google Scholar] [CrossRef]
- Sakouhi, F.; Absalon, C.; Sebei, K.; Fouquet, E.; Boukhchina, S.; Kallel, H. Gas chromatographic–mass spectrometric characterisation of triterpene alcohols and monomethylsterols in developing Olea europaea L. fruits. Food Chem. 2009, 116, 345–350. [Google Scholar] [CrossRef]
- Weete, J.D.; Kulifaj, M.; Montant, C.; Nes, W.R.; Sancholle, M. Distribution of sterols in fungi. II. Brassicasterol in Tuber and Terjezia species. Can. J. Microbiol. 2011, 31, 1127–1130. [Google Scholar] [CrossRef]
- Mo, S.; Dong, L.; Hurst, W.J.; van Breemen, R.B. Quantitative analysis of phytosterols in edible oils using APCI liquid chromatography-tandem mass spectrometry. Lipids 2013, 48, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Hammann, S.; Vetter, W. Method Development for the Determination of Free and Esterified Sterols in Button Mushrooms (Agaricus bisporus). J. Agric. Food Chem. 2016, 64, 3437–3444. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.M.; Wang, C.Y.; Zhang, A.L.; Liu, J.K. A New Trihydroxy Fatty Acid from the Ascomycete, Chinese Truffle Tuber indicum. Lipids 2001, 36, 1365–1370. [Google Scholar] [CrossRef] [PubMed]
- Jennemann, R.; Geyer, R.; Sandhoff, R.; Gschwind, R.; Levery, S.; Wiegandt, H.; Grone, H.J. Glycoinositolphospholipids (Basidiolipids) of higher mushrooms. Eur. J. Biochem. 2001, 268, 1190–1205. [Google Scholar] [CrossRef] [PubMed]
- Calpe-Berdiel, L.; Méndez-González, J.; Llaverias, G.; Escolà-Gil, J.C.; Blanco-Vaca, F. Plant sterols, cholesterol metabolism and related disorders. In Biochemical Aspects of Human Nutrition; Avigliano, L., Rossi, L., Eds.; Transworld Research Network: Kerala, India, 2010; pp. 223–242. ISBN 978-81-7895-478-3. [Google Scholar]
- Gao, J.M.; Zhu, W.M.; Zhang, S.Q.; Zhang, X.; Zhang, A.L.; Chen, H.; Sun, Y.Y.; Tang, M. Sphingolipids from the edible fungus Tuber indicum. Eur. J. Lipid Sci. Technol. 2004, 106, 815–821. [Google Scholar]
- Zhang, X.; Ye, L.; Kang, Z.; Zou, J.; Zhang, X.; Li, X. Mycorrhization of Quercusacutissima with Chinese black truffle significantly altered the host physiology and root-associated microbiomes. PeerJ 2019, 18, e6421. [Google Scholar] [CrossRef]
- Salhab, A.S.A. Minireview on Mushroom: Emphasis on the Wild Mushroom of Jordan. Jordan Med. J. 2007, 41, 170–178. [Google Scholar]
- Claus, R.; Hoppen, H.O.; Karg, H. The secret of truffles: A steroidal pheromone? Experientia 1981, 37, 1178–1179. [Google Scholar] [CrossRef]
- Villares, A.; García-Lafuente, A.; Guillamón, E.; Ramos, Á. Identification and quantification of ergosterol and phenolic compounds occurring in Tuber spp. truffles. J. Food Compos. Anal. 2012, 26, 177–182. [Google Scholar] [CrossRef]
- Zang, N.; Chen, H.; Sun, B.; Mao, X.; Zhang, Y.; Zhou, Y. Comparative Analysis of Volatile Composition in Chinese Truffles via GC × GC/HR-TOF/MS and Electronic Nose. Int. J. Mol. Sci. 2016, 17, 412. [Google Scholar] [CrossRef]
- Rodrigues, M.L. The Multifunctional Fungal Ergosterol. mBio 2018, 9, e01755-18. [Google Scholar] [CrossRef] [PubMed]
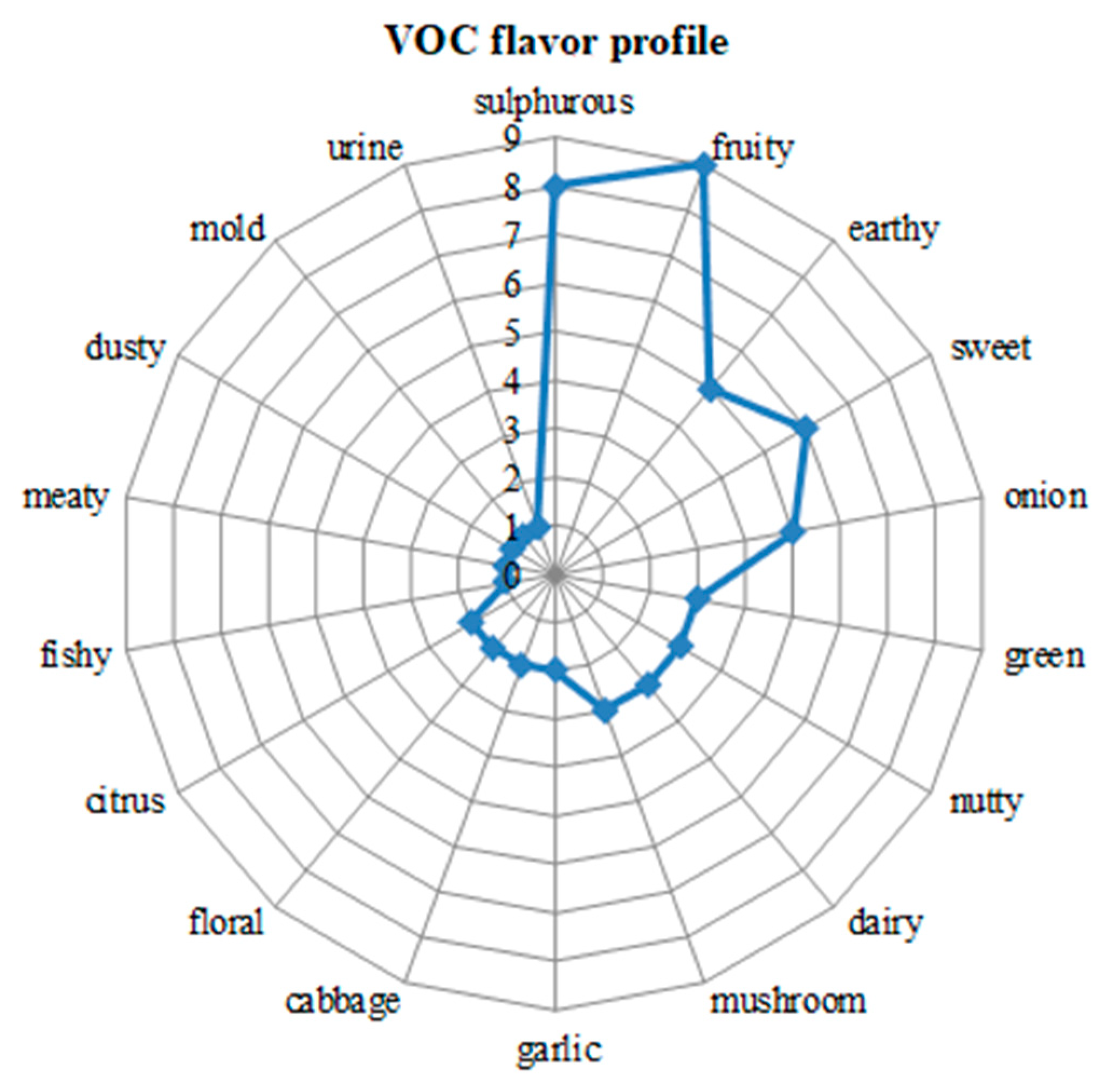
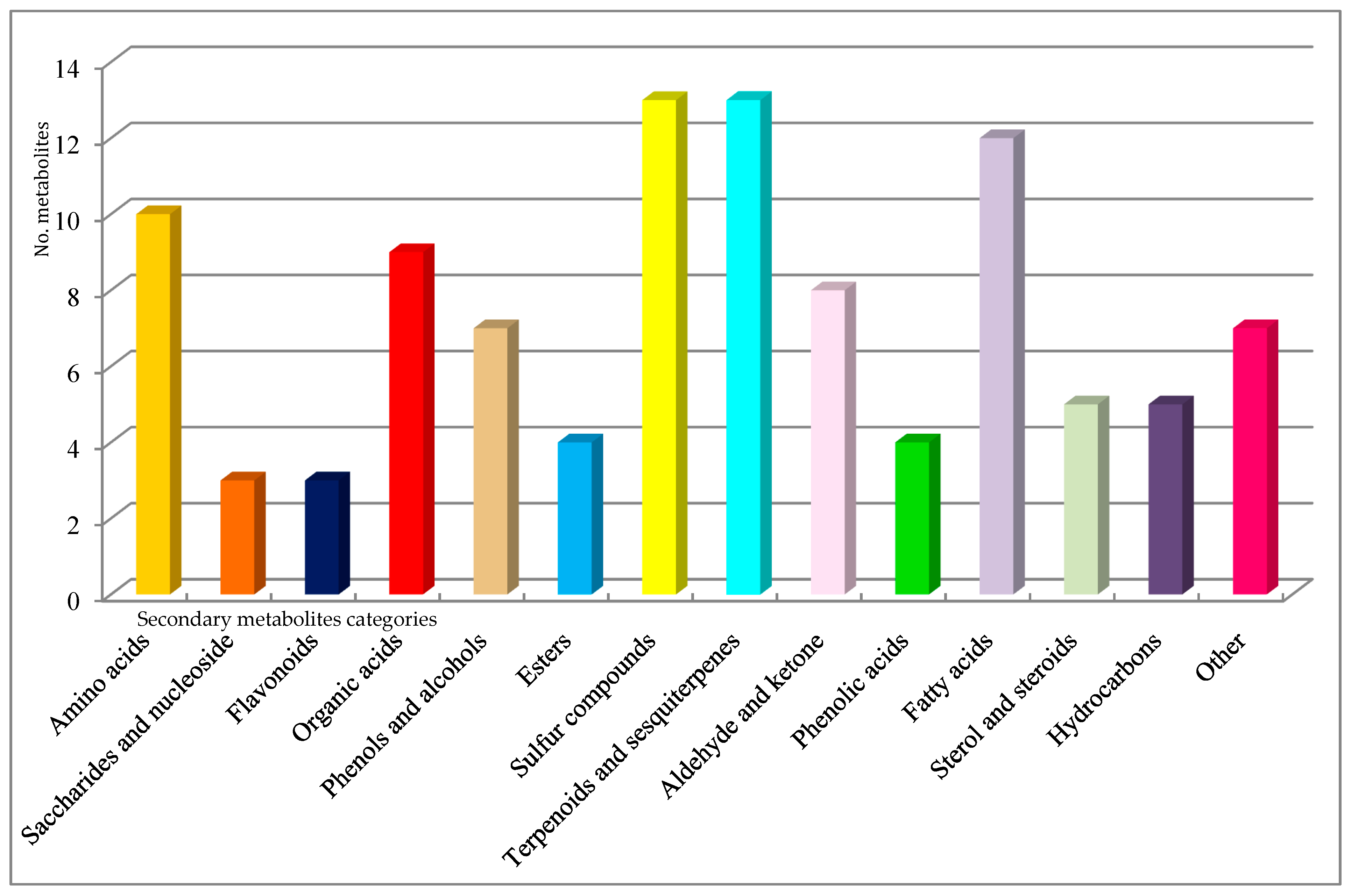
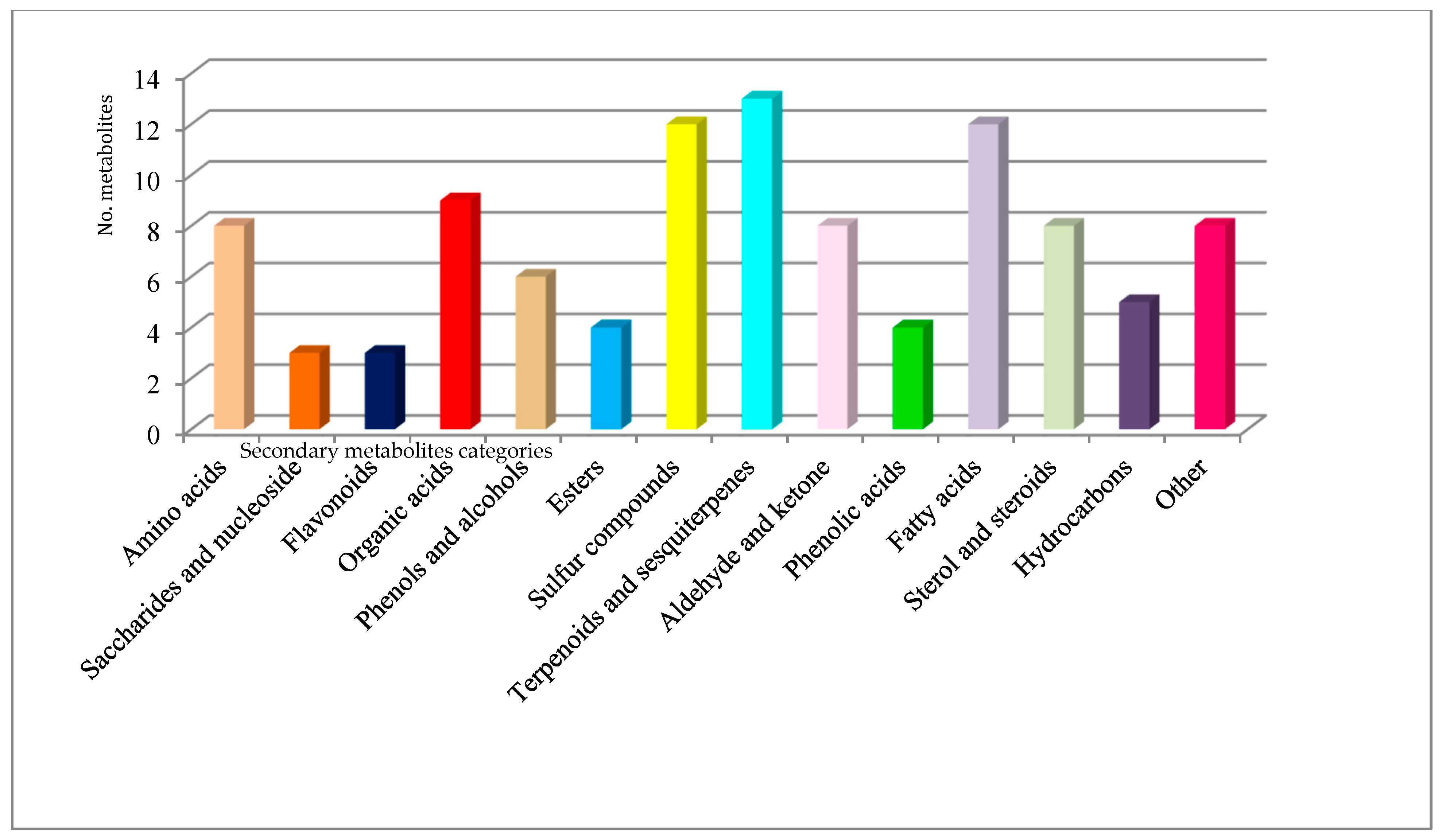
| Sample | Compounds Identified from GC-MS Library | RT | RI (Determinated) |
|---|---|---|---|
| Tuber magnatum pico | 3-octanol | 20.452 | 1087 |
| dimethyl sulfoxide | 28.769 | 516 | |
| stearic acid | 32.974 | 216 | |
| squalene | 34.536 | 2745 | |
| beta-sitosterol | 36.167 | 3292 | |
| campesterol | 36.680 | 3297 | |
| stearic acid | 38.211 | 2163 | |
| dimethyl sulfone | 51.286 | 924 | |
| benzothiazole | 55.461 | 1184 | |
| Tuber brumale | 3-octanol | 20.452 | 1087 |
| 1,2-butanediol | 21.968 | 811 | |
| lupeol | 21.971 | 3265 | |
| 2,4-octanedione | 35.445 | 1082 | |
| tris(methylthio)methane | 51.275 | 1364 | |
| ergosterol | 52.008 | 3085 |
| Sample Fraction | Compound No. | m/z Detected | Theoretic m/z | Formula | Tentative of Identification | Ref. |
|---|---|---|---|---|---|---|
| Tuber magnatum pico | 1 | 65.17 | 65.15 | C2H6S+ | dimethyl sulfide | [3,7,10,23,37] |
| 2 | 89.18 | 89.15 | C6H12O+ | isoamyl alcohol | [7,10] | |
| 3 | 89.14 | 89.12 | C4H8O2+ | 3-hydroxy-2-butanone | [38] | |
| 4 | 90.11 | 90.097 | C3H7O2+ | alanine | [7,25,39] | |
| 5 | 95.15 | 95.14 | C2H6O2S+ | dimethyl sulfone | [10,37] | |
| 6 | 95.23 | 95.20 | C2H6S2+ | dimethyl disulfide | [10,23] | |
| 7 | 99.17 | 99.15 | C6H10O | 1-hexen-3-one | [38] | |
| 8 | 105.19 | 105.18 | C4H8OS | methional | [7,10,38] | |
| 9 | 107.17 | 107.13 | C7H6O+ | benzaldehyde | [25,59] | |
| 10 | 107.20 | 107.19 | C4H10OS+ | 3-(methylthio)propanol | [38,40] | |
| 11 | 109.07 | 109.06 | C7H8O+ | methoxybenzene (anisole) | [10] | |
| 12 | 109.25 | 109.24 | C3H8S2+ | bis(methylthio)methane | [6,39] | |
| 13 | 110.15 | 110.14 | C6H7NO | 4-amino-phenol | [7,10,22] | |
| 14 | 117.17 | 117.16 | C6H12O2+ | butanoic acid ethyl ester | [39,40] | |
| 15 | 117.19 | 117.17 | C6H12O2 | ethyl butyrate | [40] | |
| 16 | 118.11 | 118.14 | C5H11NO2+ | valine | [7,25,39] | |
| 17 | 120.08 | 120.03 | C4H9NO3+ | threonine | [7,10,25,39] | |
| 18 | 121.18 | 121.16 | C8H8O+ | benzeneacetaldehyde | [38,59] | |
| 19 | 123.07 | 123.67 | C8H10O+ | 2-phenylethanol | [10] | |
| 20 | 123.10 | 123.08 | C8H10O+ | p-cresyl methyl ether | [40] | |
| 21 | 123.19 | 123.17 | C8H10O | 3-ethylphenol | [41] | |
| 22 | 125.16 | 125.15 | C7H8O2+ | 2-acetyl-5-methyl furan | [10,23,25] | |
| 23 | 125.27 | 125.24 | C9H18O+ | nonanal | [10,59] | |
| 24 | 127.16 | 127.13 | C8H14O+ | 6-methyl-5-hepten-2-one | [38] | |
| 25 | 127.23 | 127,21 | C8H14O+ | 3,4-dimethyl-3-hexen-2-one | [38] | |
| 26 | 127.29 | 127.27 | C2H6S3+ | dimethyl trisulfide | [3,38] | |
| 27 | 129.21 | 129.18 | C10H8+ | naphthalene | [10] | |
| 28 | 129.25 | 129.22 | C8H16O+ | 1-octen-3-ol | [3] | |
| 28 | 131.20 | 131.19 | C7H14O2+ | butanoic acid propyl ester | [38] | |
| 29 | 132.17 | 132.75 | C5H12N2O2+ | ornithine | [7,10,25,38,39] | |
| 30 | 132.19 | 132.18 | C6H13NO2+ | leucine | [7,10,25,38,39] | |
| 31 | 133.08 | 133.06 | C4H8O3+ | asparagine | [7,10,25,38] | |
| 32 | 135.25 | 135.23 | C10H14+ | p-cymene | [25,37,38] | |
| 33 | 136.20 | 136.19 | C7H5NS+ | benzothiazole | [37,60] | |
| 34 | 137.22 | 137.20 | C9H12O | 3-methyl-5-ethylphenol | [40,41,59] | |
| 35 | 137.26 | 137.24 | C10H16+ | D-limonene | [38] | |
| 36 | 137.27 | 137.25 | C10H14+ | cis-ocimene | [38] | |
| 37 | 141.31 | 141.29 | C3H8S3+ | methyl(methylthio)dimethyl sulfoxide | [3,38] | |
| 38 | 143.23 | 143.21 | C8H14O2+ | 2,4-octanedione | [37] | |
| 39 | 145.22 | 145.21 | C8H16O2+ | isobutyl hexanoate | [40] | |
| 40 | 147.21 | 147.19 | C6H14N2O2+ | lysine | [7,10,25] | |
| 41 | 149.19 | 149.17 | C9H8O2+ | cinnamic acid | [38] | |
| 42 | 150.21 | 150.20 | C6H11NO2S+ | methionine | [7,10,25] | |
| 43 | 151.23 | 151.22 | C10H14O+ | thymol | [21] | |
| 44 | 155.27 | 155.25 | C10H18O+ | α-terpineol | [38] | |
| 45 | 155.28 | 155.26 | C10H18O+ | eucalyptol | [38] | |
| 46 | 155.35 | 155.32 | C4H10S3+ | tris(methylthio)methane | [3,41] | |
| 47 | 156.18 | 156.16 | C6H9N3O2+ | histidine | [7,10,25,39] | |
| 48 | 157.25 | 157.23 | C9H16O2+ | 2-pentyl-3-butenoic acid | [59] | |
| 49 | 159.26 | 159.25 | C9H18O2+ | 2-isopropyl-hexanoic acid | [41] | |
| 50 | 165.19 | 165.17 | C9H8O3+ | p-coumaric acid | [22] | |
| 51 | 165.23 | 165.21 | C10H12O2+ | eugenol | [38] | |
| 52 | 169.18 | 169.16 | C8H8O4+ | homogentisic acid | [22] | |
| 53 | 169.31 | 169.29 | C11H20O+ | 2-methylisoborneol | [21] | |
| 54 | 171.15 | 171.13 | C7H6O5+ | gallic acid | [22,25] | |
| 55 | 171.28 | 171.26 | C10H18O2 | 3-methyl-2-nonenoic acid | [38,60] | |
| 56 | 171.36 | 171.34 | C12H26+ | 2,4-dimethyl-decane | [38] | |
| 57 | 173.11 | 173.15 | C10H20O2+ | capric acid | [22,25,38] | |
| 58 | 173.29 | 173.27 | C10H20O2+ | isobutyl hexanoate | [40] | |
| 59 | 177.14 | 177.13 | C6H8O6+ | ascorbic acid | [22] | |
| 60 | 179.28 | 179.24 | C11H14O2+ | benzene-1,2-dimethoxy-4-(2-propenyl) | [39] | |
| 61 | 181.19 | 181.17 | C9H8O4+ | caffeic acid | [22,25] | |
| 62 | 183.19 | 183.17 | C6H14O6+ | D-allitol | [51] | |
| 63 | 187.24 | 187.22 | C12H10O2+ | 2-naphthylacetic acid | [38] | |
| 64 | 195.21 | 195.19 | C10H10O4 | ferulic acid | [7,10,25] | |
| 65 | 205.36 | 205.35 | C15H24+ | α-cubebene | [10,38] | |
| 66 | 205.37 | 205.35 | C15H24+ | caryophyllene | [10,39] | |
| 67 | 205.38 | 205.36 | C15H24+ | β-elemene | [10,38] | |
| 68 | 217.35 | 217.33 | C12H24O3+ | triisopropyl-S-trioxane | [3,38] | |
| 69 | 227.36 | 227.35 | C14H26O2+ | 8-dodecenyl acetate | [10,38] | |
| 70 | 230.32 | 230.31 | C9H15N3O2S+ | L-ergothioneine | [7] | |
| 71 | 235.40 | 235.39 | C15H26N2+ | sparteine | [7] | |
| 72 | 239.35 | 239.34 | C16H18N2 | agroclavine | [7] | |
| 73 | 241.33 | 241.31 | C6H12N2O4S2+ | cystine | [7,10,39] | |
| 74 | 255.43 | 255.42 | C16H30O2+ | palmitoleic acid | [22,25] | |
| 75 | 257.27 | 257.25 | C16H32O2 | palmitic acid | [22] | |
| 76 | 273.45 | 272,43 | C19H28O | androstenone | [52] | |
| 77 | 278.25 | 278.24 | C9H17NO8+ | neuraminic acid | [7] | |
| 78 | 281.41 | 281.40 | C18H32O2 | linoleic acid | [22,25] | |
| 79 | 281.46 | 281.45 | C18H32O2 | octadecadienoic acid | [22,25,38] | |
| 80 | 283.51 | 283.50 | C18H34O2+ | oleic acid | [22,25] | |
| 81 | 289.47 | 289.45 | C18H36O2+ | stearic acid | [22,25] | |
| 82 | 291.11 | 291.09 | C15H14O6+ | catechin | [21] | |
| 83 | 298.30 | 298.28 | C11H15N5O5+ | 7-methylguanosine | [7] | |
| 84 | 300.27 | 300.29 | C18H37NO2+ | sphing-4-enine | [54] | |
| 85 | 303.06 | 303.05 | C20H30O2+ | eicosapentaenoic acid | [22] | |
| 86 | 305.53 | 305.51 | C20H32O2+ | arachidonic acid | [7,22] | |
| 87 | 309.53 | 309.51 | C20H36O2+ | ethyl linolate | [21,22] | |
| 88 | 322.38 | 322.36 | C11H19N3O6S | S-methyl glutathione | [1] | |
| 89 | 329.52 | 329.51 | C22H32O2+ | docosahexaenoic acid | [22] | |
| 90 | 341.35 | 341.34 | C22H44O2+ | behenic acid | [22] | |
| 91 | 343.32 | 343.31 | C12H22O11+ | trehalose | [22] | |
| 92 | 369.62 | 369.61 | C24H48O2+ | lignoceric acid | [22,25] | |
| 93 | 387.38 | 387.37 | C27H46O+ | cholesterol | [48,50,53,57,58,59,60] | |
| 94 | 401.71 | 401.69 | C28H48O | campestanol | [48,50,53,57,58,59,60] | |
| 95 | 411.74 | 411.72 | C30H50+ | squalene | [7,23,45] | |
| 96 | 413.71 | 413.70 | C29H48O+ | fucosterol | [48,50,53,57,58,59,60] | |
| 97 | 415.73 | 415.71 | C29H50O+ | beta-sitosterol | [7,45] | |
| 98 | 419.71 | 419.70 | C27H46O3 | cholest-5-en-3β,6,24S-triol | [48,50,53,57,58,59,60] | |
| 99 | 425.72 | 425.70 | C30H48O+ | lupenone | [7,22,45] | |
| 100 | 427.74 | 427.73 | C30H50O | lupeol | [7,22,45] | |
| 101 | 537.92 | 537.91 | C40H56+ | lycopene | [22] | |
| 102 | 596.51 | 586.50 | C31H24O12+ | kolaflavanone | [7] | |
| 103 | 812.72 | 812.70 | C46H89NO8 | glucosylceramide | [7,53,54] | |
| Tuber brumale | 1 | 95.23 | 95.20 | C2H6S2+ | dimethyl disulfide | [3,7,10,23,37] |
| 2 | 99.17 | 99.15 | C6H10O | 1-hexen-3-one | [38] | |
| 3 | 105.19 | 105.18 | C4H8OS | methional | [7,10,39] | |
| 4 | 107.17 | 107.13 | C7H6O+ | benzaldehyde | [25,60] | |
| 5 | 107.20 | 107.19 | C4H10OS+ | 3-(methylthio)propanol | [38,40] | |
| 6 | 109.07 | 109.06 | C7H8O+ | methoxybenzene (anisole) | [10] | |
| 7 | 109.25 | 109.24 | C3H8S2+ | bis(methylthio)methane | [6,38] | |
| 8 | 110.15 | 110.14 | C6H7NO | 4-amino-phenol | [7,10,22] | |
| 9 | 117.17 | 117.16 | C6H12O2+ | butanoic acid ethyl ester | [38,41] | |
| 10 | 117.19 | 117.17 | C6H12O2 | ethyl butyrate | [40] | |
| 11 | 118.11 | 118.14 | C5H11NO2+ | valine | [10,25,39] | |
| 12 | 120.14 | 120.13 | C4H9NO3+ | threonine | [7,10,25,39] | |
| 13 | 121.18 | 121.16 | C8H8O+ | benzeneacetaldehyde | [38,59] | |
| 14 | 123.07 | 123.67 | C8H10O+ | 2-phenylethanol | [10] | |
| 15 | 123.19 | 123.17 | C8H10O+ | 3-ethylphenol | [41] | |
| 16 | 123.10 | 123.08 | C8H10O+ | p-cresyl methyl ether | [40] | |
| 17 | 125.16 | 125.15 | C7H8O2+ | 2-acetyl-5-methylfuran | [10,23,25] | |
| 18 | 125.27 | 125.24 | C9H18O+ | nonanal | [10,59] | |
| 19 | 127.16 | 127.13 | C8H14O+ | 6-methyl-5-hepten-2-one | [38] | |
| 20 | 127.23 | 127.21 | C8H14O+ | 3,4-dimethyl-3-hexen-2-one | [38] | |
| 21 | 127.29 | 127.27 | C2H6S3+ | dimethyl trisulfide | [10,23] | |
| 22 | 129.21 | 129.18 | C10H8+ | naphthalene | [10] | |
| 23 | 129.25 | 129.22 | C8H16O+ | 1-octen-3-ol | [3] | |
| 24 | 131.20 | 131.19 | C7H14O2+ | butanoic acid propyl ester | [38] | |
| 25 | 132.17 | 132.75 | C5H12N2O2+ | ornithine | [7,10,25,38,39] | |
| 26 | 132.19 | 132.18 | C6H13NO2+ | leucine | [7,10,25,38,39] | |
| 27 | 133.08 | 133.06 | C4H8O3+ | asparagine | [7,10,25,39] | |
| 28 | 135.25 | 135.23 | C10H14+ | p-cymene | [25,37,38] | |
| 29 | 137.22 | 137.20 | C9H12O+ | 3-methyl-5-ethylphenol | [40,41,59] | |
| 30 | 137.26 | 137.24 | C10H16+ | D-limonene | [38] | |
| 31 | 137.27 | 137.25 | C10H14+ | cis-ocimene | [38] | |
| 32 | 141.31 | 141.29 | C3H8S3+ | methyl(methylthio)dimethyl sulfoxide | [3,38] | |
| 33 | 143.23 | 143.21 | C8H14O2+ | 2,4-octanedione | [37] | |
| 34 | 145.22 | 145.21 | C8H16O2 | isobutyl hexanoate | [40] | |
| 35 | 147.21 | 147.19 | C6H14N2O2+ | lysine | [7,10,25] | |
| 36 | 149.19 | 149.17 | C9H8O2+ | cinnamic acid | [39] | |
| 37 | 150.21 | 150.21 | C6H11NO2S+ | methionine | [7,10,25] | |
| 38 | 151.23 | 151.22 | C10H14O+ | thymol | [21] | |
| 39 | 155.27 | 155.25 | C10H18O+ | α-terpineol | [38] | |
| 40 | 155.28 | 155.26 | C10H18O+ | eucalyptol | [38] | |
| 41 | 155.35 | 155.32 | C4H10S3+ | tris(methylthio)methane | [3,41] | |
| 42 | 156.18 | 156.16 | C6H9N3O2+ | histidine | [7,10,25,39] | |
| 43 | 157.25 | 157.23 | C9H16O2+ | 2-pentyl-3-butenoic acid | [59] | |
| 44 | 159.26 | 159.25 | C9H18O2+ | 2-isopropyl-hexanoic acid | [41] | |
| 45 | 162.15 | 162.13 | C7H15NO3+ | carnitine | [7] | |
| 46 | 165.19 | 165.17 | C9H8O3+ | p-coumaric acid | [22] | |
| 47 | 165.23 | 165.21 | C10H12O2+ | eugenol | [38] | |
| 48 | 169.18 | 169.16 | C8H8O4+ | homogentisic acid | [22] | |
| 49 | 169.31 | 169.29 | C11H20O+ | 2-methylisoborneol | [21] | |
| 50 | 171.15 | 171.13 | C7H6O5+ | gallic acid | [22,25] | |
| 51 | 171.28 | 171.26 | C10H18O2 | 3-methyl-2-nonenoic acid | [38,59] | |
| 52 | 171.36 | 171.34 | C12H26+ | 2,4-dimethyl-decane | [38] | |
| 53 | 173.11 | 173.15 | C10H20O2+ | capric acid | [22,25,38] | |
| 54 | 173.29 | 173.27 | C10H20O2+ | isobutyl hexanoate | [40] | |
| 55 | 177.14 | 177.13 | C6H8O6+ | ascorbic acid | [22] | |
| 56 | 179.28 | 179.24 | C11H14O2+ | benzene-1,2-dimethoxy-4-(2-propenyl) | [38] | |
| 57 | 181.19 | 181.17 | C9H8O4+ | caffeic acid | [22,25] | |
| 58 | 183.19 | 183.17 | C6H14O6+ | D-allitol | [51] | |
| 59 | 183.40 | 183.38 | C6H14S3+ | dipropyl trisulfide | [10,23] | |
| 60 | 187.24 | 187.22 | C12H10O2+ | 2-naphthylacetic acid | [38] | |
| 61 | 195.21 | 195.19 | C10H10O4 | ferulic acid | [7,10,25] | |
| 62 | 205.36 | 205.35 | C15H24+ | α-cubebene | [10,38] | |
| 63 | 205.37 | 205.35 | C15H24+ | caryophyllene | [7,38] | |
| 64 | 205.38 | 205.36 | C15H24+ | β-elemene | [10,38] | |
| 65 | 217.35 | 217.33 | C12H24O3+ | triisopropyl-S-trioxane | [3,38] | |
| 66 | 227.34 | 227.30 | C10H10O2S2 | bis(2-methyl-3 furyl)disulfide | [40] | |
| 67 | 227.36 | 227.35 | C14H26O2+ | 8-dodecenyl acetate | [10,38] | |
| 68 | 230.32 | 230.31 | C9H15N3O2S+ | L-ergothioneine | [7] | |
| 69 | 235.40 | 235.39 | C15H26N2+ | sparteine | [7] | |
| 70 | 239.35 | 239.34 | C16H18N2 | agroclavine | [7] | |
| 71 | 241.03 | 241.31 | C6H12N2O4S2+ | cystine | [7,10,39] | |
| 72 | 255.43 | 255.42 | C16H30O2+ | palmitoleic acid | [22,25] | |
| 73 | 257.27 | 257.25 | C16H32O2 | palmitic acid | [22] | |
| 74 | 273.45 | 272,43 | C19H28O | androstenone | [53] | |
| 75 | 278.25 | 278.24 | C9H17NO8+ | neuraminic acid | [7] | |
| 76 | 281.41 | 281.40 | C18H32O2 | linoleic acid | [22,25] | |
| 77 | 281.46 | 281.45 | C18H32O2 | octadecadienoic acid | [22,25,38] | |
| 78 | 283.51 | 283.50 | C18H34O2+ | oleic acid | [22,25] | |
| 79 | 289.47 | 289.45 | C18H36O2+ | stearic acid | [22,25] | |
| 80 | 291.11 | 291.09 | C15H14O6+ | catechin | [21] | |
| 81 | 298.30 | 298.28 | C11H15N5O5+ | 7-methylguanosine | [7] | |
| 82 | 300.27 | 300.29 | C18H37NO2+ | sphing-4-enine | [56] | |
| 83 | 303.06 | 303.05 | C20H30O2+ | eicosapentaenoic acid | [22] | |
| 84 | 305.53 | 305.51 | C20H32O2+ | arachidonic acid | [7,22] | |
| 85 | 309.53 | 309.51 | C20H36O2+ | ethyl linolate | [21,22] | |
| 86 | 311.37 | 311.36 | C16H24NO5+ | sinapine | [7] | |
| 87 | 322.38 | 322.36 | C11H19N3O6S | S-methyl glutathione | [1] | |
| 88 | 329.52 | 329.51 | C22H32O2+ | docosahexaenoic acid | [22] | |
| 89 | 341.35 | 341.34 | C22H44O2+ | behenic acid | [22] | |
| 90 | 343.32 | 343.31 | C12H22O11+ | trehalose | [22] | |
| 91 | 369.62 | 369.61 | C24H48O2+ | lignoceric acid | [22,25] | |
| 92 | 387.38 | 387.37 | C27H46O+ | cholesterol | [48,50,53,57,58,59,60] | |
| 93 | 397.61 | 397.60 | C28H44O | ergosta-5,7,22-trien-ß-ol | [48,50,53,57,58,59,60] | |
| 94 | 397.66 | 397.65 | C28H44O | ergosterol | [51,53,57,58,59,60] | |
| 95 | 399.69 | 399.67 | C28H46O | brassicasterol | [7,45,48,50,53,57,58,59,60] | |
| 96 | 401.71 | 401.69 | C28H48O | campestanol | [7,45,48,50,53,57,58,59,60] | |
| 97 | 411.74 | 411.72 | C30H50+ | squalene | [7,23,45] | |
| 98 | 413.71 | 413.70 | C29H48O+ | fucosterol | [48,50,53,57,58,59,60] | |
| 99 | 415.73 | 415.71 | C29H50O+ | beta-sitosterol | [7,45,48,50,53,57,58,59,60] | |
| 100 | 419.71 | 419.70 | C27H46O3 | cholest-5-en-3β,6,24S-triol | [48,50,53,57,58,59,60] | |
| 101 | 425.72 | 425.70 | C30H48O+ | lupenone | [7,22,45] | |
| 102 | 427.74 | 427.73 | C30H50O | lupeol | [7,45] | |
| 103 | 537.92 | 537.91 | C40H56+ | lycopene | [22] | |
| 104 | 596.51 | 586.50 | C31H24O12+ | kolaflavanone | [7] | |
| 105 | 812.72 | 812.70 | C46H89NO8 | glucosylceramide | [1,7,54] |
| No. | VOC Name | Odor |
|---|---|---|
| 1 | dimethylsulfone | sulfuric |
| 2 | dimethylsulfide | cabbage, sulfurous onion |
| 3 | dimethyl disulfide | cabbage, onion |
| 4 | methional | mold, French fry, yeasty |
| 5 | isoamyl alcohol | alcoholic, fruity |
| 6 | 3-hydroxy-2-butanone | dairy, buttery |
| 7 | 1-hexen-3-one | vegetable metallic |
| 8 | benzaldehyde | sweet almond |
| 9 | 3-(methylthio)propanol | onion, garlic |
| 10 | methoxybenzene (anisole) | anise seed |
| 11 | bis(methylthio)methane | garlic sulfurous, mushroom |
| 12 | 4-amino-phenol | sweet, balsamic |
| 13 | butanoic acid ethyl ester | sweet, fruity (apple) |
| 14 | ethyl butyrate | fruity, sweet |
| 15 | benzeneacetaldehyde | earthy, chocolate, floral |
| 16 | 2-phenylethanol | floral |
| 17 | p-cresyl methyl ether | nutty, camphor |
| 18 | 3-ethylphenol | phenolic |
| 19 | 2-acetyl-5-methylfuran | nutty, dusty |
| 20 | nonanal | citrus |
| 21 | 6-methyl-5-hepten-2-one | citrus, green, nutty |
| 22 | 3,4-dimethyl-3-hexen-2-one | blue-cheese, nutty |
| 23 | dimethyl trisulfide | onion, leek |
| 24 | naphthalene | naphthalene |
| 25 | 1-octen-3-ol | earthy, green, mushroom |
| 26 | butanoic acid propyl ester | fruity, pineapple |
| 27 | benzothiazole | sulfurous, nutty |
| 28 | 3-methyl-5-ethylphenol | fruity |
| 29 | methyl(methylthio)dimethyl sulfoxide | sulfurous, broccoli |
| 30 | 2,4-octanedione | earthy, dill |
| 31 | isobutyl hexanoate | sweet, fruity |
| 32 | tris(methylthio)methane | earthy, mushroom |
| 33 | carnitine | fishy |
| 34 | 2-methylisoborneol | earthy, musty |
| 35 | 3-methyl-2-nonenoic acid | fruity |
| 36 | isobutyl hexanoate | fruity, green |
| 37 | benzene-1,2-dimethoxy-4-(2-propenyl) | spicy, woody |
| 38 | dipropyl trisulfide | sulfurous, garlic, pungent |
| 39 | triisopropyl-S-trioxane | dairy |
| 40 | bis(2-methyl-3 furyl)disulfide | sulfurous, meaty |
| 41 | 8-dodecenyl acetate | fruity, pineapple |
| 42 | androstenone | urine, sweet, floral |
| 43 | S-methyl glutathione | allium, sulfurous |
| Sample Fraction | Chemical Class | Metabolite Name |
|---|---|---|
| Tuber magnatum pico | Amino acids | alanine |
| valine | ||
| threonine | ||
| ornithine | ||
| leucine | ||
| asparagine | ||
| lysine | ||
| methionine | ||
| histidine | ||
| cystine | ||
| Saccharides and nucleoside | trehalose | |
| 7-methylguanosine | ||
| glucosylceramide | ||
| Flavonoids | sparteine | |
| agroclavine | ||
| kolaflavanone | ||
| Organic acids | cinnamic acid | |
| 2-pentyl-3-butenoic acid | ||
| 2-isopropyl-hexanoic acid | ||
| p-coumaric acid | ||
| 3-methyl-2-nonenoic acid | ||
| capric acid | ||
| 2-naphthylacetic acid | ||
| neuraminic acid | ||
| homogentisic acid | ||
| Phenols and alcohols | 4-amino-phenol | |
| isoamyl alcohol | ||
| D-allitol | ||
| 2-phenylethanol | ||
| 3-ethylphenol | ||
| 1-octen-3-ol | ||
| 3-methyl-5-ethylphenol | ||
| Esters | butanoic acid ethyl ester | |
| butanoic acid propyl ester | ||
| ethyl butyrate | ||
| 8-dodecenyl acetate | ||
| Sulfur compounds | dimethylsulfide | |
| dimethylsulfone | ||
| dimethyl disulfide | ||
| methional | ||
| bis(methylthio)methane | ||
| methyl(methylthio)dimethyl sulfoxide | ||
| 3-(methylthio)propanol | ||
| tris(methylthio)methane | ||
| triisopropyl-S-trioxane | ||
| L-ergothioneine | ||
| S-methyl glutathione | ||
| dimethyl trisulfide | ||
| benzothiazole | ||
| Terpenoids and sesquiterpenes | p-cymene | |
| α-terpineol | ||
| D-limonene | ||
| cis-ocimene | ||
| thymol | ||
| eucalyptol | ||
| 2-methylisoborneol | ||
| α-cubebene | ||
| caryophyllene | ||
| β-elemene | ||
| squalene | ||
| lupenone | ||
| lupeol | ||
| Aldehyde and ketone | benzaldehyde | |
| 3-hydroxy-2-butanone | ||
| benzeneacetaldehyde | ||
| nonanal | ||
| 1-Hexen-3-one | ||
| 6-methyl-5-hepten-2-one | ||
| 3,4-dimethyl-3-hexen-2-one | ||
| 2,4-octanedione | ||
| Phenolic acids | ferulic acid | |
| gallic acid | ||
| caffeic acid | ||
| catechin | ||
| Fatty acids | palmitoleic acid | |
| palmitic acid | ||
| linoleic acid | ||
| octadecadienoic acid | ||
| oleic acid | ||
| stearic acid | ||
| eicosapentaenoic acid | ||
| arachidonic acid | ||
| ethyl linolate | ||
| docosahexaenoic acid | ||
| behenic acid | ||
| lignoceric acid | ||
| Sterol and steroids | cholesterol | |
| campestanol | ||
| fucosterol | ||
| beta-sitosterol | ||
| cholest-5-en-3β,6,24S-triol | ||
| Hydrocarbons | 2,4-dimethyl-decane | |
| 2-acetyl-5-methylfuran | ||
| naphthalene | ||
| p-cymene | ||
| eugenol | ||
| Other | sphing-4-enine (ceramide) | |
| isobutyl hexanoate (fatty acid esters) | ||
| ascorbic acid (vitamin) | ||
| lycopene (carotenoid) | ||
| benzene-1,2-dimethoxy-4-(2-propenyl) | ||
| p-cresyl methyl ether | ||
| methoxybenzene (anisole) | ||
| Tuber brumale | Amino acids | valine |
| threonine | ||
| ornithine | ||
| leucine | ||
| asparagine | ||
| lysine | ||
| methionine | ||
| cystine | ||
| Saccharides and nucleoside | trehalose | |
| 7-methylguanosine | ||
| glucosylceramide | ||
| Flavonoids | sparteine | |
| agroclavine | ||
| kolaflavanone | ||
| Organic acids | cinnamic acid | |
| p-coumaric acid | ||
| 3-methyl-2-nonenoic acid | ||
| capric acid | ||
| 2-naphthylacetic acid | ||
| neuraminic acid | ||
| homogentisic acid | ||
| 2-pentyl-3-butenoic acid | ||
| 2-isopropyl-hexanoic acid | ||
| Phenols and alcohols | 4-amino-phenol | |
| 3-ethylphenol | ||
| 1-octen-3-ol | ||
| 3-methyl-5-ethylphenol | ||
| 2-phenylethanol | ||
| D-allitol | ||
| Esters | butanoic acid ethyl ester | |
| butanoic acid propyl ester | ||
| ethyl butyrate | ||
| 8-dodecenyl acetate | ||
| Sulfur compounds | dimethyl trisulfide | |
| benzothiazole | ||
| methional | ||
| bis(methylthio)methane | ||
| methyl(methylthio)dimethyl sulfoxide | ||
| 3-(methylthio)propanol | ||
| tris(methylthio)methane | ||
| triisopropyl-S-trioxane | ||
| L-ergothioneine | ||
| S-methyl glutathione | ||
| dipropyl trisulfide | ||
| bis(2-methyl-3 furyl)disulfide | ||
| Terpenoids and sesquiterpenes | p-cymene | |
| α-terpineol | ||
| D-limonene | ||
| cis-ocimene | ||
| thymol | ||
| eucalyptol | ||
| 2-methylisoborneol | ||
| α-cubebene | ||
| caryophyllene | ||
| β-elemene | ||
| squalene | ||
| lupenone | ||
| lupeol | ||
| Aldehyde and ketone | benzaldehyde | |
| 3-hydroxy-2-butanone | ||
| benzeneacetaldehyde | ||
| nonanal | ||
| 1-Hexen-3-one | ||
| 6-methyl-5-hepten-2-one | ||
| 3,4-dimethyl-3-hexen-2-one | ||
| 2,4-octanedione | ||
| Phenolic acid | gallic acid | |
| ferulic acid | ||
| caffeic acid | ||
| catechin | ||
| Hydrocarbons | 2,4-dimethyl-decane | |
| 2-acetyl-5-methylfuran | ||
| naphthalene | ||
| p-cymene | ||
| eugenol | ||
| Fatty acids | palmitoleic acid | |
| palmitic acid | ||
| linoleic acid | ||
| octadecadienoic acid | ||
| oleic acid | ||
| stearic acid | ||
| eicosapentaenoic acid | ||
| arachidonic acid | ||
| ethyl linolate | ||
| docosahexaenoic acid | ||
| behenic acid | ||
| lignoceric acid | ||
| Sterol and steroids | cholesterol | |
| campestanol | ||
| fucosterol | ||
| beta-sitosterol | ||
| cholest-5-en-3β,6,24S-triol | ||
| ergosta-5,7,22-trien-ß-ol | ||
| ergosterol | ||
| brassicasterol | ||
| Others | sphing-4-enine (ceramide) | |
| isobutyl hexanoate (fatty acid esters) | ||
| ascorbic acid (vitamins) | ||
| lycopene (carotenoid) | ||
| benzene-1,2-dimethoxy-4-(2-propenyl) | ||
| p-cresyl methyl ether | ||
| Lycopene (carotenoid) | ||
| Sinapine (alkaloid) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segneanu, A.-E.; Cepan, M.; Bobica, A.; Stanusoiu, I.; Dragomir, I.C.; Parau, A.; Grozescu, I. Chemical Screening of Metabolites Profile from Romanian Tuber spp. Plants 2021, 10, 540. https://doi.org/10.3390/plants10030540
Segneanu A-E, Cepan M, Bobica A, Stanusoiu I, Dragomir IC, Parau A, Grozescu I. Chemical Screening of Metabolites Profile from Romanian Tuber spp. Plants. 2021; 10(3):540. https://doi.org/10.3390/plants10030540
Chicago/Turabian StyleSegneanu, Adina-Elena, Melinda Cepan, Adrian Bobica, Ionut Stanusoiu, Ioan Cosmin Dragomir, Andrei Parau, and Ioan Grozescu. 2021. "Chemical Screening of Metabolites Profile from Romanian Tuber spp." Plants 10, no. 3: 540. https://doi.org/10.3390/plants10030540
APA StyleSegneanu, A.-E., Cepan, M., Bobica, A., Stanusoiu, I., Dragomir, I. C., Parau, A., & Grozescu, I. (2021). Chemical Screening of Metabolites Profile from Romanian Tuber spp. Plants, 10(3), 540. https://doi.org/10.3390/plants10030540






