Simultaneous Quantification of Four Marker Compounds in Bauhinia coccinea Extract and Their Potential Inhibitory Effects on Alzheimer’s Disease Biomarkers
Abstract
1. Introduction
2. Results
2.1. Optimization of HPLC Condition
2.2. Regression Equation, Linearity, Limits of Detection (LOD), and Limits of Quantification (LOQ)
2.3. Quantitative Analysis of the Four Marker Compounds in EEBC
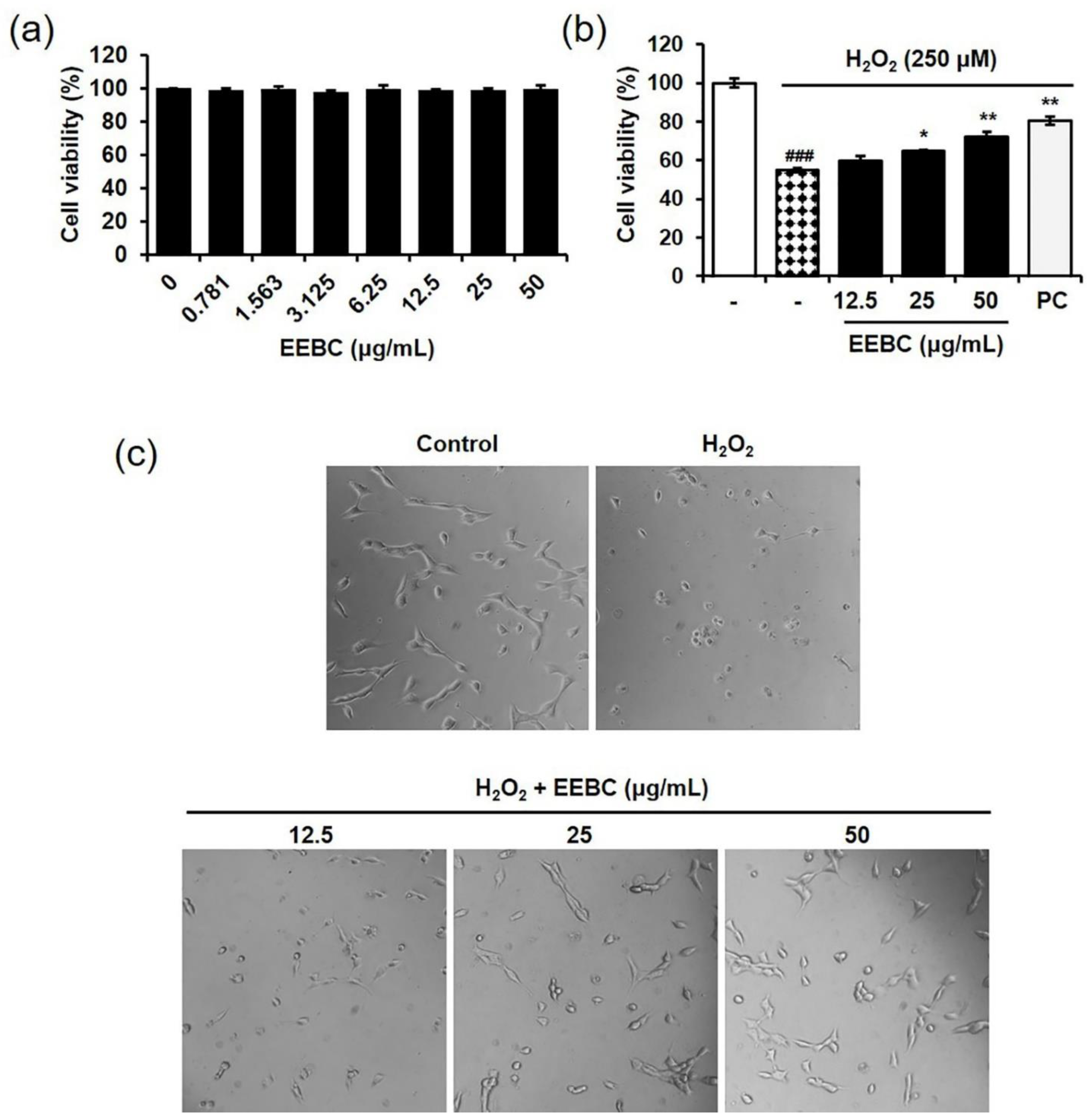
2.4. Protective Effects of EEBC in Hydrogen Peroxide (H2O2)-Induced Neuronal Cell Damage
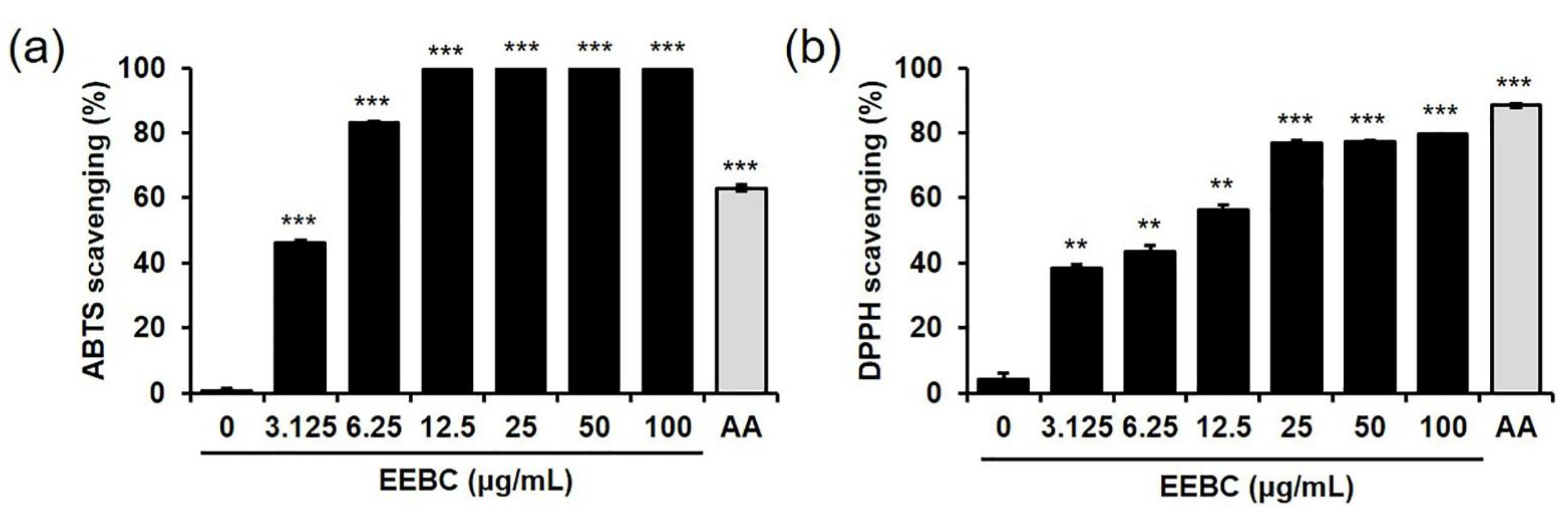
2.5. Antioxidant Activity of EEBC via Free Radical Scavenging Actions
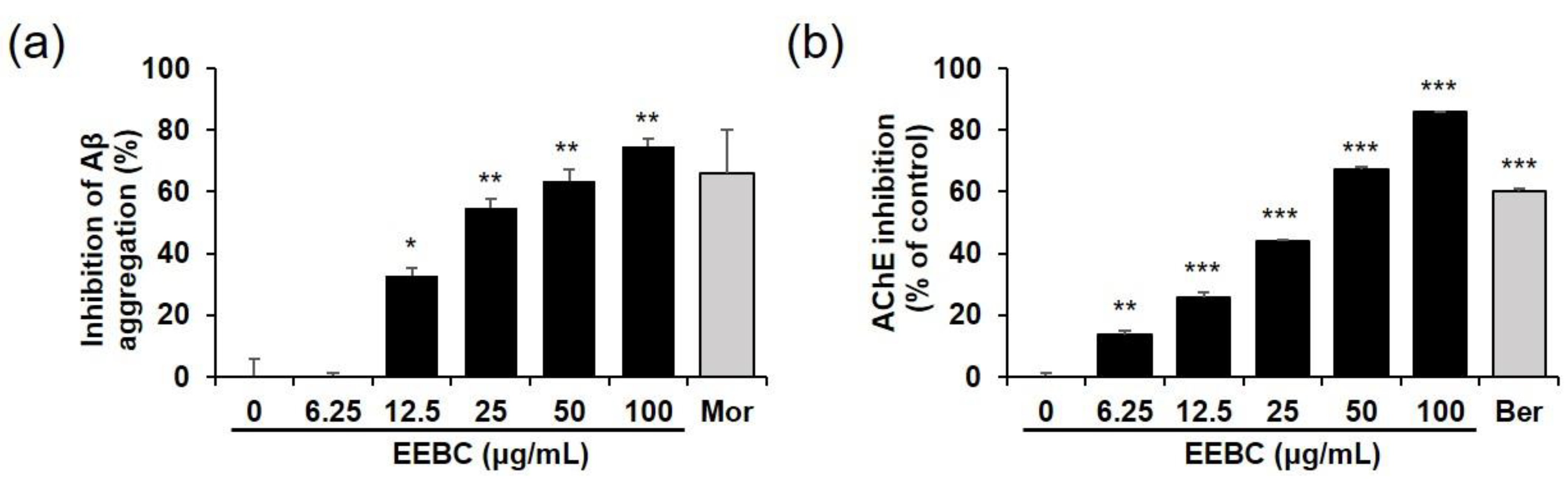
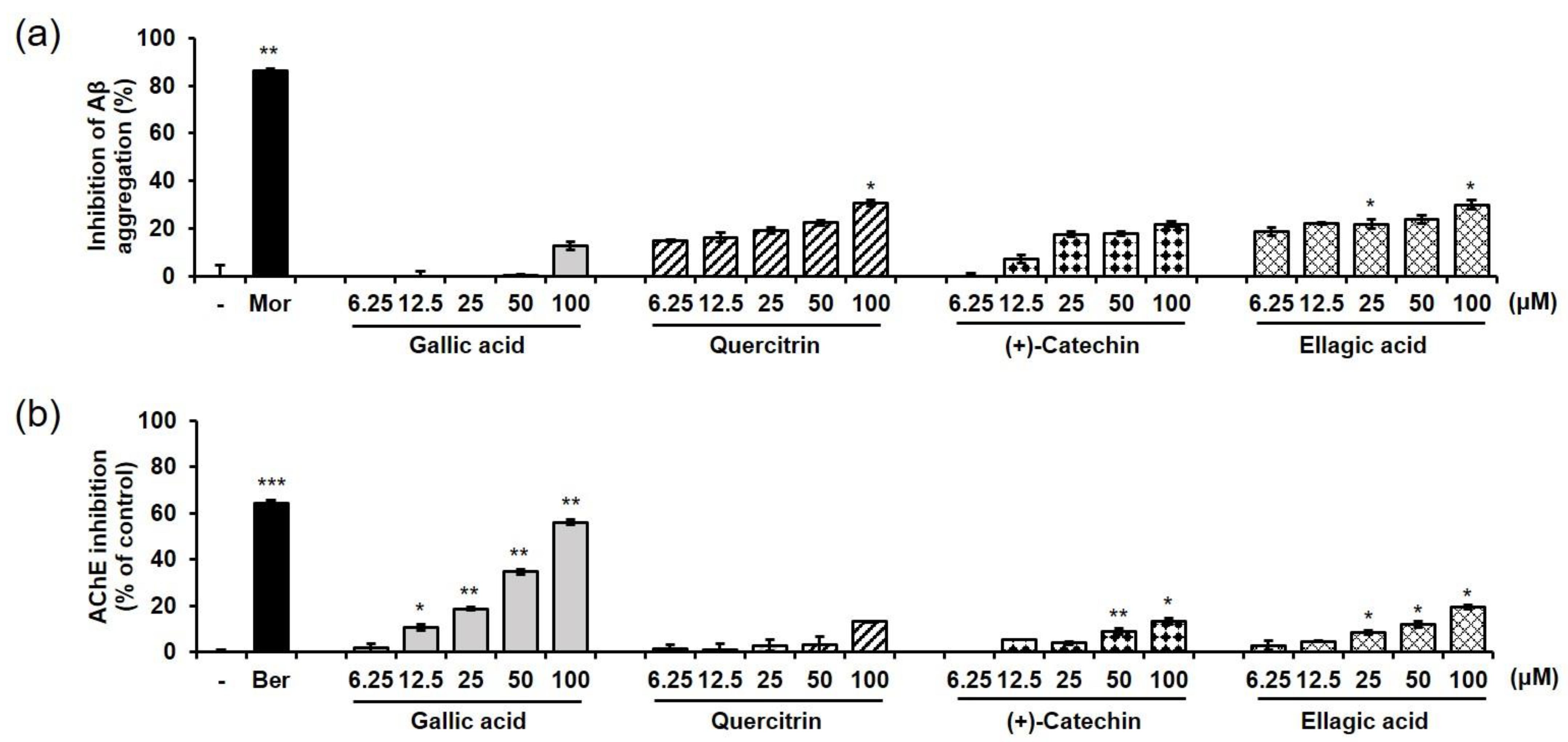
2.6. Effects of EEBC and Its Marker Compounds on AD Biomarkers
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Chemicals and Reagents
4.3. Preparation of Sample and Standard Solutions
4.4. Apparatus and Chromatographic Conditions
4.5. Calibration Curve and Limits of Detection and Quantification
4.6. Cell Culture and Drug Treatment
4.7. Cell Viability Assay
4.8. Free Radical Scavenging Assay
4.9. Aβ Aggregation Assay
4.10. AChE and BChE Activity Assay
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armanios, M.; de Cabo, R.; Mannick, J.; Partridge, L.; van Deursen, J.; Villeda, S. Translational strategies in aging and age-related disease. Nat. Med. 2015, 21, 1395–1399. [Google Scholar] [CrossRef]
- Friedman, J. Why is the nervous system vulnerable to oxidative stress? In Oxidative Stress and Free Radical Damage in Neurology; Gadoth, N., Govel, H.H., Eds.; Humana Press: Totowa, NJ, USA, 2011; pp. 19–26. [Google Scholar]
- Dong, Y.; Brewer, G.J. Global metabolic shifts in age and Alzheimer’s disease mouse brains pivot at NAD+/NADH redox sites. J. Alzheimers Dis. 2019, 71, 119–140. [Google Scholar] [CrossRef] [PubMed]
- Martínez de Toda, I.; Miguélez, L.; Vida, C.; Carro, E.; De la Fuente, M. Altered redox state in whole blood cells from patients with mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis. 2019, 71, 153–163. [Google Scholar] [CrossRef]
- Lazarevic-Pasti, T.; Leskovac, A.; Momic, T.; Petrovic, S.; Vasic, V. Modulators of acetylcholinesterase activity: From Alzheimer’s disease to anti-cancer drugs. Curr. Med. Chem. 2017, 24, 3283–3309. [Google Scholar] [CrossRef] [PubMed]
- Morsy, A.; Trippier, P.C. Current and emerging pharmacological targets for the treatment of Alzheimer’s disease. J. Alzheimers Dis. 2019, 72, S145–S176. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, L.; Faustino, C. Therapeutic strategies targeting amyloid-β in Alzheimer’s disease. Curr. Alzheimer Res. 2019, 16, 418–452. [Google Scholar] [CrossRef]
- Birks, J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev. 2006. [Google Scholar] [CrossRef]
- Alldredge, B.K.; Corelli, R.L.; Ernst, M.E.; Guglielmo, B.J.; Jacobson, P.A.; Kradjan, W.A.; Williams, B.R. Koda-Kimble and Young’s Applied Therapeutics: The Clinical Use of Drugs, 10th ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Baltimore, MD, USA, 2013; p. 2385. [Google Scholar]
- Bui, T.T.; Nguyen, T.H. Natural product for the treatment of Alzheimer’s disease. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 413–423. [Google Scholar] [CrossRef]
- Ahmed-Chaouch, M.; Cheriet, T.; Beretta, G.; Sarri, D.; Bensouici, C.; Ouelbani, R.; Mancini, I.; Sekhara, I.; Seghiri, R. Chemical composition, in vitro antioxidant, anticholinesterase and antibacterial activities of Linaria scariosa Desf. Nat. Prod. Res. 2019, 1–5. [Google Scholar] [CrossRef]
- Ali Reza, A.S.M.; Hossain, M.S.; Akhter, S.; Rahman, M.R.; Nasrin, M.S.; Uddin, M.J.; Sadik, G.; Khurshid Alam, A.H.M. In vitro antioxidant and cholinesterase inhibitory activities of Elatostema papillosum leaves and correlation with their phytochemical profiles: A study relevant to the treatment of Alzheimer’s disease. BMC Complement. Altern. Med. 2018, 18, 123. [Google Scholar] [CrossRef]
- Roskov, Y.; Kunze, T.; Orrell, T.; Abucay, L.; Paglinawan, L.; Culham, A.; Bailly, N.; Kirk, P.; Bourgoin, T.; Baillargeon, G.; et al. Species 2000 & ITIS Catalogue of Life; 2014 Annual Checklist, Species 2000/IT IS; Naturalis: Leuden, The Netherlands, 2014. [Google Scholar]
- The Legume Phylogeny Working Group (LPWG). A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon 2017, 66, 44–77. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Gupta, Y.K.; Singh, S. In vivo anti-arthritic activity of Bauhinia purpurea Linn. Bark Extract. Indian J. Pharmacol. 2019, 51, 25–30. [Google Scholar] [PubMed]
- Hisam, E.E.; Zakaria, Z.A.; Mohtaruddin, N.; Rofiee, M.S.; Hamid, H.A.; Othman, F. Antiulcer activity of the chloroform extract of Bauhinia purpurea leaf. Pharm. Biol. 2012, 50, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Shreedhara, C.S.; Vaidya, V.P.; Vagdevi, H.M.; Latha, K.P.; Muralikrishna, K.S.; Krupanidhi, A.M. Screening of Bauhinia purpurea Linn. for analgesic and anti-inflammatory activities. Indian J. Pharmacol. 2009, 41, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Pinafo, M.S.; Benedetti, P.R.; Gaiotte, L.B.; Costa, F.G.; Schoffen, J.P.F.; Fernandes, G.S.A.; Chuffa, L.G.A.; Seiva, F.R.F. Effects of Bauhinia forficata on glycaemia, lipid profile, hepatic glycogen content and oxidative stress in rats exposed to Bisphenol A. Toxicol. Rep. 2019, 6, 244–252. [Google Scholar] [CrossRef]
- Curcio, S.A.; Stefan, L.F.; Randi, B.A.; Dias, M.A.; da Silva, R.E.; Caldeira, E.J. Hypoglycemic effects of an aqueous extract of Bauhinia forficata on the salivary glands of diabetic mice. Pak. J. Pharm. Sci. 2012, 25, 493–499. [Google Scholar]
- Salgueiro, A.C.; Folmer, V.; da Silva, M.P.; Mendez, A.S.; Zemolin, A.P.; Posser, T.; Franco, J.L.; Puntel, R.L.; Puntel, G.O. Effects of Bauhinia forficata tea on oxidative stress and liver damage in diabetic mice. Oxid. Med. Cell. Longev. 2016, 2016, 8902954. [Google Scholar] [CrossRef]
- Jian, J.; Xuan, F.; Qin, F.; Huang, R. The antioxidant, anti-Inflammatory and anti-apoptotic activities of the Bauhinia Championii flavone are connected with protection against myocardial ischemia/reperfusion injury. Cell. Physiol. Biochem. 2016, 38, 1365–1375. [Google Scholar] [CrossRef]
- Ni, L.; Huang, W.; Shi, Y.; Wang, H.; Qiu, Y.; Xu, H. Chemical constituents from the bark of Bauhinia purpurea and their NO inhibitory activities. Nat. Prod. Res. 2018, 23, 1–6. [Google Scholar] [CrossRef]
- Boonphong, S.; Puangsombat, P.; Baramee, A.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Bioactive compounds from Bauhinia purpurea possessing antimalarial, antimycobacterial, antifungal, anti-inflammatory, and cytotoxic activities. J. Nat. Prod. 2007, 70, 795–801. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, G.; Sun, C.; Li, H.; Fu, Y.; Xu, W. Apoptosis Effects of Dihydrokaempferol Isolated from Bauhinia championii on Synoviocytes. Evid. Based Complement. Alternat. Med. 2018, 2018, 9806160. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.P.; Zhang, Y.Q.; Ye, M.; Xu, W.; Wang, X.Y.; Fu, Y.H.; Xu, W. A new polyoxygenated abietane diterpenoid from the rattans of Bauhinia championii (Benth.) Benth. Nat. Prod. Res. 2018, 32, 2577–2582. [Google Scholar] [CrossRef] [PubMed]
- Beber, A.P.; de Souza, P.; Boeing, T.; Somensi, L.B.; Mariano, L.N.B.; Cury, B.J.; Burci, L.M.; da Silva, C.B.; Simionatto, E.; de Andrade, S.F.; et al. Constituents of leaves from Bauhinia curvula Benth. exert gastroprotective activity in rodents: Role of quercitrin and kaempferol. Inflammopharmacology 2018, 26, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhang, J.; Cai, Y.; Huang, J.; You, L. Catechin attenuates traumatic brain injury-induced blood-brain barrier damage and improves longer-term neurological outcomes in rats. Exp. Physiol. 2017, 102, 1269–1277. [Google Scholar] [CrossRef]
- Daglia, M.; Di Lorenzo, A.; Nabavi, S.F.; Talas, Z.S.; Nabavi, S.M. Polyphenols: Well beyond the antioxidant capacity: Gallic acid and related compounds as neuroprotective agents: You are what you eat! Curr. Pharm. Biotechnol. 2014, 15, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.B.; Panchal, S.S.; Shah, A. Ellagic acid: Insights into its neuroprotective and cognitive enhancement effects in sporadic Alzheimer’s disease. Pharmacol. Biochem. Behav. 2018, 175, 33–46. [Google Scholar] [CrossRef]
- Rattanajarasroj, S.; Unchern, S. Comparable attenuation of Abeta(25-35)-induced neurotoxicity by quercitrin and 17beta-estradiol in cultured rat hippocampal neurons. Neurochem. Res. 2010, 35, 1196–1205. [Google Scholar] [CrossRef]
- Ben Othman, S.; Yabe, T. Use of hydrogen peroxide and peroxyl radicals to induce oxidative stress in neuronal cells. Rev. Agric. Sci. 2015, 3, 40–45. [Google Scholar] [CrossRef][Green Version]
- Schultz, C.; Del Tredici, K.; Braak, H. Neuropathology of Alzheimer’s Disease. In Alzheimer’s Disease Current Clinical Neurology; Richter, R.W., Richiter, B.Z., Eds.; Humana Press: Totowa, NJ, USA, 2004; pp. 21–31. [Google Scholar]
- Donev, R.; Kolev, M.; Millet, B.; Thome, J. Neuronal death in Alzheimer’s disease and therapeutic opportunities. J. Cell. Mol. Med. 2009, 13, 4329–4348. [Google Scholar] [CrossRef]
- Satyabrata Kar, S.; Slowikowski, S.P.M.; Westaway, D.; Mount, H.T.J. Interactions between β-amyloid and central cholinergic neurons: Implications for Alzheimer’s disease. J. Psychiatry Neurosci. 2004, 29, 427–441. [Google Scholar]
- Alzheimer’s Society. Drug Treatments for Alzheimer’s Disease. Available online: https://www.alzheimers.org.uk/about-dementia/treatments/drugs/drug-treatments-alzheimers-disease (accessed on 23 October 2020).
- Panza, F.; Lozupone, M.; Logroscino, G.; Imbimbo, B.P. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2019, 15, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. Anti-amyloid failures stack up as Alzheimer antibody flops. Nat. Rev. Drug Discov. 2019, 18, 327. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, R.R.; Popovic-Nikolic, M.R.; Nikolic, K.; Uliassi, E.; Bolognesi, M.L. A perspective on multi-target drug discovery and design for complex diseases. Clin. Transl. Med. 2018, 7, 3. [Google Scholar] [CrossRef]
- Nunomura, A.; Perry, G.; Aliev, G.; Hirai, K.; Takeda, A.; Balraj, E.K.; Jones, P.K.; Ghanbari, H.; Wataya, T.; Shimohama, S.; et al. Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2001, 60, 759–767. [Google Scholar] [CrossRef]
- Resende, R.; Moreira, P.I.; Proença, T.; Deshpande, A.; Busciglio, J.; Pereira, C.; Oliveira, C.R. Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free Radic. Biol. Med. 2008, 44, 2051–2057. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxid. Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef]
- Davis, J.B.; Maher, P. Protein kinase C activation inhibits glutamate-induced cytotoxicity in a neuronal cell line. Brain Res. 1994, 652, 169–173. [Google Scholar] [CrossRef]
- Morimoto, B.H.; Koshland, D.E., Jr. Induction and expression of long- and short-term neurosecretory potentiation in a neural cell line. Neuron 1990, 5, 875–880. [Google Scholar] [CrossRef]
- Park, H.J.; Kwak, M.; Baek, S.H. Neuroprotective effects of Dendropanax morbifera leaves on glutamate-induced oxidative cell death in HT22 mouse hippocampal neuronal cells. J. Ethnopharmacol. 2020, 251, 112518. [Google Scholar] [CrossRef]
- Hirata, Y.; Yamada, C.; Ito, Y.; Yamamoto, S.; Nagase, H.; Oh-Hashi, K.; Kiuchi, K.; Suzuki, H.; Sawada, M.; Furuta, K. Novel oxindole derivatives prevent oxidative stress-induced cell death in mouse hippocampal HT22 cells. Neuropharmacology 2018, 135, 242–252. [Google Scholar] [CrossRef]
- Schulz, M.; Gonzaga, L.V.; de Souza, V.; Farina, M.; Vitali, L.; Micke, G.A.; Costa, A.C.O.; Fett, R. Neuroprotective effect of juçara (Euterpe edulis Martius) fruits extracts against glutamate-induced oxytosis in HT22 hippocampal cells. Food Res. Int. 2019, 120, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafidh, K.; Mhadhbi, L.; Mezni, A.; Badreddine, S.; Beyrem, H.; Mahmoudi, E. Protective effect of Zizyphus lotus jujube fruits against cypermethrin-induced oxidative stress and neurotoxicity in mice. Biomarkers 2018, 23, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Ranawat, P.; Sharma, N.; Nehru, B. Ginkgo biloba attenuates aluminum lactate-induced neurotoxicity in reproductive senescent female rats: Behavioral, biochemical, and histopathological study. Environ. Sci. Pollut. Res. Int. 2019, 26, 27148–27167. [Google Scholar] [CrossRef] [PubMed]
- Thomaz, D.V.; Peixoto, L.F.; de Oliveira, T.S.; Fajemiroye, J.O.; da Silva Neri, H.F.; Xavier, C.H.; Costa, E.A.; Dos Santos, F.C.A.; de Souza Gil, E.; Ghedini, P.C. Antioxidant and Neuroprotective Properties of Eugenia dysenterica Leaves. Oxid. Med. Cell. Longev. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, C.; Napolitano, M.; Tedesco, I.; Moccia, S.; Milito, A.; Russo, G.L. Neuroprotective Role of Natural Polyphenols. Curr. Top. Med. Chem. 2016, 16, 1943–1950. [Google Scholar] [CrossRef]
- Haass, C.; Selkoe, D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef]
- Saxena, M.; Dubey, R. Target Enzyme in Alzheimer’s Disease: Acetylcholinesterase Inhibitors. Curr. Top. Med. Chem. 2019, 19, 264–275. [Google Scholar] [CrossRef]
- Santos, K.M.; Gonçalves, P.S.; Paiva, M.J.; Lacerda, G.A. Acetylcholinesterase inhibition starting from extracts of Bauhinia variegata L., Bauhinia var. candida (Aiton) Buch.-Ham., and Bauhinia ungulata L. Rev. Soc. Bras. Med. Trop. 2011, 44, 781–783. [Google Scholar] [CrossRef]
- da Silva, H.C.; Pinto, F.D.C.L.; de Sousa, A.F.; Pessoa, O.D.L.; Trevisan, M.T.S.; Santiago, G.M.P. Chemical constituents and acetylcholinesterase inhibitory activity from the stems of Bauhinia pentandra. Nat. Prod. Res. 2020, 28, 1–5. [Google Scholar] [CrossRef]
- Khare, N.; Maheshwari, S.K.; Jha, A.K. Screening and identification of secondary metabolites in the bark of Bauhinia variegata to treat Alzheimer’s disease by using molecular docking and molecular dynamics simulations. J. Biomol. Struct. Dyn. 2020, 28, 1–11. [Google Scholar] [CrossRef]
- Inestrosa, N.C.; Alvarez, A.; Pérez, C.A.; Moreno, R.D.; Vicente, M.; Linker, C.; Casanueva, O.I.; Soto, C.; Garrido, J. Acetylcholinesterase accelerates assembly of amyloid-beta-peptides into Alzheimer’s fibrils: Possible role of the peripheral site of the enzyme. Neuron 1996, 16, 881–891. [Google Scholar] [CrossRef]
- Carvajal, F.J.; Inestrosa, N.C. Interactions of AChE with Aβ Aggregates in Alzheimer’s Brain: Therapeutic Relevance of IDN 5706. Front. Mol. Neurosci. 2011, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Guan, X.; Lin, R.; Liu, X.; Yan, Y.; Lin, R.; Zhang, T.; Chen, X.; Huang, J.; Sun, X.; et al. Silibinin inhibits acetylcholinesterase activity and amyloid β peptide aggregation: A dual-target drug for the treatment of Alzheimer’s disease. Neurobiol. Aging 2015, 36, 1792–1807. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Murakami, K.; Uno, M.; Nakagawa, Y.; Katayama, S.; Akagi, K.; Masuda, Y.; Takegoshi, K.; Irie, K. Site-specific inhibitory mechanism for amyloid β42 aggregation by catechol-type flavonoids targeting the Lys residues. J. Biol. Chem. 2013, 288, 23212–23224. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Sobolova, K.; Hrabinova, M.; Hepnarova, V.; Kucera, T.; Kobrlova, T.; Benkova, M.; Janockova, J.; Dolezal, R.; Prchal, L.; Benek, O.; et al. Discovery of novel berberine derivatives with balanced cholinesterase and prolyl oligopeptidase inhibition profile. Eur. J. Med. Chem. 2020, 203, 112593. [Google Scholar] [CrossRef] [PubMed]
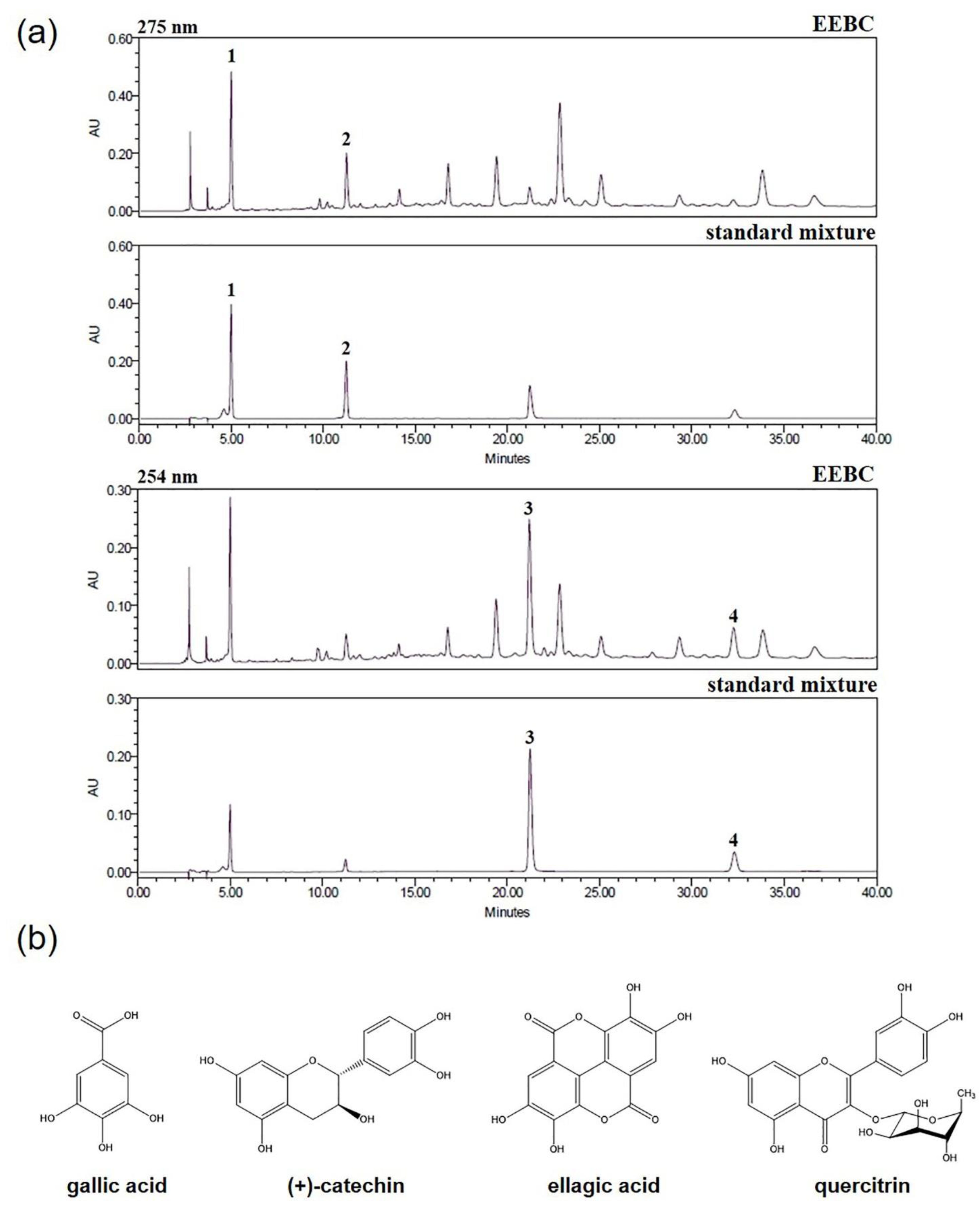
| Compound | Linear Range (μg/mL) | Regression Equation (y = ax + b) a) | r2 | LOD b) (μg/mL) | LOQ c) (μg/mL) | |
|---|---|---|---|---|---|---|
| Slope (a) | Intercept (b) | |||||
| Gallic acid | 6.25–200 | 25,130 | 1562.7 | 1.0000 | 0.726 | 2.199 |
| (+)-catechin | 12.5–400 | 8599.9 | −4988.9 | 1.0000 | 0.381 | 1.154 |
| Ellagic acid | 3.125–100 | 104,400 | −22,602 | 1.0000 | 0.315 | 0.954 |
| Quercitrin | 3.125–100 | 25,051 | −4781.8 | 1.0000 | 0.172 | 0.520 |
| Compound | Contents (mg/g) |
|---|---|
| Gallic acid | 11.757 ± 0.012 |
| (+)-catechin | 18.736 ± 0.034 |
| Ellagic acid | 2.912 ± 0.001 |
| Quercitrin | 3.897 ± 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.J.; Sohn, E.; Lim, H.-S.; Kim, Y.; Kim, J.-H.; Jeong, S.-J. Simultaneous Quantification of Four Marker Compounds in Bauhinia coccinea Extract and Their Potential Inhibitory Effects on Alzheimer’s Disease Biomarkers. Plants 2021, 10, 702. https://doi.org/10.3390/plants10040702
Kim YJ, Sohn E, Lim H-S, Kim Y, Kim J-H, Jeong S-J. Simultaneous Quantification of Four Marker Compounds in Bauhinia coccinea Extract and Their Potential Inhibitory Effects on Alzheimer’s Disease Biomarkers. Plants. 2021; 10(4):702. https://doi.org/10.3390/plants10040702
Chicago/Turabian StyleKim, Yu Jin, Eunjin Sohn, Hye-Sun Lim, Yoonju Kim, Joo-Hwan Kim, and Soo-Jin Jeong. 2021. "Simultaneous Quantification of Four Marker Compounds in Bauhinia coccinea Extract and Their Potential Inhibitory Effects on Alzheimer’s Disease Biomarkers" Plants 10, no. 4: 702. https://doi.org/10.3390/plants10040702
APA StyleKim, Y. J., Sohn, E., Lim, H.-S., Kim, Y., Kim, J.-H., & Jeong, S.-J. (2021). Simultaneous Quantification of Four Marker Compounds in Bauhinia coccinea Extract and Their Potential Inhibitory Effects on Alzheimer’s Disease Biomarkers. Plants, 10(4), 702. https://doi.org/10.3390/plants10040702






