Effect of Graft and Nano ZnO on Nutraceutical and Mineral Content in Bell Pepper
Abstract
:1. Introduction
2. Results
2.1. Graft Effect on Mineral Contents
2.2. Effect of ZnO NPs on Mineral Content
2.3. Effect of the Interaction between Grafting and ZnO NPs on Mineral Content
2.4. Effect of Grafting on Nutraceutical Content
2.5. Effect of ZnO NPs on Nutraceutical Content
2.6. Effect of the Interaction between Grafting and ZnO NPs on Nutraceutical Content
2.7. Effect of Grafting on Fruit Quality
2.8. Effect of ZnO NPs on Fruit Quality
2.9. Effect of Graft and ZnO NPs Interaction on Fruit Quality
3. Discussion
3.1. Graft Effect on Mineral Content
3.2. Effect of ZnO NPs on Mineral Content
3.3. Effect of the Interaction between Grafts and ZnO NPs on Mineral Content
3.4. Effect of the Graft on Nutraceutical Content
3.5. Effect of the ZnO NPs on Nutraceutical Content
3.6. Effect of the Interaction between Grafting and ZnO NPs on Nutraceutical Content
3.7. Effect of Grafting on Fruit Quality
3.8. Effect of ZnO NPs on Fruit Quality
3.9. Interactive Effects of Grafting and ZnO NPs on Fruit Quality
4. Materials and Methods
4.1. Plant Material and Growing Conditions
4.2. Graft
4.3. Transplant to NFT System
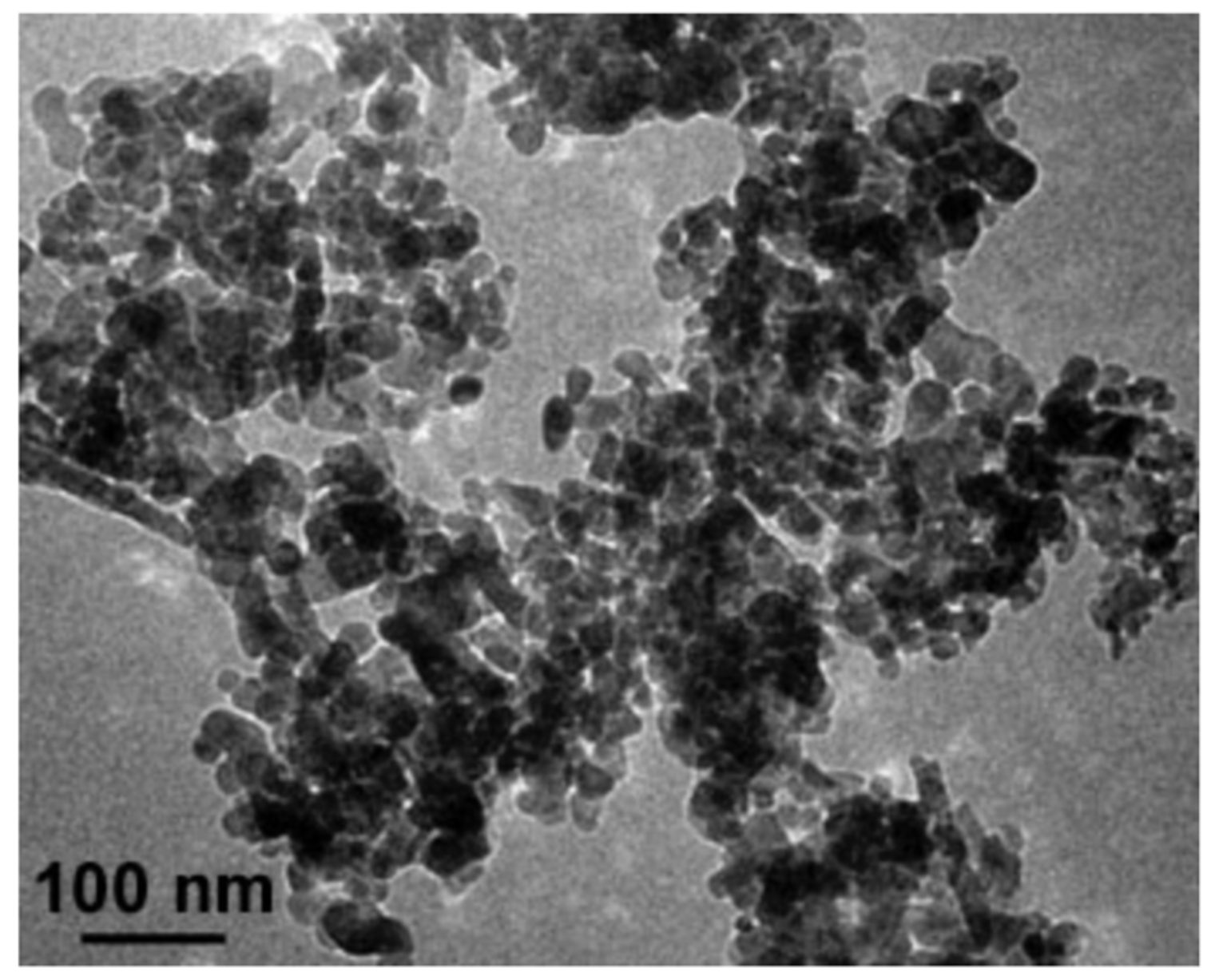
4.4. Synthesis and Characterization of ZnO NPs
- ▪
- 13.7 g of Zn(O2CCH3)2 and 600 mL of ethanol were placed in a ball flask with three necks;
- ▪
- The solution was constantly stirred at 75 °C under reflux for 2 h;
- ▪
- Then an aqueous solution of 0.22 M NaOH and an additional 100 mL of distilled H2O were added to complete the reaction mixture;
- ▪
- It was stirred for 24 h;
- ▪
- The obtained ZnO NPs were immersed in ethanol and recovered by centrifugation (15,000 rpm/5 min);
- ▪
- The precipitate was washed two times with ethanol and dried in an oven at 60 °C for 24 h;
- ▪
- The dried ZnO NPs were crushed in an agate mortar to obtain a fine powder;
- ▪
- Size and morphology of nanoparticles were measured by means of a high-resolution transmission electron microscope (HRTEM) Titan 80–300 kV (FEI Company, Hillsboro, OR, USA).
4.5. Mineral Analysis
4.5.1. Nitrogen Determination
4.5.2. Determination of N, P, K+, Ca2+, Mg2+, Mn2+, Cu2+, Fe2+, and Zn2+
4.6. Biochemical Analysis
4.6.1. Antioxidant Capacity
4.6.2. Ascorbic Acid
4.6.3. Total Phenols
4.6.4. Reduced Glutathione (GSH)
4.6.5. Total Protein
4.6.6. Fruits Firmness
4.6.7. Total Soluble Solids
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vera-Guzmán, A.M.; Aquino-Bolaños, E.N.; Heredia-García, E.; Carrillo-Rodríguez, J.C.; Hernández-Delgado, S.; Chávez-Servia, J.L. Flavonoid and Capsaicinoid Contents and Consumption of Mexican Chili Pepper (Capsicum annuum L.) Landraces. In Flavonoids—From Biosynthesis to Human Health; InTech: London, UK, 2017; pp. 405–437. [Google Scholar]
- Ganguly, S.; Praveen, P.K.; Para, P.A.; Sharma, V. Medicinal Properties of Chilli Pepper in Human Diet: An Editorial. ARC J. Public Heal. Community Med. 2017, 2, 6–7. [Google Scholar] [CrossRef]
- FAO. Food and Agriculure Organization of de United Nations. Available online: http://www.fao.org/faostat/es/#data/QC (accessed on 12 September 2020).
- SIAP. Servicio de Información Agroalimentaria y Pesquera [Agri-Food and Fisheries Information Service]. Available online: https://nube.siap.gob.mx/cierreagricola/ (accessed on 20 October 2020).
- Hara, T.; Takeda, T.; Takagishi, T.; Fukue, K.; Kambe, T.; Fukada, T. Physiological roles of zinc transporters: Molecular and genetic importance in zinc homeostasis. J. Physiol. Sci. 2017, 67, 283–301. [Google Scholar] [CrossRef] [PubMed]
- Maret, W.; Sandstead, H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, Z.A.; Salam, R.A. Global nutrition epidemiology and trends. Ann. Nutr. Metab. 2012, 61, 19–27. [Google Scholar] [CrossRef]
- Joy, E.J.M.; Ahmad, W.; Zia, M.H.; Kumssa, D.B.; Young, S.D.; Ander, E.L.; Watts, M.J.; Stein, A.J.; Broadley, M.R. Valuing increased zinc (Zn) fertiliser-use in Pakistan. Plant Soil 2017, 411, 139–150. [Google Scholar] [CrossRef] [Green Version]
- Pfeiffer, W.H.; McClafferty, B. HarvestPlus: Breeding Crops for Better Nutrition. Crop Sci. 2007, 47, S-88–S-105. [Google Scholar] [CrossRef]
- Kumar, R.; Bohra, J.S. Effect of NPKS and Zn application on growth, yield, economics and quality of baby corn. Arch. Agron. Soil Sci. 2014, 60, 1193–1206. [Google Scholar] [CrossRef]
- Noulas, C.; Tziouvalekas, M.; Karyotis, T. Zinc in soils, water and food crops. J. Trace Elem. Med. Biol. 2018, 49, 252–260. [Google Scholar] [CrossRef]
- Rehman, A.; Farooq, M.; Ozturk, L.; Asif, M.; Siddique, K.H.M. Zinc nutrition in wheat-based cropping systems. Plant Soil 2018, 422, 283–315. [Google Scholar] [CrossRef]
- Subbaiah, L.V.; Prasad, T.N.V.K.V.; Krishna, T.G.; Sudhakar, P.; Reddy, B.R.; Pradeep, T. Novel Effects of Nanoparticulate Delivery of Zinc on Growth, Productivity, and Zinc Biofortification in Maize (Zea mays L.). J. Agric. Food Chem. 2016, 64, 3778–3788. [Google Scholar] [CrossRef]
- García-Gómez, C.; García, S.; Obrador, A.F.; González, D.; Babín, M.; Fernández, M.D. Effects of aged ZnO NPs and soil type on Zn availability, accumulation and toxicity to pea and beet in a greenhouse experiment. Ecotoxicol. Environ. Saf. 2018, 160, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Kumar, P.; Chaudhari, S.; Edelstein, M. Tomato Grafting: A Global Perspective. HortScience 2017, 52, 1328–1336. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Liao, S.; Lv, H.; Khaldun, A.B.M.; Wang, Y. Characterization of the growth and fruit quality of tomato grafted on a woody medicinal plant, Lycium chinense. Sci. Hortic. 2015, 197, 447–453. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Colla, G. Vegetable Grafting: A Toolbox for Securing Yield Stability under Multiple Stress Conditions. Front. Plant Sci. 2018, 8, 10–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Kalunke, R.M.; Colla, G. Insight into the role of grafting and arbuscular mycorrhiza on cadmium stress tolerance in tomato. Front. Plant Sci. 2015, 6, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, U.J.; Bahadur, V.; Prasad, V.M.; Mishra, S.; Shukla, P.K. Effect of Different Concentrations of Iron Oxide and Zinc Oxide Nanoparticles on Growth and Yield of Strawberry (Fragaria x ananassa Duch) cv. Chandler. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2440–2445. [Google Scholar] [CrossRef]
- Amiri, M.E.; Fallahi, E.; Safi-Songhorabad, M. Influence of Rootstock on Mineral Uptake and Scion Growth of “Golden Delicious” and “Royal Gala” Apples. J. Plant Nutr. 2014, 37, 16–29. [Google Scholar] [CrossRef]
- He, X.; Deng, H.; Hwang, H. min The current application of nanotechnology in food and agriculture. J. Food Drug Anal. 2019, 27, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Auffan, M.; Rose, J.; Bottero, J.-Y.; Lowry, G.V.; Jolivet, J.-P.; Wiesner, M.R. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef]
- Hajra, A.; Mondal, N.K. Effects of ZnO and TiO2 nanoparticles on germination, biochemical and morphoanatomical attributes of Cicer arietinum L. Energy, Ecol. Environ. 2017, 2, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Jalali, M.; Ghanati, F.; Modarres-Sanavi, A.M.; Khoshgoftarmanesh, A.H. Physiological effects of repeated foliar application of magnetite nanoparticles on maize plants. J. Agron. Crop Sci. 2017, 203, 593–602. [Google Scholar] [CrossRef]
- Da Costa, M.V.J.; Sharma, P.K. Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica 2016, 54, 110–119. [Google Scholar] [CrossRef]
- Ebbs, S.D.; Bradfield, S.J.; Kumar, P.; White, J.C.; Musante, C.; Ma, X. Accumulation of zinc, copper, or cerium in carrot (Daucus carota) exposed to metal oxide nanoparticles and metal ions. Environ. Sci. Nano 2016, 3, 114–126. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, A.; Peralta-Videa, J.R.; Bandyopadhyay, S.; Rico, C.M.; Zhao, L.; Gardea-Torresdey, J.L. Physiological effects of nanoparticulate ZnO in green peas (Pisum sativum L.) cultivated in soil. Metallomics 2014, 6, 132–138. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, H.; Lal, R. Effects of Stabilized Nanoparticles of Copper, Zinc, Manganese, and Iron Oxides in Low Concentrations on Lettuce (Lactuca sativa) Seed Germination: Nanotoxicants or Nanonutrients? Water. Air. Soil Pollut. 2016, 227. [Google Scholar] [CrossRef]
- Navarro, E.; Baun, A.; Behra, R.; Hartmann, N.B.; Filser, J.; Miao, A.J.; Quigg, A.; Santschi, P.H.; Sigg, L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 2008, 17, 372–386. [Google Scholar] [CrossRef] [Green Version]
- Raliya, R.; Tarafdar, J.C. ZnO Nanoparticle Biosynthesis and Its Effect on Phosphorous-Mobilizing Enzyme Secretion and Gum Contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2013, 2, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Velasco-Alvarado, M.; Castro-Brindis, R.; Castillo-González, A.M.; Avitia-García, E.; Lobato-Ortiz, J.; Sahagún-Castellanos, R. Composición mineral, biomasa y rendimiento en tomate (Solanum lycopersicum L.) injertado. Interciencia 2016, 41, 703–708. [Google Scholar]
- Pilli, K.; Samant, P.K.; Naresh, P.; Acharya, G.C. Influence of organic and inorganic nutrients on nutrient accumulation in grafted tomato. Pharma Innov. 2018, 7, 27–33. [Google Scholar]
- Tagliavini, M.; Scudellari, D.; Marangoni, B.; Bastianel, A.; Franzin, F.; Zamborlini, M. Leaf mineral composition of apple tree: Sampling date and effects of cultivar and rootstock. J. Plant Nutr. 1992, 15, 605–619. [Google Scholar] [CrossRef]
- Rivero, R.M.; Ruiz, J.M.; Romero, L. Role of grafting. J. Food, Agric. Environ. 2003, 1, 70–74. [Google Scholar]
- Castle, W.S.; Krezdorn, A.H. Effect of citrus rootstocks on root distribution and leaf mineral content of‘Orlando’tangelo trees. J. Am. Soc. Hortic. Sci. 1975, 100, 1–4. [Google Scholar]
- Gautier, A.; Cookson, S.J.; Hevin, C.; Vivin, P.; Lauvergeat, V.; Mollier, A. Phosphorus acquisition efficiency and phosphorus remobilization mediate genotype-specific differences in shoot phosphorus content in grapevine. Tree Physiol. 2018, 38, 1742–1751. [Google Scholar] [CrossRef]
- Pererira-Días, L.; Lopéz-Serrano, L.; Castell-Zeising, V.; Lopéz-Galarza, S.; San Bautista, A.; Calatayud, Á.; Fita, A. Different Root Morphological Responses to Phosphorus Supplies in Grafted Pepper. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Hortic. 2018, 75, 59. [Google Scholar] [CrossRef]
- Godoy, H.; Ramos, J.; González, G.; Sandoval, M.; José De Ramos, J. Efecto Del Injerto Y Nutrición De Tomate Sobre Rendimiento, Materia Seca Y Extracción De Nutrimentos. Terra Latinoam. 2009, 27, 1–9. [Google Scholar]
- Mayer, N.A.; Ueno, B.; Nava, G.; Neves, T.R. das Leaf nutrient content on seven plum cultivars with grafted by budding or own-rooted trees. Rev. Bras. Frutic. 2018, 40. [Google Scholar] [CrossRef] [Green Version]
- Milenković, L.; Mastilović, J.; Kevrešan, Ž.; Bajić, A.; Gledić, A.; Stanojević, L.; Cvetković, D.; Šunić, L.; Ilić, Z.S. Effect of shading and grafting on yield and quality of tomato. J. Sci. Food Agric. 2020, 100, 623–633. [Google Scholar] [CrossRef]
- Gregory, P.J.; Atkinson, C.J.; Bengough, A.G.; Else, M.A.; Fernández-Fernández, F.; Harrison, R.J.; Schmidt, S. Contributions of roots and rootstocks to sustainable, intensified crop production. J. Exp. Bot. 2013, 64, 1209–1222. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.N.; Hayat, F.; Asim, M.; Iqbal, S.; Ashraf, T.; Asghar, S. Influence of citrus rootstocks on growth performance and leaf mineral nutrition of “Salustiana” sweet orange [Citrus sinensis (L.). obsek]. J. Pure Appl. Agric. 2020, 5, 2617–8680. [Google Scholar]
- Ceylan, Ş.; Alan, Ö.; Elmacı, Ö.L. Karpuzda Aşılamanın Besin Element İçeriği ve Verim Üzerine Etkisi. Ege Üniversitesi Ziraat Fakültesi Derg. 2018, 55, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Awasthi, O.P.; Dubey, A.K.; Pandey, R.; Sharma, V.K.; Mishra, A.K.; Sharma, R.M. Root morphology and the effect of rootstocks on leaf nutrient acquisition of Kinnow mandarin (Citrus nobilis Loureiro × Citrus reticulata Blanco). J. Hortic. Sci. Biotechnol. 2018, 93, 100–106. [Google Scholar] [CrossRef]
- Gai, A.P.C.; dos Santos, D.S.; Vieira, E.A. Effects of zinc excess on antioxidant metabolism, mineral content and initial growth of Handroanthus impetiginosus (Mart. ex DC.) Mattos and Tabebuia roseoalba (Ridl.) Sandwith. Environ. Exp. Bot. 2017, 144, 88–99. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; White, J.C.; Elmer, W.H.; Gardea-Torresdey, J. Nanoparticle and ionic Zn promote nutrient loading of sorghum grain under low NPK fertilization. J. Agric. Food Chem. 2017, 65, 8552–8559. [Google Scholar] [CrossRef]
- Tarafdar, J.C.; Claassen, N. Organic phosphorus utilization by wheat plants under sterile conditions. Biol. Fertil. Soils 2003, 39, 25–29. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Singh, U.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Zinc oxide nanoparticles alleviate drought-induced alterations in sorghum performance, nutrient acquisition, and grain fortification. Sci. Total Environ. 2019, 688, 926–934. [Google Scholar] [CrossRef]
- Kuhn, A.J.; Schröder, W.H.; Bauch, J. The kinetics of calcium and magnesium entry into mycorrhizal spruce roots. Planta 2000, 210, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Bala, R.; Kalia, A.; Dhaliwal, S.S. Evaluation of Efficacy of ZnO Nanoparticles as Remedial Zinc Nanofertilizer for Rice. J. Soil Sci. Plant Nutr. 2019, 19, 379–389. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; Zia ur Rehman, M.; Waris, A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef]
- Hong, J.; Peralta-Videa, J.R.; Rico, C.; Sahi, S.; Viveros, M.N.; Bartonjo, J.; Zhao, L.; Gardea-Torresdey, J.L. Evidence of translocation and physiological impacts of foliar applied CeO2 nanoparticles on cucumber (Cucumis sativus) plants. Environ. Sci. Technol. 2014, 48, 4376–4385. [Google Scholar] [CrossRef]
- da Cruz, T.N.M.; Savassa, S.M.; Montanha, G.S.; Ishida, J.K.; de Almeida, E.; Tsai, S.M.; Lavres Junior, J.; Pereira de Carvalho, H.W. A new glance on root-to-shoot in vivo zinc transport and time-dependent physiological effects of ZnSO4 and ZnO nanoparticles on plants. Sci. Rep. 2019, 9, 10416. [Google Scholar] [CrossRef] [Green Version]
- Bandyopadhyay, S.; Peralta-Videa, J.R.; Plascencia-Villa, G.; José-Yacamán, M.; Gardea-Torresdey, J.L. Comparative toxicity assessment of CeO2 and ZnO nanoparticles towards Sinorhizobium meliloti, a symbiotic alfalfa associated bacterium: Use of advanced microscopic and spectroscopic techniques. J. Hazard. Mater. 2012, 241–242, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B.J. Zinc in soils and crop production. Int. Fertil. Ind. Assoc. Paris 2008, 139. [Google Scholar]
- Faizan, M.; Sehar, S.; Rajput, V.D.; Faraz, A.; Afzal, S.; Minkina, T.; Sushkova, S.; Adil, M.F.; Yu, F.; Alatar, A.A.; et al. Modulation of Cellular Redox Status and Antioxidant Defense System after Synergistic Application of Zinc Oxide Nanoparticles and Salicylic Acid in Rice (Oryza sativa) Plant under Arsenic Stress. Plants 2021, 10, 2254. [Google Scholar] [CrossRef]
- Salama, D.M.; Osman, S.A.; Abd El-Aziz, M.E.; Abd Elwahed, M.S.A.; Shaaban, E.A. Effect of zinc oxide nanoparticles on the growth, genomic DNA, production and the quality of common dry bean (Phaseolus vulgaris). Biocatal. Agric. Biotechnol. 2019, 18, 101083. [Google Scholar] [CrossRef]
- Shebl, A.; Hassan, A.A.; Salama, D.M.; Abd El-Aziz, M.E.; Abd Elwahed, M.S.A. Green synthesis of nanofertilizers and their application as a foliar for Cucurbita pepo L. J. Nanomater. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Bihmidine, S.; Hunter, C.T.; Johns, C.E.; Koch, K.E.; Braun, D.M. Regulation of assimilate import into sink organs: Update on molecular drivers of sink strength. Front. Plant Sci. 2013, 4, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garciá-López, J.I.; Zavala-Garcia, F.; Olivares-Saénz, E.; Lira-Saldivar, R.H.; Barriga-Castro, E.D.; Ruiz-Torres, N.A.; Ramos-Cortez, E.; Vázquez-Alvarado, R.; Ninõ-Medina, G. Zinc Oxide nanoparticles boosts phenolic compounds and antioxidant activity of Capsicum Annuum L. during germination. Agronomy 2018, 8, 215. [Google Scholar] [CrossRef] [Green Version]
- Djidonou, D.; Simonne, A.H.; Koch, K.E.; Brecht, J.K.; Zhao, X. Nutritional quality of field-grown tomato fruit as affected by grafting with interspecific hybrid rootstocks. HortScience 2016, 51, 1618–1624. [Google Scholar] [CrossRef] [Green Version]
- Zi-kum, Z.; Li, H.; Yu, Z.; Zhi-jun, H.; Kun, C.; Shi-qi, L. Grafting Enhances Copper Tolerance of Cucumber Through Regulating Nutrient Uptake and Antioxidative System. Agric. Sci. China 2010, 9, 1758–1770. [Google Scholar] [CrossRef]
- Zhao, H.; Song, X.; Yang, G.; Li, Z.; Zhang, D.; Feng, H. Monitoring of nitrogen and grain protein content in winter wheat based on Sentinel-2A data. Remote Sens. 2019, 11, 1724. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Torres, P.; Raigón, M.D.; Gammoudi, N.; Gisbert, C. Effects of grafting combinations on the nutritional composition of pepper fruit. Fruits 2016, 71, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Diao, X.; Wang, T.; Chen, G.; Lin, Q.; Yang, X.; Xu, J. Phylogenetic diversity and antioxidant activities of culturable fungal endophytes associated with the mangrove species Rhizophora stylosa and R. mucronata in the South China Sea. PLoS ONE 2018, 13, e0197359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davarpanah, S.; Tehranifar, A.; Davarynejad, G.; Abadía, J.; Khorasani, R. Effects of foliar applications of zinc and boron nano-fertilizers on pomegranate (Punica granatum cv. Ardestani) fruit yield and quality. Sci. Hortic. 2016, 210, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Esfandiari, E.; Abdoli, M.; Mousavi, S.B.; Sadeghzadeh, B. Impact of foliar zinc application on agronomic traits and grain quality parameters of wheat grown in zinc deficient soil. Indian J. Plant Physiol. 2016, 21, 263–270. [Google Scholar] [CrossRef]
- García-Gómez, C.; Obrador, A.; González, D.; Babín, M.; Fernández, M.D. Comparative effect of ZnO NPs, ZnO bulk and ZnSO4 in the antioxidant defences of two plant species growing in two agricultural soils under greenhouse conditions. Sci. Total Environ. 2017, 589, 11–24. [Google Scholar] [CrossRef]
- Thwala, M.; Musee, N.; Sikhwivhilu, L.; Wepener, V. The oxidative toxicity of Ag and ZnO nanoparticles towards the aquatic plant Spirodela punctuta and the role of testing media parameters. Environ. Sci. Process. Impacts 2013, 15, 1830–1843. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Mishra, R.K.; Singh, S.; Singh, S.; Vishwakarma, K.; Sharma, S.; Singh, V.P.; Singh, P.K.; Prasad, S.M.; Dubey, N.K.; et al. Nitric oxide ameliorates zinc oxide nanoparticles phytotoxicity in wheat seedlings: Implication of the ascorbate–glutathione cycle. Front. Plant Sci. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chan, Z. Proteomic responses of fruits to environmental stresses. Front. Plant Sci. 2013, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Priyanka, N.; Venkatachalam, P. Biofabricated zinc oxide nanoparticles coated with phycomolecules as novel micronutrient catalysts for stimulating plant growth of cotton. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 045018. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, S.K.; Malodia, L. Biosynthesis of zinc oxide nanoparticles using leaf extract of calotropis gigantea: Characterization and its evaluation on tree seedling growth in nursery stage. Appl. Nanosci. 2017, 7, 501–512. [Google Scholar] [CrossRef]
- Raliya, R.; Nair, R.; Chavalmane, S.; Wang, W.N.; Biswas, P. Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 2015, 7, 1584–1594. [Google Scholar] [CrossRef]
- Servin, A.; Elmer, W.; Mukherjee, A.; De la Torre-Roche, R.; Hamdi, H.; White, J.C.; Bindraban, P.; Dimkpa, C. A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. J. Nanoparticle Res. 2015, 17, 92. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M. del C.; Moreno, D.A.; Carvajal, M. The physiological importance of glucosinolates on plant response to abiotic stress in Brassica. Int. J. Mol. Sci. 2013, 14, 11607–11625. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, Y.; Ding, Z.; Wang, H.; Song, L.; Jia, S.; Ma, D. Zinc stress affects ionome and metabolome in tea plants. Plant Physiol. Biochem. 2017, 111, 318–328. [Google Scholar] [CrossRef]
- Botella, M.Á.; Arévalo, L.; Mestre, T.C.; Rubio, F.; García-Sánchez, F.; Rivero, R.M.; Martínez, V. Potassium fertilization enhances pepper fruit quality. J. Plant Nutr. 2017, 40, 145–155. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, L.; Kong, Q.; Cheng, F.; Niu, M.; Xie, J.; Muhammad Azher Nawaz; Bie, Z. Comprehensive Mineral Nutrition Analysis of Watermelon Grafted onto Two Different Rootstocks. Hortic. Plant J. 2016, 2, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Lakshmi, S.J.; Bai, R.R.S.; Sharanagouda, H.; Ramachandra, C.T.; Nadagouda, S.; Nidoni, U. Effect of biosynthesized zinc oxide nanoparticles coating on quality parameters of fig (Ficus carica L.) fruit. J. Pharmacogn. Phytochem. 2018, 7, 10–14. [Google Scholar]
- López-Herrera, A.; Castillo-González, A.M.; Trejo-Téllez, L.I.; Avitia-García, E.; Valdez-Aguilar, L.A. Respuesta de fresa cv. Albión a dosis crecientes de zinc. Rev. Mex. Ciencias Agrícolas 2018, 9, 1591–1601. [Google Scholar] [CrossRef] [Green Version]
- Tsegay, D.; Tesfaye, B.; Mohammed, A.; Yirga, H.; Bayleyegn, A. Effects of harvesting stage and storage duration on postharvest quality and shelf life of sweet bell pepper (Capsicum annuum L.) varieties under passive refrigeration system. Int. J. Biotechnol. Mol. Biol. Res. 2013, 4, 60–70. [Google Scholar] [CrossRef]
- Yfran, M.D.M.; Chabbal, M.D.; Píccoli, A.B.; Giménez, L.I.; Rodríguez, V.A. Fertilización Foliar Con Potasio, Calcio Y Boro. Incidencia Sobre La Nutrición Y Calidad De Frutos En Mandarino ‘ Nova.’ Cultiv. Trop. 2017, 38, 22–29. [Google Scholar] [CrossRef]
- Lee, J.-M. Cultivation of grafted vegetables I. Current status, grafting methods, and benefits. HortScience 1994, 29, 235–239. [Google Scholar] [CrossRef]
- Steiner, A.A. A universal method for preparing nutrient solutions of a certain desired composition. Plant Soil 1961, 15, 134–154. [Google Scholar] [CrossRef] [Green Version]
- Betancourt, G.R.; Berlanga, D.M.L.; Puente, U.B.; Rodríguez, F.O.; Sánchez-Valdes, S. Surface modification of ZnO nanoparticles. Mater. Sci. Forum 2010, 644, 61–64. [Google Scholar] [CrossRef]
- Gutiérrez-Ramírez, J.A.; Betancourt-Galindo, R.; Aguirre-Uribe, L.A.; Cerna-Chávez, E.; Sandoval-Rangel, A.; Castro-del Ángel, E.; Chacón-Hernández, J.C.; García-López, J.I.; Hernández-Juárez, A. Insecticidal effect of zinc oxide and titanium dioxide nanoparticles against Bactericera cockerelli sulc. (hemiptera: Triozidae) on tomato Solanum lycopersicum. Agronomy 2021, 11, 1460. [Google Scholar] [CrossRef]
- Raliya, R.; Saharan, V.; Dimkpa, C.; Biswas, P. Nanofertilizer for Precision and Sustainable Agriculture: Current State and Future Perspectives. J. Agric. Food Chem. 2018, 66, 6487–6503. [Google Scholar] [CrossRef]
- Rodríguez-Fuentes, H.; Rodríguez-Absi, J. Metodos de Analisis de Suelos y Plantas: Criterios de Interpretacion; Editorial Trillas SA de CV: Ciudad de México, México, 2015. [Google Scholar]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Levine, M.; Dutta, A.; et al. Vitamin C as an Antioxidant: Evaluation of Its Role in Disease Prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Xue, T.; Hartikainen, H.; Piironen, V. Antioxidative and growth-promoting effect of selenium on senescing lettuce. Plant Soil 2001, 237, 55–61. [Google Scholar] [CrossRef]
- Sahoo, S.; Awasthi, J.P.; Sunkar, R. Plant Stress Tolerance; Methods in Molecular Biology; Sunkar, R., Ed.; Springer New York: New York, NY, USA, 2017; Volume 1631, ISBN 978-1-4939-7134-3. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]



| Factor | N mg g−1 | P mg g−1 | K+ mg g−1 | Ca2+ mg g−1 | Mg2+ mg g−1 | Mn2+ mg g−1 | Zn2+ mg g−1 | Fe2+ mg g−1 | Cu2+ mg g−1 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Grafted | 36.2 a | 12.6 a | 40.4 a | 13.8 a | 7.4 a | 0.033 a | 0.092 a | 0.050 a | 0.008 a | |
| Ungrafted | 31.8 a | 10.6 b | 29.6 b | 11.3 a | 5.9 b | 0.021 b | 0.074 b | 0.041 b | 0.007 b | |
| Control | 32.8 bc | 10.6 b | 28.9 b | 12.1 a | 5.5 c | 0.021 c | 0.073 b | 0.032 c | 0.009 a | |
| 10 mg L−1 | 32.2 c | 10.4 b | 37.6 ab | 10.8 a | 5.5 c | 0.022 c | 0.082 b | 0.040 bc | 0.008 ab | |
| 20 mg L−1 | 33.7 b | 11.1 b | 33.1 ab | 11 a | 6.9 b | 0.028 b | 0.077 b | 0.049 ab | 0.008 ab | |
| 30 mg L−1 | 37.4 a | 14.3 a | 40.4 a | 16.3 a | 8.7 a | 0.037 a | 0.100 a | 0.059 a | 0.009 a | |
| Grafted | Control | 35.6 b | 11.3 bc | 36.5 abc | 11.7 b | 5.8 bc | 0.025 bc | 0.084 bc | 0.040 abc | 0.009 a |
| 10 mg L−1 | 34.5 b | 10.8 c | 52.2 a | 8.7 b | 5.4 c | 0.027 bc | 0.094 ab | 0.050 ab | 0.008 ab | |
| 20 mg L−1 | 36 b | 12.3 b | 35.3 abc | 12.1 b | 7.3 b | 0.032 b | 0.081 bc | 0.051 ab | 0.008 ab | |
| 30 mg L−1 | 38.6 a | 16 a | 36.7 abc | 22.7 a | 11 a | 0.047 a | 0.109 a | 0.059 a | 0.009 a | |
| Ungrafted | Control | 29.9 c | 10 c | 21.2 c | 12.5 b | 5.3 c | 0.017 c | 0.090 ab | 0.025 c | 0.007 ab |
| 10 mg L−1 | 29.8 c | 10.8 c | 23 c | 12.9 b | 5.7 c | 0.017 c | 0.070 bc | 0.031 bc | 0.007 b | |
| 20 mg L−1 | 31.3 c | 10 bc | 30 bc | 9.9 b | 6.4 bc | 0.024 bc | 0.073 bc | 0.047 ab | 0.007 ab | |
| 30 mg L−1 | 36.2 b | 12.5 bc | 44.2 ab | 10 b | 6.4 bc | 0.027 bc | 0.090 ab | 0.060 a | 0.008 ab | |
| Grafted | *** | ** | ** | ns | *** | *** | *** | * | *** | |
| ZnO NPs | *** | *** | * | ns | *** | *** | ** | *** | ns | |
| Graft x NPs | * | ns | ** | ** | *** | * | ns | ns | ns | |
| CV % | 2.96 | 13.32 | 21.77 | 33.10 | 10.45 | 15.32 | 12.91 | 20.29 | 7.19 | |
| SD | 0.32 | 0.24 | 1.19 | 0.55 | 0.19 | 8.83 | 10.96 | 14.45 | 0.80 | |
| Factor | Antioxidant Capacity (µMTrolox EQ 100/g DW) | Ascorbic Acid (g/kg DW) | Total Phenols (g/kg DW) | Reduced Glutathione (mmol 100 g −1 DW) | Total Protein (g/kg DW) | |
|---|---|---|---|---|---|---|
| Grafted | 0.82 a | 1033.55 a | 9.77 a | 7.24 a | 56.64 a | |
| Ungrafted | 0.80 b | 985.70 b | 9.64 b | 6.72 b | 55.93 b | |
| Control | 0.74 d | 848.70 d | 9.05 d | 6.41 b | 52.92 d | |
| 10 mg L−1 | 0.80 c | 950.69 c | 9.74 c | 6.97 a | 55.32 c | |
| 20 mg L−1 | 0.82 b | 1080.21 b | 9.88 b | 6.89 a | 57.43 b | |
| 30 mg L−1 | 0.86 a | 1158.90 a | 10.16 a | 7.24 a | 59.47 a | |
| Grafted | Control | 0.76 d | 864.28 f | 9.12 e | 6.51 bc | 53.42 e |
| 10 mg L−1 | 0.80 c | 984.21 d | 9.79 cd | 7.22 a | 55.49 d | |
| 20 mg L−1 | 0.83 bc | 1103.50 b | 9.92 bc | 7.07 ab | 57.79 bc | |
| 30 mg L−1 | 0.88 a | 1182.19 a | 10.27 a | 7.32 a | 59.86 a | |
| Ungrafted | Control | 0.73 d | 833.12 f | 8.99 e | 6.30 c | 52.42 e |
| 10 mg L−1 | 0.80 c | 917.16 e | 9.70 d | 6.72 abc | 55.15 d | |
| 20 mg L−1 | 0.82 bc | 1056.92 c | 9.84 cd | 6.70 abc | 57.07 c | |
| 30 mg L−1 | 0.84 b | 1135.61 b | 10.04 b | 7.16 ab | 59.09 ab | |
| Grafted | ** | *** | *** | * | ** | |
| ZnO Nps | *** | *** | *** | *** | *** | |
| Graft x NPs | * | * | * | ns | ns | |
| CV % | 1.99 | 1.77 | 0.74 | 4.71 | 1.27 | |
| SD | 0.05 | 124.14 | 0.42 | 0.46 | 2.58 | |
| Factor | Fruits Firmness (Kg) | Sugar Content (°Brix) | |
|---|---|---|---|
| Grafted | 2.38 a | 5.26 a | |
| Ungrafted | 2.17 b | 5.11 a | |
| Control | 2.09 b | 6.67 a | |
| 10 mg L−1 | 2.29 b | 4.66 bc | |
| 20 mg L−1 | 2.18 b | 5.05 b | |
| 30 mg L−1 | 2.54 a | 4.36 c | |
| Grafted | Control | 2.10 b | 6.84 a |
| 10 mg L−1 | 2.34 b | 4.78 bc | |
| 20 mg L−1 | 2.20 b | 4.80 bc | |
| 30 mg L−1 | 2.88 a | 4.22 c | |
| Ungrafted | Control | 2.08 b | 6.50 a |
| 10 mg L−1 | 2.24 b | 4.54 bc | |
| 20 mg L−1 | 2.16 b | 5.30 b | |
| 30 mg L−1 | 2.20 b | 4.10 c | |
| Grafted | ** | ns | |
| ZnO NPs | *** | *** | |
| Graft × NPs | ** | ns | |
| CV % | 8.69 | 9.64 | |
| SD | 0.30 | 1.03 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uresti-Porras, J.-G.; Cabrera-De-La Fuente, M.; Benavides-Mendoza, A.; Olivares-Sáenz, E.; Cabrera, R.I.; Juárez-Maldonado, A. Effect of Graft and Nano ZnO on Nutraceutical and Mineral Content in Bell Pepper. Plants 2021, 10, 2793. https://doi.org/10.3390/plants10122793
Uresti-Porras J-G, Cabrera-De-La Fuente M, Benavides-Mendoza A, Olivares-Sáenz E, Cabrera RI, Juárez-Maldonado A. Effect of Graft and Nano ZnO on Nutraceutical and Mineral Content in Bell Pepper. Plants. 2021; 10(12):2793. https://doi.org/10.3390/plants10122793
Chicago/Turabian StyleUresti-Porras, José-Gerardo, Marcelino Cabrera-De-La Fuente, Adalberto Benavides-Mendoza, Emilio Olivares-Sáenz, Raul I. Cabrera, and Antonio Juárez-Maldonado. 2021. "Effect of Graft and Nano ZnO on Nutraceutical and Mineral Content in Bell Pepper" Plants 10, no. 12: 2793. https://doi.org/10.3390/plants10122793
APA StyleUresti-Porras, J.-G., Cabrera-De-La Fuente, M., Benavides-Mendoza, A., Olivares-Sáenz, E., Cabrera, R. I., & Juárez-Maldonado, A. (2021). Effect of Graft and Nano ZnO on Nutraceutical and Mineral Content in Bell Pepper. Plants, 10(12), 2793. https://doi.org/10.3390/plants10122793









