Trophic Transfer without Biomagnification of Cadmium in a Soybean-Dodder Parasitic System
Abstract
:1. Introduction
2. Results
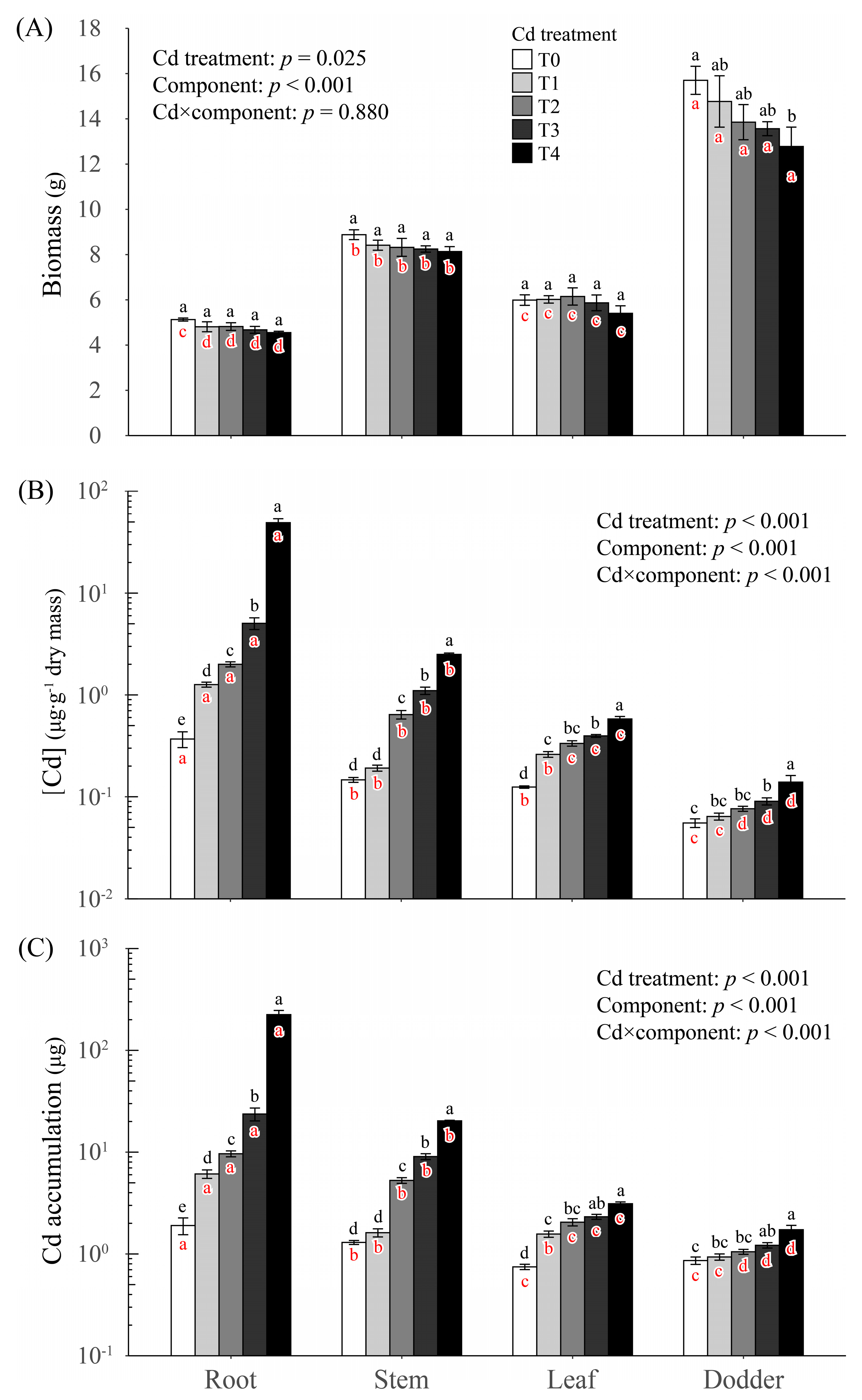
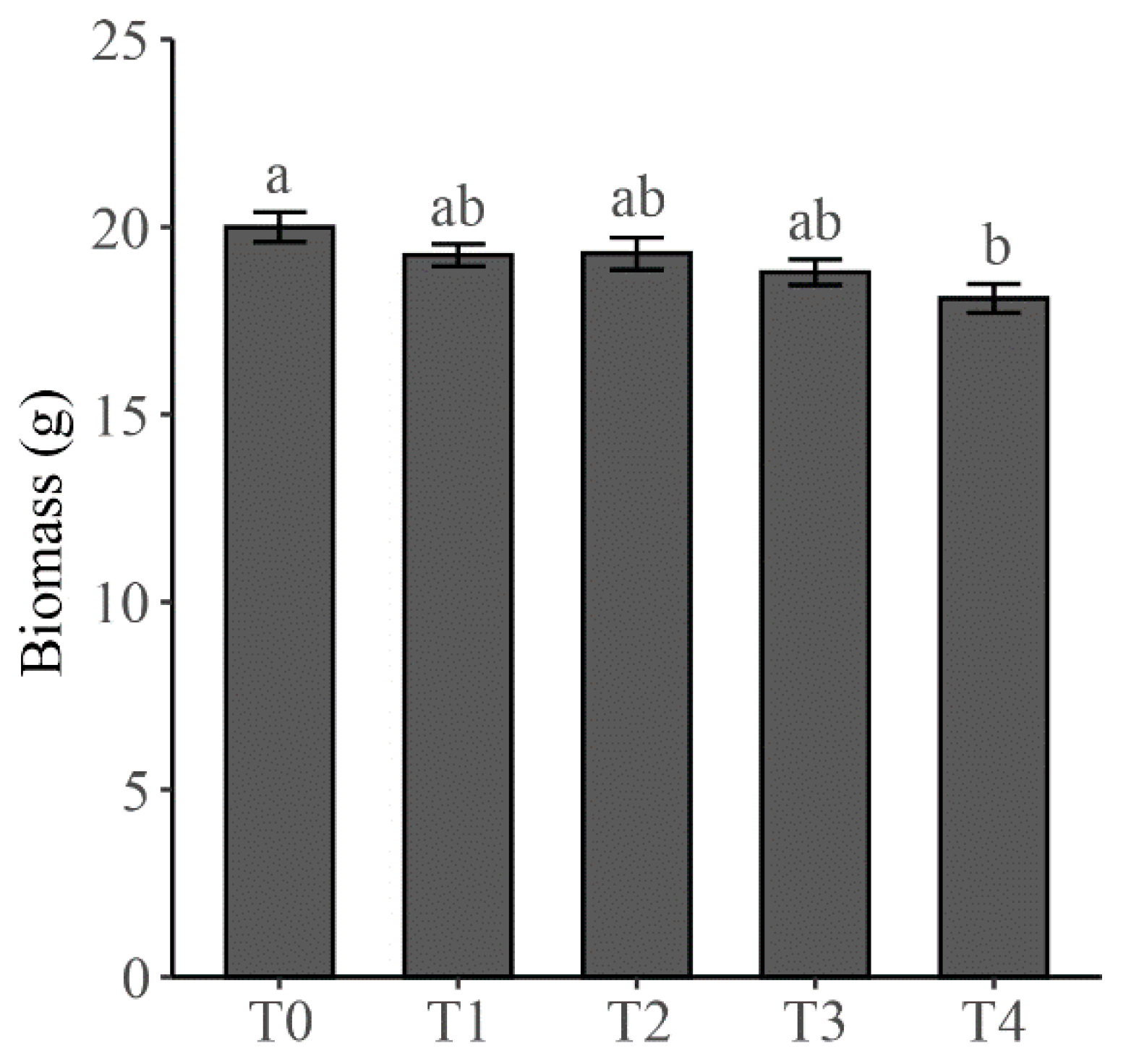
2.1. Biomass
2.2. Cd Concentration
2.3. Cd Transfer Coefficient
2.4. Cd Accumulation
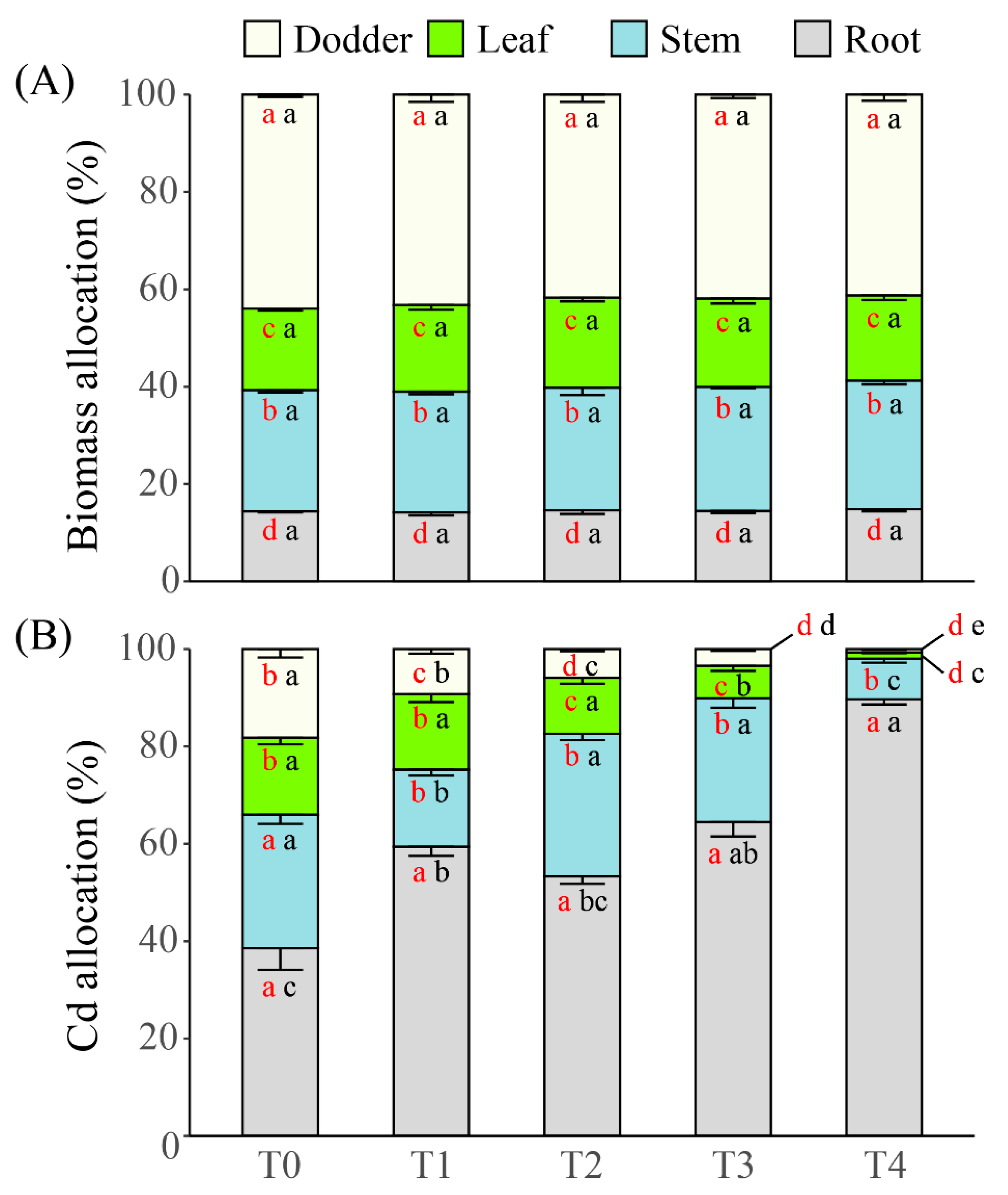
2.5. Allocations of Biomass and Cd
3. Discussion
4. Materials & Methods
4.1. Plant Materials
4.2. Experimental Design
4.3. Harvest and Measurements
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, M.K. Understanding Environmental Pollution, 4th ed.; Cambridge University Press: Cambridge, UK, 2020. [Google Scholar]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [Green Version]
- Gratão, P.L.; Polle, A.; Lea, P.J.; Azevedo, R.A. Making the life of heavy metal-stressed plants a little easier. Funct. Plant Ecol. 2005, 32, 481–494. [Google Scholar] [CrossRef]
- Shanmugaraj, B.M.; Malla, A.; Ramalingam, S. Cadmium stress and toxicity in plants: An overview. In Cadmium Toxicity and Tolerance in Plants: From Physiology to Remediation; Hasanuzzaman, M., Prasad, M.N.V., Fujita, M., Eds.; Academic Press: London, UK, 2019; pp. 1–17. [Google Scholar]
- Wu, G.; Kang, H.; Zhang, X.; Shao, H.; Chu, L.; Ruan, C. A critical review on the bio-removal of hazardous heavy metals from contaminated soils: Issues, progress, eco-environmental concerns and opportunities. J. Hazard. Mater. 2010, 174, 1–8. [Google Scholar] [CrossRef]
- Shahid, M.; Khalid, S.; Abbas, G.; Shahid, N.; Nadeem, M.; Sabir, M.; Aslam, M.; Dumat, C. Heavy metal stress and crop productivity. In Crop Production and Global Environmental Issues; Hakeem, K.R., Ed.; Springer: Cham, Switzerland, 2015; pp. 1–25. [Google Scholar]
- Suhani, I.; Sahab, S.; Srivastava, V.; Singh, R.P. Impact of cadmium pollution on food safety and human health. Cur. Opin. Toxicol. 2021, 27, 1–7. [Google Scholar] [CrossRef]
- McLaughlin, M.J.; Singh, B.R. Cadmium in soils and plants: A global perspective. In Cadmium in Soils and Plants; McLaughlin, M.J., Singh, B.R., Eds.; Springer: Dordrecht, The Netherlands, 1999; pp. 1–9. [Google Scholar]
- Sterckeman, T.; Thomine, S. Mechanisms of cadmium accumulation in plants. Crit. Rev. Plant Sci. 2020, 39, 322–359. [Google Scholar] [CrossRef]
- Abedi, T.; Mojiri, A. Cadmium uptake by wheat (Triticum aestivum L.): An overview. Plants 2020, 9, 500. [Google Scholar] [CrossRef] [Green Version]
- Waalkes, M.P. Cadmium carcinogenesis in review. J. Inorg. Biochem. 2000, 79, 241–244. [Google Scholar] [CrossRef]
- Hunter, B.A.; Johnson, M.S. Food chain relationships of copper and cadmium in contaminated grassland ecosystems. Oikos 1982, 38, 108–117. [Google Scholar] [CrossRef]
- Li, T.; Chang, Q.; Yuan, X.; Li, J.; Ayoko, G.A.; Frost, R.L.; Chen, H.; Zhang, X.; Song, Y.; Song, W. Cadmium transfer from contaminated soils to the human body through rice consumption in southern Jiangsu Province, China. Environ. Sci. Process. Impacts 2017, 19, 843–850. [Google Scholar] [CrossRef]
- Smith, T.M.; Smith, R.L. Elements of Ecology: International Edition, 6th ed.; Pearson Education, Benjamin Cummings: San Francisco, CA, USA, 2006. [Google Scholar]
- Pennings, S.C.; Callaway, R.M. Parasitic plants: Parallels and contrasts with herbivores. Oecologia 2002, 131, 479–489. [Google Scholar] [CrossRef]
- Twyford, A.D. Parasitic plants. Cur. Biol. 2018, 28, R857–R859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, C. Parasitic weeds: A world challenge. Weed Sci. 2012, 60, 269–276. [Google Scholar] [CrossRef]
- Kim, G.; Westwood, J.H. Macromolecule exchange in Cuscuta–host plant interactions. Curr. Opin. Plant Biol. 2015, 26, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Furuhashi, T.; Furuhashi, K.; Weckwerth, W. The parasitic mechanism of the holostemparasitic plant Cuscuta. J. Plant Interact. 2011, 6, 207–219. [Google Scholar] [CrossRef] [Green Version]
- Vurro, E.; Ruotolo, R.; Ottonello, S.; Elviri, L.; Maffini, M.; Falasca, G.; Zanella, L.; Altamura, M.M.; Sanità di Toppi, L. Phytochelatins govern zinc/copper homeostasis and cadmium detoxification in Cuscuta campestris parasitizing Daucus carota. Environ. Exp. Bot. 2011, 72, 26–33. [Google Scholar] [CrossRef]
- Touchette, B.W.; Feely, S.; McCabe, S. Elevated nutrient content in host plants parasitized by swamp dodder (Cuscuta gronovii): Evidence of selective foraging by a holoparasitic plant? Plant Biosyst. 2021. [Google Scholar] [CrossRef]
- Vaughn, K.C. Conversion of the searching hyphae of dodder into xylic and phloic hyphae: A cytochemical and immunocytochemical investigation. Int. J. Plant Sci. 2006, 167, 1099–1114. [Google Scholar] [CrossRef]
- Birschwilks, M.; Haupt, S.; Hofius, D.; Neumann, S. Transfer of phloem-mobile substances from the host plants to the holoparasite Cuscuta sp. J. Exp. Bot. 2006, 57, 911–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, H.; Hong, L.; Ye, W.; Cao, H.; Wang, Z. The influence of the holoparasitic plant Cuscuta campestris on the growth and photosynthesis of its host Mikania micrantha. J. Exp. Bot. 2007, 58, 2929–2937. [Google Scholar] [CrossRef] [Green Version]
- Furuhashi, T.; Kojima, M.; Sakakibara, H.; Fukushima, A.; Hirai, M.Y.; Furuhashi, K. Morphological and plant hormonal changes during parasitization by Cuscuta japonica on Momordica charantia. J. Plant Interact. 2014, 9, 220–232. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, H.; Li, J.; Song, J.; Hettenhausen, C.; Schuman, M.C.; Sun, G.; Zhang, C.; Li, J.; Song, D.; Wu, J. Aphid (Myzus persicae) feeding on the parasitic plant dodder (Cuscuta australis) activates defense responses in both the parasite and soybean host. New Phytol. 2018, 218, 1586–1596. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Shen, G.; Xu, Y.; Liu, H.; Zhang, J.; Li, S.; Li, J.; Zhang, C.; Qi, J.; Wang, L.; et al. Extensive inter-plant protein transfer between Cuscuta parasites and their host plants. Mol. Plant 2020, 13, 573–585. [Google Scholar] [CrossRef]
- Zagorchev, L.; Stöggl, W.; Teofanova, D.; Li, J.; Kranner, I. Plant parasites under pressure: Effects of abiotic stress on the interactions between parasitic plants and their hosts. Int. J. Mol. Sci. 2021, 22, 7418. [Google Scholar] [CrossRef]
- Srivastava, S.; Tripathi, R.D.; Dwivedi, U.N. Synthesis of phytochelatins and modulation of antioxidants in response to cadmium stress in Cuscuta reflexa—An angiospermic parasite. J. Plant Physiol. 2004, 161, 665–674. [Google Scholar] [CrossRef]
- Naikoo, M.I.; Raghib, F.; Dar, M.I.; Khan, F.A.; Hessini, K.; Ahmad, P. Uptake, accumulation and elimination of cadmium in a soil—Faba bean (Vicia faba)—Aphid (Aphis fabae)—Ladybird (Coccinella transversalis) food chain. Chemosphere 2021, 279, 130522. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Garland, T.R.; Wildung, R.E. Cadmium distribution and chemical fate in soybean plants. Plant Physiol. 1981, 68, 835–839. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, S.; Goldsbrough, P.; Carpena, R.O. Assessing the relative contributions of phytochelatins and the cell wall to cadmium resistance in white lupin. Physiol. Plant. 2006, 128, 487–495. [Google Scholar] [CrossRef]
- Konopka, J.K.; Hanyu, K.; Macfie, S.M.; McNeil, J.N. Does the response of insect herbivores to cadmium depend on their feeding strategy? J. Chem. Ecol. 2013, 39, 546–554. [Google Scholar] [CrossRef] [Green Version]
- Butt, A.; Quratul, A.; Rehman, K.; Khan, M.X.; Hesselberg, T. Bioaccumulation of cadmium, lead, and zinc in agriculture-based insect food chains. Environ. Monit. Assess. 2018, 190, 698. [Google Scholar] [CrossRef]
- Dawson, J.H.; Musselman, L.J.; Wolswinkel, P.; Dörr, I. Biology and control of Cuscuta. Rev. Weed Sci. 1994, 6, 265–317. [Google Scholar]
- Boyd, R.S.; Martens, S.N.; Davis, M.A. The nickel hyperaccumulator Streptanthus polygaloides (Brassicaceae) is attacked by the parasitic plant Cuscuta californica (Cuscutaceae). Madroño 1999, 46, 92–99. [Google Scholar]
- Lin, Y.-F.; Hassan, Z.; Talukdar, S.; Schat, H.; Aarts, M.G.M. Expression of the ZNT1 zinc transporter from the metal hyperaccumulator Noccaea caerulescens confers enhanced zinc and cadmium tolerance and accumulation to Arabidopsis thaliana. PLoS ONE 2016, 11, e0149750. [Google Scholar] [CrossRef]
- Weggler, K.; McLaughlin, M.J.; Graham, R.D. Effect of chloride in soil solution on the plant availability of biosolid-borne cadmium. J. Environ. Qual. 2004, 33, 496–504. [Google Scholar] [CrossRef]
- He, L.; Wang, X.; Feng, R.; He, Q.; Wang, S.; Liang, C.; Yan, L.; Bi, Y. Alternative pathway is involved in nitric oxide-enhanced tolerance to cadmium stress in barley roots. Plants 2019, 8, 557. [Google Scholar] [CrossRef] [Green Version]
- Mendoza-Cózatl, D.; Loza-Tavera, H.; Hernández-Navarro, A.; Moreno-Sánchez, R. Sulfur assimilation and glutathione metabolism under cadmium stress in yeast, protists and plants. FEMS Microbiol. Rev. 2005, 29, 653–671. [Google Scholar] [CrossRef] [Green Version]
- Schutzendubel, A.; Schwanz, P.; Teichmann, T.; Gross, K.; Langenfeld-Heyser, R.; Godbold, D.L.; Polle, A. Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in scots pine roots. Plant Physiol. 2001, 127, 887–898. [Google Scholar] [CrossRef]
- Xue, Z.-C.; Gao, H.-Y.; Zhang, L.-T. Effects of cadmium on growth, photosynthetic rate and chlorophyll content in leaves of soybean seedlings. Biol. Plant. 2013, 57, 587–590. [Google Scholar] [CrossRef]
- Balestrasse, K.B.; Gallego, S.M.; Tomaro, M.L. Cadmium-induced senescence in nodules of soybean (Glycine max L.) plants. Plant Soil 2004, 262, 373–381. [Google Scholar] [CrossRef]
- Finger-Teixeira, A.; Lucio Ferrarese, M.d.L.; Ricardo Soares, A.; da Silva, D.; Ferrarese-Filho, O. Cadmium-induced lignification restricts soybean root growth. Ecotoxicol. Environ. Saf. 2010, 73, 1959–1964. [Google Scholar] [CrossRef]
- Chen, Y.X.; He, Y.F.; Yang, Y.; Yu, Y.L.; Zheng, S.J.; Tian, G.M.; Luo, Y.M.; Wong, M.H. Effect of cadmium on nodulation and N2-fixation of soybean in contaminated soils. Chemosphere 2003, 50, 781–787. [Google Scholar] [CrossRef]
- Alyemeni, M.N.; A., A.M.; Wijaya, L.; Alam, P.; Ahmad, P. Contrasting tolerance among soybean genotypes subjected to different levels of cadmium stress. Pak. J. Bot. 2017, 49, 903–911. [Google Scholar]
- Fang, X.; Zhao, Y.; Ma, Q.; Huang, Y.; Wang, P.; Zhang, J.; Nian, H.; Yang, C. Identification and comparative analysis of cadmium tolerance-associated miRNAs and their targets in two soybean genotypes. PLoS ONE 2013, 8, e81471. [Google Scholar] [CrossRef] [Green Version]
- Cui, G.; Ai, S.; Chen, K.; Wang, X. Arbuscular mycorrhiza augments cadmium tolerance in soybean by altering accumulation and partitioning of nutrient elements, and related gene expression. Ecotoxicol. Environ. Saf. 2019, 171, 231–239. [Google Scholar] [CrossRef]
- Zagorchev, L.; Albanova, I.; Tosheva, A.; Li, J.; Teofanova, D. Salinity effect on Cuscuta campestris Yunck. parasitism on Arabidopsis thaliana L. Plant Physiol. Biochem. 2018, 132, 408–414. [Google Scholar] [CrossRef]
- Fang, R.-C.; Staples, G. Convolvulaceae. In Flora of China, Volume 16: Gentianaceae through Boraginaceae; Wu, C.Y., Raven, P.H., Eds.; Missouri Botanical Garden Press: St. Louis, MI, USA, 1995; pp. 271–325. [Google Scholar]
- Bao, X.; Wang, Z.; Fang, J.; Li, X. Structural features of an immunostimulating and antioxidant acidic polysaccharide from the seeds of Cuscuta chinensis. Planta Med. 2002, 68, 237–243. [Google Scholar] [CrossRef]
- Donnapee, S.; Li, J.; Yang, X.; Ge, A.-H.; Donkor, P.O.; Gao, X.-M.; Chang, Y.-X. Cuscuta chinensis Lam.: A systematic review on ethnopharmacology, phytochemistry and pharmacology of an important traditional herbal medicine. J. Ethnopharmacol. 2014, 157, 292–308. [Google Scholar] [CrossRef]
- Chen, B.J.W.; Huang, L.; During, H.J.; Wang, X.; Wei, J.; Anten, N.P.R. No neighbour-induced increase in root growth of soybean and sunflower in mesh-divider experiments after controlling for nutrient concentration and soil volume. AoB Plants 2021, 13, plab020. [Google Scholar] [CrossRef]
- Hutchison, J.M.; Ashton, F.M. Effect of desiccation and scarification on the permeability and structure of the seed coat of Cuscuta campestris. Am. J. Bot. 1979, 66, 40–46. [Google Scholar] [CrossRef]
- Wang, L.; Cui, X.; Cheng, H.; Chen, F.; Wang, J.; Zhao, X.; Lin, C.; Pu, X. A review of soil cadmium contamination in China including a health risk assessment. Environ. Sci. Pollut. Res. 2015, 22, 16441–16452. [Google Scholar] [CrossRef]
- Han, G.-Z.; Zhang, G.-L.; Gong, Z.-T.; Wang, G.-F. Pedotransfer functions for estimating soil bulk density in China. Soil Sci. 2012, 177, 158–164. [Google Scholar] [CrossRef]
- National Health Commission of the PRC; National Medical Products Administration of the PRC. China National Food Safety Standard. In Determination of Elements in Food; GB5009.268-2016; 2016; Available online: https://sppt.cfsa.net.cn:8086/staticPages/DBE4CA28-C983-42EC-AE60-5EB9A0ED008A.html?clicks=384 (accessed on 6 October 2020).
- State Environmental Protection Administration of the PRC; State Bureau of Quality and Technical Supervision of the PRC. Environmental Quality Standard for Soils. 1995. GB15618-1995. Available online: https://www.chinesestandard.net/Related.aspx/GB15618-1995 (accessed on 6 October 2020).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage Publishing: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, A.; Ransijn, J. LMERConvenienceFunctions: Model Selection and Post-Hoc Analysis for (G)LMER Models. R package version 3.0. Available online: https://CRAN.R-project.org/package=LMERConvenienceFunctions (accessed on 6 October 2020).
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.6.3. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 29 September 2021).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]

| Cd Treatment | Root-Stem | Stem-Leaf | Stem-Dodder |
|---|---|---|---|
| T0 | 0.44ab (0.08) | 0.85ba (0.04) | 0.38ab (0.03) |
| T1 | 0.15cdc (0.01) | 1.40aa (0.19) | 0.34abb (0.04) |
| T2 | 0.32abb (0.03) | 0.54ca (0.07) | 0.12bc (0.02) |
| T3 | 0.23bcb (0.02) | 0.37cda (0.03) | 0.08cc (0.01) |
| T4 | 0.05db (0.01) | 0.23da (0.02) | 0.06cb (0.01) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.J.W.; Xu, J.; Wang, X. Trophic Transfer without Biomagnification of Cadmium in a Soybean-Dodder Parasitic System. Plants 2021, 10, 2690. https://doi.org/10.3390/plants10122690
Chen BJW, Xu J, Wang X. Trophic Transfer without Biomagnification of Cadmium in a Soybean-Dodder Parasitic System. Plants. 2021; 10(12):2690. https://doi.org/10.3390/plants10122690
Chicago/Turabian StyleChen, Bin J. W., Jing Xu, and Xinyu Wang. 2021. "Trophic Transfer without Biomagnification of Cadmium in a Soybean-Dodder Parasitic System" Plants 10, no. 12: 2690. https://doi.org/10.3390/plants10122690
APA StyleChen, B. J. W., Xu, J., & Wang, X. (2021). Trophic Transfer without Biomagnification of Cadmium in a Soybean-Dodder Parasitic System. Plants, 10(12), 2690. https://doi.org/10.3390/plants10122690





