Chemical Composition and Biological Activities of Tunisian Ziziphus lotus Extracts: Evaluation of Drying Effect, Solvent Extraction, and Extracted Plant Parts
Abstract
:1. Introduction
2. Results
2.1. Extraction Yields
2.2. Chemical Compositions of Z. lotus Extracts
2.3. Antioxidant Activity
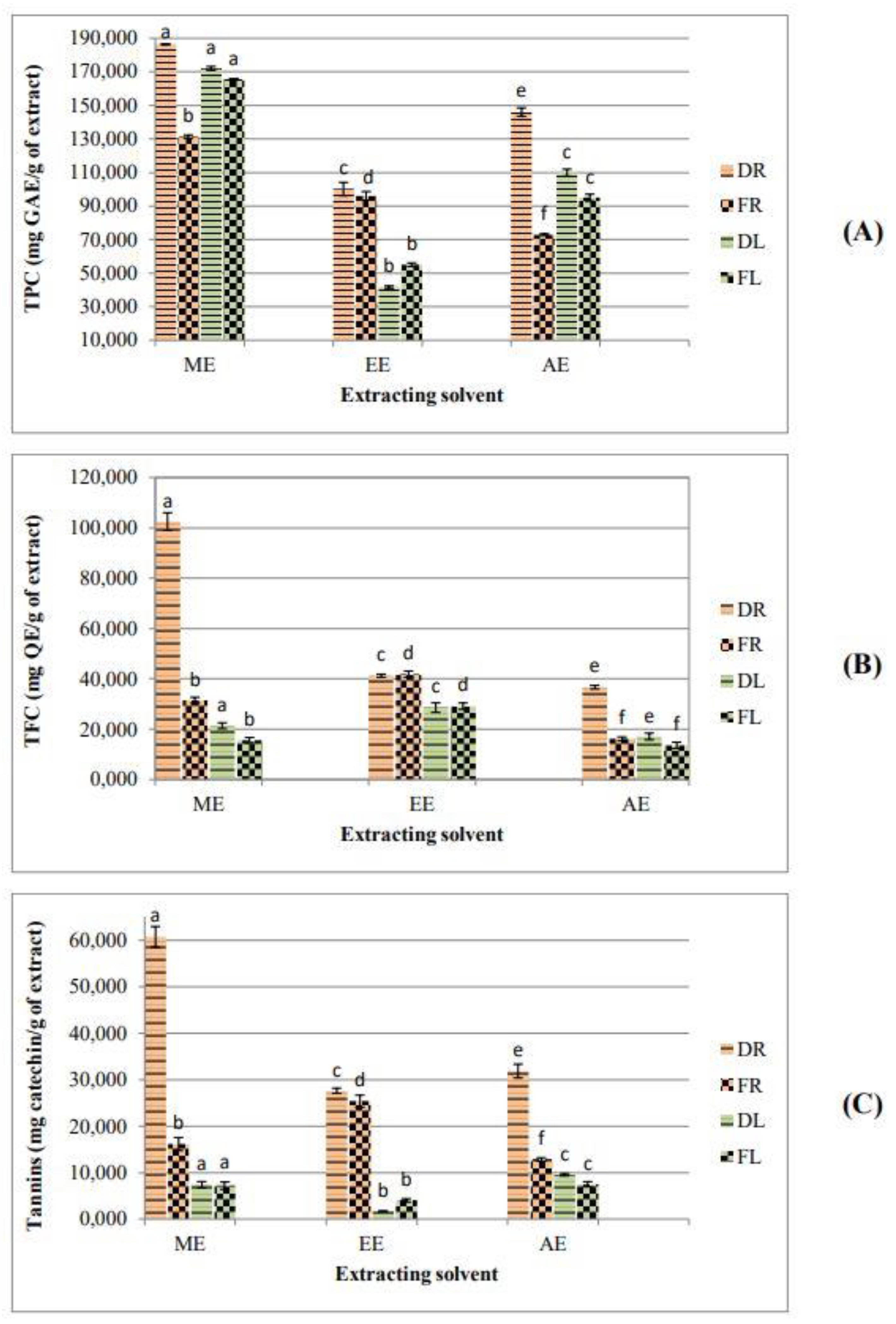
2.4. Drying Effect on Phytochemical Composition and Antioxidant Activity
2.5. Chemical Characterization of the Dried Root Petroleum Ether and Dichloromethane Extracts: GC-MS Analysis and Thin Layer Chromatography
2.6. Cytotoxic Activity
3. Discussion
4. Materials and Methods
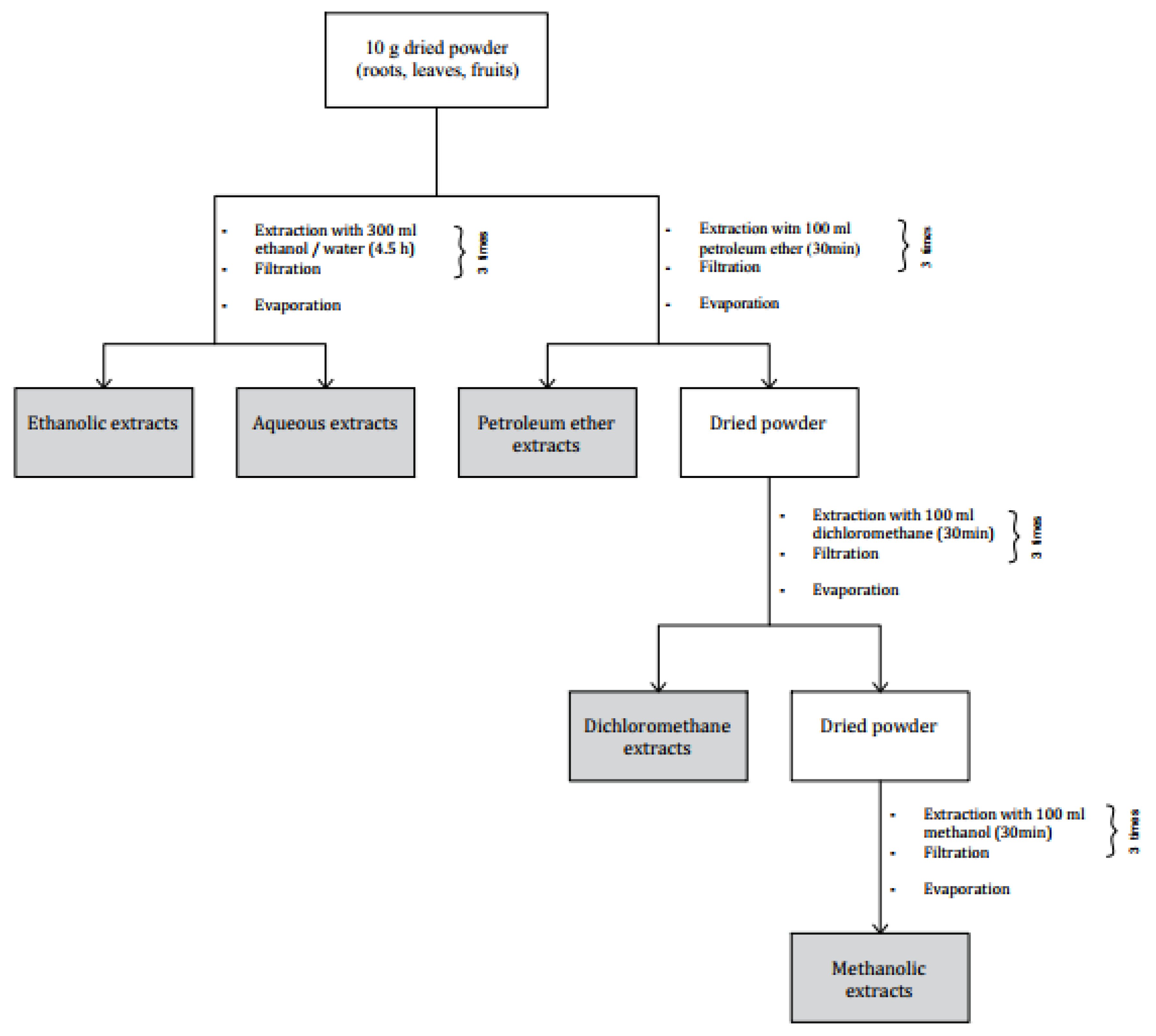
4.1. Preparation of Samples and Extracts
- w: the weight of residue in grams;
- W: the weight of dried plant material in grams.
4.2. Total Phenolic Content
4.3. Total Flavonoid Content
4.4. Tannin Content
4.5. DPPH• Scavenging Activity
- A (blank): the absorbance of the prepared DPPH• solution without the sample extract;
- A (sample): the absorbance of the sample after the reaction with the DPPH• solution.
4.6. ABTS•+ Scavenging Activity
4.7. Total Antioxidant Capacity (TAC)
4.8. Thin Layer Chromatography (TLC)
4.9. GC-MS/GC-FID Analysis
4.10. Cytotoxic Activity
4.10.1. Cell Cultures
4.10.2. MTT
- A(treated): absorbance mean of the treated cells
- A(control): absorbance mean of the untreated cells
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jain, C.; Khatana, S. Rekha Vijayvergia Bioactivity of secondary metabolites of various plants: A review. Int. J. Pharm. Sci. Res. 2019, 10, 494–504. [Google Scholar] [CrossRef]
- Mera, I.F.G.; Falconí, D.E.G.; Córdova, V.M. Secondary metabolites in plants: Main classes, phytochemical analysis and pharmacological activities. Rev. Bionatura 2019, 4, 1000–1009. [Google Scholar] [CrossRef]
- Abdullahi, R. Abubakar and Mainul Haque Preparation of Medicinal Plants: Basic Extraction and Fractionation Procedures for Experimental Purposes. J. Pharm. Bioallied Sci. 2020, 12, 1–10. [Google Scholar]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Yonbawi, A.R.; Abdallah, H.M.; Alkhilaiwi, F.A.; Koshak, A.E.; Heard, C.M. Anti-Proliferative, Cytotoxic and Antioxidant Properties of the Methanolic Extracts of Five Saudi Arabian Flora with Folkloric Medicinal Use: Aizoon canariense, Citrullus colocynthis, Maerua crassifolia, Rhazya stricta and Tribulus macropterus. Plants 2021, 10, 2073. [Google Scholar] [CrossRef] [PubMed]
- Ghedira, K. Zizyphus lotus (L.) Desf. (Rhamnaceae): Jujubier sauvage. Phytotherapie 2013, 11, 149–153. [Google Scholar] [CrossRef]
- San, B.; Yildirim, A.N.; Polat, M.; Yildirim, F. Mineral composition of leaves and fruits of some promising jujube (zizyphus jujuba miller) genotypes. Asian J. Chem. 2009, 21, 2898–2902. [Google Scholar]
- Maraghni, M.; Gorai, M.; Neffati, M. The influence of water-deficit stress on growth, water relations and solute accumulation in wild Jujube. J. Ornam. Hortic. Plants 2011, 1, 63–72. [Google Scholar]
- Guirado, E.; Tabik, S.; Alcaraz-Segura, D.; Cabello, J.; Herrera, F. Deep-learning Versus OBIA for scattered shrub detection with Google Earth Imagery: Ziziphus lotus as case study. Remote Sens. 2017, 9, 1220. [Google Scholar] [CrossRef] [Green Version]
- Maraghni, M.; Gorai, M.; Neffati, M. Seed germination at different temperatures and water stress levels, and seedling emergence from different depths of Ziziphus lotus. S. Afr. J. Bot. 2010, 76, 453–459. [Google Scholar] [CrossRef] [Green Version]
- Hammi, K.M.; Jdey, A.; Abdelly, C.; Majdoub, H.; Ksouri, R. Optimization of ultrasound-assisted extraction of antioxidant compounds from Tunisian Zizyphus lotus fruits using response surface methodology. Food Chem. 2015, 184, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Boussaid, M.; Taïbi, K.; Ait Abderrahim, L.; Ennajah, A. Genetic diversity of Ziziphus lotus natural populations from Algeria based on fruit morphological markers. Arid L. Res. Manag. 2018, 32, 184–197. [Google Scholar] [CrossRef]
- Khouchlaa, A.; Talbaoui, A.; El Yahyaoui El Idrissi, A.; Bouyahya, A.; Ait Lahsen, S.; Kahouadji, A.; Tijane, M. Determination of phenol content and evaluation of in vitro litholytic effects on urolithiasis of moroccan Zizyphus lotus L. extract. Phytotherapie 2020, 16, s1. [Google Scholar] [CrossRef]
- Borgi, W.; Bouraoui, A.; Chouchane, N. Antiulcerogenic activity of Zizyphus lotus (L.) extracts. J. Ethnopharmacol. 2007, 112, 228–231. [Google Scholar] [CrossRef]
- Benammar, C.; Hichami, A.; Yessoufou, A.; Simonin, A.M.; Belarbi, M.; Allali, H.; Khan, N.A. Zizyphus lotus L. (Desf.) modulates antioxidant activity and human T-cell proliferation. BMC Complement. Altern. Med. 2010, 10, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahlou, M.; Mahi, J.H. Évaluation des activités antifongique et molluscicide de Zizyphus lotus (L.) Desf. du Maroc. Ann. Pharm. françaises 2002, 60, 410–414. [Google Scholar]
- Ghedira, K.; Chemli, R.; Richard, B.; Nuzillard, J.M.; Zeches, M.; Men-Olivier, L. Le Two cyclopeptide alkaloids from Zizyphus lotus. Phytochemistry 1993, 32, 1591–1594. [Google Scholar] [CrossRef]
- Climati, E.; Mastrogiovanni, F.; Valeri, M.; Salvini, L.; Bonechi, C.; Mamadalieva, N.Z.; Egamberdieva, D.; Taddei, A.R.; Tiezzi, A. Methyl carnosate, an antibacterial diterpene isolated from salvia officinalis leaves. Nat. Prod. Commun. 2013, 8, 429–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin-Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Ghalem, M.; Merghache, S.; Belarbi, M. Study on the antioxidant activities of root extracts of Zizyphus lotus from the western region of Algeria. Pharmacogn. J. 2014, 6, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Elaloui, M.; Ennajah, A.; Ghazghazi, H.; Ben Youssef, I.; Ben Othman, N.; Hajlaoui, M.R.; Khouja, A.; Laamouri, A. Quantification of total phenols, flavonoides and tannins from Ziziphus jujuba (mill.) and Ziziphus lotus (l.) (Desf). Leaf extracts and their effects on antioxidant and antibacterial activities. Int. J. Second. Metab. 2016, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Abderrahim, B.; Harrar, A.; Gul, F.; Demirtas, I. Phenolic Compounds, Antioxidant and Antibacterial Activities of Zizyphus lotus L. Leaves Extracts. Nat. Prod. J. 2017, 7, 316–322. [Google Scholar]
- Olajuyigbe, O.O.; Afolayan, A.J. Phenolic content and antioxidant property of the bark extracts of Ziziphus mucronata Willd. subsp. mucronata Willd. BMC Complement. Altern. Med. 2011, 11, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchaillot, A.; Caffin, N.; Bhandari, B. Drying of lemon myrtle (Backhousia citriodora) leaves: Retention of volatiles and color. Dry. Technol. 2009, 27, 445–450. [Google Scholar] [CrossRef]
- Esparza-Martínez, F.J.; Miranda-López, R.; Guzman-Maldonado, S.H. Effect of air-drying temperature on extractable and non-extractable phenolics and antioxidant capacity of lime wastes. Ind. Crops Prod. 2016, 84, 1–6. [Google Scholar] [CrossRef]
- Ghazouani, N.; Abderrabba, M.; Bouajila, J. Teucrium ramosissimum (Lamiaceae): Volatile Composition, Seasonal Variation, and Pharmaceutical Activity. Anal. Lett. 2016, 49, 1258–1271. [Google Scholar] [CrossRef]
- Olufunmilayo, D.A.; Oyetola, O.; Shaid, R.O. Phytochemical Analysis and Antioxidant Activities of Dry and Fresh Leaves of Petivera alliacea and Ocimum. Int. J. Sci. Basic Appl. Res. 2015, 24, 1–13. [Google Scholar]
- El Cadi, H.; Bouzidi, H.E.L.; Selama, G.; El Cadi, A.; Ramdan, B.; Oulad, Y.; Majdoub, E.; Alibrando, F.; Dugo, P.; Mondello, L.; et al. Physico-Chemical and Phytochemical Characterization of Moroccan Wild Jujube “Zizyphus lotus (L.)” Fruit Crude Extract and Fractions. Molecules 2020, 25, 5237. [Google Scholar] [CrossRef]
- Ravi, L.; Krishnan, K. Cytotoxic Potential of N-hexadecanoic Acid Extracted from Kigelia pinnata Leaves. Asian J. Cell Biol. 2016, 12, 20–27. [Google Scholar] [CrossRef]
- Harada, H.; Yamashita, U.; Kurihara, H.; Fukushi, E.; Kawabata, J.; Kamei, Y. Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Anticancer Res. 2002, 22, 2587–2590. [Google Scholar]
- Rached, W.; Barros, L.; Ziani, B.E.C.; Bennaceur, M.; Calhelha, R.C.; Heleno, S.A.; Alves, M.J.; Marouf, A.; Ferreira, I.C.F.R. HPLC-DAD-ESI-MS/MS screening of phytochemical compounds and the bioactive properties of different plant parts of: Zizyphus lotus (L.) Desf. Food Funct. 2019, 10, 5898–5909. [Google Scholar] [CrossRef] [Green Version]
- Christine, U.; Ruxandra, P.; Benedikt, G.; Daniela, L.; Susanne, H.; Mareike, S.; Rene, D.; Bruno, W.; Gerda, E.; Melanie, H.; et al. The dichloromethane extract of the ethnomedicinal plant Neurolaena lobata inhibits NPM/ALK expression which is causal for anaplastic large cell lymphomagenesis. Int. J. Oncol. 2013, 42, 338–348. [Google Scholar]
- Yahyaoui, M.; Ghazouani, N.; Saoudi, S.; Sifaoui, I.; Chammem, N.; Abderrabba, M. Experimental design methodologyapplication in the optimization of phytochemical compoundsextraction and antioxidant activity of Thymelaea hirsuta L. extracts. J. Mater. Environ. Sci. 2018, 9, 1551–1561. [Google Scholar] [CrossRef]
- Tlili, N.; Mejri, H.; Yahia, Y.; Saadaoui, E.; Rejeb, S.; Khaldi, A.; Nasri, N. Phytochemicals and antioxidant activities of Rhus tripartitum (Ucria) fruits depending on locality and different stages of maturity. Food Chem. 2014, 160, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Ovidi, E.; Garzoli, S.; Laghezza Masci, V.; Turchetti, G.; Tiezzi, A. GC-MS investigation and antiproliferative activities of extracts from male and female flowers of Schinus molle L. Nat. Prod. Res. 2021, 35, 1923–1927. [Google Scholar] [CrossRef] [PubMed]


| Extracts | Extraction Yield (%) | |
|---|---|---|
| Roots | PEE | 0.50 |
| DE | 1.30 | |
| ME | 29.80 | |
| EE | 5.58 | |
| AE | 7.07 | |
| Leaves | PEE | 4.20 |
| DE | 2.80 | |
| ME | 15.10 | |
| EE | 9.23 | |
| AE | 12.97 | |
| Fruits | ME | 25.30 |
| AE | 48.00 | |
| Samples | TPC (mg GAE/g DW) | TFC (mg QE/g DW) | Tannins (mg CE /g DW) | |
|---|---|---|---|---|
| Roots | PEE | 39.22 ± 0.62 a | 19.63 ± 0.12 a | 9.14 ± 0.90 a |
| DE | 30.15 ± 0.13 a | 18.50 ± 0.88 a | 6.90 ± 1.41 a | |
| ME | 186.44 ± 0.26 b | 102.50 ± 3.53 b | 60.71 ± 2.20 b | |
| EE | 100.13 ± 4.02 c | 41.25 ± 0.63 c | 27.70 ± 0.57 b | |
| AE | 146.06 ± 2.50 d | 36.70 ± 0.72 a | 31.86 ± 1.49 b | |
| Leaves | PEE | 12.81 ± 0.10 e | 3.06 ± 0.12 d | 2.50 ± 0.37 c |
| DE | 11.16 ± 0.45 e | 3.44 ± 0.06 d | 3.44 ± 0.47 c | |
| ME | 171.99 ± 1.14 f | 21.35 ± 1.19 e | 7.41 ± 0.68 d | |
| EE | 41.70 ± 0.70 g | 28.54 ± 1.89 f | 1.66 ± 0.09 d | |
| AE | 109.87 ± 2.07 h | 17.10 ± 1.30 d | 9.54 ± 0.26 d | |
| Fruits | ME | 26.12 ± 0.73 i | 0.75 ± 0.13 g | 1.00 ± 0.170 e |
| AE | 82.12 ± 1.70 j | 13.40 ± 0.72 h | 1.02 ± 0.10 e | |
| Samples | Antioxidant Assay | |||
|---|---|---|---|---|
| ABTS•+ IC50 (mg/L) | DPPH• IC50 (mg/L) | TAC (mg AAE/mg Extract) | ||
| Roots | PEE | 14.76 ± 0.02 a | 101.06 ± 0.40 a | 105.56 ± 0.37 a |
| DE | 136.58 ± 0.41 b | 192.33 ± 0.60 b | 91.11 ± 2.20 a | |
| ME | 14.31 ± 0.13 c | 18.03 ± 0.61 c | 304.07 ± 1.11 b | |
| EE | 27.42 ± 0.32 d | 39.50 ± 0.49 d | 167.41 ± 7.40 c | |
| AE | 8.96 ± 0.38 e | 16.46 ± 0.60 e | 191.85 ± 0.00 d | |
| Leaves | PEE | 28.98 ± 0.06 f | NA | NA |
| DE | 29.51 ± 1.23 g | NA | 154.44 ± 6.20 e | |
| ME | 23.48 ± 0.63 h | 33.66 ± 0.11 f | 142.47 ± 0.85 f | |
| EE | 249.37 ± 1.26 i | 375.50 ± 1.50 g | 173.09 ± 2.99 g | |
| AE | 29.01 ± 0.44 j | 64.80 ± 0.36 h | 99.26 ± 4.62 h | |
| Fruits | ME | 173.93 ± 0.88 k | 343.00 ± 1.32 i | 26.42 ± 2.26 i |
| AE | 342.25 ± 1.25 l | 383.33 ± 0.29 j | 40.74 ± 3.39 j | |
| DR-PEE | DR-DE | |||
|---|---|---|---|---|
| After 24 h of Treatment | After 48 h of Treatment | After 24 h of Treatment | After 48 h of Treatment | |
| IC50 (µg/mL) | 184.413 ± 4.77 | 20.941 ± 1.16 | 16.148 ± 0.93 | 7.341 ± 1.98 |
| Component | LRI 1 | LRI 2 | DR-PEE (%) | DR-DE (%) | A Fraction (%) | B Fraction (%) |
|---|---|---|---|---|---|---|
| Ethyl tridecanoate | 1944 | 1943 | 0.2 | 6.7 | - | 14.9 |
| 2-pentadecanone | 2026 | 2028 | 0.8 | - | - | - |
| Tetradecanoic acid, ethyl ester | 2055 | 2059 | - | 72.8 | - | - |
| Pentadecanoic acid, ethyl ester | 2178 | 2179 | 2.2 | - | - | - |
| 13-epimanool | 2670 | 2676 * | 0.8 | 20.5 | 100.0 | 85.1 |
| Tetradecanoic acid | 2680 | 2679 | 5.4 | - | - | - |
| n-hexadecanoic acid | 2943 | 2946 | 90.6 | - | - | - |
| Total | 100.0 | 100.0 | 100.0 | 100.0 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Letaief, T.; Garzoli, S.; Laghezza Masci, V.; Mejri, J.; Abderrabba, M.; Tiezzi, A.; Ovidi, E. Chemical Composition and Biological Activities of Tunisian Ziziphus lotus Extracts: Evaluation of Drying Effect, Solvent Extraction, and Extracted Plant Parts. Plants 2021, 10, 2651. https://doi.org/10.3390/plants10122651
Letaief T, Garzoli S, Laghezza Masci V, Mejri J, Abderrabba M, Tiezzi A, Ovidi E. Chemical Composition and Biological Activities of Tunisian Ziziphus lotus Extracts: Evaluation of Drying Effect, Solvent Extraction, and Extracted Plant Parts. Plants. 2021; 10(12):2651. https://doi.org/10.3390/plants10122651
Chicago/Turabian StyleLetaief, Touka, Stefania Garzoli, Valentina Laghezza Masci, Jamel Mejri, Manef Abderrabba, Antonio Tiezzi, and Elisa Ovidi. 2021. "Chemical Composition and Biological Activities of Tunisian Ziziphus lotus Extracts: Evaluation of Drying Effect, Solvent Extraction, and Extracted Plant Parts" Plants 10, no. 12: 2651. https://doi.org/10.3390/plants10122651
APA StyleLetaief, T., Garzoli, S., Laghezza Masci, V., Mejri, J., Abderrabba, M., Tiezzi, A., & Ovidi, E. (2021). Chemical Composition and Biological Activities of Tunisian Ziziphus lotus Extracts: Evaluation of Drying Effect, Solvent Extraction, and Extracted Plant Parts. Plants, 10(12), 2651. https://doi.org/10.3390/plants10122651









