Alkaloid Biosynthesis in the Early Stages of the Germination of Argemone mexicana L. (Papaveraceae)
Abstract
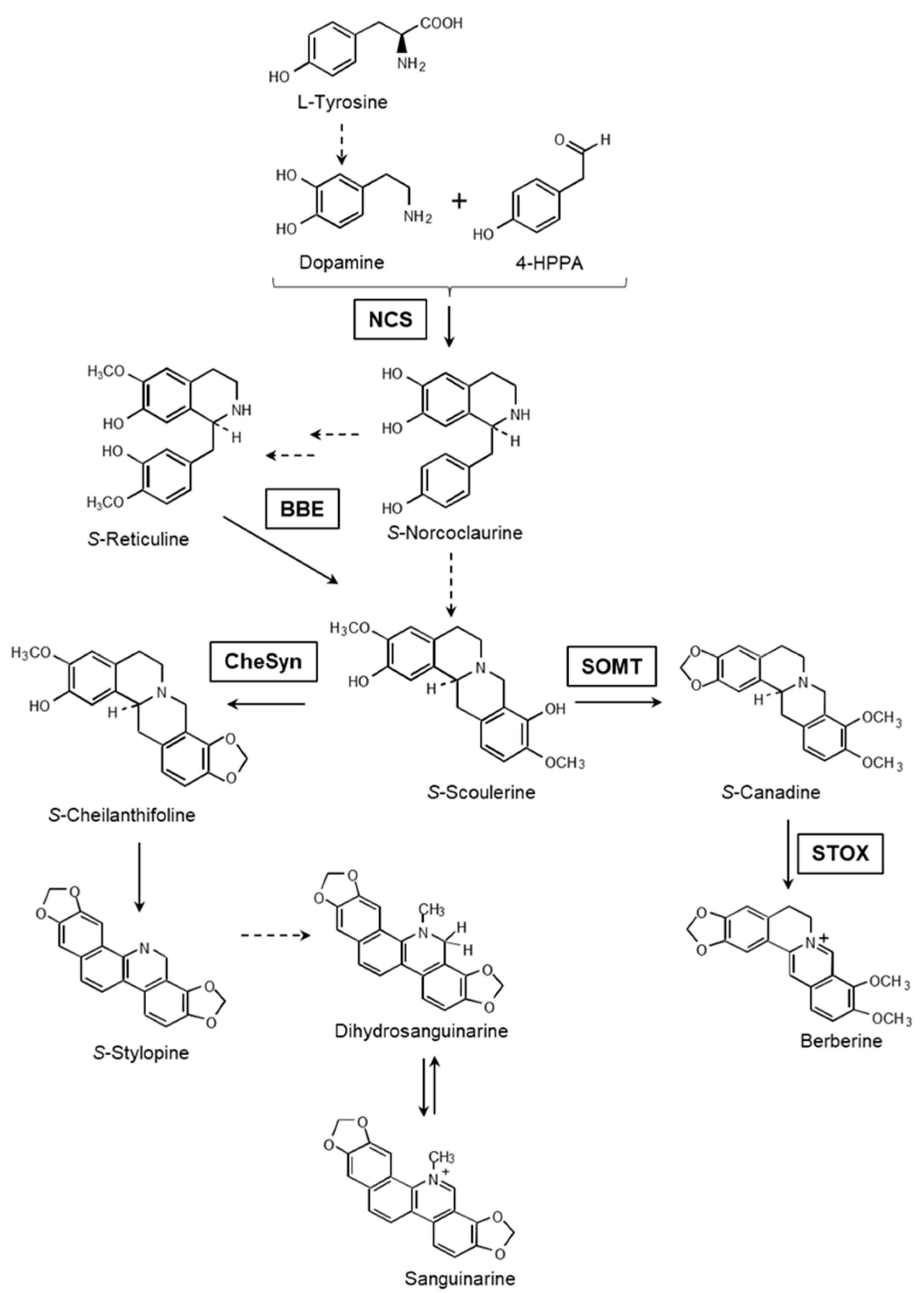
1. Introduction
2. Results
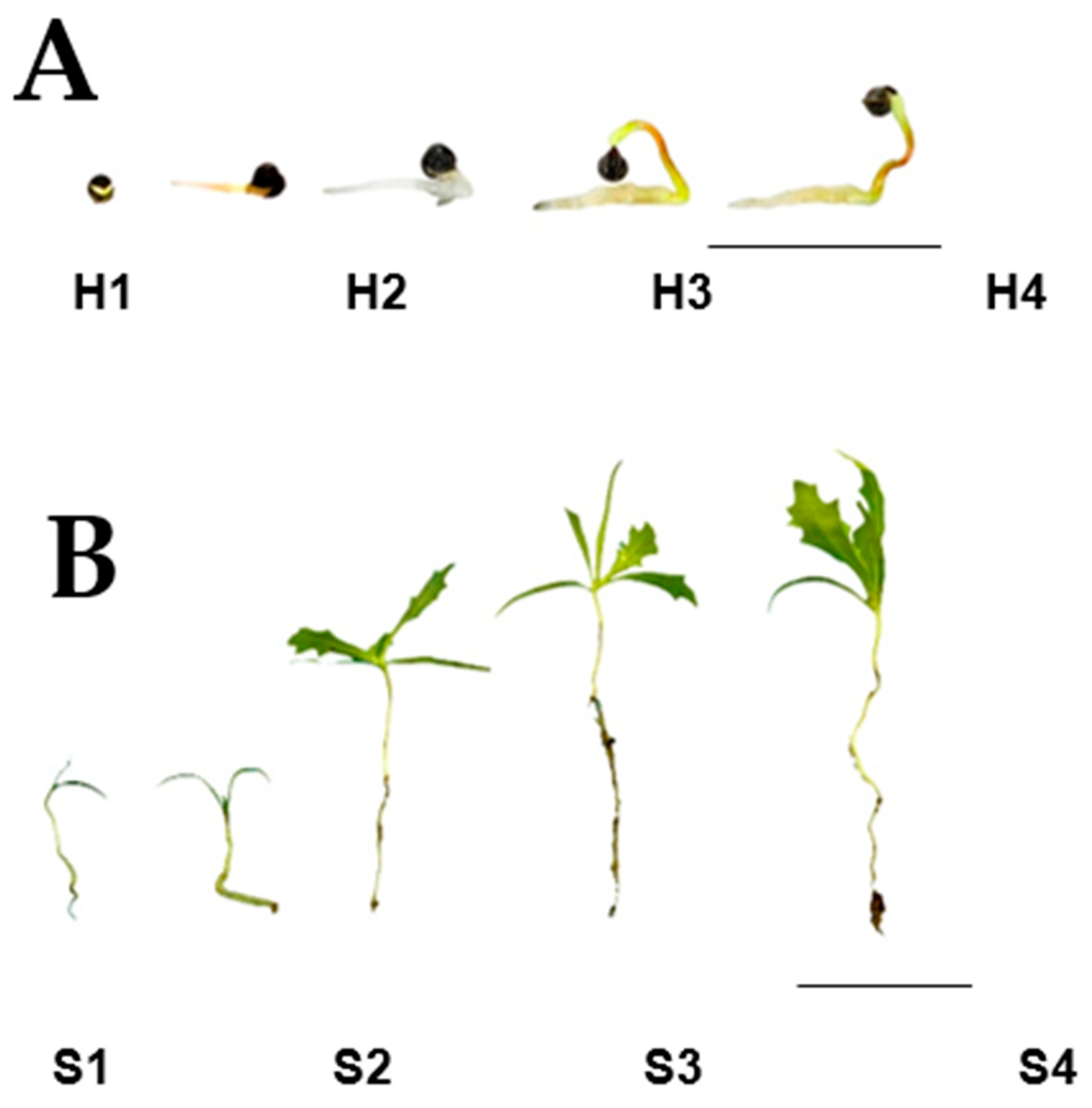
2.1. Hypocotyl and Seedling Development
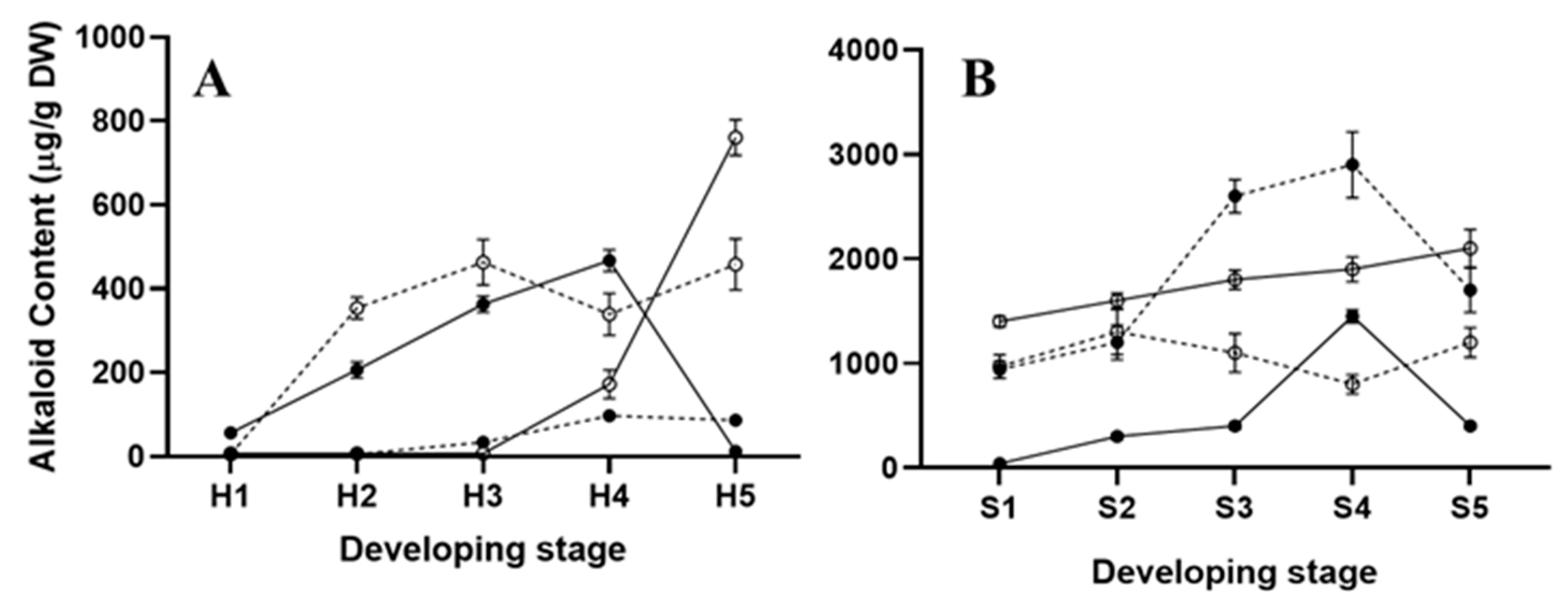
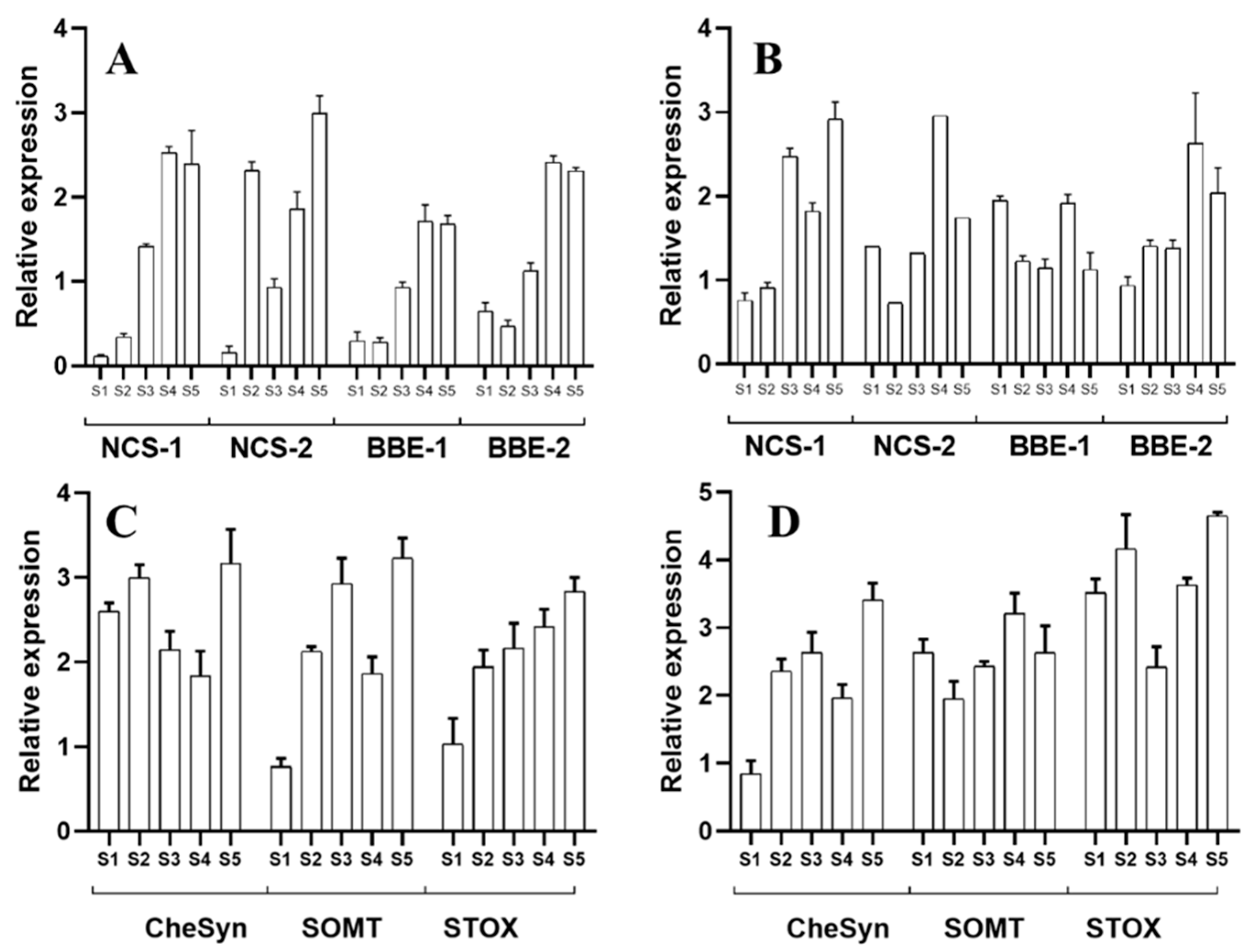
2.2. Alkaloid Synthesis at the Hypocotyl Stage
2.3. Alkaloid Synthesis at the Seedling Stage
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Nucleic Acid Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Gene | Direction 1 | Sequence (5′–3′) |
|---|---|---|
| ACTIN | Fwd | CACIACTACTGCTAAACGGGAAA |
| Rvs | ACATCTGCTGGAAGGTGCTG | |
| TyDC | Fwd | GTTGAACCAGGTTATTTACG |
| Rvs | CGTATTCTTTCGCAACCTC | |
| NCS-1 | Fwd | CATCGCTAATTACGTTCTCAAGAATCA |
| Rvs | ATAGTAGTACATGGAATTACCTGGATGGGA | |
| NCS-2 | Fwd | CGTACCATTGAAATCCATGTCAGAA |
| Rvs | CATCGGACGGTAATTACCCATG | |
| BBE-1 | Fwd | CATCTTTGTTCATCATCATCTTCTTCTTCTTCTT |
| Rvs | GATCCTCTTGTGCAACATCTAACGGT | |
| BBE-2 | Fwd | CTCATCTTTGTTCATCTTCTTTTCTGTGC |
| Rvs | GATCCTCTTGTGCAACATCTAACGGT | |
| CHESYN | Fwd | AGGTCTTCAAGGTGTTGCCC |
| (CYP719A14) | Rvs | TCTTTTCCCGCCCGTAACAT |
| SOMT | Fwd | ATCCTATCCATGTCTACGAGGGCTATT |
| Rvs | CCAGTACCACCACCAACATCTAACA | |
| STOX | Fwd | GGTTAGGAAATATGGACTTG |
| Rvs | ATAACATTGCTGGTGAATCT |
References
- Hildreth, S.B.; Gehman, E.A.; Yang, H.; Lu, R.-H.; Ritesh, K.C.; Harich, K.C.; Yu, S.; Lin, J.; Sandoe, J.L.; Okumoto, S.; et al. Tobacco nicotine uptake permease (NUP1) affects alkaloid metabolism. Proc. Natl. Acad. Sci. USA 2011, 108, 18179–18184. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, J.; Facchini, P.J. Alkaloid Biosynthesis: Metabolism and Trafficking. Annu. Rev. Plant Biol. 2008, 59, 735–769. [Google Scholar] [CrossRef] [PubMed]
- Schilmiller, A.L.; Last, R.L.; Pichersky, E. Harnessing plant trichome biochemistry for the production of useful compounds. Plant J. 2008, 54, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Schwarzbach, A.E.; Kadereit, J.W. Phylogeny of prickly poppies, Argemone (Papaveraceae), and the evolution of morphological and alkaloid characters based on ITS nrDNA sequence variation. Plant Syst. Evol. 1999, 218, 257–279. [Google Scholar] [CrossRef]
- Rubio-Piña, J.; Vázquez-Flota, F. Pharmaceutical applications of the benzylisoquinoline alkaloids from Argemone mexicana L. Curr. Top. Med. Chem. 2013, 13, 2200–2207. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Flota, F.; Rubio-Piña, J.; Xool-Tamayo, J.; Vergara-Olivares, M.; Tamayo-Ordoñez, Y.; Monforte-González, M.; Guízar-González, C.; Mirón-López, G. Tissue distribution of transcripts involved in benzylisoquinoline alkaloid biosynthesis in Argemone mexicana L. (Papaveraceae). Rev. Fitotec. Mex. 2018, 41, 13–21. [Google Scholar] [CrossRef][Green Version]
- Xool-Tamayo, J.F.; Monforte-González, M.; Rubio-Piña, J.; Mirón-López, G.; Vázquez-Flota, F. Early developmental onset of alkaloid biosynthesis in Mexican poppy (Argemone mexicana L.) Papaveraceae. Phytochem. Lett. 2017, 20, 300–305. [Google Scholar] [CrossRef]
- Xool-Tamayo, J.; Serrano-Gamboa, G.; Monforte-González, M.; Mirón-López, G.; Vázquez-Flota, F. Development of newly sanguinarine biosynthetic capacity in in vitro rootless shoots of Argemone mexicana L. Mexican prickly poppy. Biotechnol. Lett. 2017, 39, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Monforte-González, M.; Serrano-Gamboa, J.G.; Guízar-González, C.; Miranda-Ham, M.L.; Vázquez-Flota, F.A. Alkaloid synthesis is coupled to shoot morphogenesis in Argemone mexicana L. (Papaveraceae) in vitro cultures. Vitr. Cell. Dev. Biol. Plant 2019, 55, 695–701. [Google Scholar] [CrossRef]
- Sullivan, J.; Deng, X.W. From seed to seed: The role of photoreceptors in Arabidopsis development. Dev. Biol. 2003, 260, 289–297. [Google Scholar] [CrossRef]
- Harada, J.J. Role of Arabidopsis LEAFY COTYLEDON genes in seed development. J. Plant Physiol. 2001, 158, 405–409. [Google Scholar] [CrossRef]
- Yang, N.; Ye, Z.; Liu, J.; Liu, Y.; Chen, Q.; Wang, H.; Guo, X.; Herppich, W.B.; Tang, Z. Network during light-induced cotyledons opening and greening in Astragalus membranaceus. J. Plant Interact. 2020, 15, 358–370. [Google Scholar] [CrossRef]
- Hardtke, C.S.; Berlett, T. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J. 1998, 17, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Langel, D.; Ober, D.; Pelser, P.B. The evolution of pyrrolizidine alkaloid biosynthesis and diversity in the Senecioneae. Phytochem. Rev. 2011, 10, 3–74. [Google Scholar] [CrossRef]
- Shitan, N.; Bazin, I.; Dan, K.; Obata, K.; Kigawa, K.; Ueda, K.; Sato, F.; Forestier, C.; Yazaki, K. Involvement of CjMDR1, a plant multidrug-resistance-type ATP-binding cassette protein, in alkaloid transport in Coptis japonica. Proc. Natl. Acad. Sci. USA 2003, 100, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Monforte-González, M.; Guízar-González, C.; Rubio-Piña, J.; Carrillo-Pech, M.; Vázquez-Flota, F. Berberine and sanguinarine quantitation in Argemone mexicana L. (Papaveraceae) tissues by TLC-in situ fluorography. J. Planar Chromatogr. Mod. TLC 2012, 25, 358–360. [Google Scholar] [CrossRef]
- Rubio-Piña, J.; Vázquez-Flota, F. Isolation of functional total RNA from Argemone mexicana tissues. Electron. J. Biotechnol. 2008, 11, 1–5. [Google Scholar] [CrossRef]
- Chávez, M.L.D.; Rolf, M.; Gesell, A.; Kutchan, T.M. Characterization of two methylenedioxy bridge-forming cytochrome P450-dependent enzymes of alkaloid formation in the Mexican prickly poppy Argemone mexicana. Arch. Biochem. Biophys. 2011, 507, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Gesell, A.; Díaz-Chávez, M.L.; Kramell, R.; Piotrowski, M.; Macheroux, P.; Kutchan, T.M. Heterologous expression of two FAD-dependent oxidases with (S)-tetrahydroprotoberberine oxidase activity from Argemone mexicana and Berberis wilsoniae in insect cells. Planta 2011, 233, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Simon, P. Q-Gene: Processing quantitative real-time RT-PCR data. Bioinformatics 2003, 19, 1439–1440. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xool-Tamayo, J.; Tamayo-Ordoñez, Y.; Monforte-González, M.; Muñoz-Sánchez, J.A.; Vázquez-Flota, F. Alkaloid Biosynthesis in the Early Stages of the Germination of Argemone mexicana L. (Papaveraceae). Plants 2021, 10, 2226. https://doi.org/10.3390/plants10102226
Xool-Tamayo J, Tamayo-Ordoñez Y, Monforte-González M, Muñoz-Sánchez JA, Vázquez-Flota F. Alkaloid Biosynthesis in the Early Stages of the Germination of Argemone mexicana L. (Papaveraceae). Plants. 2021; 10(10):2226. https://doi.org/10.3390/plants10102226
Chicago/Turabian StyleXool-Tamayo, Jorge, Yahaira Tamayo-Ordoñez, Miriam Monforte-González, José Armando Muñoz-Sánchez, and Felipe Vázquez-Flota. 2021. "Alkaloid Biosynthesis in the Early Stages of the Germination of Argemone mexicana L. (Papaveraceae)" Plants 10, no. 10: 2226. https://doi.org/10.3390/plants10102226
APA StyleXool-Tamayo, J., Tamayo-Ordoñez, Y., Monforte-González, M., Muñoz-Sánchez, J. A., & Vázquez-Flota, F. (2021). Alkaloid Biosynthesis in the Early Stages of the Germination of Argemone mexicana L. (Papaveraceae). Plants, 10(10), 2226. https://doi.org/10.3390/plants10102226






