The Impact of Light Spectrum and Intensity on the Growth, Physiology, and Antioxidant Activity of Lettuce (Lactuca sativa L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Condition
2.1.1. First Experiment
2.1.2. Second Experiment
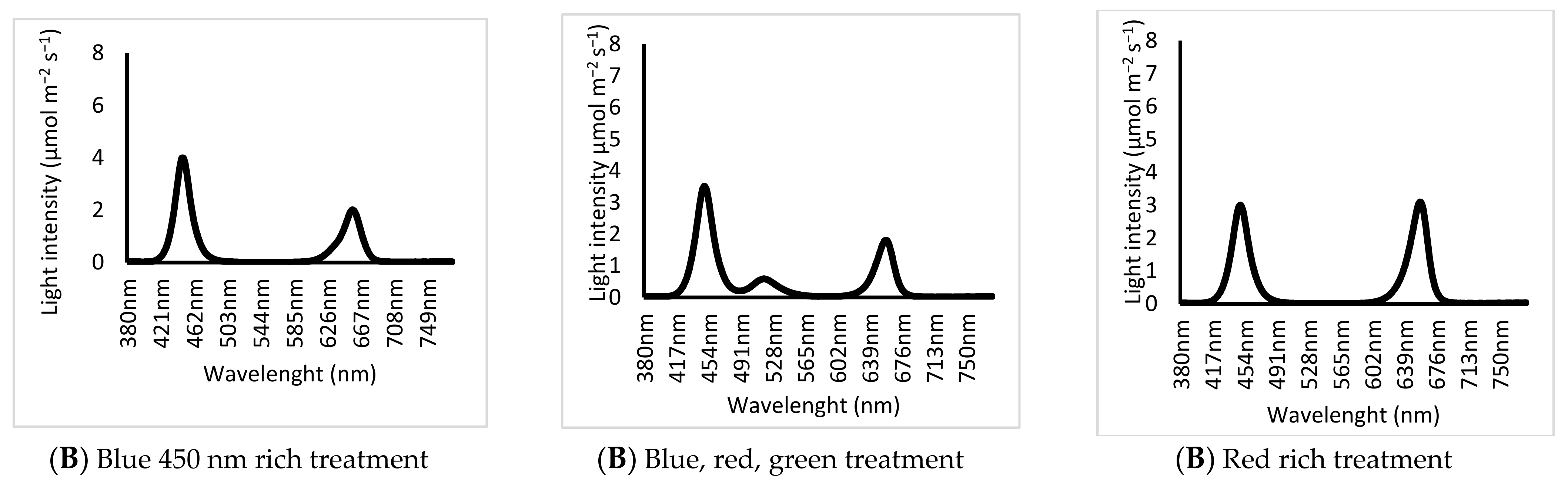
- (1)
- Blue 450 nm rich treatment: Blue/Red treatment (B-rich/R). Blue rich spectrum with 450 nm wavelength used as a source of blue (B/R: 1.25 ± 0.1) (Blue (450 nm) to Red (663) peak ratio, 1.6:1).
- (2)
- Blue, red, green treatment: Blue/Red/Green treatment (B/R/G). Blue rich spectrum with 450 nm wavelength used as a source of blue with (B/R/G: 1.25/1/0.35) (Blue (435 nm) to Red (663) peak ratio, 1.6:1).
- (3)
- Red rich treatment: Red (663 nm) rich light spectrum with 450 nm wavelength used as a source of blue (B/R: 0.72) (Blue Red peak ratio 1:1.2).
2.2. Physiological Parameters Measurements
2.3. Determination of Plant Morphology
2.4. Antioxidant Activity Analysis
2.5. Statistical Analysis
3. Results
3.1. Assimilation Rate, Stomatal Conductance and Transpiration at High Lighting Intensity
3.2. Growth and Morphology at High Lighting Intensity
3.3. Assimilation Rate, Stomatal Conductance and Transpiration at Low Lighting Intensity
3.4. Growth and Morphology at Low Lighting Intensity
3.5. Second Experiment
3.5.1. Assimilation Rate, Stomatal Conductance and Transpiration under B-rich/R, B-rich/R/G and B/R-rich
3.5.2. Growth and Morphology under B-rich/R, B-rich/R/G and B/R-rich
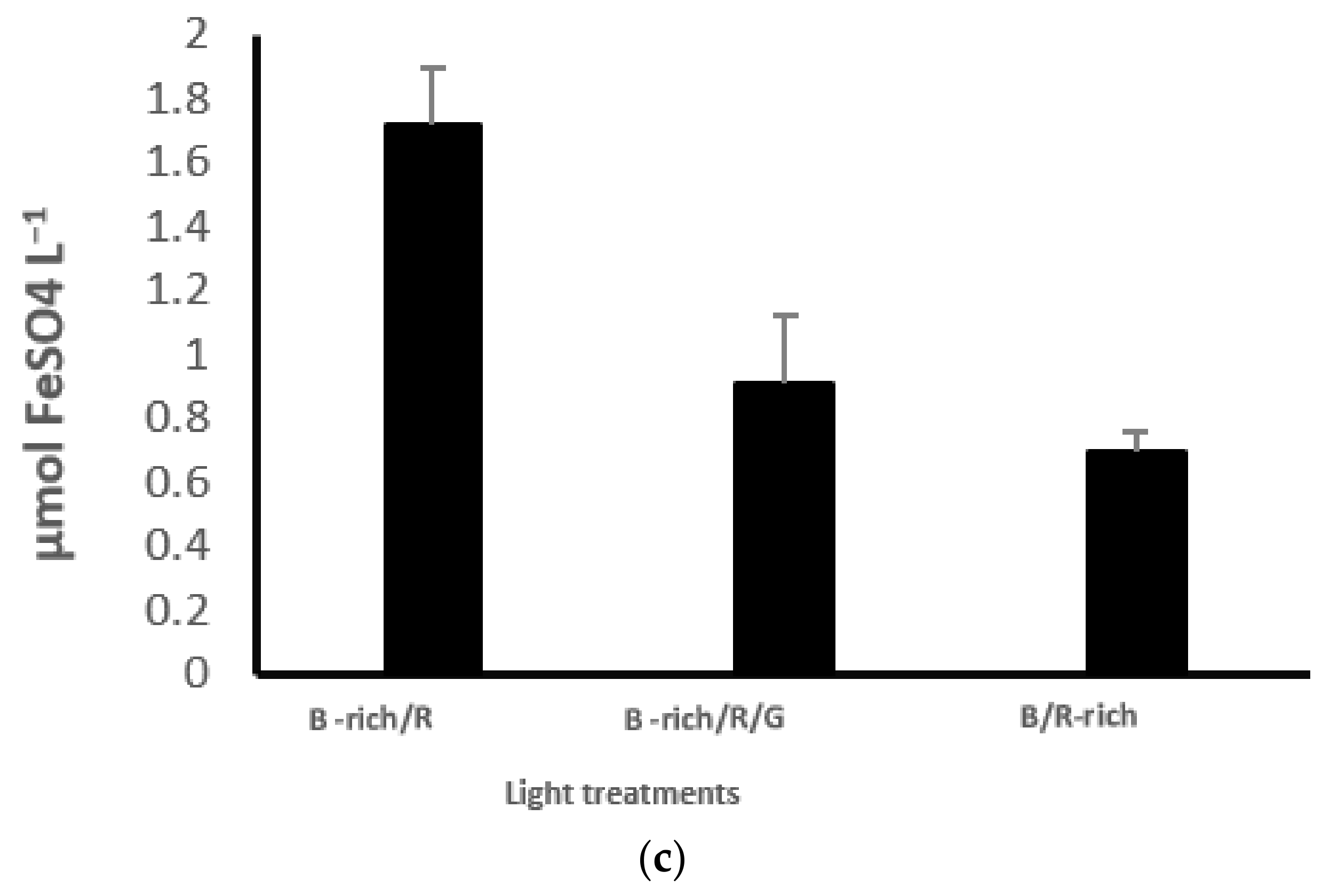
3.6. Antioxidant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chon, S.-U.; Jang, H.-G.; Kim, D.-K.; Kim, Y.-M.; Boo, H.-O. Allelopathic potential in lettuce (Lactuca sativa L.) plants. Sci. Hortic. 2005, 106, 309–317. [Google Scholar] [CrossRef]
- Briskin, D.P. Medicinal Plants and Phytomedicines. Linking Plant Biochemistry and Physiology to Human Health. Plant Physiol. 2000, 124, 507–514. [Google Scholar] [CrossRef]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [PubMed]
- Teodoro, A.J. Bioactive Compounds of Food: Their Role in the Prevention and Treatment of Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R.; Deng, Z. Separation procedures for naturally occurring antioxidant phytochemicals. J. Chromatogr. B 2004, 812, 85–99. [Google Scholar] [CrossRef]
- Lee, J.G.; Lee, B.Y.; Lee, H.J. Accumulation of phytotoxic organic acids in reused nutrient solution during hydroponic cultivation of lettuce (Lactuca sativa L.). Sci. Hortic. 2006, 110, 119–128. [Google Scholar] [CrossRef]
- Yan, Z.; He, D.; Niu, G.; Zhou, Q.; Qu, Y. Growth, Nutritional Quality, and Energy Use Efficiency of Hydroponic Lettuce as Influenced by Daily Light Integrals Exposed to White versus White Plus Red Light-emitting Diodes. HortScience 2019, 54, 1737–1744. [Google Scholar] [CrossRef]
- Amoozgar, A.; Mohammadi, A.; Sabzalian, M.R. Impact of light-emitting diode irradiation on photosynthesis, phytochemical composition and mineral element content of lettuce cv. Grizzly. Photosynthetica 2016, 55, 85–95. [Google Scholar] [CrossRef]
- Shams, M.; Ekinci, M.; Turan, M.; Dursun, A.; Kul, R.; Yildirim, E. Growth, nutrient uptake and enzyme activity response of Lettuce (Lactuca sativa L.) to excess copper. Environ. Sustain. 2019, 2, 67–73. [Google Scholar] [CrossRef]
- Pennisi, G.; Orsini, F.; Blasioli, S.; Cellini, A.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C. Resource use efficiency of indoor lettuce (Lactuca sativa L.) cultivation as affected by red: Blue ratio provided by LED lighting. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Shimizu, H.; Saito, Y.; Nakashima, H.; Miyasaka, J.; Ohdoi, K. Light Environment Optimization for Lettuce Growth in Plant Factory. IFAC Proc. Vol. 2011, 44, 605–609. [Google Scholar] [CrossRef]
- Saito, K.; Ishigami, Y.; Goto, E. Evaluation of the Light Environment of a Plant Factory with Artificial Light by Using an Optical Simulation. Agronomy 2020, 10, 1663. [Google Scholar] [CrossRef]
- Goins, G.D.; Ruffe, L.M.; Cranston, N.A.; Yorio, N.C.; Wheeler, R.M.; Sager, J.C. Salad Crop Production under Different Wavelengths of Red Light-Emitting Diodes (LEDs). SAE Technical Paper (Canada). 2001. Available online: https://www.sae.org/publications/technical-papers/content/2001-01-2422/ (accessed on 29 September 2021).
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf Morphology, Photosynthetic Performance, Chlorophyll Fluorescence, Stomatal Development of Lettuce (Lactuca sativa L.) Exposed to Different Ratios of Red Light to Blue Light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Azad, O.K. Blue Light added with Red LEDs Enhance Growth Characteristics, Pigments Content, and Antioxidant Capacity in Lettuce, Spinach, Kale, Basil, and Sweet Pepper in a Controlled Environment. Plants 2019, 8, 93. [Google Scholar] [CrossRef]
- Wang, X.Y.; Xu, X.M.; Cui, J. The importance of blue light for leaf area expansion, development of photosynthetic apparatus, and chloroplast ultrastructure of Cucumis sativus grown under weak light. Photosynthetica 2014, 53, 213–222. [Google Scholar] [CrossRef]
- Tang, Y.K.; Guo, S.S.; Ai, W.D.; Qin, L.F. Effects of Red and Blue Light Emitting Diodes (LEDs) on the Growth and Development of Lettuce (var. Youmaicai). SAE Technical Paper (Canda). 2009. Available online: https://www.sae.org/publications/technical-papers/content/2009-01-2565/ (accessed on 29 September 2021).
- Kim, H.-H.; Goins, G.D.; Wheeler, R.M.; Sager, J.C. Green-light Supplementation for Enhanced Lettuce Growth under Red- and Blue-light-emitting Diodes. HortScience 2004, 39, 1617–1622. [Google Scholar] [CrossRef]
- Folta, K.M.; Maruhnich, S.A. Green light: A signal to slow down or stop. J. Exp. Bot. 2007, 58, 3099–3111. [Google Scholar] [CrossRef]
- Saito, Y.; Shimizu, H.; Nakashima, H.; Miyasaka, J.; Ohdoi, K. The effect of light quality on growth of lettuce. IFAC Proc. Vol. 2010, 43, 294–298. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, Y.H. Growth and Anthocyanins of Lettuce Grown under Red or Blue Light-emitting Diodes with Distinct Peak Wavelength. Korean J. Hortic. Sci. Technol. 2014, 32, 330–339. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Rihan, H.Z.; Aldarkazali, M.; Mohamed, S.J.; McMulkin, N.B.; Jbara, M.H.; Fuller, M.P. A Novel New Light Recipe Significantly Increases the Growth and Yield of Sweet Basil (Ocimum basilicum) Grown in Plant Factory System. Agronomy 2020, 10, 934. [Google Scholar] [CrossRef]
- Landi, M.; Zivcak, M.; Sytar, O.; Brestic, M.; Allakhverdiev, S.I. Plasticity of photosynthetic processes and the accumulation of secondary metabolites in plants in response to monochromatic light environments: A review. Biochim. Biophys. Acta (BBA) Bioenerg. 2020, 1861, 148131. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-L.; Xue, X.-Z.; Guo, W.-Z.; Wang, L.-C.; Qiao, X.-J. Growth and nutritional properties of lettuce affected by mixed irradiation of white and supplemental light provided by light-emitting diode. Sci. Hortic. 2016, 200, 111–118. [Google Scholar] [CrossRef]
- Hiyama, A.; Takemiya, A.; Munemasa, S.; Okuma, E.; Sugiyama, N.; Tada, Y.; Murata, Y.; Shimazaki, K.-I. Blue light and CO2 signals converge to regulate light-induced stomatal opening. Nat. Commun. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Savvides, A.; Fanourakis, D.; van Ieperen, W. Co-ordination of hydraulic and stomatal conductances across light qualities in cucumber leaves. J. Exp. Bot. 2011, 63, 1135–1143. [Google Scholar] [CrossRef]
- Whitelam, C.G.; Halliday, K.J. Annual Plant Reviews, Light and Plant Development; John Wiley & Sons: Hoboken, NJ, USA, 2008; Volume 30. [Google Scholar]
- Inoue, S.-I.; Kinoshita, T.; Matsumoto, M.; Nakayama, K.I.; Doi, M.; Shimazaki, K.-I. Blue light-induced autophosphorylation of phototropin is a primary step for signaling. Proc. Natl. Acad. Sci. USA 2008, 105, 5626–5631. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gu, M.; Cui, J.; Shi, K.; Zhou, Y.; Yu, J. Effects of light quality on CO2 assimilation, chlorophyll-fluorescence quenching, expression of Calvin cycle genes and carbohydrate accumulation in Cucumis sativus. J. Photochem. Photobiol. B Biol. 2009, 96, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; KrishnaKumar, S.; Atulba, S.L.S.; Jeong, B.R.; Hwang, S.J. Light intensity and photoperiod influence the growth and development of hydroponically grown leaf lettuce in a closed-type plant factory system. Hortic. Environ. Biotechnol. 2013, 54, 501–509. [Google Scholar] [CrossRef]
- Kang, W.H.; Park, J.S.; Park, K.S.; Son, J.E. Leaf photosynthetic rate, growth, and morphology of lettuce under different fractions of red, blue, and green light from light-emitting diodes (LEDs). Hortic. Environ. Biotechnol. 2016, 57, 573–579. [Google Scholar] [CrossRef]
- Zivcak, M.; Brückova, K.; Sytar, O.; Brestic, M.; Olsovska, K.; Allakhverdiev, S.I. Lettuce flavonoids screening and phe-notyping by chlorophyll fluorescence excitation ratio. Planta 2017, 245, 1215–1229. [Google Scholar] [CrossRef]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the en-igmatic question of why leaves are green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef]
- Olle, M.; Viršilė, A. The effects of light-emitting diode lighting on greenhouse plant growth and quality. Agric. Food Sci. 2013, 22, 223–234. [Google Scholar] [CrossRef]
- Ohtake, N.; Ishikura, M.; Suzuki, H.; Yamori, W.; Goto, E. Continuous Irradiation with Alternating Red and Blue Light Enhances Plant Growth While Keeping Nutritional Quality in Lettuce. HortScience 2018, 53, 1804–1809. [Google Scholar] [CrossRef]
- Shin, Y.-S.; Lee, M.-J.; Lee, E.-S.; Ahn, J.-H.; Kim, M.-K.; Lee, J.-E.; Do, H.-W.; Cheung, J.-D.; Park, J.-U.; Um, Y.-G.; et al. Effect of Light Emitting Diodes Treatment on Growth and Quality of Lettuce (Lactuca sativa L. ‘Oak Leaf’). J. Life Sci. 2014, 24, 148–153. [Google Scholar] [CrossRef][Green Version]
- Sytar, O.; Zivcak, M.; Neugart, S.; Toutounchi, P.M.; Brestic, M. Precultivation of young seedlings under different color shades modifies the accumulation of phenolic compounds in Cichorium leaves in later growth phases. Environ. Exp. Bot. 2019, 165, 30–38. [Google Scholar] [CrossRef]
- Sellaro, R.; Crepy, M.; Trupkin, S.A.; Karayekov, E.; Buchovsky, A.S.; Rossi, C.; Casal, J.J. Cryptochrome as a Sensor of the Blue/Green Ratio of Natural Radiation in Arabidopsis. Plant Physiol. 2010, 154, 401–409. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development; Sinauer Associates Incorporated: Sunderland, MA, USA, 2015. [Google Scholar]
- Shao, M.; Liu, W.; Zha, L.; Zhou, C.; Zhang, Y.; Li, B. Differential effects of high light duration on growth, nutritional quality, and oxidative stress of hydroponic lettuce under red and blue LED irradiation. Sci. Hortic. 2020, 268, 109366. [Google Scholar] [CrossRef]
- Li, L.; Tong, Y.X.; Lu, J.L.; Li, Y.M.; Yang, Q.C. Lettuce growth, nutritional quality, and energy use efficiency as affected by red–blue light combined with different monochromatic wavelengths. HortScience 2020, 55, 613–620. [Google Scholar] [CrossRef]
- Zhang, T.; Maruhnich, S.A.; Folta, K.M. Green Light Induces Shade Avoidance Symptoms. Plant Physiol. 2011, 157, 1528–1536. [Google Scholar] [CrossRef]
- Sytar, O.; Zivcak, M.; Bruckova, K.; Brestic, M.; Hemmerich, I.; Rauh, C.; Simko, I. Shift in accumulation of flavonoids and phenolic acids in lettuce attributable to changes in ultraviolet radiation and temperature. Sci. Hortic. 2018, 239, 193–204. [Google Scholar] [CrossRef]
- Son, K.-H.; Lee, J.-H.; Oh, Y.; Kim, D.; Oh, M.-M.; In, B.-C. Growth and Bioactive Compound Synthesis in Cultivated Lettuce Subject to Light-quality Changes. HortScience 2017, 52, 584–591. [Google Scholar] [CrossRef]
- Son, K.-H.; Park, J.-H.; Kim, D.; Oh, M.-M. Leaf Shape Index, Growth, and Phytochemicals in Two Leaf Lettuce Cultivars Grown under Monochromatic Light-emitting Diodes. Korean J. Hortic. Sci. Technol. 2012, 30, 664–672. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Taulavuori, K.; Hyöky, V.; Oksanen, J.; Taulavuori, E.; Julkunen-Tiitto, R. Species-specific differences in synthesis of flavonoids and phenolic acids under increasing periods of enhanced blue light. Environ. Exp. Bot. 2016, 121, 145–150. [Google Scholar] [CrossRef]
- Jiao, Y.; Lau, O.S.; Deng, X.W. Light-regulated transcriptional networks in higher plants. Nat. Rev. Genet. 2007, 8, 217–230. [Google Scholar] [CrossRef]



| Light Treatment | Harvest Stage | Growth and Physiological Parameters | ||||||
|---|---|---|---|---|---|---|---|---|
| Assimilation Rate (μmol m−2 s−1) | Stomatal Conductance (mmol m−2 s−1) | Transpiration Rate (mmol m−2 s−1) | Shoot Fresh Weight (g) | Leaves Number | Root Fresh Weight (g) | |||
| Blue/Red (B435/R663) | Harvest stage 1 | 6.58 ± 0.31 | 0.5 ± 0.150 | 1.19 ± 0.021 | 84.9 ± 6.84 | 39.37 ± 4.30 | 8.6 ± 0.67 | |
| Harvest stage 2 | 3.94 ± 0.33 | 0.38 ± 0.054 | 1.54 ± 0.106 | 165.30 ± 2.44 | 69.67 ± 6.09 | 11.62 ± 1 | ||
| Blue/Red (B450/R663) | Harvest stage 1 | 5.03 ± 0.43 | 0.23 ± 0.024 | 1.20 ± 0.066 | 77.36 ± 8.83 | 25.25 ± 3.03 | 3.96 ± 0.87 | |
| Harvest stage 2 | 1.95 ± 0.31 | 0.12 ± 0.012 | 1.23 ± 0.092 | 119.22 ± 2.45 | 67.83 ± 6.24 | 7.61 ± 0.64 | ||
| Blue/Red treatment (B/R-rich) | Harvest stage 1 | 6.90 ± 0.47 | 1.09 ± 0.162 | 1.48 ± 0.074 | 60.31 ± 11.98 | 24.5 ± 4.52 | 4.22 ± 0.37 | |
| Harvest stage 2 | 2.76 ± 0.36 | 0.20 ± 0.017 | 2.05 ± 0.126 | 137.01 ± 2.44 | 71.67 ± 8.86 | 8.83 ± 1.19 | ||
| LSD | Harvest stage | 2.41 | 0.40 | Not significant | 40.49 | 10.17 | 4.39 | |
| Light treatment | 1.96 | 0.33 | 1.25 | 33.06 | Not significant | Not significant | ||
| Interaction between light treatment and harvest stage | Not significant | 0.23 | 0.70 | Not significant | Not significant | Not significant | ||
| Light Treatment | Harvest Stage | Growth and Physiological Parameters | ||||||
|---|---|---|---|---|---|---|---|---|
| Assimilation Rate (μmol m−2 s−1) | Stomatal Conductance (mmol m−2 s−1) | Transpiration Rate (mmol m−2 s−1) | Shoot Fresh Weight (g) | Leaves Number | Root Fresh Weight (g) | |||
| Blue/Red (B435/R663) | Harvest stage 1 | 3.18 ± 0.64 | 0.16 ± 0.016 | 3.83 ± 0.81 | 29.6 ± 2.85 | 23.25 ± 2.37 | 7.57 ± 1.19 | |
| Harvest stage 2 | 2.68 ± 0.62 | 0.09 ± 0.011 | 1.45 ± 0.1 | 75.87 ± 2.45 | 40.83 ± 3.81 | 10.18 ± 0.81 | ||
| Blue/Red (B450/R663) | Harvest stage 1 | 2.44 ± 0.31 | 0.26 ± 0.019 | 2.38 ± 0.10 | 22.69 ± 3.27 | 21.5 ± 1.90 | 1.87 ± 0.22 | |
| Harvest stage 2 | 1.04 ± 0.11 | 0.07 ± 0.007 | 0.93 ± 0.07 | 73.99 ± 2.45 | 46 ± 2.84 | 7.58 ± 0.61 | ||
| Blue/Red treatment (B/R-rich) | Harvest stage 1 | 3.12 ± 0.37 | 0.16 ± 0.016 | 3.01 ± 0.69 | 26.62 ± 2.55 | 24.12 ± 2.41 | 3.79 ± 0.36 | |
| Harvest stage 2 | 1.85 ± 0.30 | 0.11 ± 0.009 | 1.46 ± 0.10 | 44.98 ± 2.45 | 28.83 ± 3.92 | 9.05 ± 0.9 | ||
| LSD | Harvest stage | 2.41 | 0.073 | 0.54 | 30.19 | 5.06 | 2.55 | |
| Light treatment | 1.96 | Not significant | 0.44 | Not significant | Not significant | 2.08 | ||
| Interaction between light treatment and harvest stage | Not significant | 0.04 | 0.31 | Not significant | 13.01 | Not significant | ||
| Light Treatment | Harvest Stage | Growth and Physiological Parameters | ||||||
|---|---|---|---|---|---|---|---|---|
| Assimilation Rate (μmol m−2 s−1) | Stomatal Conductance (mmol m−2 s−1) | Transpiration Rate (mmol m−2 s−1) | Shoot Fresh Weight (g) | Leaves Number | Root Fresh Weight (g) | |||
| Blue/Red (B435/R663) | Harvest stage 1 | 3.30 ± 0.74 | 0.15 ± 0.01 | 1.58 ± 0.16 | 37.72 ± 2.72 | 21.62 ± 1.13 | 5.24 ± 1.04 | |
| Harvest stage 2 | 2.76 ± 0.36 | 0.21 ± 0.017 | 2.05 ± 0.126 | 100.5 ± 2.45 | 46.5 ± 5.46 | 10.67 ± 0.57 | ||
| Blue/Red (B450/R663) | Harvest stage 1 | 4.04 ± 0.37 | 0.19 ± 0.027 | 1.20 ± 0.066 | 33.81 ± 3.36 | 23 ± 2.84 | 1.89 ± 0.14 | |
| Harvest stage 2 | 2.27 ± 0.27 | 0.15 ± 0.016 | 1.41 ± 0.085 | 95.33 ± 2.45 | 50.33 ± 4.66 | 6.04 ± 0.43 | ||
| Blue/Red treatment (B/R-rich) | Harvest stage 1 | 6.39 ± 0.45 | 0.25 ± 0.010 | 3.84 ± 0.729 | 24.87 ± 4.79 | 24.75 ± 1.77 | 2.26 ± 0.51 | |
| Harvest stage 2 | 2.05 ± 0.20 | 0.22 ± 0.020 | 1.89 ± 0.091 | 82.21 ± 2.45 | 36 ± 2.73 | 10.23 ± 1.04 | ||
| LSD | Harvest stage | 2.26 | Not significant | 1.42 | 43.05 | 5.30 | 3.35 | |
| Light treatment | Not significant | 0.09 | 1.16 | Not significant | Not significant | Not significant | ||
| Interaction between light treatment and harvest stage | 1.31 | Not significant | 0.82 | Not significant | 14.71 | Not significant | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, S.J.; Rihan, H.Z.; Aljafer, N.; Fuller, M.P. The Impact of Light Spectrum and Intensity on the Growth, Physiology, and Antioxidant Activity of Lettuce (Lactuca sativa L.). Plants 2021, 10, 2162. https://doi.org/10.3390/plants10102162
Mohamed SJ, Rihan HZ, Aljafer N, Fuller MP. The Impact of Light Spectrum and Intensity on the Growth, Physiology, and Antioxidant Activity of Lettuce (Lactuca sativa L.). Plants. 2021; 10(10):2162. https://doi.org/10.3390/plants10102162
Chicago/Turabian StyleMohamed, Shiren J., Hail Z. Rihan, Naofel Aljafer, and Michael P. Fuller. 2021. "The Impact of Light Spectrum and Intensity on the Growth, Physiology, and Antioxidant Activity of Lettuce (Lactuca sativa L.)" Plants 10, no. 10: 2162. https://doi.org/10.3390/plants10102162
APA StyleMohamed, S. J., Rihan, H. Z., Aljafer, N., & Fuller, M. P. (2021). The Impact of Light Spectrum and Intensity on the Growth, Physiology, and Antioxidant Activity of Lettuce (Lactuca sativa L.). Plants, 10(10), 2162. https://doi.org/10.3390/plants10102162








