Encapsulation of Cinnamic Acid on Plant-Based Proteins: Evaluation by HPLC, DSC and FTIR-ATR
Abstract
:1. Introduction
2. Results and Discussion
2.1. HPLC Analysis of Protein/Cinnamic Acid Complexes
2.2. DSC Analysis of Protein/Cinnamic Acid Complexes
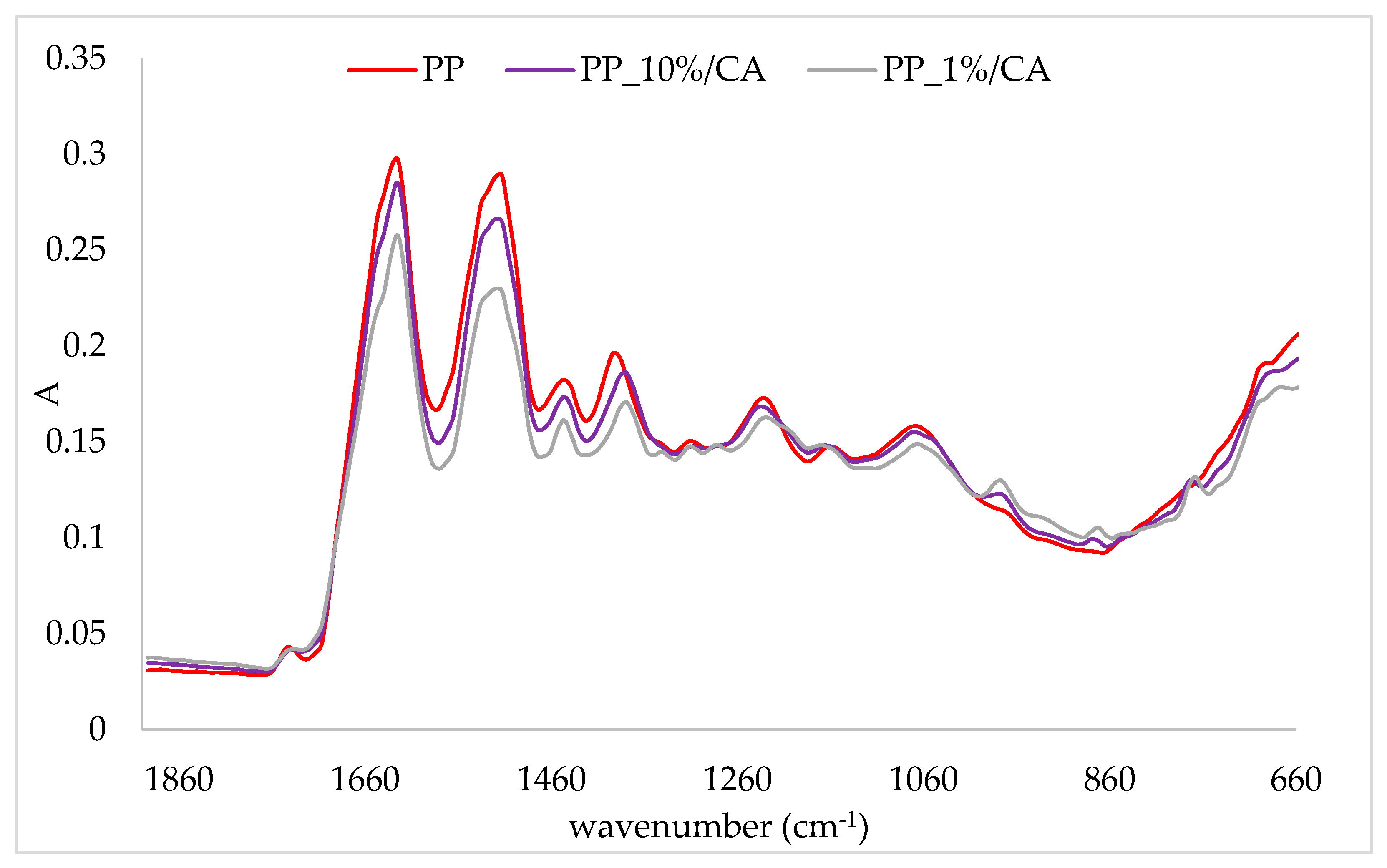
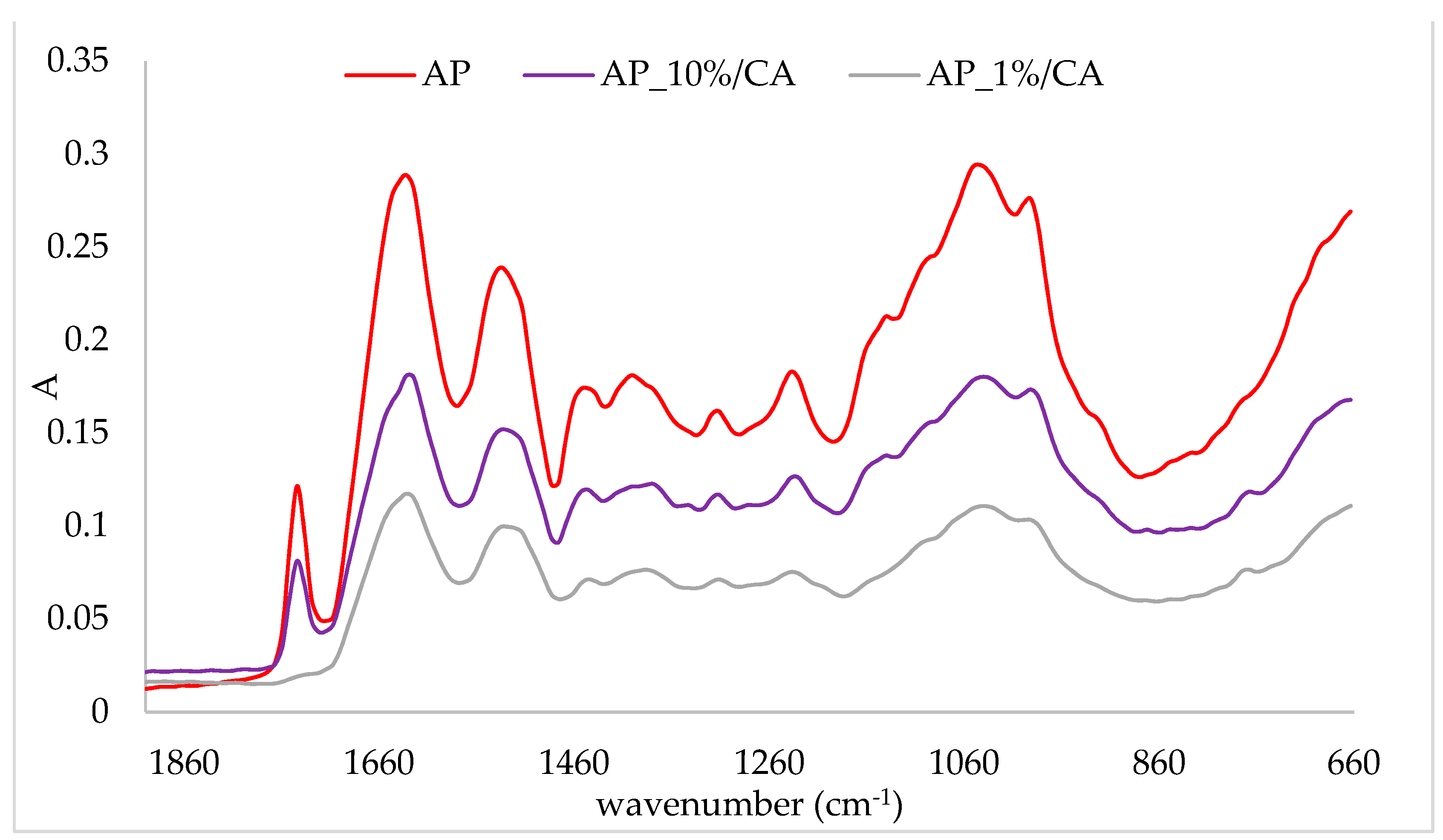
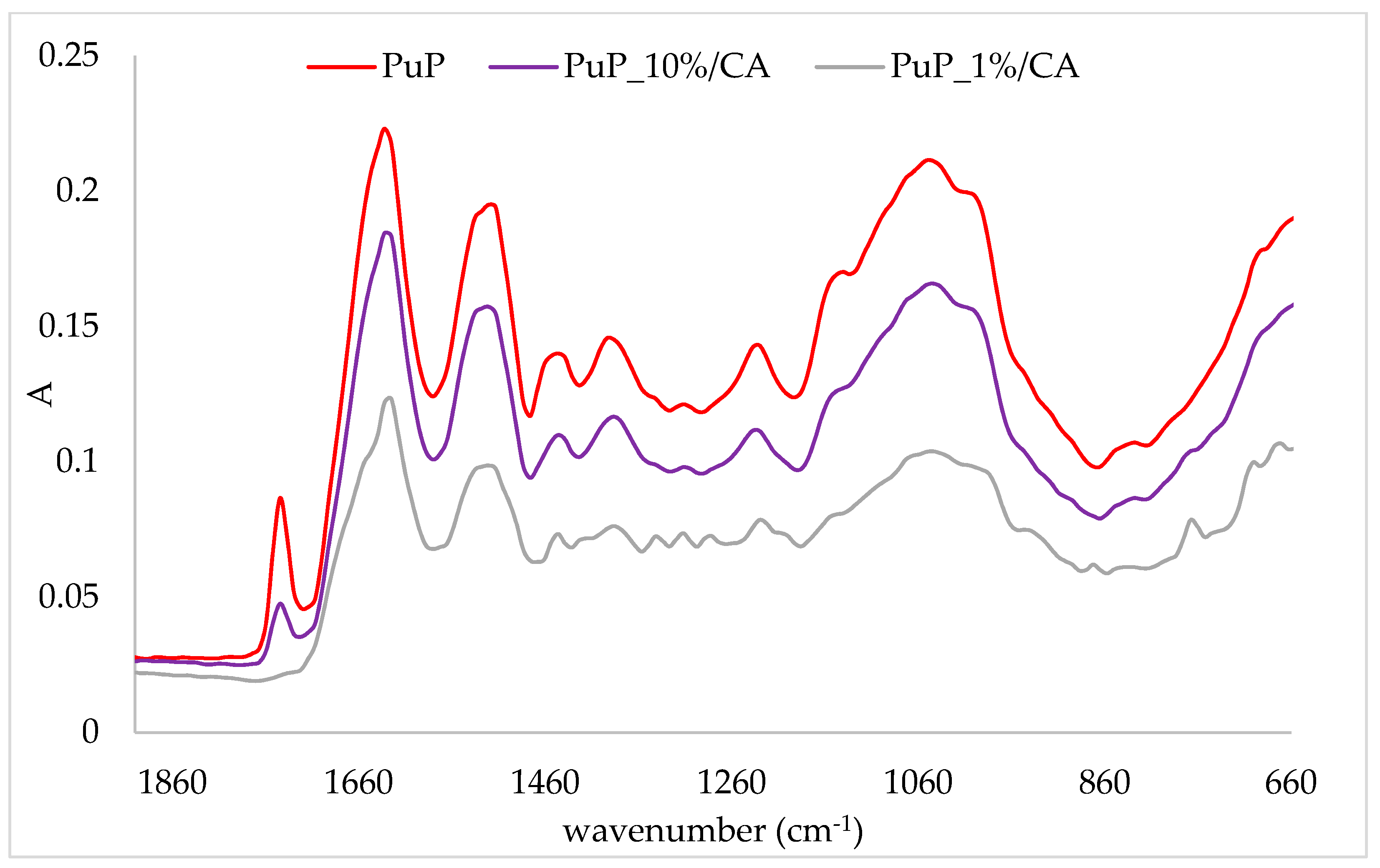
2.3. FTIR-ATR Analysis of Protein/Cinnamic Acid Complexes
3. Materials and Methods
3.1. Materials
3.2. Formulation of Protein/Cinnamic Acid Complexes
3.3. Reverse-Phase High Performance Liquid Chromatography (RP-HPLC)
3.4. Analysis by Differential Scanning Calorimetry (DSC)
3.5. Recording of IR Spectra by Fourier-Transform Infrared Spectroscopy-Attenuated Total Reflectance (FTIR-ATR)
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Czubinski, J.; Dwiecki, K. A review of methods used for investigation of protein–phenolic compound interactions. Int. J. Food Sci. Technol. 2017, 52, 573–585. [Google Scholar] [CrossRef]
- Kroll, J.; Rawel, H.M.; Rohn, S. Reactions of plant phenolics with food proteins and enzymes under special consideration of covalent bonds. Food Sci. Technol. Res. 2003, 9, 205–218. [Google Scholar] [CrossRef] [Green Version]
- Tomas-Barberan, F.A.; Andres-Lacueva, C. Polyphenols and health: Current state and progress. J. Agri. Food Chem. 2012, 60, 8773–8775. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein-phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Gonzales, G.B.; Smagghe, G.; Grootaert, C.; Zotti, M.; Raes, K.; Van Camp, J. Flavonoid interactions during digestion, absorption, distribution and metabolism: A sequential structureactivity/property relationship-based approach in the study of bioavailability and bioactivity. Drug Metab. Rev. 2015, 47, 175–190. [Google Scholar] [CrossRef]
- Grace, M.H.; Guzman, I.; Roopchand, D.E.; Moskal, K.; Cheng, D.M.; Pogrebnyak, N.; Raskin, I.; Howell, A.; Lila, M.A. Stable binding of alternative protein-enriched food matrices with concentrated cranberry bioflavonoids for functional food applications. J. Agric. Food Chem. 2013, 61, 6856–6864. [Google Scholar] [CrossRef] [Green Version]
- Bordenave, N.; Hamaker, B.R.; Ferruzzi, M.G. Nature and consequences of non-covalent interactions between flavonoids and macronutrients in foods. Food Funct. 2014, 5, 18–34. [Google Scholar] [CrossRef]
- Lila, M.A.; Schneider, M.; Devlin, A.; Plundrich, N.; Lasterc, S.; Foegeding, E.A. Polyphenol-enriched berry extracts naturally modulate reactive proteins in model foods. Food Funct. 2017, 8, 4760–4767. [Google Scholar] [CrossRef]
- Bohin, M.C.; Vincken, J.-P.; van der Hijden, H.T.W.M.; Gruppen, H. Efficacy of food proteins as carriers for flavonoids. J. Agric. Food Chem. 2012, 60, 4136–4143. [Google Scholar] [CrossRef]
- Roopchand, D.; Grace, M.H.; Kuhen, P.; Cheng, D.; Plundrich, N.; Pouleva, A.; Lila, M.A. Efficient sorption of polyphenols to soybean four enables natural fortification of foods. Food Chem. 2012, 131, 1193–1200. [Google Scholar] [CrossRef] [Green Version]
- Nishinari, K.; Fang, Y.; Guo, S.; Phillips, G.O. Soy proteins: A review on composition, aggregation and emulsification. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]
- Plundrich, N.J.; White, B.L.; Dean, L.L.; Davis, J.P.; Foegeding, E.A.; Lila, M.A. Stability and immunogenicity of hypoallergenic peanut protein–polyphenol complexes during in vitro pepsin digestion. Food Funct. 2015, 6, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Plundrich, N.J.; Kulis, M.; White, B.L.; Grace, M.H.; Guo, R.; Burks, W.; Davis, J.P.; Lila, M.A. Novel strategy to create hypoallergenic peanut protein−polyphenol edible matrices for oral immunotherapy. J. Agric. Food Chem. 2014, 62, 7010–7021. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.T.; Bourbon, A.I.; Pinheiro, A.C.; Fasolin, L.H.; Vicente, A.A. Protein-based structures for food applications: From macro to nanoscale. Front. Sustain. Food Syst. 2018, 2, 77. [Google Scholar] [CrossRef]
- Han, J.; Chang, Y.; Britten, M.; St-Gelais, D.; Champagne, C.P.; Fustier, P.; Lacroix, M. Interactions of phenolic compounds with milk proteins. Eur. Food Res. Technol. 2019, 245, 1881–1888. [Google Scholar] [CrossRef]
- Liu, J.; Yong, H.; Yao, X.; Hu, H.; Yun, D.; Xiao, L. Recent advances in phenolic–protein conjugates: Synthesis, characterization, biological activities and potential applications. RSC Adv. 2019, 9, 35825. [Google Scholar] [CrossRef] [Green Version]
- Quiroz, J.Q.; Velazquez, V.; Corrales-Garcia, L.L.; Torres, J.D.; Delgado, E.; Ciro, G.; Rojas, J. Use of plant proteins as microencapsulating agents of bioactive compounds extracted from annatto seeds (Bixa orellana L.). Antioxidants 2020, 9, 310. [Google Scholar] [CrossRef] [PubMed]
- Kelemen, V.; Pichler, A.; Ivić, I.; Buljeta, I.; Šimunović, J.; Kopjar, M. Brown rice proteins as delivery system of phenolic and volatile compounds of raspberry juice. Int. J. Food Sci. Technol. 2021, 1–9. [Google Scholar] [CrossRef]
- Abd El-Raouf, O.M.; El-Sayed, M.; Manie, M.F. Cinnamic acid and cinnamaldehyde ameliorate cisplatin-induced splenotoxicity in rats. J. Biochem. Mol. Toxicol. 2015, 29, 426–431. [Google Scholar] [CrossRef]
- Wang, S.R.; Yang, W.; Fan, Y.; Dehaen, W.; Li, Y.; Li, H.; Wang, W.; Zheng, Q.; Huai, Q. Design and synthesis of the novel oleanolic acid-cinnamic acid ester derivatives and glycyrrhetinic acid-cinnamic acid ester derivatives with cytotoxic properties. Bioorg. Chem. 2019, 88, 102951. [Google Scholar] [CrossRef]
- Guo, S.; Zhen, Y.; Zhu, Z.; Zhou, G.; Zheng, X. Cinnamic acid rescues behavioral deficits in a mouse model of traumatic brain injury by targeting MiR-455-3p/HDAC2. Life Sci. 2019, 235, 116819. [Google Scholar] [CrossRef]
- Ruwizhi, N.; Aderibigbe, B.A. Review cinnamic acid derivatives and their biological efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Świeca, M.; Kapusta, I.; Gawlik-Dziki, U. Protein-phenolic interactions as a factor affecting the physicochemical properties of white bean proteins. Molecules 2019, 24, 408. [Google Scholar] [CrossRef] [Green Version]
- Rawel, H.M.; Czajka, D.; Rohn, S.; Kroll, J. Interactions of different phenolic acids and flavonoids with soy proteins. Int. J. Biol. Macromol. 2002, 30, 137–150. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Renard, C.M.G.C. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, C.D.; Hasni, I.; Bourassa, P.; Tarantilis, P.A.; Polissiou, M.G.; Tajmir-Riahi, H.-A. Milk β-lactoglobulin complexes with tea polyphenols. Food Chem. 2011, 127, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Yildirim-Elikoglu, S.; Erdem, K.E. Interactions between milk proteins and polyphenols: Binding mechanisms, related changes and the future trends in dairy industry. Food Rev. Int. 2018, 34, 665–697. [Google Scholar] [CrossRef]
- Cao, Y.; Xiong, Y.L. Interaction of whey proteins with phenolic derivatives under neutral and acidic pH conditions. J. Food Sci. 2017, 82, 409–419. [Google Scholar] [CrossRef]
- Wu, W.; Clifford, M.; Howell, N.K. The effect of instant green tea on the foaming and rheological properties of egg albumen proteins. J. Sci. Food Agric. 2007, 87, 1810–1819. [Google Scholar] [CrossRef]
- von Staszewskia, M.; Jaraa, F.L.; Ruizb, A.L.T.G.; Jagusa, R.J.; Carvalhob, J.E.; Pilosof, A.M.R. Nanocomplex formation between β-lactoglobulin or caseinomacropeptide and green tea polyphenols: Impact on protein gelation and polyphenols antiproliferative activity. J. Funct. Foods 2012, 4, 800–8009. [Google Scholar] [CrossRef]
- Prigent, S.V.E.; Gruppen, H.; Visser, A.J.W.G.; van Koningsveld, G.A.; de Jong, D.A.H.; Voragen, A.G.J. Effects of non-covalent interactions with 5-O-caffeoylquinic acid (chlorogenic acid) on the heat denaturation and solubility of globular proteins. J. Agric. Food Chem. 2003, 51, 5088–5095. [Google Scholar] [CrossRef] [PubMed]
- Prigent, S.V.E.; Voragen, A.G.J.; Visser, A.J.W.G.; van Koningsveld, G.A.; Gruppen, H. Covalent interactions between proteins and oxidation products of caffeoylquinic acid (chlorogenic acid). J. Sci. Food Agric. 2007, 87, 2502–2510. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; ur Rehman, I. Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Carton, I.; Böcker, U.; Ofstad, R.; Sørheim, O.; Kohler, A. Monitoring secondary structural changes in salted and smoked salmon muscle myofiber proteins by FT-IR microspectroscopy. J. Agric. Food Chem. 2009, 57, 3563–3570. [Google Scholar] [CrossRef] [Green Version]
- Jia, Z.; Zheng, M.; Tao, F.; Chen, W.; Huang, G.; Jiang, J. Effect of covalent modification by (-)-epigallocatechin-3-gallate on physicochemical and functional properties of whey protein isolate. LWT-Food Sci. Technol. 2016, 66, 305–310. [Google Scholar] [CrossRef]
- Xiang, H.; Sun-Waterhouse, D.; Cui, C.; Wang, W.; Dong, K. Modification of soy protein isolate by glutaminase for nanocomplexation with curcumin. Food Chem. 2018, 268, 504–512. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N. Influence of kidney bean, field pea and amaranth protein isolates on the characteristics of starch based gluten free muffins. Int. J. Food Sci. Technol. 2014, 49, 2237–2244. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Kaur, A.; Rana, C.J. Structural and functional characterization of kidney bean and field pea protein isolates: A comparative study. Food Hydrocoll. 2015, 43, 679–689. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, D.; Sun, J.; Guo, H.; Ding, Q.; Liu, R.; Ren, F. Interaction of milk whey protein with common phenolic acids. J. Mol. Struct. 2014, 1058, 228–233. [Google Scholar] [CrossRef]
- Xue, F.; Li, C.; Wang, S. Effects of high-intensity ultrasound treatment on physiochemical properties of caseins-cyanidin-3-galactoside conjugates. Int. J. Food Sci. Technol. 2021, 56, 3378–3387. [Google Scholar] [CrossRef]

| Amount of Protein Matrix | Protein Matrix | ||
|---|---|---|---|
| Pea | Almond | Pumpkin | |
| 1% | 35.82 ± 0.05 a | 30.37 ± 0.47 a | 41.15 ± 0.11 a |
| 2% | 34.95 ± 0.24 a | 30.49 ± 0.13 a | 34.13 ± 0.67 b |
| 5% | 32.99 ± 0.16 b | 23.63 ± 0.17 b | 29.05 ± 0.02 c |
| 10% | 30.69 ± 0.63 c | 20.67 ± 0.01 c | 25.58 ± 0.02 d |
| Amount of Protein Matrix | Protein Matrix | ||
|---|---|---|---|
| Pea | Almond | Pumpkin | |
| 100% | 88.60 ± 0.47 a | 85.24 ± 0.07 d | 87.44 ± 0.27 a |
| 1% | 85.15 ± 0.39 c | 88.18 ± 0.08 a | 85.11 ± 0.25 c |
| 2% | 85.26 ± 0.44 c | 87.19 ± 0.20 b | 86.13 ± 0.47 b |
| 5% | 85.66 ± 0.38 c | 86.73 ± 0.12 b,c | 86.30 ± 0.34 b |
| 10% | 86.88 ± 0.33 b | 86.38 ± 0.36 c | 86.69 ± 0.39 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopjar, M.; Buljeta, I.; Jelić, I.; Kelemen, V.; Šimunović, J.; Pichler, A. Encapsulation of Cinnamic Acid on Plant-Based Proteins: Evaluation by HPLC, DSC and FTIR-ATR. Plants 2021, 10, 2158. https://doi.org/10.3390/plants10102158
Kopjar M, Buljeta I, Jelić I, Kelemen V, Šimunović J, Pichler A. Encapsulation of Cinnamic Acid on Plant-Based Proteins: Evaluation by HPLC, DSC and FTIR-ATR. Plants. 2021; 10(10):2158. https://doi.org/10.3390/plants10102158
Chicago/Turabian StyleKopjar, Mirela, Ivana Buljeta, Ivana Jelić, Vanja Kelemen, Josip Šimunović, and Anita Pichler. 2021. "Encapsulation of Cinnamic Acid on Plant-Based Proteins: Evaluation by HPLC, DSC and FTIR-ATR" Plants 10, no. 10: 2158. https://doi.org/10.3390/plants10102158
APA StyleKopjar, M., Buljeta, I., Jelić, I., Kelemen, V., Šimunović, J., & Pichler, A. (2021). Encapsulation of Cinnamic Acid on Plant-Based Proteins: Evaluation by HPLC, DSC and FTIR-ATR. Plants, 10(10), 2158. https://doi.org/10.3390/plants10102158







