High Phenotypic Plasticity in a Prominent Plant Invader along Altitudinal and Temperature Gradients
Abstract
:1. Introduction
2. Results
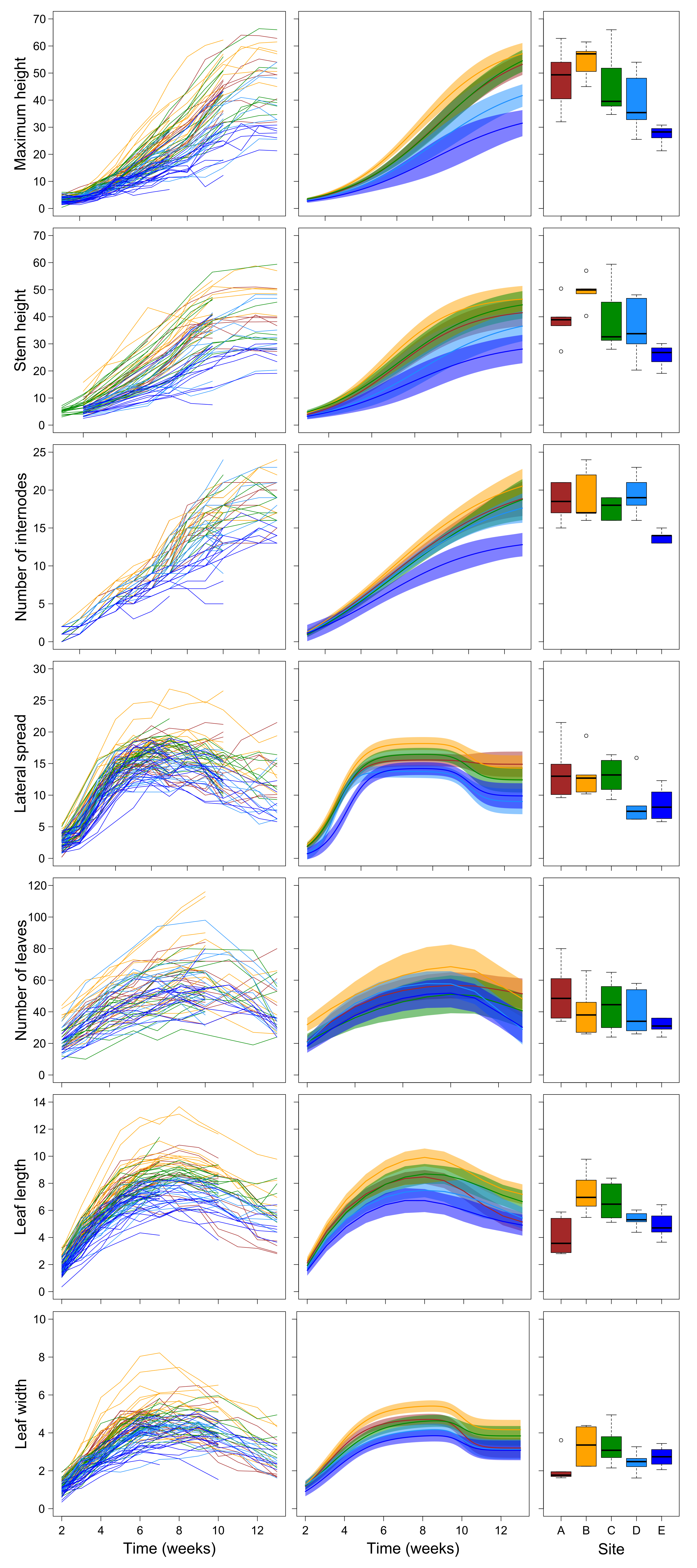
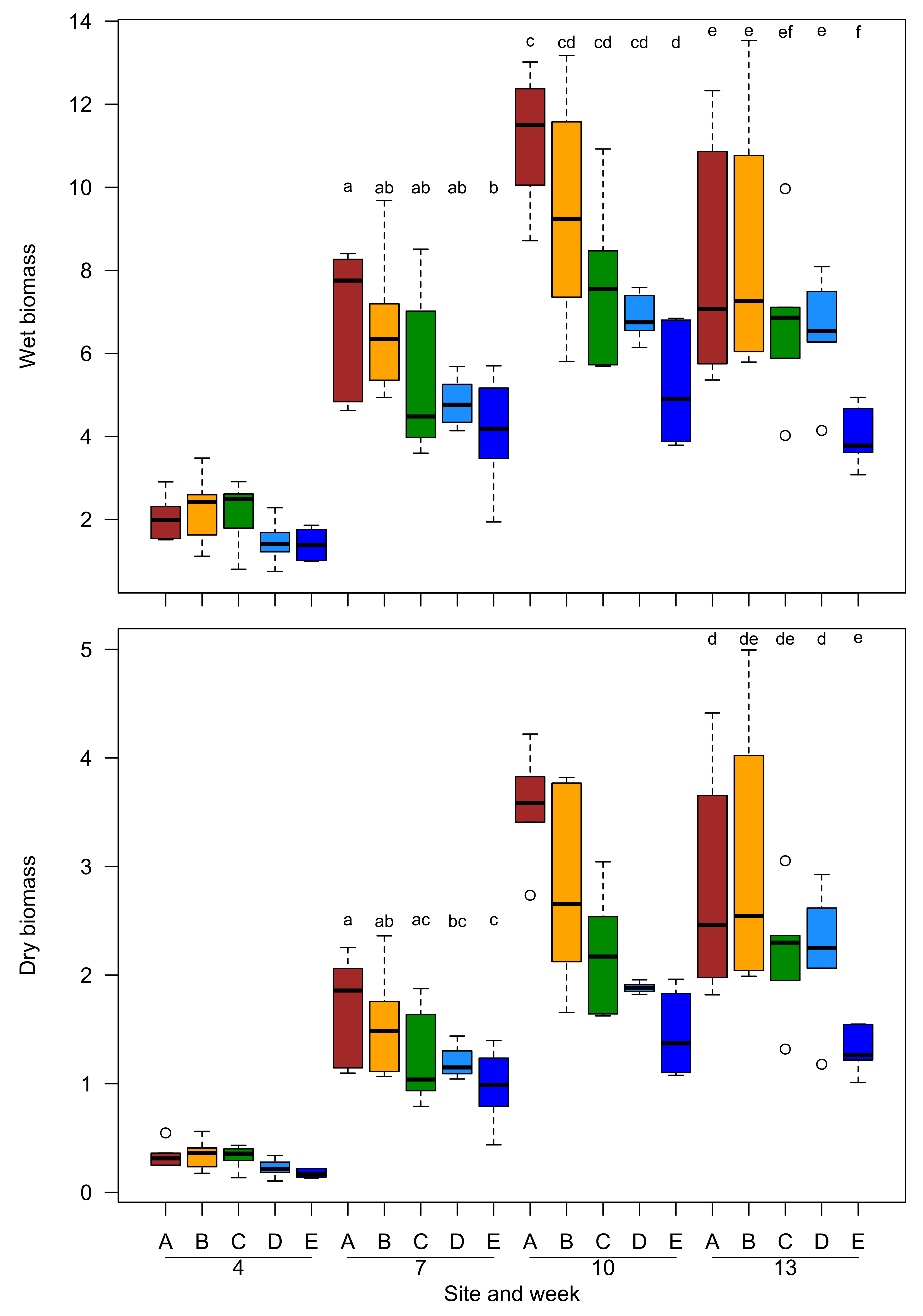
2.1. Growth Trajectories and Biomass of Plant Grown in Field Conditions
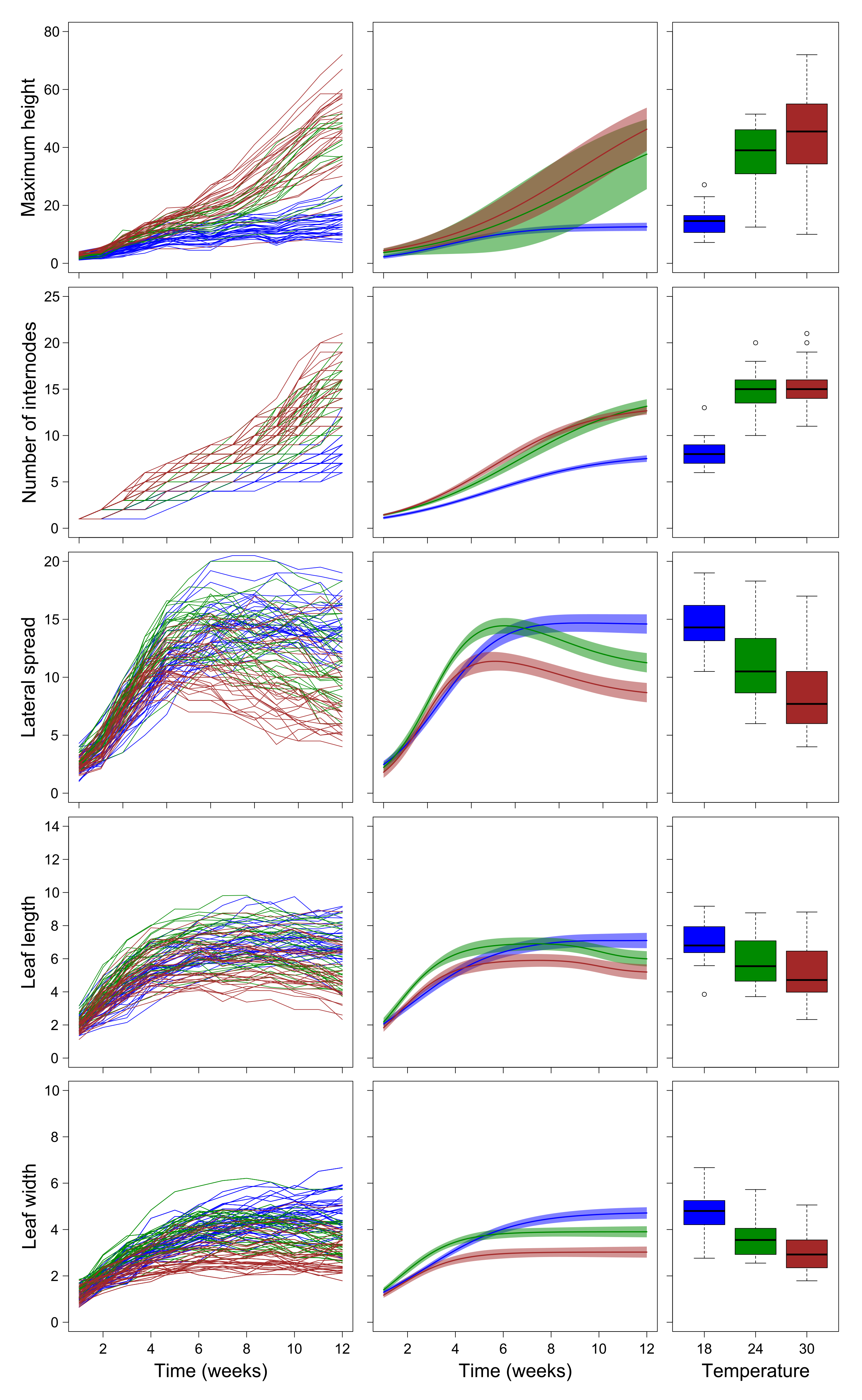
2.2. Growth Trajectories of Plants Grown in Laboratory Conditions
2.3. Biomass and Reproductive Parameters of Plants Grown in Laboratory Conditions
3. Discussion
3.1. Field Experiment
3.2. Laboratory Experiment
3.3. Implication for the Invasion Syndrome of Common Ragweed
4. Material and Methods
4.1. Plant Material and Preliminary Germination
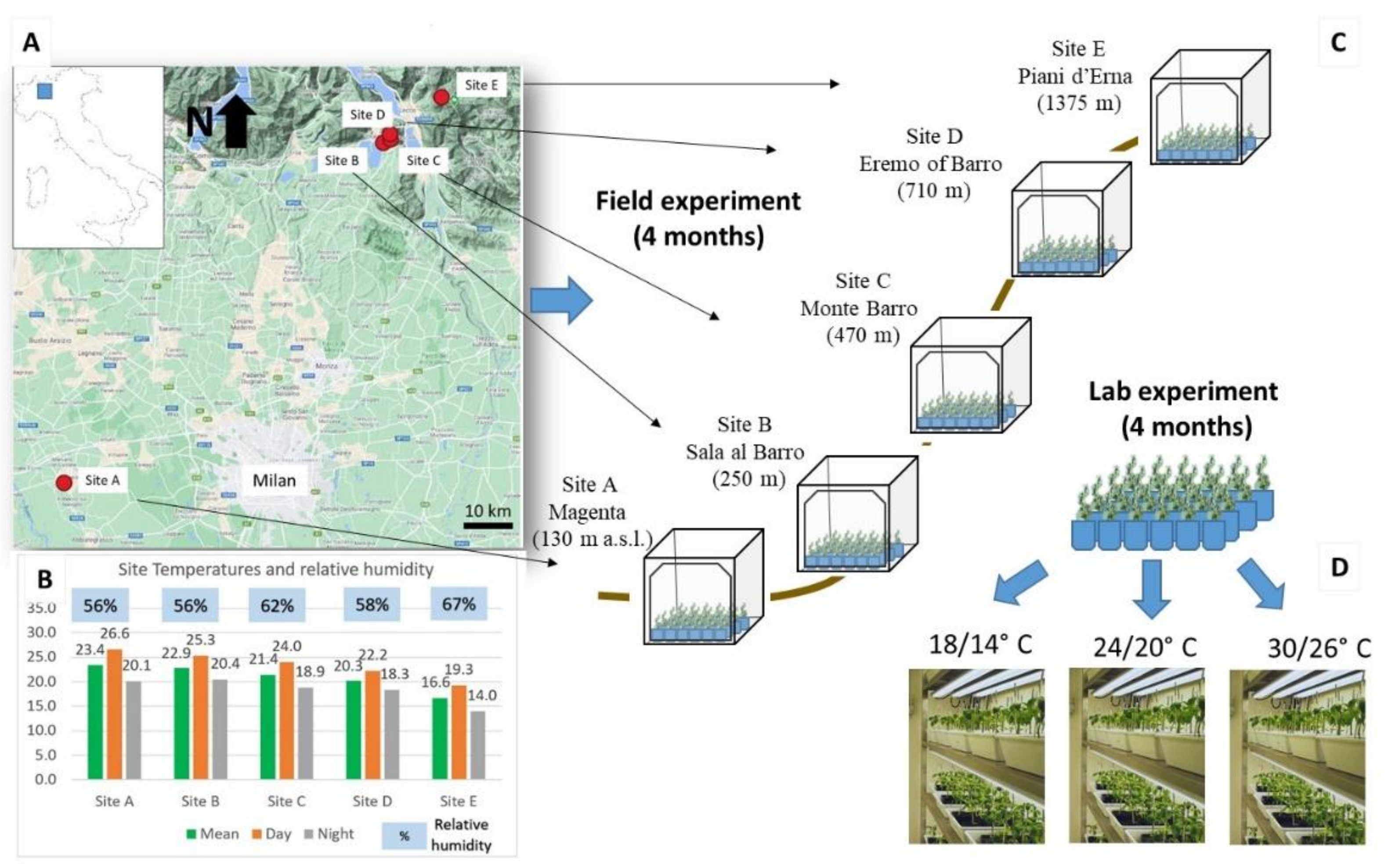
4.2. Field Experiment
4.3. Lab Experiment
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pfennigwerth, A.A.; Bailey, J.K.; Schweitzer, J.A. Trait variation along elevation gradients in a dominant woody shrub is population-specific and driven by plasticity. AoB Plants 2017, 9, plx027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Körner, C. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef]
- Pellissier, L.; Fournier, B.; Guisan, A.; Vittoz, P. Plant traits covary with altitude in grasslands and forests in the European Alps. Plant. Ecol. 2010, 211, 351–365. [Google Scholar] [CrossRef] [Green Version]
- Št’astná, P.; Klimešová, J.; Doležal, J. Altitudinal changes in the growth and allometry of Rumex alpinus. Alp. Bot. 2012, 122, 35–44. [Google Scholar] [CrossRef]
- Midolo, G.; De Frenne, P.; Hölzel, N.; Wellstein, C. Global patterns of intraspecific leaf trait responses to elevation. Glob. Change Biol. 2019, 25, 2485–2498. [Google Scholar] [CrossRef]
- Jösson, K.I.; Tuomi, J. Costs of reproduction in a historical perspective. Trends Ecol. Evol. 1994, 9, 304–307. [Google Scholar] [CrossRef]
- Von Arx, G.; Edwards, P.J.; Diez, H. Evidence for life history changes in high-altitude populations of three perennial forbs. Ecology 2006, 87, 665–674. [Google Scholar] [CrossRef] [Green Version]
- Gienapp, P.; Teplitsky, C.; Alho, J.S.; Mills, J.A.; Merilä, J. Climate change and evolution: Disentangling environmental and genetic responses. Mol. Ecol. 2008, 17, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiari, M.; Formenti, L.; Caggìa, V.; Glauser, G.; Rasmann, S. Variable effects on growth and defense traits for plant ecotypic differentiation and phenotypic plasticity along elevation gradients. Ecol. Evol. 2019, 9, 3740–3755. [Google Scholar] [CrossRef]
- Spencer, W.E.; Teeri, J.; Wetzel, R.G. Acclimation of photosynthetic phenotype to environmental heterogeneity. Ecology 1994, 75, 301–314. [Google Scholar] [CrossRef]
- Roach, D.A.; Gampe, J. Age-specific demography in Plantago: Uncovering age-dependent mortality in a natural population. Am. Nat. 2004, 164, 60–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, S.; Wu, N.; Sun, S. Within-twig biomass allocation in subtropical evergreen broad-leaved species along an altitudinal gradient: Allometric scaling analysis. Trees 2009, 23, 637–647. [Google Scholar] [CrossRef]
- Alexander, J.M.; Kueffer, C.; Daehler, C.C.; Edwards, P.J.; Pauchard, A.; Seipel, T.; Arevalo, J.; Cavieres, L.; Dietz, H.; Jakobs, G.; et al. Assembly of nonnative floras along elevational gradients explained by directional ecological filtering. Proc. Natl. Acad. Sci. USA 2011, 108, 656–661. [Google Scholar] [CrossRef] [Green Version]
- March-Salas, M.; Pertierra, L.R. Warmer and less variable temperatures favour an accelerated plant phenology of two invasive weeds across sub-Antarctic Macquarie Island. Austral. Ecol. 2020, 45, 572–585. [Google Scholar] [CrossRef]
- Davidson, A.M.; Jennions, M.; Nicotra, A.B. Do invasive species show higher phenotypic plasticity than native species and if so, is it adaptive? A meta-analysis. Ecol. Lett. 2011, 14, 419–431. [Google Scholar] [CrossRef]
- Knop, E.; Reusser, N. Jack-of-all-trades: Phenotypic plasticity facilitates the invasion of an alien slug species. Proc. R. Soc. B 2012, 279, 4668–4676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pyšek, P.; Jarošík, V.; Pergl, J.; Wild, J. Colonization of high altitudes by alien plants over the last two centuries. Proc. Natl. Acad. Sci. USA 2011, 108, 439–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelley, A.L. The role thermal physiology plays in species invasion. Conserv. Physiol. 2014, 2, cou045. [Google Scholar] [CrossRef] [Green Version]
- Hou, Q.-Q.; Chen, B.-M.; Peng, S.-L.; Chen, L.-Y. Effects of extreme temperature on seedling establishment of nonnative invasive plants. Biol. Invasions 2014, 16, 2049–2061. [Google Scholar] [CrossRef]
- Fenollosa, E.; Munné-Bosch, S. Increased chilling tolerance of the invasive species Carpobrotus edulis may explain its expansion across new territories. Conserv. Physiol. 2019, 7, coz075. [Google Scholar] [CrossRef]
- Galera, H.; Chwedorzewska, K.J.; Wo´dkiewicz, M. Response of Poa annua to extreme conditions: Comparison of morphological traits between populations from cold and temperate climate conditions. Polar Biol. 2015, 38, 1657–1666. [Google Scholar] [CrossRef]
- Hellmann, J.J.; Byers, J.E.; Bierwagen, B.G.; Dukes, J.S. Five potential consequences of climate change for invasive species. Conserv. Biol. 2008, 22, 534–543. [Google Scholar] [CrossRef]
- Beaury, E.M.; Fusco, E.J.; Jackson, M.R.; Laginhas, B.B.; Morelli, T.L.; Allen, J.; Pasquarella, V.J.; Bradley, B.A. Incorporating climate change into invasive species management: Insights from managers. Biol. Invasions 2020, 22, 233–252. [Google Scholar] [CrossRef] [Green Version]
- Morelli, T.L.; Brown-Lima, C.J.; Allen, J.M.; Beaury, E.M.; Fusco, E.J.; Barker-Plotkin, A.; Laginhas, B.B.; Quirion, B.R.; Griffin, B.; McLaughlin, B.; et al. Translational invasion ecology: Bridging research and practice to address one of the greatest threats to biodiversity. Biol. Invasions 2021. [Google Scholar] [CrossRef]
- Morais, M.C.; Cabral, J.A.; Gonçalves, B. Seasonal variation in the leaf physiology of co-occurring invasive (Hakea sericea) and native (Pinus pinaster) woody species in a Mediterranean-type ecosystem. For. Ecol. Manag. 2021, 480, 118662. [Google Scholar] [CrossRef]
- Augustinus, B.; Sun, Y.; Beuchat, C.; Schaffner, U.; Müller-Schärer, H. Predicting impact of a biocontrol agent: Integrating distribution modeling with climate-dependent vital rates. Ecol. Appl. 2019, 30, e02003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, A.R.; Fourie, A.; Krug, R.M.; Gordon, A.J. A guide to the biological control of alien invasive hakea species. In Plant Protection Research Institute Handbook No. 22; Agricultural Research Council: Pretoria, South Africa, 2021; pp. 1–68. [Google Scholar]
- Falster, D.S.; Duursma, R.A.; FitzJohn, R.G. How functional traits influence plant growth and shade tolerance across the life cycle. Proc. Natl. Acad. Sci. USA 2018, 115, E6789–E6798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kühn, P.; Ratier Backes, A.; Römermann, C.; Bruelheide, H.; Haider, S. Contrasting patterns of intraspecific trait variability in native and non-native plant species along an elevational gradient on Tenerife, Canary Islands. Ann. Bot. 2021, 127, 565–576. [Google Scholar] [CrossRef]
- Alexander, J.M.; Edwards, P.J.; Poll, M.; Parks, C.G.; Dietz, H. Establishment of parallel altitudinal clines in traits of native and introduced forbs. Ecology 2009, 90, 612–622. [Google Scholar] [CrossRef] [Green Version]
- Montagnani, C.; Gentili, R.; Smith, M.; Guarino, M.; Citterio, S. The Worldwide Spread, Success, and Impact of Ragweed (Ambrosia spp.). Crit. Rev. Plant Sci. 2017, 36, 139–178. [Google Scholar] [CrossRef]
- Ghiani, A.; Ciappetta, S.; Gentili, R.; Asero, R.; Citterio, S. Is ragweed pollen allergenicity governed by environmental conditions during plant growth and flowering? Sci. Rep. 2016, 6, 30438. [Google Scholar] [CrossRef] [Green Version]
- Schaffner, U.; Steinbach, S.; Sun, Y.; Skjøth, C.; de Weger, L.A.; Lommen, S.T.; Augustinus, B. Müller-Schärer, H. Biological weed control to relieve millions of allergy sufferers in Europe. Nat. Comm. 2020, 11, 1745. [Google Scholar] [CrossRef] [Green Version]
- Ciappetta, S.; Ghiani, A.; Gilardelli, F.; Bonini, M.; Citterio, S.; Gentili, R. Invasion of Ambrosia artemisiifolia in Italy: Assessment via analysis of genetic variability and herbarium data. Flora 2016, 223, 106–111. [Google Scholar] [CrossRef]
- Fumanal, B.; Girod, C.; Fried, G.; Bretagnolle, F.; Chauvel, B. Can the large ecological amplitude of Ambrosia artemisiifolia explain its invasive success in France? Weed Res. 2008, 48, 349–359. [Google Scholar] [CrossRef]
- Gentili, R.; Montagnani, C.; Gilardelli, F.; Guarino, M.F.; Citterio, S. Let native species take their course: Ambrosia artemisiifolia replacement during natural or “artificial” succession. Acta Oecol. 2017, 82, 32–40. [Google Scholar] [CrossRef]
- Cunze, S.; Leiblein, M.C.; Tackenberg, O. Range expansion of Ambrosia artemisiifolia in Europe is promoted by climate change. ISRN Ecol. 2013, 2013, 610126. [Google Scholar] [CrossRef] [Green Version]
- Gentili, R.; Gilardelli, F.; Bona, E.; Prosser, F.; Selvaggi, A.; Alessandrini, A.; Martini, F.; Nimis, P.L.; Wilhalm, T.; Adorni, M.; et al. Distribution map of Ambrosia artemisiifolia L. (Asteraceae) in Italy. Plant Biosyst. 2017, 151, 381–386. [Google Scholar] [CrossRef] [Green Version]
- Dickerson, C.T.; Sweet, R.D. Common ragweed ecotypes. Weed Sci. 1971, 19, 64–66. [Google Scholar] [CrossRef]
- Leiblein-Wild, M.C.; Tackenberg, O. Phenotypic variation of 38 European Ambrosia artemisiifolia populations measured in a common garden experiment. Biol. Invasions 2014, 16, 2003–2015. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Bossdorf, O.; Grados, R.D.; Liao, Z.; Müller-Schärer, H. Rapid genomic and phenotypic change in response to climate warming in a widespread plant invader. Glob. Change Biol. 2020, 26, 6511–6522. [Google Scholar] [CrossRef]
- Kawano, T.; Wallbridge, N.; Plummer, C. Logistic Models for Simulating the Growth of Plants by Defining the Maximum Plant Size as the Limit of Information Flow. Plant Signal. Behav. 2020, 15, 1709718. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Frelich, L.E. Flowering phenology and height growth pattern are associated with maximum plant height, relative growth rate and stem tissue mass density in herbaceous grassland species. J. Ecol. 2011, 99, 991–1000. [Google Scholar] [CrossRef]
- Moles, A.T.; Warton, D.I.; Warman, L.; Swenson, N.G.; Laffan, S.W.; Zanne, A.E.; Pitman, A.; Hemmings, F.A.; Leishman, M.R. Global patterns in plant height. J. Ecol. 2009, 97, 923–932. [Google Scholar] [CrossRef]
- Nabeshima, E.; Hiura, T. Size-dependency in hydraulic and photosynthetic properties of three Acer species having different maximum sizes. Ecol. Res. 2008, 23, 281–288. [Google Scholar] [CrossRef]
- Thomas, S.C. Photosynthetic capacity peaks at intermediate size in temperate deciduous trees. Tree Physiol. 2010, 30, 555–573. [Google Scholar] [CrossRef] [Green Version]
- Bucher, S.F.; Römermann, C. The timing of leaf senescence relates to flowering phenology and functional traits in 17 herbaceous species along elevational gradients. J. Ecol. 2021, 109, 1537–1548. [Google Scholar] [CrossRef]
- Falster, D.S.; Westoby, M. Plant height and evolutionary games. Trends Ecol. Evol. 2003, 18, 337–343. [Google Scholar] [CrossRef]
- Tracey, A.J.; Aarssen, L.W. Competition and body size in plants: The between-species trade-off for maximum potential versus minimum reproductive threshold size. J. Plant Ecol. 2011, 4, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Turnbull, L.A.; Rees, M.; Crawley, M.J. Seed mass and the competition/colonization trade-off: A sowing experiment. J. Ecol. 1999, 87, 899–912. [Google Scholar] [CrossRef]
- Bazzaz, F.A. Ecophysiology of Ambrosia artemisiifolia: A successional dominant. Ecology 1974, 55, 112–119. [Google Scholar] [CrossRef]
- Gentili, R.; Ambrosini, R.; Montagnani, C.; Caronni, S.; Citterio, S. Effect of soil pH on the growth, reproductive investment and pollen allergenicity of Ambrosia artemisiifolia L. Front. Plant Sci. 2018, 9, 1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Z.-Y.; Scheepens, J.F.; Li, W.-T.; Wang, R.-F.; Zheng, Y.-L.; Feng, Y.-L. Biomass reallocation and increased plasticity might contribute to successful invasion of Chromolaena odorata. Flora 2019, 256, 79–84. [Google Scholar] [CrossRef]
- Alexander, J.M.; Lembrechts, J.J.; Cavieres, L.A.; Daehler, C.C.; Haider, S.; Kueffer, C.; Liu, G.; McDougall, K.; Milbau, A.; Pauchard, A.; et al. Plant invasions into mountains and alpine ecosystems: Current status and future challenges. Alp. Bot. 2016, 126, 89–103. [Google Scholar] [CrossRef]
- Clements, D.R.; Jones, V.L. Rapid Evolution of Invasive Weeds Under Climate Change: Present Evidence and Future Research Needs. Front. Agron. 2021, 3, 664034. [Google Scholar] [CrossRef]
- Poorter, H.; Fiorani, F.; Pieruschka, R.; Wojciechowski, T.; van der Putten, W.H.; Kleyer, M.; Schurr, U.; Postma, J. Pampered inside, pestered outside? Differences and similarities between plants growing in controlled conditions and in the field. New Phytol. 2016, 212, 838–855. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yan, L.; Xia, J. A threefold difference in plant growth response to nitrogen addition between the laboratory and field experiments. Ecosphere 2019, 10, e02572. [Google Scholar] [CrossRef]
- Pescador, D.S.; de Bello, F.; Valladares, F.; Escudero, A. Plant Trait Variation along an Altitudinal Gradient in Mediterranean High Mountain Grasslands: Controlling the Species Turnover Effect. PLoS ONE 2015, 10, e0118876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baronetti, A.; González-Hidalgo, J.C.; Vicente-Serrano, S.M.; Acquaotta, F.; Fratianni, S. A weekly spatio-temporal distribution of drought events over the Po Plain (North Italy) in the last five decades. Int. J. Climatol. 2020, 40, 4463–4476. [Google Scholar] [CrossRef]
- Gentili, R.; Schaffner, U.; Martinoli, A.; Citterio, S. Invasive alien species and biodiversity: Impacts and management. Biodiversity 2021, 22, 1–3. [Google Scholar] [CrossRef]
- Banfi, E.; Galasso, G. La Flora Esotica Lombarda; Museo di Storia Naturale di Milano—Regione Lombardia, Sistemi Verdi e Paesaggio: Milano, Italy, 2010; pp. 1–139. [Google Scholar]
- Müller-Schärer, H.; Lommen, S.T.E.; Rossinelli, M.; Bonini, M.; Boriani, M.; Bosio, G.; Schaffner, U. Ophraella communa, the ragweed leaf beetle, has successfully landed in Europe: Fortunate coincidence or threat? Weed Res. 2014, 54, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models; R Package Version 3.1-153; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://CRAN.R-project.org/package=nlme (accessed on 26 September 2021).
- Oswald, S.A.; Nisbet, I.C.T.; Chiaradia, A.; Arnold, J.M. FlexParamCurve: R package for flexible fitting of nonlinear parametric curves. Methods Ecol. Evol. 2012, 3, 1073–1077. [Google Scholar] [CrossRef]
- Yin, X.; Goudriaan, J.; Lantinga, E.A.; Vos, J.; Spiertz, H.J. A flexible sigmoid function of determinate growth. Ann. Bot. 2003, 91, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Paine, C.E.T.; Marthew, T.R.; Vogt, D.R.; Purves, D.; Rees, M.; Hector, A.; Turnbull, L.A. How to fit nonlinear plant growth models and calculate growth rates: An update for ecologists. Methods Ecol. Evol. 2012, 3, 245–256. [Google Scholar] [CrossRef]
- Vasseur, F.; Fouqueau, L.; de Vienne, D.; Nidelet, T.; Violle, C.; Weigel, D. Nonlinear phenotypic variation uncovers the emergence of heterosis in Arabidopsis thaliana. PLoS Biol. 2019, 17, e3000214. [Google Scholar] [CrossRef] [PubMed]
- Morganti, M.; Rubolini, D.; Åkesson, S.; Bermejo, A.; De La Puente, J.; Lardelli, R.; Liechti, F.; Boano, G.; Tomassetto, E.; Ferri, M.; et al. Effect of light-level geolocators on apparent survival of two highly aerial swift species. J. Avian Biol. 2017, 49, jav-01521. [Google Scholar] [CrossRef]
- Sicurella, B.; Caprioli, M.; Romano, A.; Romano, M.; Rubolini, D.; Saino, N.; Ambrosini, R. Hayfields enhance colony size of the barn swallow Hirundo rustica in northern Italy. Bird Conserv. Int. 2014, 24, 17–31. [Google Scholar] [CrossRef]
- Bollen, K.A.; Jackman, R.W. Regression Diagnostics: An Expository Treatment of Outliers and Influential Cases. In Modern Methods of Data Analysis; Fox, J., Long, J.S., Eds.; Sage Publications: Newbury Park, CA, USA, 1990; pp. 1–446. [Google Scholar]
- Fossard, J.; Renaud, O. Permutation Tests for Regression, ANOVA and Comparison of Signals: The Permuco Package. R Package Version 1.1.0. 2019. Available online: https://cran.r-project.org/web/packages/permuco/index.html (accessed on 3 July 2021).
- Lenth, R.V. emmeans: Estimated Marginal Means, aka Least-Squares Means. 2020. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 3 July 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 26 September 2021).

| Parameter | F | df | p |
|---|---|---|---|
| Maximum height (three-parameter logistic curve, 867 observations and 89 plants) | |||
| K | 14.539 | 4 | <0.001 |
| i | 1.7 | 4 | 0.148 |
| s | 1.914 | 4 | 0.162 |
| Residual df = 764; φ = 0.578; AICc = 3702.3 | |||
| Stem height (three-parameter logistic curve, 502 observations and 65 plants) | |||
| K | 6.229 | 4 | <0.001 |
| i | 2.281 | 4 | 0.060 |
| s | 4.192 | 4 | 0.002 |
| Residual df = 423; φ = 0.609; AICc = 2242.9 | |||
| Number of internodes (four-parameter logistic curve, 849 observations and 89 plants) | |||
| L | 0.951 | 4 | 0.434 |
| K | 6.303 | 4 | <0.001 |
| i | 0.483 | 4 | 0.748 |
| s | 0.432 | 4 | 0.785 |
| Residual df = 741; φ = 0.558; AICc = 2438.2 | |||
| Lateral spread (double-Richards curve #31, 740 observations and 86 plants) | |||
| K | 11.949 | 4 | <0.001 |
| r | 3.600 | 4 | 0.006 |
| i | 11.191 | 4 | <0.001 |
| K′ | 2.699 | 4 | 0.030 |
| Residual df = 635; φ = 0.761; AICc = 2791.1 | |||
| m = 1.233, r′ = 2.073, i′ = 10, m′ = 0.963 | |||
| Number of leaves (double-Richards curve #31, 453 observations and 59 plants) | |||
| K | 1.379 | 4 | 0.241 |
| r | 1.864 | 4 | 0.116 |
| i | 1.035 | 4 | 0.389 |
| K′ | 4.844 | 4 | 0.001 |
| Residual df = 375; φ = 0.737; AICc = 3050.7 | |||
| m = −0.123, r′ = 1.438, i′ = 12, m′ = 0.944 | |||
| Leaf length (double-Richards curve #34, 773 observations and 89 plants) | |||
| K | 6.145 | 4 | <0.001 |
| r | 1.677 | 4 | 0.086 |
| i | 0.104 | 4 | 0.606 |
| r′ | 2.271 | 4 | 0.229 |
| i′ | 1.283 | 4 | 0.275 |
| Residual df = 660; φ = 0.637; AICc = 1351.5 | |||
| i = −0.924, K′ = −4.645, m′ = 0.880 | |||
| Leaf width (double-Richards curve #31, 773 observations and 89 plants) | |||
| K | 12.171 | 4 | <0.001 |
| r | 2.047 | 4 | 0.086 |
| i | 0.68 | 4 | 0.606 |
| K′ | 1.14 | 4 | 0.229 |
| Residual df = 665; φ = 0.792; AICc = 855.3 | |||
| m = 0.938, r′ = 2.580, i′ = 10, m′ = 0.819 | |||
| Parameter | F | df | p |
|---|---|---|---|
| Wet biomass (n = 120) | |||
| Time | 38.318 | 3 | <0.001 |
| Site | 2.464 | 4 | 0.05 |
| Time × Site | 3.087 | 12 | 0.001 |
| Dry biomass (n = 120) | |||
| Time | 51.068 | 3 | <0.001 |
| Site | 2.755 | 4 | 0.032 |
| Time × Site | 4.143 | 12 | <0.001 |
| Parameter | F | df | p |
|---|---|---|---|
| Maximum height (three-parameter logistic curve, 982 observations and 76 plants) | |||
| K | 83.642 | 2 | <0.001 |
| i | 78.978 | 2 | <0.001 |
| s | 25.544 | 2 | <0.001 |
| Residual df = 898; φ = 0.904; AICc = 3974.1 | |||
| Number of internodes (three-parameter logistic curve, 1096 observations and 85 plants) | |||
| K | 64.147 | 2 | <0.001 |
| i | 5.007 | 2 | 0.007 |
| s | 3.484 | 2 | 0.003 |
| Residual df = 741; φ = 0.558; AICc = 2438.2 | |||
| Lateral spread (double-Richards curve #31, 1182 observations and 96 plants) | |||
| K | 16.213 | 2 | <0.001 |
| r | 26.439 | 2 | <0.001 |
| i | 17.121 | 2 | <0.001 |
| K′ | 13.381 | 2 | <0.001 |
| Residual df = 1075; φ = 0.726; AICc = 3398.5 | |||
| m = 1.228, r′ = 0.542, Ri = 7.739, m′ = 1.000 | |||
| Leaf length (double-Richards curve #31, 453 observations and 59 plants) | |||
| K | 11.727 | 2 | <0.001 |
| r | 49.162 | 2 | <0.001 |
| i | 16 | 2 | <0.001 |
| K′ | 7.138 | 2 | 0.001 |
| Residual df = 997; φ = 0.704; AICc = 1376.1 | |||
| m = 0.572, r′ = 1.372, i′ = 10, m′ = 0.998 | |||
| Leaf width (three-parameter logistic curve, 1119 observations and 96 plants) | |||
| K | 46.151 | 2 | <0.001 |
| i | 48.659 | 2 | <0.001 |
| s | 27.652 | 2 | <0.001 |
| Residual df = 1015; φ = 0.615; AICc = 421.4 | |||
| Parameter | F | df | p |
|---|---|---|---|
| Dry biomass (76 plants) | |||
| Temperature | 4.841 | 2 | 0.011 |
| Residual df = 73; AICc = 148.4 | |||
| Day of emission of female flowers (71 plants) | |||
| Temperature | 105.800 | 2 | <0.001 |
| Centred dry biomass | 0.421 | 2 | 0.519 |
| Temp. x c. dry biomass | 0.007 | 2 | 0.992 |
| Residual df = 65; AICc = 277.8 | |||
| Day of emission of male flowers (72 plants) | |||
| Temperature | 110.462 | 2 | <0.001 |
| Centred dry biomass | 2.851 | 2 | 0.096 |
| Temp. x c. dry biomass | 1.027 | 2 | 0.364 |
| Residual df = 66; AICc = −225.7 | |||
| Spike dry weight (72 plants) | |||
| Temperature | 1.872 | 2 | 0.162 |
| Centered dry biomass | 294.893 | 2 | <0.001 |
| Temp. x c. dry biomass | 1.347 | 2 | 0.267 |
| Residual df = 65; AICc = −141.6 | |||
| Pollen weight (70 plants) | |||
| Temperature | 34.639 | 2 | <0.001 |
| Centered dry biomass | 24.125 | 2 | <0.001 |
| Temp. x c. dry biomass | 7.147 | 2 | <0.001 |
| Residual df = 64; AICc = −345.0 | |||
| Parameter | F | df | p |
|---|---|---|---|
| Number of male flowers (Asymptotic regression 73 plants) | |||
| K | 0.75 | 2 | 0.477 |
| L′ | 37.75 | 2 | <0.001 |
| r | 1.211 | 2 | 0.305 |
| Residual df = 64; AICc = 237.3.8 | |||
| Growth Curve | Equation | Morphological Trait |
|---|---|---|
| (1) Linear | Reproductive parameters except for the number of male flowers | |
| (2) Asymptotic | Number of male flowers | |
| (3) Three-parameter logistic | Plant height, stem height | |
| (4) Four-parameter logistic | Number of leaves | |
| (5) Double-Richards | Lateral spread, leaf length, leaf width | |
| Parameter | Description | |
| Intercept, corresponding to mean value if is centred | ||
| r and r′ | Growth rates | |
| s | Scale parameter replacing the growth rate () in the parameterization of Equations (2) and (3) of SSlogis and SSfpl (used to fit them) | |
| K | Upper asymptote | |
| L and L′ | Lower asymptote or initial value | |
| m and m′ | Shape parameters of the generalized logistic curves, values > 1, imply that the inflection points are realized sooner than i or i′ and the growth rates at i or i′ are lower than r or r′; values < 1 imply the opposite | |
| i and i′ | Inflection points, i.e., time at which the fastest growth/recession is attained | |
| K′ | Difference between asymptotes of the curve before and after recession |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gentili, R.; Ambrosini, R.; Augustinus, B.A.; Caronni, S.; Cardarelli, E.; Montagnani, C.; Müller-Schärer, H.; Schaffner, U.; Citterio, S. High Phenotypic Plasticity in a Prominent Plant Invader along Altitudinal and Temperature Gradients. Plants 2021, 10, 2144. https://doi.org/10.3390/plants10102144
Gentili R, Ambrosini R, Augustinus BA, Caronni S, Cardarelli E, Montagnani C, Müller-Schärer H, Schaffner U, Citterio S. High Phenotypic Plasticity in a Prominent Plant Invader along Altitudinal and Temperature Gradients. Plants. 2021; 10(10):2144. https://doi.org/10.3390/plants10102144
Chicago/Turabian StyleGentili, Rodolfo, Roberto Ambrosini, Benno A. Augustinus, Sarah Caronni, Elisa Cardarelli, Chiara Montagnani, Heinz Müller-Schärer, Urs Schaffner, and Sandra Citterio. 2021. "High Phenotypic Plasticity in a Prominent Plant Invader along Altitudinal and Temperature Gradients" Plants 10, no. 10: 2144. https://doi.org/10.3390/plants10102144
APA StyleGentili, R., Ambrosini, R., Augustinus, B. A., Caronni, S., Cardarelli, E., Montagnani, C., Müller-Schärer, H., Schaffner, U., & Citterio, S. (2021). High Phenotypic Plasticity in a Prominent Plant Invader along Altitudinal and Temperature Gradients. Plants, 10(10), 2144. https://doi.org/10.3390/plants10102144








