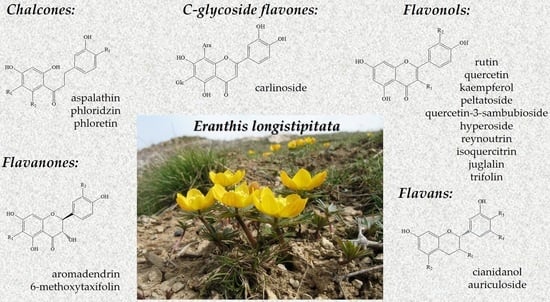
Identification of Flavonoids in the Leaves of Eranthis longistipitata (Ranunculaceae) by Liquid Chromatography with High-Resolution Mass Spectrometry (LC-HRMS)
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material and Preparation of the Extract
3.2. MS Settings and the Spectral Library
3.3. Flavonoid Analysis by High-Performance Liquid Chromatography
3.4. Chemicals
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erst, A.S.; Sukhorukov, A.P.; Mitrenina, E.Y.; Skaptsov, M.V.; Kostikova, V.A.; Chernisheva, O.A.; Troshkina, V.; Kushunina, M.; Krivenko, D.A.; Ikeda, H.; et al. An integrative taxonomic approach reveals a new species of Eranthis (Ranunculaceae) in North Asia. PhytoKeys 2020, 140, 75–100. [Google Scholar] [CrossRef]
- Xiang, K.L.; Erst, A.S.; Yang, J.; Peng, H.W.; Ortiz, R.D.C.; Jabbour, F.; Erst, T.V.; Wang, W. Biogeographic diversification of Eranthis (Ranunculaceae) reflects the geological history of the three great Asian plateaus. Proc. R. Soc. B 2021, 288, 20210281. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, B.D. Eranthis Salisb. In Flora of North America North of Mexico; Oxford University Press: New York, NY, USA; Oxford, MS, USA, 1997; Volume 3, p. 97. [Google Scholar]
- Huang, Z.; Ren, Y.; Zhang, X. Morphological and numerical variation patterns of floral organs in two species of Eranthis. Flora 2021, 276, 151785. [Google Scholar] [CrossRef]
- Tamura, M. Eranthis and Shibateranthis. Acta Phytotax. Geobot. 1987, 38, 96–97. [Google Scholar]
- Tamura, M. Eranthis. In Die Natürlichen Pflanzenfamilien; Duncker und Humblot: Berlin, Germany, 1995; Volume 17, pp. 253–255. [Google Scholar]
- Park, S.Y.; Jeon, M.J.; Ma, S.H.; Wahlsteen, E.; Amundsen, K.; Kim, J.H.; Suh, J.K.; Chang, J.S.; Joung, Y.H. Phylogeny and genetic variation in the genus Eranthis using nrITS and cpIS singlenucleotide polymorphisms. Hortic. Environ. Biotechnol. 2019, 60, 239–252. [Google Scholar] [CrossRef]
- Hao, D.C. Ranunculales Medicinal Plants: Biodiversity, Chemodiversity and Pharmacotherapy; Academic Press: London, UK, 2018. [Google Scholar]
- Malik, J.; Tauchen, J.; Landa, P.; Kutil, Z.; Marsik, P.; Kloucek, P.; Havlik, J.; Kokoska, L. In vitro antiinflammatory and antioxidant potential of root extracts from Ranunculaceae species. S. Afr. J. Bot. 2017, 109, 128–137. [Google Scholar] [CrossRef]
- Watanabe, K.; Mimaki, Y.; Fukaya, H.; Matsuo, Y. Cycloartane and oleanane glycosides from the tubers of Eranthis cilicica. Molecules 2019, 24, 69. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, M.; Uchida, S.; Watanabe, K.; Mimaki, Y. Chromones from the tubers of Eranthis cilicica and their antioxidant activity. Phytochemistry 2009, 70, 288–293. [Google Scholar] [CrossRef]
- McConnell, M.-T.; Lisgarten, D.R.; Byrne, L.J.; Harvey, S.C.; Bertolo, E. Winter Aconite (Eranthis hyemalis) lectin as a cytotoxic effector in the lifecycle of Caenorhabditis elegans. PeerJ. 2015, 3, e1206. [Google Scholar] [CrossRef] [Green Version]
- Djafari, J.; McConnell, M.T.; Santos, H.M.; Capelo, J.L.; Bertolo, E.; Harvey, S.C.; Lodeiro, C.; Fernández-Lodeiro, J. Synthesis of Gold Functionalised Nanoparticles with the Eranthis hyemalis Lectin and Preliminary Toxicological Studies on Caenorhabditis elegans. Materials 2018, 11, 1363. [Google Scholar] [CrossRef] [Green Version]
- Bespalov, V.G.; Alexandrov, V.A.; Vysochina, G.I.; Kostikova, V.A.; Semenov, A.L.; Baranenko, D. Inhibitory effect of Filipendula ulmaria on mammary carcinogenesis induced by local administration of methylnitrosourea to target organ in rats. Anticancer Agents Med. Chem. 2018, 18, 1177–1183. [Google Scholar] [CrossRef]
- Alamgir, A.N.M. Therapeutic Use of Medicinal Plants and Their Extracts; Springer: Cham, Switzerland, 2018; Volume 2. [Google Scholar]
- Alzaabi, M.M.; Hamdy, R.; Ashmawy, N.S.; Hamoda, A.M.; Alkhayat, F.; Khademi, N.N.; Al Joud, S.M.; El-Keblawy, A.A.; Soliman, S.S. Flavonoids are promising safe therapy against COVID-19. Phytochem. Rev. 2021, 22, 1–22. [Google Scholar] [CrossRef]
- Braunberger, C.; Zehl, M.; Conrad, J.; Wawrosch, C.; Strohbach, J.; Beifuss, U.; Krenn, L. Flavonoids as chemotaxonomic markers in the genus Drosera. Phytochemistry 2015, 118, 74–82. [Google Scholar] [CrossRef]
- Kostikova, V.A.; Erst, A.S.; Kuznetsov, A.A.; Gureyeva, I.I. Levels of phenolic compounds in leaves of Eranthis sibirica, E. stellata, and E. tanhoensis (Ranunculaceae). Ukr. J. Ecol. 2020, 10, 232–237. [Google Scholar] [CrossRef]
- Erst, A.A.; Erst, A.S. Features of in vitro seed germination of Eranthis longistipitata, an endemic plant of Central Asia. Plant Cell Biotechnol. Mol. Biol. 2019, 20, 611–616. [Google Scholar]
- Valant-Vetschera, K.M. C-Glycosylflavones as an accumulation tendency: A critical review. Bot. Rev. 1985, 51, 1–52. [Google Scholar] [CrossRef]
- Zhou, C.Y.; Xie, Z.S.; Lei, Z.X.; Huang, Y.C.; Wei, G. Simultaneous identification and determination of flavonoids in Dendrobium officinale. Chem. Cent. J. 2018, 12, 40. [Google Scholar] [CrossRef] [Green Version]
- Isemura, M. Catechin in human health and disease. Molecules 2019, 24, 528. [Google Scholar] [CrossRef] [Green Version]
- Sahai, R.; Agarwal, S.K.; Rastogi, R.P. Auriculoside, a new flavan glycosides from Acacia auriculiformis. Phytochemistry 1980, 19, 1560–1562. [Google Scholar] [CrossRef]
- Ranga, N.K.; Samanta, S.; Pradhan, K.K. A comprehensive review on phytopharmacological investigations of Acacia auriculiformis A. Cunn. ex Benth. Asian Pac. J. Trop. Biomed. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Puri, B.; Hall, A. Phytochemical Dictionary: A Handbook of Bioactive Compounds from Plant; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Zhang, W.Y.; Lee, J.J.; Kim, I.S.; Kim, Y.; Myung, C.S. Stimulation of glucose uptake and improvement of insulin resistance by aromadendrin. Pharmacology 2011, 88, 266–274. [Google Scholar] [CrossRef]
- Lee, H.S.; Jeong, G.S. Aromadendrin inhibits T-cell activation via regulation of calcium influx and NFAT-activity. Molecules 2020, 25, 4590. [Google Scholar] [CrossRef]
- Johnson, R.; de Beer, D.; Dludla, P.V.; Ferreira, D.; Muller, C.J.F.; Joubert, E. Aspalathin from Rooios (Aspalathus linearis): A bioactive C-glycosyl dihidrochalcone with potential to target the metabolic syndrome. Planta Med. 2018, 84, 568–583. [Google Scholar] [CrossRef] [Green Version]
- Mazibuko-Mbeje, S.E.; Dludla, P.V.; Johnson, R.; Joubert, E.; Louw, J.; Ziqubu, K.; Tiano, L.; Silvestri, S.; Orlando, P.; Opoku, A.R.; et al. Aspalathin, a natural product with the potential to reverse hepatic insulin resistance by improving energy metabolism and mitochondrial respiration. PLoS ONE 2019, 14, e0216172. [Google Scholar] [CrossRef] [Green Version]
- Falcone Ferreyra, M.L.; Rius, S.; Casati, P. Flavonoids: Biosynthesis, biological functions and biotechnological applications. Front. Plant. Sci. 2012, 3, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaprometov, M.N. Phenolic Compounds: Distribution, Metabolism and Function in Plants; Publishing House Nauka: Moscow, Russia, 1993. (In Russian) [Google Scholar]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Xu, X.; Sun, H.; Tang, J.; Ouyang, Z. Pharmacological activities and pharmacokinetic study of hyperoside: A short review. Trop. J. Pharm. Res. 2017, 16, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- van Beek TA Chemical analysis of Ginkgo biloba leaves and extracts. J. Chromatogr. A. 2002, 967, 21–55. [CrossRef]


| ID | tR (min) | Calculated Mass | Measured Mass | Delta Mass [Da] | Delta Mass [ppm] | MzCloud Score | Mode | Identified Compounds |
|---|---|---|---|---|---|---|---|---|
| Flavonols | ||||||||
| 1 | 10.13 | 596.13773 | 596.13689 | −0.00084 | −1.42 | ‒ | Positive | Peltatoside (quercetin-3-(6-O-α-L- arabinopyranosyl)-β-D- glucopyranoside)) |
| 2 | 10.95 | 464.09548 | 464.09524 | −0.00024 | −0.51 | 98.2 | Positive | Hyperoside * (quercetin 3-O-β-D- galactoside) |
| 3 | 10.95 | 434.08491 | 434.08460 | −0.00031 | −0.72 | 98.7 | Positive | Reynoutrin (quercetin-3-O-β-D- xylopyranoside) |
| 4 | 11.29 | 302.04265 | 302.04231 | −0.00035 | −1.15 | 99.9 | Positive | Quercetin * |
| 5 | 11.66 | 596.13773 | 596.13714 | −0.00060 | −1.00 | 98.1 | Negative | Quercetin 3-sambubioside (quercetin-3-O-[β-D-xylosyl- (1→2)-β-D-glucoside]) |
| 6 | 11.90 | 286.04774 | 286.04751 | −0.00023 | −0.80 | 99.0 | Positive | Kaempferol * |
| 7 | 11.90 | 418.09000 | 418.08966 | −0.00033 | −0.79 | 79.8 | Positive | Juglalin (kaempferol 3-O-α-L- arabinopyranoside) |
| 8 | 11.90 | 448.10056 | 448.10032 | −0.00024 | −0.54 | 98.5 | Positive | Trifolin (kaempferol-3-O-β-D- galactoside) |
| 9 | 13.01 | 464.09548 | 464.09582 | 0.00035 | 0.75 | 99.2 | Negative | Isoquercitrin (quercetin-3-O-β-D- glucoside) |
| 10 | 12.48 | 610.15338 | 610.15249 | −0.00089 | −1.46 | 98.9 | Positive | Rutin * (quercetin 3-O-β-D- rutinoside) |
| Flavones | ||||||||
| 11 | 12.56 | 580.14282 | 580.14259 | −0.00023 | −0.40 | ‒ | Negative | Carlinoside (luteolin 6-C-β-D- glucopyranoside-8-C-α-L- arabinopyranoside) |
| Flavans | ||||||||
| 12 | 16.53 | 290.07904 | 290.07898 | −0.00003 | −0.11 | ‒ | Positive | Cianidanol ((+)-catechin) |
| 13 | 19.78 | 450.15260 | 450.15215 | −0.00045 | −0.99 | ‒ | Positive | Auriculoside (7,3′,5′-trihydroxy-4′-methoxyflavan-3′-glucoside) |
| Flavanones | ||||||||
| 14 | 14.55 | 334.06887 | 334.06903 | 0.00016 | 0.48 | ‒ | Negative | 6-Methoxytaxifolin |
| 15 | 21.50 | 288.06339 | 288.06336 | −0.00003 | −0.11 | ‒ | Positive | Aromadendrin ((+)-dihydrokaempferol) |
| Chalcones | ||||||||
| 16 | 15.45 | 452.13186 | 452.13172 | −0.00014 | −0.32 | ‒ | Positive | Aspalathin |
| 17 | 16.23 | 436.13695 | 436.13681 | −0.00013 | −0.30 | ‒ | Positive | Phloridzin (phloretin-2′-O-β-glucoside) |
| 18 | 20.80 | 274.08412 | 274.08393 | −0.00019 | −0.69 | ‒ | Positive | Phloretin (dihydroxy naringenin) |
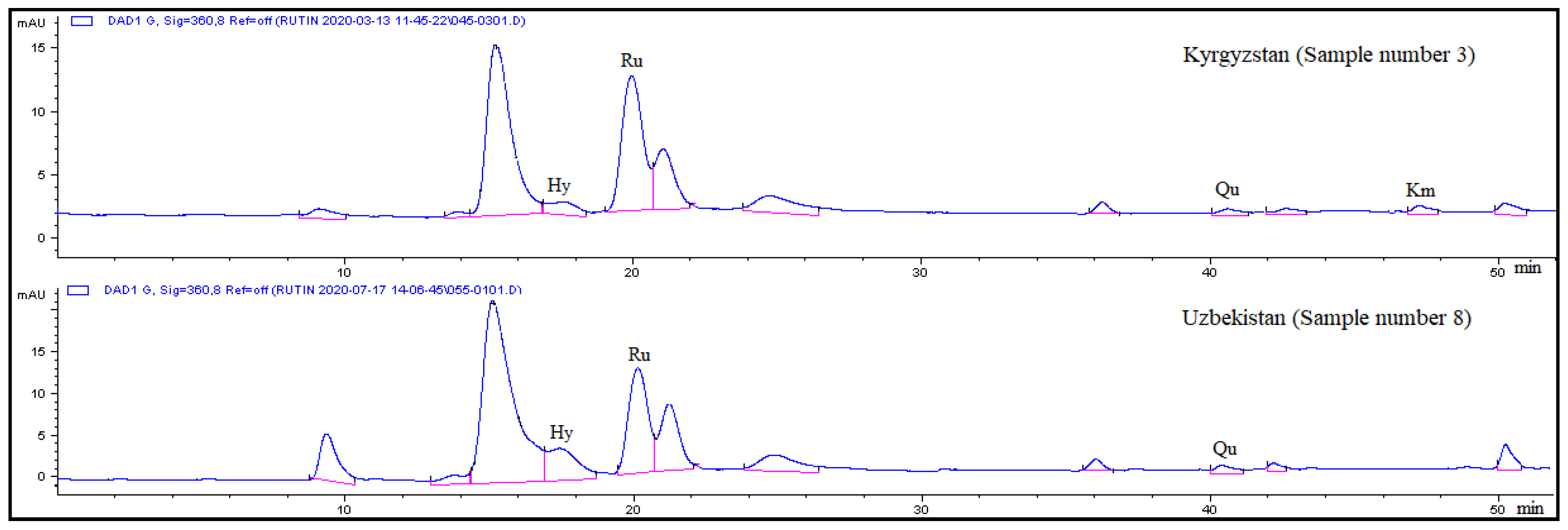
| Sample No. | Hyperoside (tR = 17.5 min) | Rutin (tR = 20.1 min) | Quercetin (tR = 40.6 min) | Kaempferol (tR = 47.0 min) |
|---|---|---|---|---|
| 1 | 1.01 ± 0.04 c | 2.49 ± 0.09 b | 0.62 ± 0.02 a | 0.55 ± 0.02 a |
| 2 | 0.88 ± 0.03 d | 2.46 ± 0.09 b | 0.12 ± 0.00 g | 0.20 ± 0.01 c |
| 3 | 0.79 ± 0.03 d | 3.20 ± 0.12 a | 0.27 ± 0.01 ef | 0.28 ± 0.01 b |
| 4 | 0.97 ± 0.04 c | 2.09 ± 0.08 c | 0.41 ± 0.02 c | – |
| 5 | 1.33 ± 0.05 b | 3.01 ± 0.11 a | 0.55 ± 0.02 b | – |
| 6 | 0.58 ± 0.02 e | 1.50 ± 0.06 d | 0.28 ± 0.01 e | 0.10 ± 0.00 e |
| 7 | 1.54 ± 0.06 a | 2.28 ± 0.08 bc | 0.23 ± 0.01 f | 0.13 ± 0.00 d |
| 8 | 1.49 ± 0.06 a | 2.04 ± 0.08 c | 0.33 ± 0.01 d | – |
| Sample No. | Locality; Coordinates | Habitat | Date |
|---|---|---|---|
| 1 | Kyrgyzstan, Chuya region, Issyk-atinskii district, Niczniaya Serafimovka village; 42°45′02″ N, 74°51′37″ E | foot of the mount | 19.03.2019 |
| 2 | Kyrgyzstan, Chuya region, Issyk-atinskii district, Karandolot tract; 42°44′22″ N, 74°55′50″ E | foot of the mount | 22.03.2019 |
| 3 | Kyrgyzstan, Talas region, Kara-Buurinskii district, west of Kirovskoe reservoir; 42°37′57″ N, 71°34′47″ E | steppe | 26.03.2019 |
| 4 | Uzbekistan, Andijan region, Khojaabad district, east-southeastern part of Fergana valley, Kyrtashtau mountains, near Imamat village; 40°32′27″ N, 72°36′28″ E | mossy stony slope | 12.03.2020 |
| 5 | Uzbekistan, Samarkand region, Urgut district, western Pamir-Alai, Gissar-Alai, western part of the Zeravshan ridge, right bank of Amankutansai river, near Amankutan kishlak; 39°18′16″ N, 66°55′45″ E | juniper forest on the slope | 14.03.2020 |
| 6 | Uzbekistan, Tashkent region, Bostanlyk district, western Tian Shan, spurs of northwestern part of Chatkal ridge, Galvasay river valley—left tributary Chirchik river, left bank; 41°32′20″ N, 69°53′03″ E | walnut grove on the slope | 16.03.2020 |
| 7 | Uzbekistan, Tashkent region, Bostanlyk district, Western Tian Shan, north-western part of Chatkal ridge, foot of Big Chimgan mountain, area between Galvasay and Mramornaya rivers, on road from Uchterek tract to Chimgan tract; 41°31′05″ N, 69°59′15″ E | bushy slope | 16.03.2020 |
| 8 | Uzbekistan, Jizzakh region, Zaamin district, western Pariro-Alai, Gissar-Alai, northern macroslope of Turkestan ridge, Zaamin forestry enterprise, Usman tract, 39°43′26″ N, 68°27′54″ E | mountain slope | 20.03.2020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostikova, V.A.; Chernonosov, A.A.; Kuznetsov, A.A.; Petrova, N.V.; Krivenko, D.A.; Chernysheva, O.A.; Wang, W.; Erst, A.S. Identification of Flavonoids in the Leaves of Eranthis longistipitata (Ranunculaceae) by Liquid Chromatography with High-Resolution Mass Spectrometry (LC-HRMS). Plants 2021, 10, 2146. https://doi.org/10.3390/plants10102146
Kostikova VA, Chernonosov AA, Kuznetsov AA, Petrova NV, Krivenko DA, Chernysheva OA, Wang W, Erst AS. Identification of Flavonoids in the Leaves of Eranthis longistipitata (Ranunculaceae) by Liquid Chromatography with High-Resolution Mass Spectrometry (LC-HRMS). Plants. 2021; 10(10):2146. https://doi.org/10.3390/plants10102146
Chicago/Turabian StyleKostikova, Vera A., Alexander A. Chernonosov, Alexander A. Kuznetsov, Natalia V. Petrova, Denis A. Krivenko, Olga A. Chernysheva, Wei Wang, and Andrey S. Erst. 2021. "Identification of Flavonoids in the Leaves of Eranthis longistipitata (Ranunculaceae) by Liquid Chromatography with High-Resolution Mass Spectrometry (LC-HRMS)" Plants 10, no. 10: 2146. https://doi.org/10.3390/plants10102146
APA StyleKostikova, V. A., Chernonosov, A. A., Kuznetsov, A. A., Petrova, N. V., Krivenko, D. A., Chernysheva, O. A., Wang, W., & Erst, A. S. (2021). Identification of Flavonoids in the Leaves of Eranthis longistipitata (Ranunculaceae) by Liquid Chromatography with High-Resolution Mass Spectrometry (LC-HRMS). Plants, 10(10), 2146. https://doi.org/10.3390/plants10102146