Exogenous Application of Polycationic Nanobactericide on Tomato Plants Reduces the Candidatus Liberibacter Solanacearum Infection
Abstract
:1. Introduction
2. Results
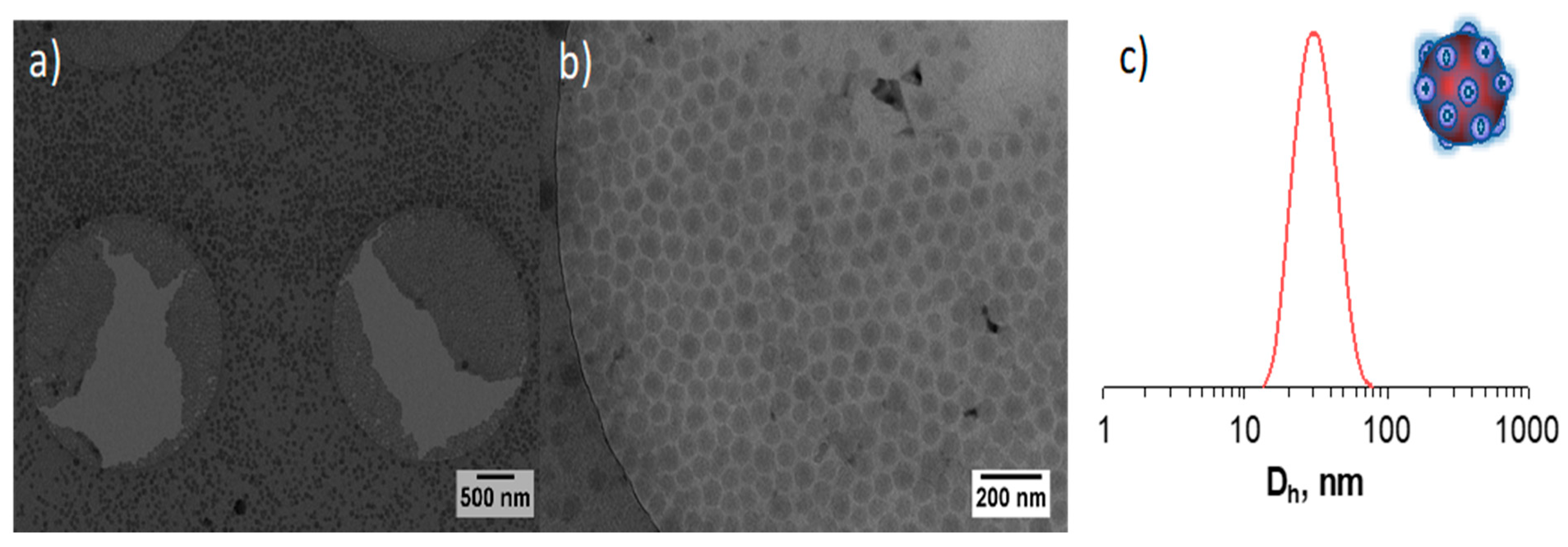
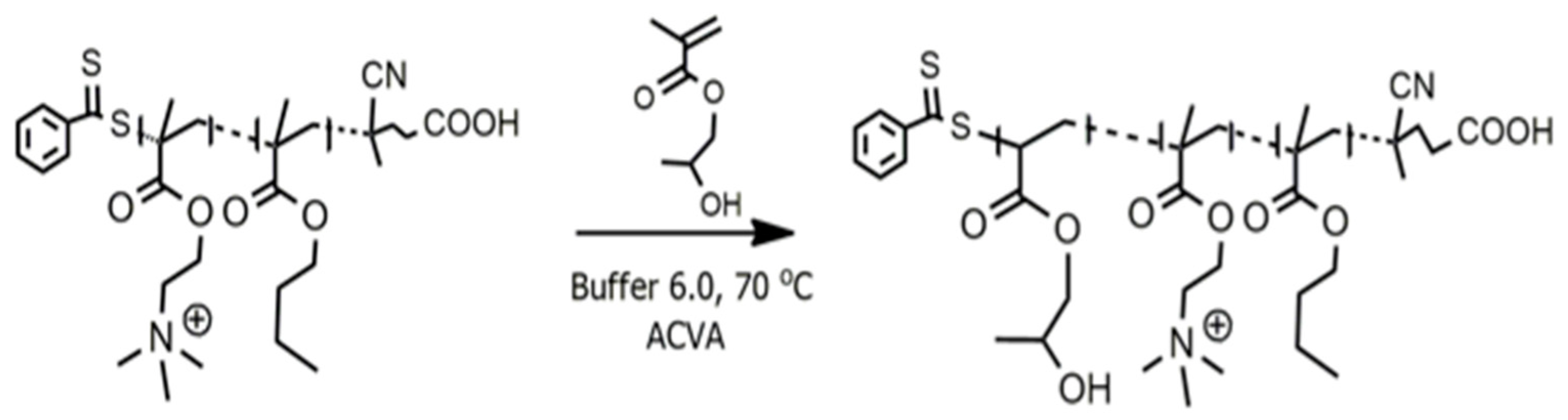
2.1. Nanobactericide Preparation
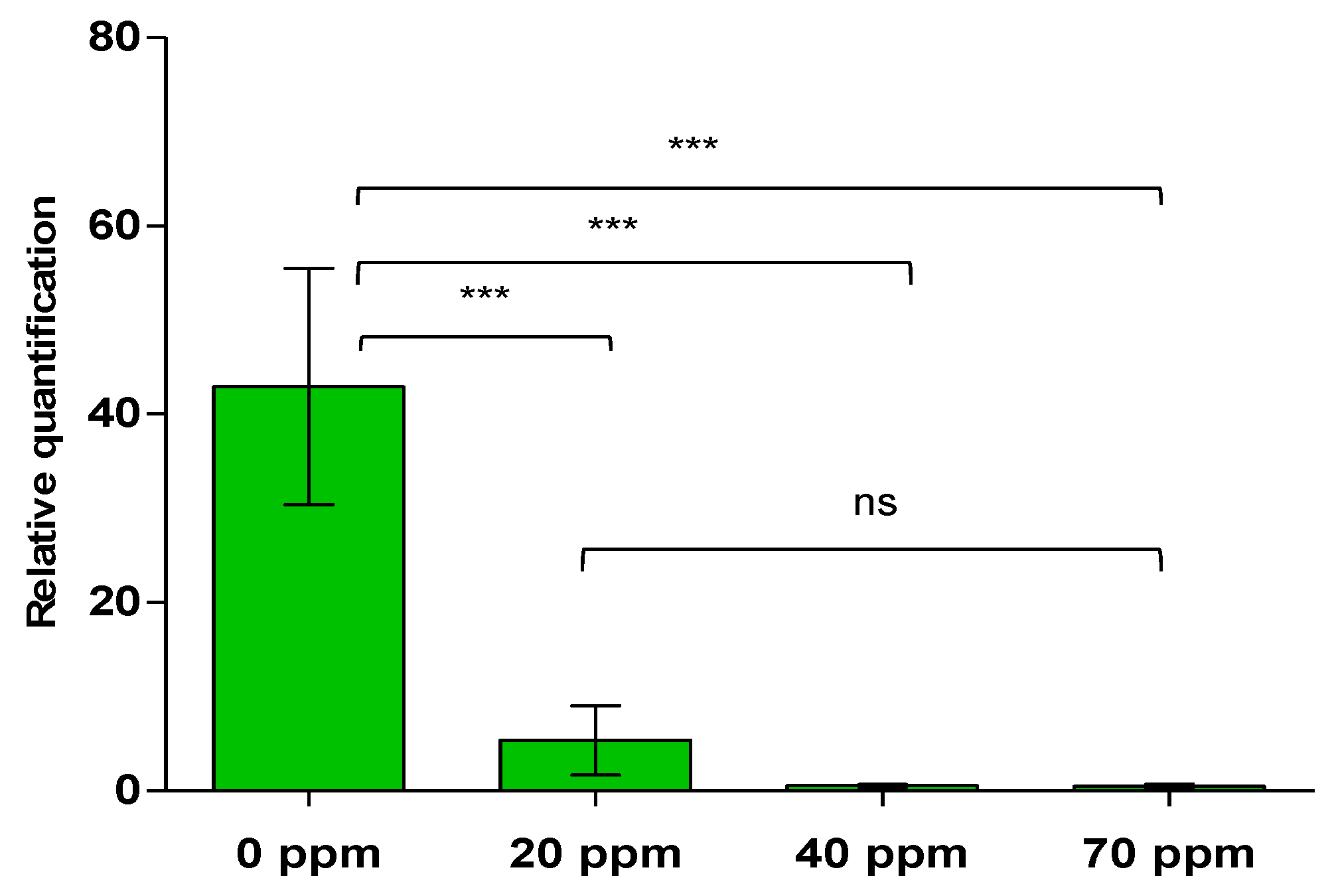
2.2. Effect of Different Doses of the PNB in Tomato Plants Infected by CaLso
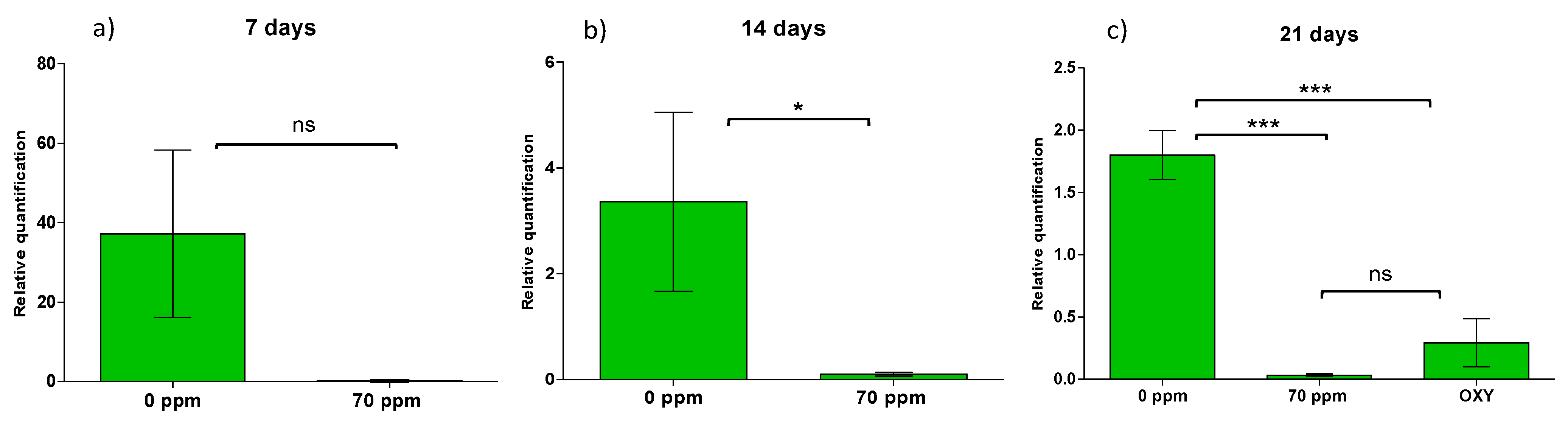
2.3. Time of Efficiency in CaLso Reduction by PNB Treatment
3. Discussion
4. Materials and Methods
4.1. Material
4.2. Synthesis of Nanobactericide
4.3. RAFT Synthesis of P(DMAEMA-stat-BMA) Copolymer
4.4. Quaternization of P(DMAEMA-stat-BMA) Copolymer
4.5. Synthesis of Nanobactericide as a Nanoparticle by Polymerization-Induced Self-Assembly (PISA)
4.6. Polymer Characterization Methods
4.7. Plant Growth Conditions and Insects
4.8. Plant Treatments
4.8.1. DNA Isolation
4.8.2. Endpoint and Quantitative Real-Time PCR
4.9. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghatak, A.; Chaturvedi, P.; Paul, P.; Agrawal, G.K.; Rakwal, R.; Kim, S.T.; Weckwerth, W.; Gupta, R. Proteomics survey of Solanaceae family: Current status and challenges ahead. J. Proteom. 2017, 169, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Salim, M.M.R.; Rashid, M.H.; Hossain, M.M.; Zakaria, M. Morphological characterization of tomato (Solanum lycopersicum L.) genotypes. J. Saudi Soc. Agric. Sci. 2018, 19, 233–240. [Google Scholar] [CrossRef]
- WANG, Y.; ZHANG, Y.; GAO, Z.; YANG, W. Breeding for Resistance to Tomato Bacterial Diseases in China: Challenges and Prospects. Hortic. Plant J. 2018, 4, 193–207. [Google Scholar] [CrossRef]
- Fennell, J.T.; Fountain, M.T.; Paul, N.D. Direct effects of protective cladding material on insect pests in crops. Crop Prot. 2019, 121, 147–156. [Google Scholar] [CrossRef]
- Blancard, D. The Tomato Plant and its Culture. In Tomato Diseases, 2nd. ed; Academic Press: San Diego, CA, USA, 2012; pp. 17–34. ISBN 978-0-12-387737-6. [Google Scholar]
- Munyaneza, J.E. Zebra Chip Disease of Potato: Biology, Epidemiology, and Management. Am. J. Potato Res. 2012, 89, 329–350. [Google Scholar] [CrossRef] [Green Version]
- Sengoda, V.G.; Cooper, W.R.; Swisher, K.D.; Henne, D.C.; Munyaneza, J.E. Latent Period and Transmission of “Candidatus Liberibacter solanacearum” by the Potato Psyllid Bactericera cockerelli (Hemiptera: Triozidae). PLoS ONE 2014, 9, e93475. [Google Scholar] [CrossRef] [Green Version]
- Crosslin, J.M.; Lin, H.; Munyaneza, J.E. Detection of ‘Candidatus Liberibacter Solanacearum’ in the Potato Psyllid, Bactericera cockerelli (Sulc), by Conventional and Real-Time PCR. Southwest. Entomol. 2011, 36, 125–135. [Google Scholar] [CrossRef]
- Avila, C.A.; Arévalo-Soliz, L.M.; Jia, L.; Navarre, D.A.; Chen, Z.; Howe, G.A.; Meng, Q.-W.; Smith, J.E.; Goggin, F.L. Loss of function of FATTY ACID DESATURASE7 in tomato enhances basal aphid resistance in a salicylate-dependent manner. Plant Physiol. 2012, 158, 2028–2041. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.-F.; Zhang, Z.-Q.; Beggs, J.R.; Paderes, E.; Zou, X.; Wei, X.-Y. Lethal and sublethal effects of entomopathogenic fungi on tomato/potato psyllid, Bactericera cockerelli (Šulc) (Hemiptera: Triozidae) in capsicum. Crop Prot. 2020, 129, 105023. [Google Scholar] [CrossRef]
- Lacey, L.A.; Liu, T.-X.; Buchman, J.L.; Munyaneza, J.E.; Goolsby, J.A.; Horton, D.R. Entomopathogenic fungi (Hypocreales) for control of potato psyllid, Bactericera cockerelli (Šulc) (Hemiptera: Triozidae) in an area endemic for zebra chip disease of potato. Biol. Control 2011, 56, 271–278. [Google Scholar] [CrossRef]
- De Lourdes Ramírez-Ahuja, M.; Rodríguez-Leyva, E.; Lomeli-Flores, J.R.; Torres-Ruiz, A.; Guzmán-Franco, A.W. Evaluating combined use of a parasitoid and a zoophytophagous bug for biological control of the potato psyllid, Bactericera cockerelli. Biol. Control 2017, 106, 9–15. [Google Scholar] [CrossRef]
- Pérez-Aguilar, D.A.; Martínez, A.M.; Viñuela, E.; Figueroa, J.I.; Gómez, B.; Morales, S.I.; Tapia, A.; Pineda, S. Impact of the zoophytophagous predator Engytatus varians (Hemiptera: Miridae) on Bactericera cockerelli (Hemiptera: Triozidae) control. Biol. Control 2019, 132, 29–35. [Google Scholar] [CrossRef]
- Tamayo-Mejía, F.; Tamez-Guerra, P.; Guzmán-Franco, A.W.; Gomez-Flores, R. Can Beauveria bassiana Bals. (Vuill) (Ascomycetes: Hypocreales) and Tamarixia triozae (Burks) (Hymenoptera: Eulophidae) be used together for improved biological control of Bactericera cockerelli (Hemiptera: Triozidae)? Biol. Control 2015, 90, 42–48. [Google Scholar] [CrossRef]
- McVay, J.; Sun, X.; Jones, D.; Urbina, H.; Aldeek, F.; Cook, J.M.; Jeyaprakash, A.; Hodges, G.; Smith, T. Limited persistence of residues and metabolites in fruit and juice following penicillin trunk infusion in citrus affected by Huanglongbing. Crop Prot. 2019, 125, 104753. [Google Scholar] [CrossRef]
- Kranjc, E.; Drobne, D. Nanomaterials in Plants: A Review of Hazard and Applications in the Agri-Food Sector. Nanomaterials 2019, 9, 1094. [Google Scholar] [CrossRef] [Green Version]
- Nwugo, C.C.; Lin, H.; Duan, Y.; Civerolo, E.L. The effect of ‘Candidatus Liberibacter asiaticus’ infection on the proteomic profiles and nutritional status of pre-symptomatic and symptomatic grapefruit (Citrus paradisi) plants. BMC Plant Biol. 2013, 13, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puttamuk, T.; Zhang, S.; Duan, Y.; Jantasorn, A.; Thaveechai, N. Effect of chemical treatments on ‘Candidatus Liberibacter asiaticus’ infected pomelo (Citrus maxima). Crop Prot. 2014, 65, 114–121. [Google Scholar] [CrossRef]
- Nelson, R. Controversial antibiotic use for crops approved in Florida. Lancet Infect. Dis. 2019, 19, 697–698. [Google Scholar] [CrossRef]
- Kumar, D.; Braun, C.; Terrazas Portillo, J.C.; Tapia Ramos, E.; Cambero Ramírez, L.M. Use of Isotianil for Control of Zebra Chip Disease. U.S. Patent Application No. WO/2017/055342, 2017. [Google Scholar]
- Yañez-Macías, R.; Muñoz-Bonilla, A.; De Jesús-Tellez, A.M.; Maldonado-Textle, H.; Guerrero-Sánchez, C.; Schubert, S.U.; Guerrero-Santos, R. Combinations of Antimicrobial Polymers with Nanomaterials and Bioactives to Improve Biocidal Therapies. Polymers 2019, 11, 1789. [Google Scholar] [CrossRef] [Green Version]
- Yañez-Macías, R. Study of the Structure-Property Relationship of Antibacterial and Thermo-Responsive Copolymers Synthesized by RAFT Polymerization. Ph.D. Thesis, Centro de Investigación en Química Aplicada, Saltillo, Coahuila, Mexico, 2017. [Google Scholar]
- Guerrero-Santos, R.; Valenzuela-Soto, J.H.; Yañez-Macías, R.; García-Sánchez, A.N.; Lugo de León, P. Process for Obtaining and Using a Phytoformulation with Dual Action to Eliminate Pathogenic Microorganisms in Agricultural Crops and Reduce Their Resistance. Mexico Patent Application No. MX/a/2020/012942, 2020. [Google Scholar]
- St Thomas, C.; Guerrero-Santos, R.; D’Agosto, F. Alkoxyamine-functionalized latex nanoparticles through RAFT polymerization-induced self-assembly in water. Polym. Chem. 2015, 6, 5405–5413. [Google Scholar] [CrossRef]
- Armes, S.P.; Perrier, S.; Zetterlund, P.B. Introduction to polymerisation-induced self assembly. Polym. Chem. 2021, 12, 8–11. [Google Scholar] [CrossRef]
- Yildirim, T.; Rinkenauer, A.C.; Weber, C.; Traeger, A.; Schubert, S.; Schubert, U.S. RAFT made methacrylate copolymers for reversible pH-responsive nanoparticles. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2711–2721. [Google Scholar] [CrossRef]
- Szczepaniec, A.; Varela, K.A.; Kiani, M.; Paetzold, L.; Rush, C.M. Incidence of resistance to neonicotinoid insecticides in Bactericera cockerelli across Southwest U. S. Crop Prot. 2019, 116, 188–195. [Google Scholar] [CrossRef]
- Cázares Alonso, N.P.; Verde Star, M.J.; López Arroyo, J.I.; Almeyda León, I.H. Evaluación de diferentes extractos vegetales contra el psílido asiático de los cítricos Diaphorina citri (Hemiptera: Liviidae). Rev. Colomb. Entomol. 2014, 40, 67–73. [Google Scholar]
- Zhang, M.; Powell, C.A.; Zhou, L.; He, Z.; Stover, E.; Duan, Y. Chemical Compounds Effective Against the Citrus Huanglongbing Bacterium ‘Candidatus Liberibacter asiaticus’ In Planta. Phytopathology 2011, 101, 1097–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yañez-Macias, R.; Alvarez-Moises, I.; Perevyazko, I.; Lezov, A.; Guerrero-Santos, R.; Schubert, U.S.; Guerrero-Sanchez, C. Effect of the Degree of Quaternization and Molar Mass on the Cloud Point of Poly[2-(dimethylamino)ethyl methacrylate] Aqueous Solutions: A Systematic Investigation. Macromol. Chem. Phys. 2017, 218, 1700065. [Google Scholar] [CrossRef]
- Mayo-Hernández, J.; Ramírez-Chávez, E.; Molina-Torres, J.; de Lourdes Guillén-Cisneros, M.; Rodríguez-Herrera, R.; Hernández-Castillo, F.; Flores-Olivas, A.; Valenzuela-Soto, J.H. Effects of Bactericera cockerelli Herbivory on Volatile Emissions of Three Varieties of Solanum lycopersicum. Plants 2019, 8, 509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Sánchez, A.N.; Yáñez-Macias, R.; Hernández-Flores, J.L.; Álvarez-Morales, A.; Valenzuela-Soto, J.H.; Guerrero-Sanchez, C.; Guerrero-Santos, R. Exogenous Application of Polycationic Nanobactericide on Tomato Plants Reduces the Candidatus Liberibacter Solanacearum Infection. Plants 2021, 10, 2096. https://doi.org/10.3390/plants10102096
García-Sánchez AN, Yáñez-Macias R, Hernández-Flores JL, Álvarez-Morales A, Valenzuela-Soto JH, Guerrero-Sanchez C, Guerrero-Santos R. Exogenous Application of Polycationic Nanobactericide on Tomato Plants Reduces the Candidatus Liberibacter Solanacearum Infection. Plants. 2021; 10(10):2096. https://doi.org/10.3390/plants10102096
Chicago/Turabian StyleGarcía-Sánchez, Adela Nazareth, Roberto Yáñez-Macias, José Luis Hernández-Flores, Ariel Álvarez-Morales, José Humberto Valenzuela-Soto, Carlos Guerrero-Sanchez, and Ramiro Guerrero-Santos. 2021. "Exogenous Application of Polycationic Nanobactericide on Tomato Plants Reduces the Candidatus Liberibacter Solanacearum Infection" Plants 10, no. 10: 2096. https://doi.org/10.3390/plants10102096
APA StyleGarcía-Sánchez, A. N., Yáñez-Macias, R., Hernández-Flores, J. L., Álvarez-Morales, A., Valenzuela-Soto, J. H., Guerrero-Sanchez, C., & Guerrero-Santos, R. (2021). Exogenous Application of Polycationic Nanobactericide on Tomato Plants Reduces the Candidatus Liberibacter Solanacearum Infection. Plants, 10(10), 2096. https://doi.org/10.3390/plants10102096






