Cultivar Differences in the Biochemical and Physiological Responses of Common Beans to Aluminum Stress
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Growth Conditions
4.2. Root Elongation
4.3. Dry Weight
4.4. Enzymes Assays
4.5. Data Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhan, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies on trackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef]
- Rengel, Z. Soil pH, soil health and climate change. In Soil Health and Climate Change; Singh, B., Cowie, A., Chan, K., Eds.; Spinger: Berlin/Heidelberg, Germany, 2011; Volume 29, pp. 69–85. [Google Scholar] [CrossRef]
- Tóth, B.; Moloi, M. The interdependence between low pH and heavy metal stress and its crucial role in crop production efficiency. In Contemporary Studies in Sciences; Efe, R., Cürebal, I., Eds.; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2020; pp. 2–17. ISBN 1527554244. [Google Scholar]
- Smith, W.H. Pollution, Overview. Encylo. Biodiver. 2001, 731–743. [Google Scholar] [CrossRef]
- Lake Scientist. Available online: https://www.lakescientist.com/acid-rain/ (accessed on 16 August 2021).
- EPA. United States Environmental Protection Agency: Effects of Acid Rain. Available online: https://www.epa.gov/acidrain/effects-acid-rain (accessed on 16 August 2021).
- Bojórquez-Quintal, E.; Escalante-Magana, C.; Echevarría-Machado, I.; Martínez-Estévez, M. Aluminum, a friend or foe of higher plants in acid soils. Front. Plant Sci. 2017, 8, 1767. [Google Scholar] [CrossRef]
- Nguyen, T.; Tran, T.T.H. The contribution of various components to pH buffering capacity of Acrisols in southeastern Vietnam. Commun. Soil Sci. Plant Anal. 2019, 50, 1170–1177. [Google Scholar] [CrossRef]
- Lindsay, W.L. Chemical Equilibria in Soils; John Wiley and Sons: New York, NY, USA, 1979; p. 449. ISBN 0471027049. [Google Scholar]
- Wright, R.J. Soil aluminum toxicity and plant growth. Commun. Soil Sci. Plant Anal. 1989, 20, 1479–1497. [Google Scholar] [CrossRef]
- University of Georgia, Agricultural and Environmental Services Laboratories. Available online: https://aesl.ces.uga.edu/publications/plant/Nutrient.html#:~:text=Aluminum%20is%20not%20considered%20a,is%20not%20required%20by%20plants.&text=Aluminum%20levels%20in%20excess%20of,is%20less%20than%200.10%20ppm (accessed on 9 September 2021).
- Jansen, S.; Broadley, M.R.; Robbrecht, E.; Smets, E. Aluminum hyperaccumulation in angiosperms: A review of if phylogenetic significance. Bot. Rev. 2002, 68, 235–269. [Google Scholar] [CrossRef]
- Ma, J.F.; Ryan, P.R.; Delhaize, E. Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 2001, 6, 273–278. [Google Scholar] [CrossRef]
- Vlamis, J.; Williams, D. Liming reduces aluminum and manganese toxicity in acid soils. Calif. Agr. 1962, 16, 6–7. [Google Scholar]
- Cármaco, M.P.; Reyes-Díaz, M.; Rengel, Z.; Alberdi, M.; Omen-Garcia, R.P.; Nunes-Nesi, A.; Inostroza-Blancheteau, C. Aluminum stress differentially affects physiological performance and metabolic compounds in cultivars of highbush blueberry. Sci. Rep. 2019, 9, 11275. [Google Scholar]
- Rodrigues, A.A.; Filho, S.C.V.; Müller, C.; Rodrigues, D.A.; Sales, J.F.; Zuchi, J.; Costa, A.C.; Rodrigues, C.L.; da Silva, A.A.; Barbosa, D.P. Tolerance of Eugenia dysenterica to aluminum: Germination and plant growth. Plants 2019, 8, 317. [Google Scholar] [CrossRef]
- Ojeda-Rivera, J.O.; Oropeza-Aburto, A.; Herrera-Estrella, L. Dissection of root transcriptional responses to low pH, aluminum toxicity and iron excess under pi-liming conditions in Arapidopsis wild-type and stop1 seedlings. Front. Plant Sci. 2020, 11, 01200. [Google Scholar] [CrossRef]
- Vance, W.; Pradeep, K.; Strachan, S.R.; Diffey, S.; Bell, R.W. Novel sources of tolerance of aluminium toxicity in wild cicer (Cicer reticulatum and Cicer echinospermum) collections. Front. Plant Sci. 2021, 12, 678211. [Google Scholar] [CrossRef]
- Singh, C.K.; Singh, D.; Sharma, S.; Chandra, S.; Taunk, J.; Konjengbam, N.S.; Singh, D.; Kumar, A.; Upadhyaya, K.C.; Pal, M. Morpho-physiological characterization coupled with expresssional accord of exlusion mechanism in wild and cultivated lentil under aluminum stress. Protoplasma 2021, 258, 1029–1045. [Google Scholar] [CrossRef]
- Delhaize, E.; Ryan, P.R. Aluminum toxicity and tolerance in plants. Plant Physiol. 1995, 107, 315–321. [Google Scholar] [CrossRef]
- Zhou, G.; Delhaize, E.; Zhou, M.; Ryan, P.R. Biotechnological solutions for enhancing the aluminium resistance of crop plants. In Abiotic Stress in Plants-Mechanisms and Adaptations; Shanker, A., Venkateswarlu, B., Eds.; InTech: Rijeka, Croatia, 2011. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; Blamey, F.P.C. Kinetic and nature of aluminium rhizotoxic effects: A review. J. Exp. Bot. 2016, 67, 4451–4467. [Google Scholar] [CrossRef]
- Delhaize, E.; Ryan, P.R.; Randall, P. Aluminium tolerance in wheat (Triticum aestivum L.) II. Aluminium-stimulated excretion of malic acid from root apices. Plant Physiol. 1993, 103, 695–702. [Google Scholar] [CrossRef]
- Kuo, M.C.; Kao, C.H. Aluminum effects on lipid peroxidation and antioxidative enzyme activities in rice leaves. Biol. Plant. 2003, 46, 149–152. [Google Scholar] [CrossRef]
- Kochian, L.V. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995, 46, 237–260. [Google Scholar] [CrossRef]
- Mukhopadyay, M.; Bantawa, P.; Das, A.; Sarkar, B.; Bera, B.; Ghosh, P.; Mondal, T.K. Changes of growth, photosynthesis and alteration of leaf antioxidative defence system of tea [Camellia sinensis (L.) O. Kuntze] seedlings under aluminum stress. Biometals 2012, 25, 1141–1154. [Google Scholar] [CrossRef] [PubMed]
- Lazarević, B.; Horvat, T.; Poljak, M. Effect of Acid Aluminous Soil on Photosynthetic Parameters of Potato (Solanum tuberosum L.). Potato Res. 2014, 57, 33–46. [Google Scholar] [CrossRef]
- Awasthi, J.P.; Saha, B.; Panigrahi, J.; Yanase, E.; Koyama, H.; Panda, S.K. Redox balance, metabolic fingerprint and physiological characterization in contrasting North East Indian rice for aluminum stress tolerance. Sci. Rep. 2019, 9, 8681. [Google Scholar] [CrossRef]
- Pradhan, B.; Patra, S.; Dash, S.R.; Maharana, S.; Behera, C.; Jena, M. Antioxidant responses against aluminum metal stress in Geitlerinema amphibium. SN Appl. Sci. 2020, 2, 800. [Google Scholar] [CrossRef]
- Devi, S.S.; Saha, B.; Awasthi, J.P.; Regon, P.; Panda, S.K. Redox status and oxalate exudation determines the differential tolerance of two contrasting varieties of ‘Assam tea’ [Camelia sinensis (L.) O. Kuntz] in response to aluminum toxicity. Hortic. Environ. Biotechnol. 2020, 61, 485–499. [Google Scholar] [CrossRef]
- Silva, S. Aluminum toxicity targets in plants. J. Bot. 2012, 2012, 219462. [Google Scholar] [CrossRef]
- Ribeiro, C.; Cambraia, J.; Peixoto, P.H.P.; da Fonseca, É.M., Jr. Antioxidant system response induced by aluminum in two rice cultivars. Braz. Soc. Plant Physiol. 2012, 24, 107–116. [Google Scholar] [CrossRef][Green Version]
- You, J.; Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef]
- Ou-Yang, C.; Gao, S.; Mei, L.J.; Chung, T.W.; Tang, L.; Wang, S.H.; Chen, F. Effects of aluminum toxicity on the growth and antioxidant status of Jatropha curcas seedlings. J. Med. Plant Res. 2013, 8, 178–185. Available online: https://academicjournals.org/article/article1390386071_Ou-yang%20et%20al.pdf (accessed on 12 July 2021).
- Nasr, N.; Carapetian, J.; Heidari, R.; Asri Rezaei, S.; Abbaspour, N.; Darvishzadeh, R.; Ghezelbash, F. The effect of aluminium on enzymes activities in two wheat cultivars. Afr. J. Biotechnol. 2011, 10, 3354–3364. [Google Scholar]
- Nogueirol, R.C.; Monteiro, F.A.; Azevedo, R.A. Tropical soils cultivated with tomato: Fractionation and speciation of Al. Eviron. Monit. Assess. 2015, 187, 160. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Nian, H.; Zhang, Z.; Yang, C. Effects of aluminum on superoxide dismutase and peroxidase activities, and lipid peroxidation in the roots and calluses of soybeans differing in aluminum tolerance. Acta Physiol. Plant. 2010, 32, 883–890. [Google Scholar] [CrossRef]
- Saxena, S.C.; Joshi, P.; Grimm, B.; Arora, S. Alleviation of ultraviolet C induced oxidative through overexpression of cytosolic ascorbate peroxidase. Biologia 2011, 66, 1052–1059. [Google Scholar] [CrossRef]
- Rajput, P.; Chaudhary, M.; Sharma, R.A. Comparative non-enzymatic and enzymatic antioxidant potential screening in species of genus Urtica. IJPSR 2021, 12, 2876–2883. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol. 2001, 42, 1265–1273. [Google Scholar] [CrossRef]
- Sairam, R.K.; Deshmukh, P.S.; Saxena, D.C. Role of antioxidant systems in wheat genotypes tolerance to water stress. Biol. Plant. 1998, 41, 387–394. [Google Scholar] [CrossRef]
- Mittler, R.; Zilinskas, B.A. Regulation of pea cytosolic ascorbate peroxidase and other antioxidant enzymes during the progression of drought stress and following recovery from drought. Plant J. 1994, 5, 397–405. [Google Scholar] [CrossRef]
- Darkó, É.; Ambrus, H.; Stefanovits-Bányai, É.; Fodor, J.; Bakos, F.; Barnabás, B. Aluminium toxicity, Al tolerance and oxidative stress in an Al-sensitive wheat genotype and in Al-tolerant lines developed by in vitro microspore selection. Plant Sci. 2004, 166, 583–591. [Google Scholar] [CrossRef]
- Shigeoka, S.; Ishikawa, T.; Tamoi, M.; Miyagawa, Y.; Takeda, T.; Yabuta, Y.; Yoshimura, K. Regulation and function of ascorbate peroxidase isoenzymes. J. Exp. Bot. 2002, 53, 1305–1319. [Google Scholar] [CrossRef]
- Boscolo, P.R.S.; Menossi, M.; Jorge, R.A. Aluminum-induced oxidative stress in maize. Phytochemistry 2003, 62, 181–189. [Google Scholar] [CrossRef]
- Li, W.; Mo, W.; Ashraf, U.; Li, G.; Wen, T.; Abrar, M.; Gao, L.; Liu, J.; Hu, J. Evaluation of physiological indices of waterlogging tolerance of different maize varieties in South China. Appl. Ecol. Environ. Res. 2018, 16, 2059–2072. [Google Scholar] [CrossRef]
- Alzahrani, S.M.; Alaraidh, I.A.; Migdadi, H.; Alghamdi, S.; Khan, M.A.; Ahmad, P. Physiological, biochemical, and antioxidant properties of two genotypes of Vicia faba grown under salinity stress. Pak. J. Bot. 2019, 51, 786–798. [Google Scholar] [CrossRef]
- Hussain, H.A.; Men, S.; Hussain, S.; Chen, Y.; Ali, S.; Zhang, S.; Zhang, K.; Li, Y.; Xu, Q.; Liao, C. Interactive effects of drought and heat stresses on morpho-physiological attributes, yield, nutrient uptake and oxidative status in maize hybrids. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, M.; Lu, M.; Sehar, S.; Holford, P.; Wu, F. Comparison of biochemical, anatomical, morphological, and physiological responses to salinity stress in wheat and barley genotypes deferring in salinity tolerance. Agronomy 2020, 10, 127. [Google Scholar] [CrossRef]
- Chahardoli, A.; Karimi, N.; Ma, X.; Qalekhani, F. Effects of engineered aluminum and nickel oxide nanoparticles on the growth and antioxidant defense system of Nigella arvensis L. Sci. Rep. 2020, 10, 3847. [Google Scholar] [CrossRef]
- Messina, V. Nutritional and health benefits of dried beans. Am. J. Clin. Nutr. 2014, 100, 437S–442S. [Google Scholar] [CrossRef]
- Broughton, W.J.; Hernández, G.; Blair, M.; Beebe, S.; Gepts, P.; Vanderleyden, J. Beans (Phaseolus ssp.)—Model food legumes. Plant Soil 2003, 252, 55–128. [Google Scholar] [CrossRef]
- Alemu, H. Review paper on breeding common bean (Phaseolus vulgaris L.) genotypes for acidic soil tolerance. Int. J. Adv. Res. Publ. 2017, 1, 39–46. Available online: http://www.ijarp.org/published-research-papers/sep2017/Review-Paper-On-Breeding-Common-Bean-phaseolus-Vulgaris-L-Genotypes-For-Acidic-Soil-Tolerance.pdf (accessed on 12 July 2021).
- dos Santos Neto, J.; Delfini, J.; Willian Silva, T.; Akihide Hirose, A.; Marcos Novais, J.; Simoes Azeredo Goncalves, L.; Moda-Cirino, V. Response of common bean cultivars and lines to aluminum toxicity. Agronomy 2020, 10, 296. [Google Scholar] [CrossRef]
- Freddi, O.S.; Tavanti, R.F.R.; Carvalho, M.P.; Montanari, R.; Andreotti, M. Restrictions of the common bean productivity in direct seedling system in the Brazilian cerrado. Nativa 2017, 5, 237–243. [Google Scholar] [CrossRef][Green Version]
- Birachi, E.A.; Ochieng, J.; Wozemba, D.; Ruraduma, C.; Niyuhire, M.C.; Ochieng, D. Factors influencing smallholder farmers’ bean production and supply to market in Burundi. Afr. Crop Sci. J. 2011, 19, 335–342. Available online: https://www.ajol.info/index.php/acsj/article/view/74193 (accessed on 12 July 2021).
- Rao, I.M. Role of physiology in improving crop adaptation to abiotic stresses in the tropics: The case of common bean and tropical forages. In Handbook of Plant and Crop Physiology; Pessarakli, M., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Rao, I.; Wenzl, P.; Arango, A.; Miles, J.; Watanabe, T.; Shinano, T.; Osaki, M.; Wagatsuma, T.; Manrique, G.; Beebe, S.; et al. Advances in developing screening methods and improving aluminum resistance in common bean and Brachiaria. Rev. Bras. Agric. 2008, 14, 1–7. [Google Scholar]
- Blair, M.W.; López-Marín, H.D.; Rao, I.M. Identification of aluminum resistant Andean common bean (Phaseolus vulgaris L.) genotypes. Braz. J. Plant Physiol. 2009, 21, 291–300. Available online: https://www.scielo.br/j/bjpp/a/pNvBpL7ptWmFdKD5c9txjCw/?lang=en&format=pdf (accessed on 12 July 2021). [CrossRef]
- Bitocchi, E.; Nanni, L.; Bellucci, E.; Rossi, M.; Giardini, A.; Zeuli, P.S.; Logozzo, G.; Stougaard, J.; McClean, P.; Attene, G.; et al. Mesoamerican origin of the common bean (Phaseolus vulgaris L.) is revealed by sequence data. Proc. Natl. Acad. Sci. USA 2012, 109, E788–E796. [Google Scholar] [CrossRef] [PubMed]
- Domingues, A.M.; da Silva, E.; Freitas, G.; Gananca, J.F.; Nóbrega, H.; Slaski, J.J.; de Carvalho, M.A.P. Aliminum tolerance in bean traditional cultivars from Maderira. Rev. De Ciências Agrárias 2013, 36, 148–156. [Google Scholar]
- Fageria, N.; Nascente, A.S. Management of soil acidity of South American soils for sustainable crops production. Adv. Agron. 2014, 128, 221–275. [Google Scholar] [CrossRef]
- Lunze, L.; Kimani, P.M.; Ngatoluwa, R.; Rabary, B.; Rachier, G.O.; Ugen, M.M.; Ruganza, V.; Awad Elkarim, E.E. Bean improvement for low soil fertility in adaptation in Eastern and Central Africa. In Advances in Integrated Soil Fertility Management in Sub-Saharan Africa: Challenges and Opportunities; Springer: Dordrecht, The Netherlands, 2007; pp. 325–332. [Google Scholar] [CrossRef]
- Horneck, D.S.; Ellsworth, J.W.; Hopkins, B.G.; Sullivan, D.M.; Stevens, R.G. Managing Salt-Affected Soils for Crop Production. PNW 601-E. Oregon State University, University of Idaho, Washington State University, 2007. Available online: https://catalog.extension.oregonstate.edu/sites/catalog/files/project/pdf/pnw601.pdf (accessed on 10 September 2021).
- Horst, W.J.; Wang, Y.X.; Eticha, D. The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminum resistance of plants: A review. Ann. Bot. 2010, 106, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Llugany, M.; Poschenrieder, C.; Barceló, J. Monitoring of aluminum-induced inhibition of root elongation in four maize cultivars differing in tolerance to aluminum and proton toxicity. Physiol. Plant 1995, 93, 265–277. [Google Scholar] [CrossRef]
- Yang, Z.B.; Eticha, D.; Albacete, A.; Rao, I.M.; Roitsch, T.; Horst, W.J. Physiological and molecular interaction between aluminium toxicity and drought stress in common bean (Phaseolus vulgaris). J. Exp. Bot. 2012, 63, 3109–3125. [Google Scholar] [CrossRef]
- Massot, N.; Llugany, M.; Poschenrieder, C.; Barceló, J. Callose production as indicator of aluminum toxicity in bean cultivars. J. Plant Nutr. 1999, 22, 1–10. [Google Scholar] [CrossRef]
- Ma, J.F.; Nagao, S.; Sato, K.; Ito, H.; Furukawa, J.; Takeda, K. Molecular mapping of a gene responsible for Al-activated secretion of citrate in barley. J. Exp. Bot. 2004, 55, 1335–1341. [Google Scholar] [CrossRef]
- Rhind, S.M. Anthropogenic pollutants: A threat to ecosystem sustainability? Philos. Trans. R. Soc. B. 2009, 364, 1534. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.J.; Garnett, T. Food security and sustainable intensification. Philos. Trans. R. Soc. B. 2014, 369, 1639. [Google Scholar] [CrossRef] [PubMed]
- Kochian, L.V.; Piñeros, M.A.; Hoekenga, O.A. The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil 2005, 274, 175–195. [Google Scholar] [CrossRef]
- Zhang, J.; He, Z.; Tian, H.; Zhu, G.; Peng, X. Identification of aluminium-responsive genes in rice cultivars with different aluminium sensitivities. J. Exp. Bot. 2007, 58, 2269–2278. [Google Scholar] [CrossRef]
- Kochian, L.V.; Piñeros, M.A.; Liu, J.; Magalhaes, J.V. Plant adaptation to acid soils: The molucular basis for crop aluminum resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, J.L.; He, L.S.; Li, Y.Y.; Zheng, S.J. Effect of aluminum on cell wall, plasma membrane, antioxidants and root elongation in triticale. Biol. Plant. 2008, 52, 87–92. [Google Scholar] [CrossRef]
- Jones, D.L.; Blancaflor, E.B.; Kochian, L.V.; Gilroy, S. Spatial coordinatation of aluminum uptake, production of reactive oxygen species, callose production and wall rigidification in maize roots. Plant Cell Environ. 2006, 29, 1309–1318. [Google Scholar] [CrossRef]
- Haug, A.; Weis, C. Aluminum-induced changes in calmodulin. In Moleculars and Cellular Aspects of Calcium in Plant Development; Trewavas, A.J., Ed.; Springer: Boston, MA, USA. [CrossRef]
- Rahman, M.A.; Lee, S.-H.; Ji, H.C.; Kabir, A.H.; Jones, C.S.; Lee, K.W. Importance of Mineral Nutrition for Mitigating Aluminum Toxicity in Plants on Acidic Soils: Current Status and Opportunities. Int. J. Mol. Sci. 2018, 19, 3073. [Google Scholar] [CrossRef]
- Rangel, A.F.; Rao, I.M.; Horst, W.J. Spatial aluminium sensitivity of root apices of two common bean (Phaseolus vulgaris L.) genotypes with contrasting aluminium resistance. J. Exp. Bot. 2007, 58, 3895–3904. [Google Scholar] [CrossRef]
- Rangel, A.F.; Mobin, M.; Rao, I.M.; Horst, W.J. Proton toxicity interferes with the screening of common bean (Phaseolus vulgaris L.) genotypes for aluminum resistance in nutrient solution. J. Plant Nutr. Soil Sci. 2005, 168, 607–616. [Google Scholar] [CrossRef]
- Zheng, S.J.; Yang, J.L. Target sites of aluminum phytotoxicity. Biol. Plant. 2005, 49, 321–331. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Nahar, K.; Hasanuzzaman, M.; Suzuki, T.; Fujita, M. Polyamines-induced aluminum tolerance in mung bean: A study on antioxidant defence and methylglyoxal detoxification systems. Ecotoxicology 2017, 26, 58–73. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kobayashi, Y.; Devi, S.R.; Rikiishi, S.; Matsumoto, H. Aluminum toxicity is associated with mitochondrial dysfunction and the production of reactive oxygen species in plant cells. Plant Physiol. 2002, 128, 63–72. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC148944/ (accessed on 12 July 2021). [CrossRef] [PubMed]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.M.; Qian, P.; Xin, W.; Li, H.Y.; Burritt, D.J.; Fujita, M.; Tran, L.S.P. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 1–19. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Meng, Q.; Zou, J.; Jiang, W.; Liu, D. Effects of aluminum on nucleoli in root tip cells, root growth and the antioxidant defence system in Vicia faba L. Acta Biol. Crac. Ser. Bot. 2009, 51, 99–106. [Google Scholar]
- Chen, Q.; Wu, K.H.; Zhang, Y.N.; Phan, X.H.; Li, K.Z.; Yu, Y.X.; Chen, L.M. Physiological and molecular responses of broad bean (Vicia faba L.) to aluminum stress. Acta Physiol. Plant 2012, 34, 2251–2263. [Google Scholar] [CrossRef]
- Rangel, A.F.; Rao, I.M.; Braun, H.P.; Horst, W.J. Aluminum resistance in common bean (Phaseolus vulgaris) involves induction and maintenance of citrate exudation from root apices. Physiol. Plant. 2010, 138, 176–190. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kobayashi, Y.; Matsumoto, H. Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol. 2001, 125, 199–208. [Google Scholar] [CrossRef]
- Horst, W.J.; Asher, C.J.; Cakmak, I.; Szulkiewica, P.; Wissemeier, A.H. Short-term response of soybean roots to aluminum. J. Plants Physiol. 1992, 140, 174–178. [Google Scholar] [CrossRef]
- Kinraide, T.B. Alumnium enhancement of plant growth in acid rooting media. A case of reciprocal alleviation of toxicity by two toxic cations. Physiol. Plant. 1993, 88, 619–625. [Google Scholar] [CrossRef]
- Birouste, M.; Zamora-Ledezma, E.; Bossard, C.; Pérez-Ramos, I.M.; Roumet, C. Measurement of fine root tissue density: A comparison of three methods reveals the potential of root dry matter content. Plant Soil 2014, 374, 299–313. [Google Scholar] [CrossRef]
- Giannopolities, C.H.; Ries, S.K. Superoxide Dismutase I. Occurrence in Higher Plant. Plant Physiol. 1977, 59, 309–314. Available online: https://shibbolethsp.jstor.org/start?entityID=https%3A%2F%2Fidp.unideb.hu%2Fsimplesaml%2Fsaml2%2Fidp%2Fmetadata.php&dest=https://www.jstor.org/stable/4264724&site=jstor (accessed on 12 July 2021). [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Zeislin, N.; Ben-Zaken, R. Peroxidases, phenylalanine ammonia-lyase and lignification in peduncles of rose flowers. Plant Physiol. Biochem. 1991, 29, 147–151. [Google Scholar]
- Keller, A.; Mohamed, A.; Drose, S.; Brandt, U.; Fleming, I.; Brandes, R.P. Analysis of dichlorodihydrofluorescein and dihydrocalcein as probes for the detection of intracellular reactive oxygen species. Free Radic. Res. 2004, 38, 1257–1267. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Ghasemi, A.; Zahediasl, S. Normality Tests for Statistical Analysis: A Guide for Non-Statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Nachar, N. The Mann-Whitney U: A Test for Assessing whether Two Independent Samples Come from the Same Distribution. Tutor. Quant. Methods Psychol. 2008, 4, 13–30. Available online: https://www.tqmp.org/RegularArticles/vol04-1/p013/p013.pdf (accessed on 12 July 2021). [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficient: Appropriate use and interpretation. Anesth. Analg. 2018, 12, 1763–1768. [Google Scholar] [CrossRef]
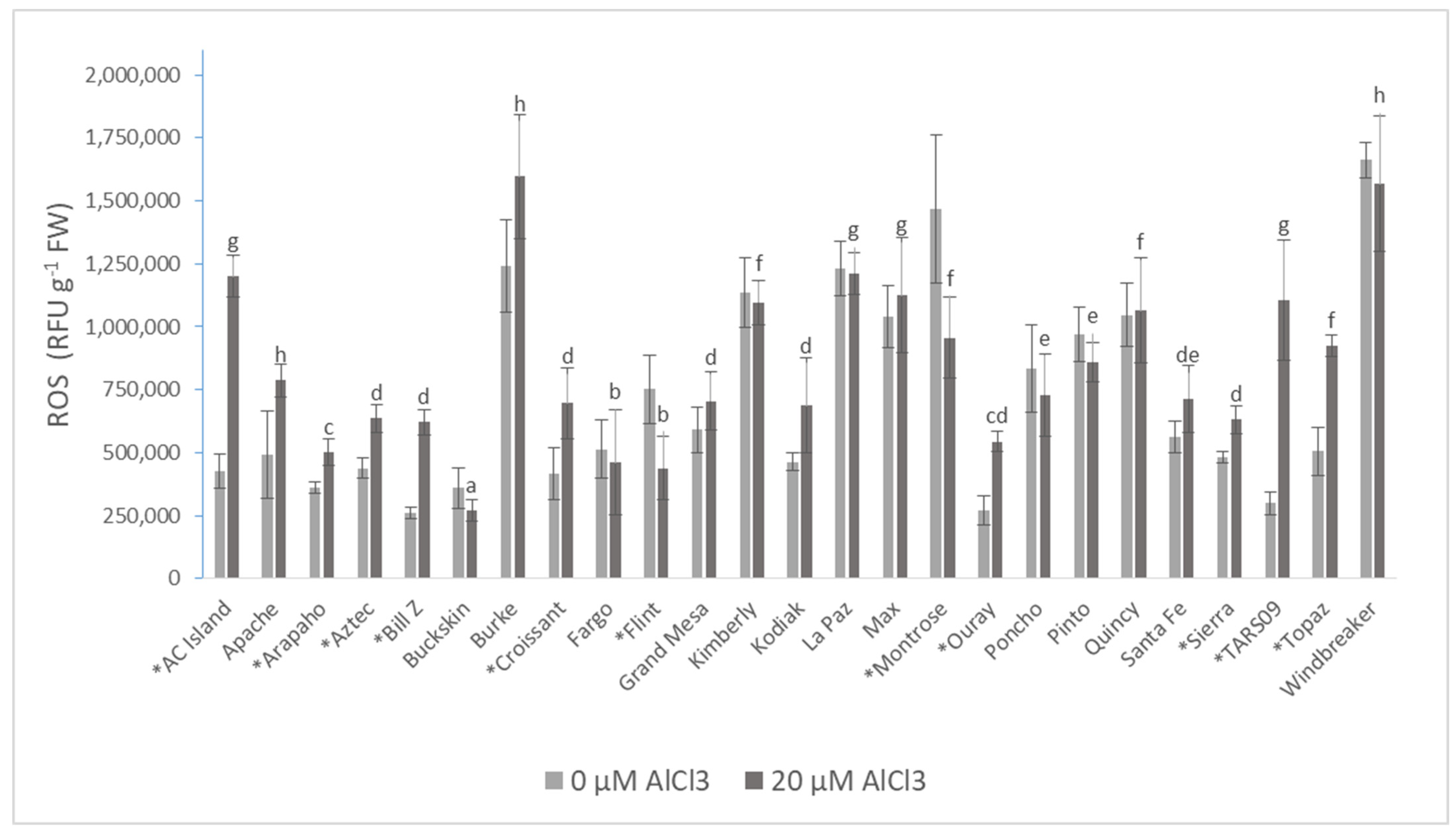
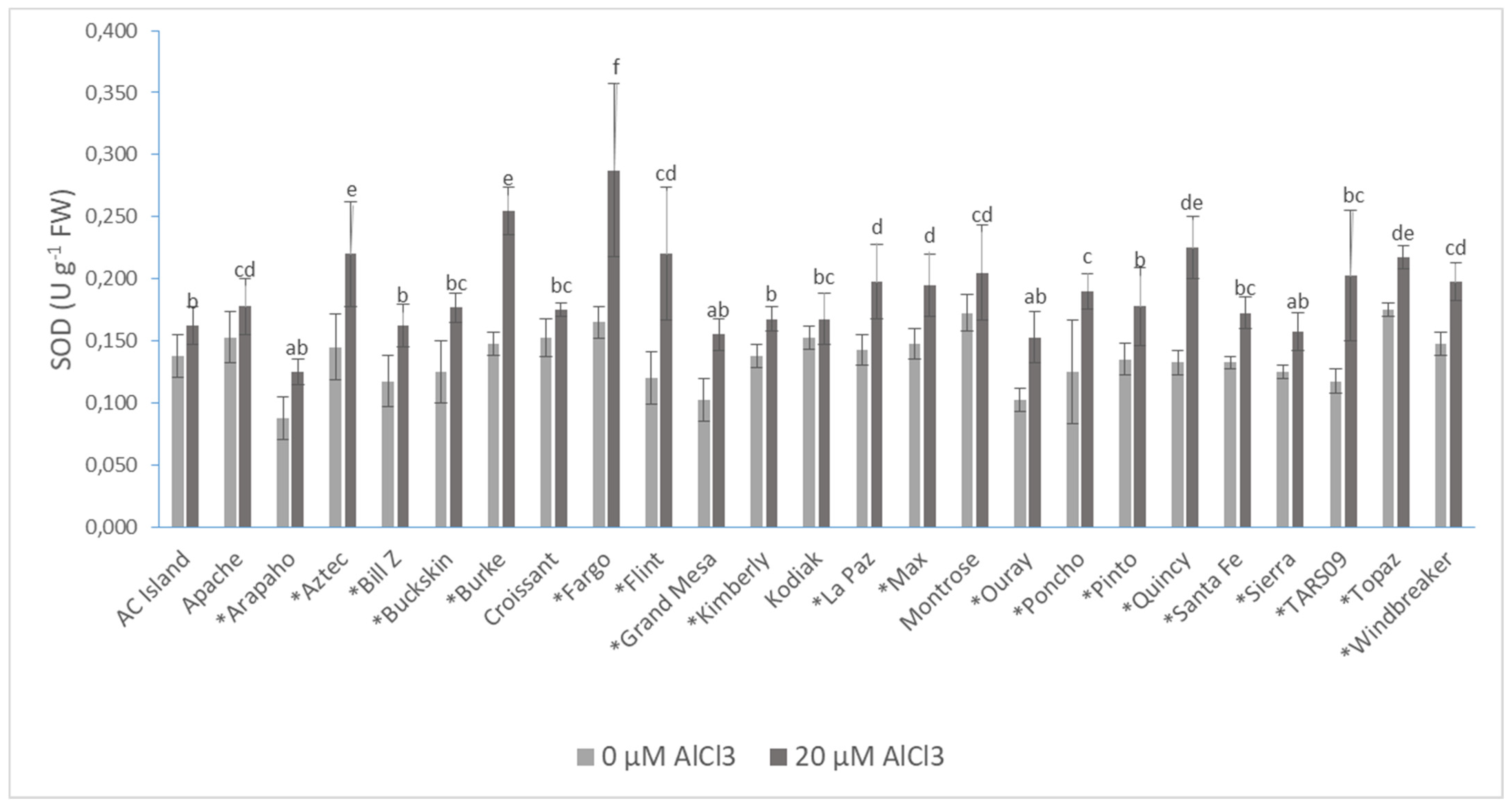
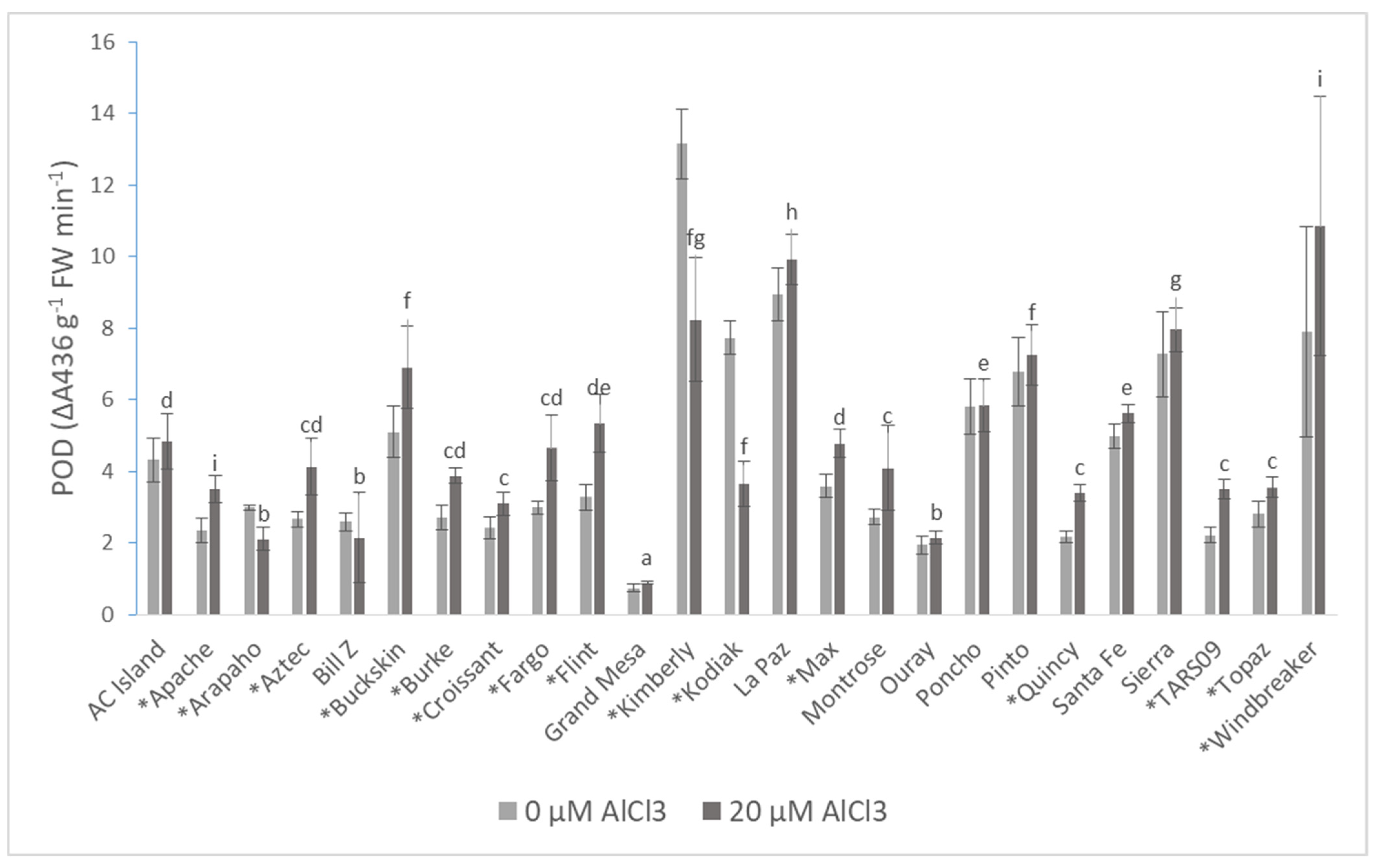
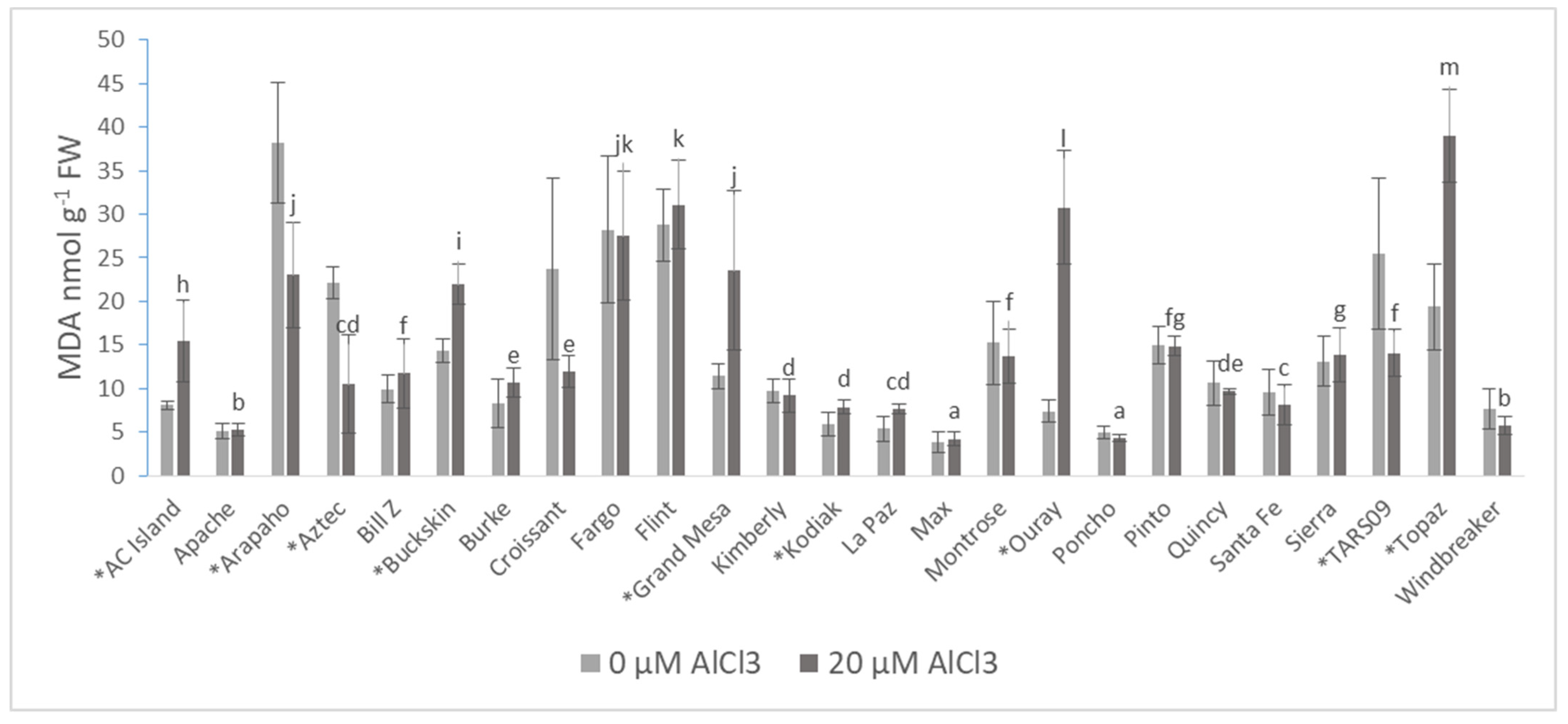
| Cultivars | Root DW (mg·plant−1) | Shoot DW (mg·plant−1) | Root/Shoot Ratio | |||
|---|---|---|---|---|---|---|
| 0 µM Al | 20 µM Al | 0 µM Al | 20 µM Al | 0 µM Al | 20 µM Al | |
| AC Island | 76 ± 18 | 69 ± 11 | 273 ± 52 | 282 ± 28 | 0.28 | 0.24 |
| Apache | 77 ± 2 | 41 ± 4 * | 269 ± 33 | 267 ± 52 | 0.28 | 0.15 * |
| Arapaho | 71 ± 27 | 52 ± 11 * | 227 ± 44 | 238 ± 48 | 0.26 | 0.22 |
| Aztec | 71 ± 7 | 31 ± 1 * | 261 ± 20 | 227 ± 38 * | 0.27 | 0.14 * |
| Bill Z | 85 ± 22 | 53 ± 5 * | 224 ± 46 | 240 ± 40 | 0.38 | 0.22 * |
| Buckskin | 80 ± 24 | 45 ± 6 * | 271 ± 70 | 254 ± 18 | 0.29 | 0.18 * |
| Burke | 64 ± 4 | 31 ± 5 * | 263 ± 21 | 272 ± 37 | 0.24 | 0.11 * |
| Croissant | 104 ± 15 | 46 ± 1 * | 268 ± 36 | 250 ± 24 | 0.39 | 0.18 * |
| Flint | 58 ± 13 | 33 ± 6 * | 208 ± 47 | 237 ± 44 | 0.28 | 0.14 * |
| Fargo | 90 ± 15 | 57 ± 7 * | 271 ± 59 | 303 ± 26 | 0.33 | 0.19 * |
| Grand Mesa | 77 ± 11 | 49 ± 3 * | 241 ± 24 | 246 ± 8 | 0.32 | 0.09 * |
| Kimberly | 60 ± 15 | 26 ± 6 * | 307 ± 45 | 288 ± 19 | 0.19 | 0.09 * |
| Kodiak | 93 ± 14 | 53 ± 14 * | 305 ± 34 | 264 ± 40 | 0.30 | 0.20 * |
| Max | 103 ± 12 | 59 ± 12 * | 271 ± 28 | 270 ± 5 | 0.38 | 0.22 * |
| Montrose | 59 ± 10 | 32 ± 6 * | 250 ± 19 | 28 ± 6 * | 0.24 | 0.11 * |
| La Paz | 75 ± 11 | 45 ± 6 * | 206 ± 19 | 229 ± 37 | 0.36 | 0.20 * |
| Ouray | 103 ± 19 | 78 ± 15 * | 237 ± 45 | 251 ± 25 | 0.43 | 0.31 * |
| Poncho | 78 ± 20 | 48 ± 7 * | 304 ± 48 | 326 ± 52 | 0.26 | 0.15 * |
| Pinto | 65 ± 10 | 35 ± 2 * | 247 ± 30 | 268 ± 26 | 0.26 | 0.13 * |
| Quincy | 67 ± 5 | 26 ± 4 * | 276 ± 25 | 271 ± 37 | 0.24 | 0.10 * |
| Santa Fe | 93 ± 11 | 53 ± 1 * | 308 ± 36 | 279 ± 17 | 0.30 | 0.19 * |
| Sierra | 89 ± 13 | 50 ± 6 * | 240 ± 21 | 236 ± 19 | 0.37 | 0.21 * |
| TARS-09 | 68 ± 7 | 30 ± 8 * | 189 ± 34 | 221 ± 38 | 0.36 | 0.14 * |
| Topaz | 78 ± 4 | 46 ± 1 * | 234 ± 26 | 247 ± 35 | 0.33 | 0.19 * |
| Windbreaker | 72 ± 7 | 46 ± 1 | 258 ± 29 | 264 ± 2 | 0.28 | 0.18 * |
| Cultivars | % Δ of Root Volume | ||
|---|---|---|---|
| 24 h after Al Treatment | 48 h after Al Treatment | 72 h after Al Treatment | |
| AC Island | −41 | −53 | −65 |
| Apache | −68 | −72 | −83 |
| Arapaho | +11 | −39 | −53 |
| Aztec | −49 | −62 | −77 |
| Bill Z | −47 | −53 | −66 |
| Buckskin | −70 | −58 | −69 |
| Burke | −82 | −84 | −89 |
| Croissant | −81 | −80 | −83 |
| Fargo | −54 | −67 | −69 |
| Flint | −55 | −70 | −69 |
| Grand Mesa | −35 | −53 | −59 |
| Kimberly | −73 | −90 | −92 |
| Kodiak | −46 | −60 | −71 |
| La Paz | −61 | −72 | −77 |
| Max | −69 | −74 | −76 |
| Montrose | −56 | −73 | −80 |
| Ouray | −46 | −59 | −63 |
| Poncho | −63 | −73 | −81 |
| Pinto | −65 | −82 | −87 |
| Quincy | −26 | −79 | −86 |
| Santa Fe | −31 | −60 | −76 |
| Sierra | −36 | −63 | −68 |
| TARS-09 | −69 | −85 | −85 |
| Topaz | −37 | −69 | −66 |
| Windbreaker | −55 | −68 | −73 |
| Cultivars | Al-Induced Root Length Inhibition as a Percentage (%) | ||||
|---|---|---|---|---|---|
| 0–4 h | 4–8 h | 8–24 h | 24–48 h | 48–72 h | |
| AC Island | 30 | 58 | 19 | 31 | 56 |
| Apache | 55 | 74 | 81 | 89 | 91 |
| Arapaho | 49 | 48 | −6 | 8 | 35 |
| Aztec | 62 | 46 | 82 | 95 | 97 |
| Bill Z | 30 | 36 | 7 | 22 | 51 |
| Buckskin | 72 | 79 | 84 | 85 | 77 |
| Burke | 15 | 76 | 78 | 87 | 96 |
| Croissant | 46 | 77 | 68 | 86 | 75 |
| Fargo | 40 | 80 | 83 | 74 | 81 |
| Flint | 65 | 69 | 86 | 81 | 82 |
| Grand Mesa | 54 | 46 | 26 | 31 | 39 |
| Kimberly | 67 | 85 | 90 | 93 | 96 |
| Kodiak | 39 | 78 | 68 | 78 | 71 |
| La Paz | 37 | 71 | 73 | 75 | 79 |
| Max | 57 | 68 | 77 | 84 | 82 |
| Montrose | 53 | 77 | 80 | 92 | 88 |
| Ouray | 24 | 55 | 21 | 19 | 48 |
| Poncho | 39 | 79 | 9 | 94 | 92 |
| Pinto | 42 | 57 | 85 | 85 | 76 |
| Quincy | 51 | 7 | 76 | 75 | 78 |
| Santa Fe | 56 | 83 | 76 | 79 | 74 |
| Sierra | 62 | 84 | 80 | 90 | 87 |
| TARS-09 | 56 | 77 | 80 | 42 | 89. |
| Topaz | 35 | 86 | 77 | 94 | 90 |
| Windbreaker | 49 | 85 | 82 | 78 | 86 |
| Treatment | ||
|---|---|---|
| 0 µM AlCl3 | 20 µM AlCl3 | |
| Root DW | 0.08 ± 0.02 | 0.05 ± 0.02 * |
| Shoot DW | 0.26 ± 0.05 | 0.26 ± 0.04 ns |
| Root:shoot | 0.31 ± 0.07 | 0.18 ± 0.06 * |
| Δ root volume cm3/24 h | 0.29 ± 0.15 | 0.14 ± 0.11 * |
| Δ root volume cm3/48 h | 0.40 ± 0.16 | 0.11 ± 0.09 * |
| Δ root volume cm3/72 h | 0.34 ± 0.17 | 0.06 ± 0.05 * |
| PRG 4 h after Al (mm/h) | 5.14 ± 2.14 | 2.58 ± 1.44 * |
| PRG 8 h after Al (mm/h) | 7.99 ± 3.25 | 2.68 ± 1.51 * |
| PRG 24 h after Al (mm/h) | 27.68 ± 3.96 | 9.27 ± 8.99 * |
| PRG 48 h after Al (mm/h) | 42.64 ± 6.97 | 11.53 ± 11.61 * |
| PRG 72 h after Al (mm/h) | 41.20 ± 6.78 | 9.56 ± 8.66 * |
| SOD | 0.14 ± 0.03 | 0.19 ± 0.04 * |
| POD | 4.41 ± 2.89 | 4.88 ± 2.61 * |
| ROS | 712,864.92 ± 407,069.50 | 849,014.63 ± 352,566.80 * |
| MDA | 13.97 ± 9.61 | 15.12 ± 10.05 ns |
| Char I | Characteristics II | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Root DW | Root Shoot | Root Volume 24 h | Root Volume 48 h | Root Volume 72 h | Root Length 4 h | Root Length 8 h | Root Length 24 h | Root Length 48 h | Root Length 72 h | ROS | SOD | POD | MDA | |
| Root DW | 1 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | +0.151 * |
| Root:Shoot | 1 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | |
| Root volume 24 h | 1 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ||
| Root volume 48 h | 1 | +0.233 * | ns | ns | ns | ns | ns | ns | ns | ns | ns | |||
| Root volume 72 h | 1 | ns | ns | ns | ns | ns | ns | ns | ns | +0.169 * | ||||
| Root length 4 h | 1 | +0.231 *** | +0.206 ** | ns | −0.345 *** | +0.165 * | ns | ns | ns | |||||
| Root length 8 h | 1 | +0.173 * | +0.135 * | ns | +0.121 * | ns | ns | ns | ||||||
| Root length 24 h | 1 | ns | ns | +0.179 * | ns | ns | ns | |||||||
| Root length 48 h | 1 | ns | ns | ns | ns | ns | ||||||||
| Root length 72 h | 1 | ns | ns | +0.126 * | ns | |||||||||
| ROS | 1 | +0.195 ** | +0.179 * | +0.202 ** | ||||||||||
| SOD | 1 | +0.256 *** | ns | |||||||||||
| POD | 1 | −0.430 ** | ||||||||||||
| MDA | 1 | |||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tóth, B.; Moloi, M.J.; Szőke, L.; Danter, M.; Grusak, M.A. Cultivar Differences in the Biochemical and Physiological Responses of Common Beans to Aluminum Stress. Plants 2021, 10, 2097. https://doi.org/10.3390/plants10102097
Tóth B, Moloi MJ, Szőke L, Danter M, Grusak MA. Cultivar Differences in the Biochemical and Physiological Responses of Common Beans to Aluminum Stress. Plants. 2021; 10(10):2097. https://doi.org/10.3390/plants10102097
Chicago/Turabian StyleTóth, Brigitta, Makoena Joyce Moloi, Lóránt Szőke, Mátyás Danter, and Michael A. Grusak. 2021. "Cultivar Differences in the Biochemical and Physiological Responses of Common Beans to Aluminum Stress" Plants 10, no. 10: 2097. https://doi.org/10.3390/plants10102097
APA StyleTóth, B., Moloi, M. J., Szőke, L., Danter, M., & Grusak, M. A. (2021). Cultivar Differences in the Biochemical and Physiological Responses of Common Beans to Aluminum Stress. Plants, 10(10), 2097. https://doi.org/10.3390/plants10102097








