Impact of Metal-Based Nanoparticles on Cambisol Microbial Functionality, Enzyme Activity, and Plant Growth
Abstract
1. Introduction
2. Results
2.1. Influence of CuNPs, NiNPs, and ZnNPs on Microbiological Indicators of Cambisols
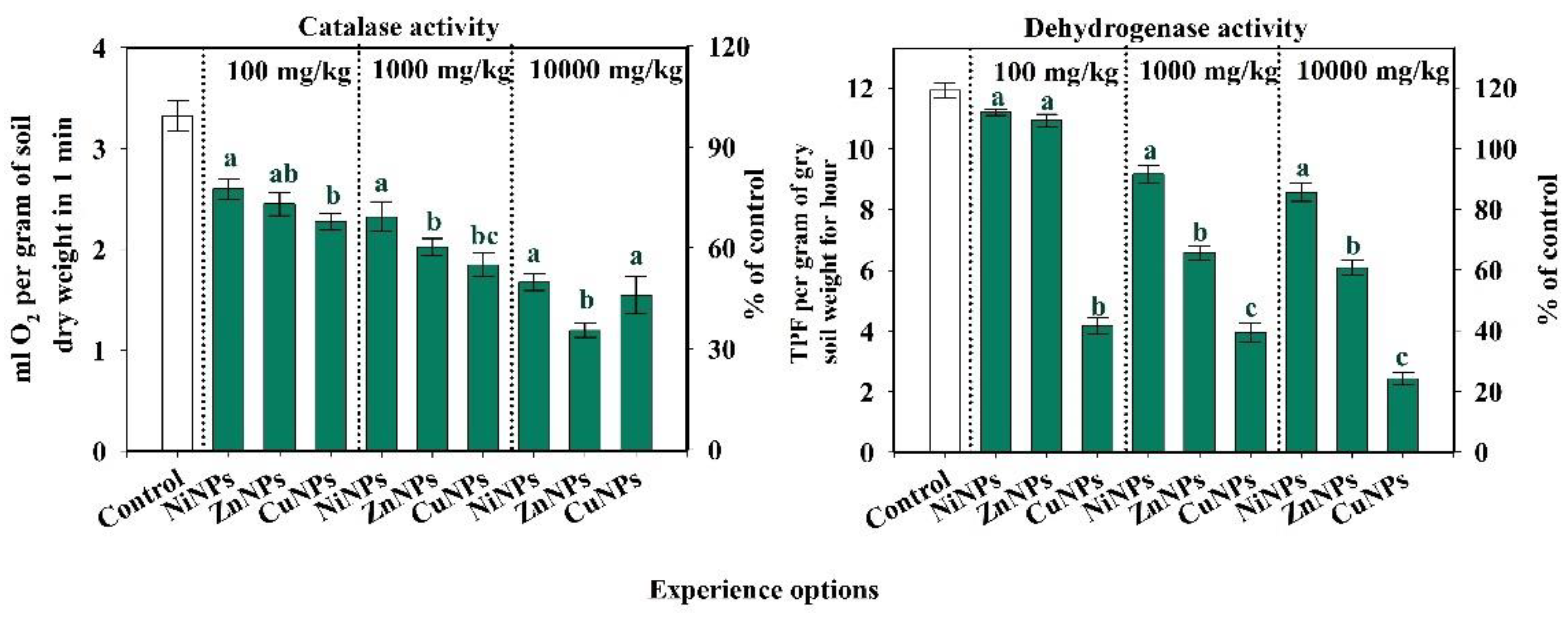
2.2. Influence of CuNPs, NiNPs, and ZnNPs on the Activity of Enzymes of Cambisols
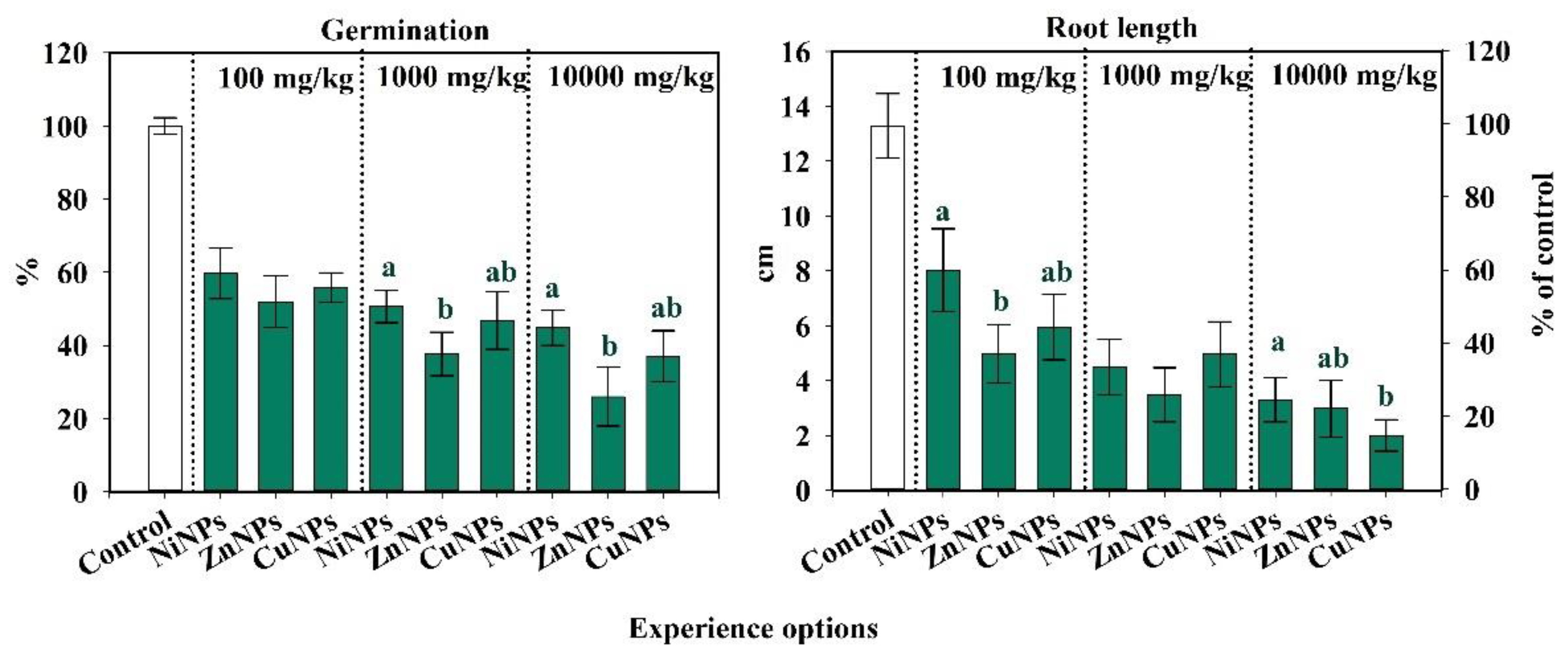
2.3. Influence of CuNPs, NiNPs, and ZnNPs on Radish Germination and Root Length of Cambisol
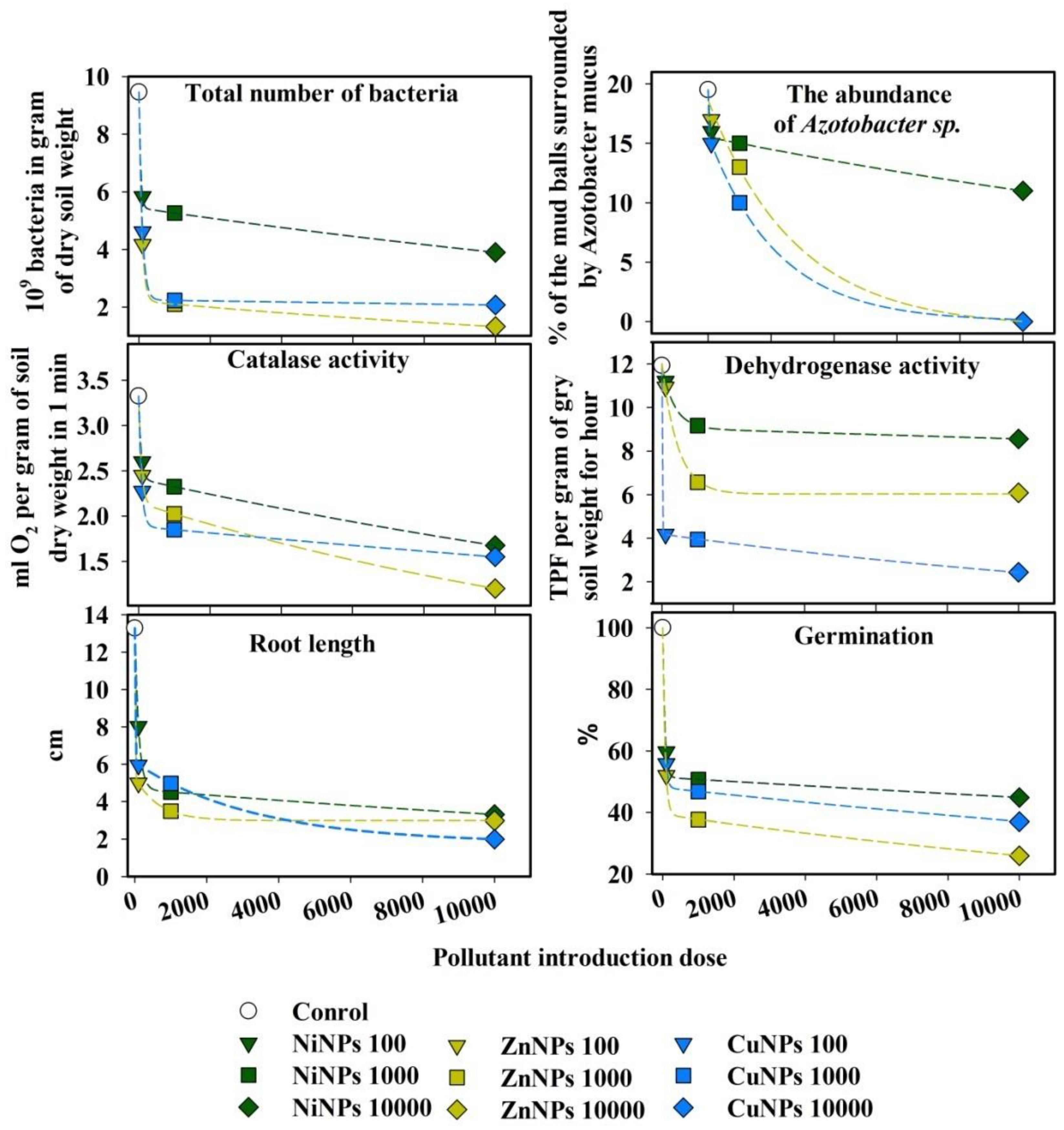
2.4. Assessment of the Relationship between Biological Parameters and the Dose of Nanoparticles
2.5. Integrated Index of the Biological State (IIBS) of Cambisols Contaminated by CuNPs, NiNPs, and ZnNPs
3. Discussion
4. Materials and Methods
4.1. Study Site
4.2. Experimental Details
4.3. Measurement Procedures for Biological Indicators
4.3.1. Measurement of Cambisols’ Organic Matter and pH
4.3.2. Measurement of the Total Number of Bacteria of Cambisols
4.3.3. Measurement of Azotobacter sp. Abundance
4.3.4. Measurement of the Activity of Catalase and Dehydrogenases of Cambisols
4.3.5. Measurement of Germination Rate and Length of Radish Roots
4.4. Data Analysis
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adams, M.B.; Kelly, C.; Kabrick, J.; Schuler, J. Chapter 6—Temperate forests and soils. Dev. Soil Sci. 2019, 36, 83–108. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, update 2015. International soil classification system for naming soils and creating legends for soil maps. 2015. [Google Scholar]
- Nafisi, S.; Maibach, H.I. Nanotechnology in cosmetics. Cosmet. Sci.Technol. Theor. Princ. Appl. 2017, 337–369. [Google Scholar] [CrossRef]
- The National Nanotechnology Initiative Supplement to the President’s 2021 Budget. 2020. Available online: https://www.nano.gov/nanodashboard (accessed on 12 August 2021).
- Global Nanotechnology Market Outlook 2020–2025 Segmented by Type, Applications & End-User Industries, Business Wire. 11 August 2020.
- Abbas, Q.; Yousaf, B.; Ali, A.M.U.; Munir, M.A.M.; El-Naggar, A.; Rinklebe, J.; Naushad, M. Transformation pathways and fate of engineered nanoparticles (ENPs) in distinct interactive environmental compartments: A review. Environ. Int. 2020, 138, 105646. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Khan, M.S.; Musarrat, J. Toxicity assessment of metal oxide nano-pollutants on tomato (Solanum lycopersicon): A study on growth dynamics and plant cell death. Environ. Pollut. 2018, 240, 802–816. [Google Scholar] [CrossRef] [PubMed]
- Lead, J.R.; Batley, G.E.; Alvarez, P.J.J.; Croteau, M.N.; Handy, R.D.; McLaughlin, M.J.; Judy, J.D.; Schirmer, K. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects—An updated review. Environ. Toxicol. Chem. 2018, 37, 2029–2063. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Minkina, T.; Fedorenko, A.; Mandzhieva, S.; Sushkova, S.; Lysenko, N.D.V.; Azarov, A.; Chokheli, V. Destructive effect of copper oxide nanoparticles on ultrastructure of chloroplast, plastoglobules and starch grains in spring barley (Hordeum sativum). Int. J. Agric. Biol. 2019, 21, 171–174. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, Z.; Zhang, Z.; Fu, H.; White, J.C.; Lynch, I. Nanomaterial transformation in the soil-plant system: Implications for food safety and application in agriculture. Small 2020, 16, 2000705. [Google Scholar] [CrossRef]
- Abd-Elsalam, K.A. (Ed.) Zinc-Based Nanostructures for Environmental and Agricultural Applications; Elsevier: Amsterdam, The Netherlands, 2021; Volume 676, ISBN 9780128236567. [Google Scholar]
- Sanzari, I.; Leone, A.; Ambrosone, A. Nanotechnology in plant science: To make a long story short. Front. Bioeng. Biotechnol. 2019, 7, 120. [Google Scholar] [CrossRef]
- Soltanian, S.; Sheikhbahaei, M.; Mohamadi, N.; Pabarja, A.; Abadi, M.F.S.; Tahroudi, M.H.M. Biosynthesis of Zinc Oxide Nanoparticles Using Hertia intermedia and Evaluation of its Cytotoxic and Antimicrobial Activities. BioNanoScience 2021, 11, 245–255. [Google Scholar] [CrossRef]
- Rajput, V.; Minkina, T.; Fedorenko, A.; Sushkova, S.; Mandzhieva, S.; Lysenko, V.; Duplii, N.; Fedorenko, G.; Dvadnenko, K.; Ghazaryan, K. Toxicity of copper oxide nanoparticles on spring barley (Hordeum sativum distichum). Sci. Total Environ. 2018, 645, 1103–1113. [Google Scholar] [CrossRef]
- Kolesnikov, S.I.; Kazeev, K.S.; Akimenko, Y.V. Development of regional standards for pollutants in the soil using biological parameters. Environ. Monit. Assess. 2019, 191, 544. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Peng, C.; Sun, L.; Zhang, S.; Huang, H.; Chen, Y.; Shi, J. Distinctive effects of TiO2 and CuO nanoparticles on soil microbes and their community structures in flooded paddy soil. Soil Biol. Biochem. 2015, 86, 24–33. [Google Scholar] [CrossRef]
- Ameen, K.I.; Alabdullatif, J.A.; AL-Nadhari, S. A review on metal-based nanoparticles and their toxicity to beneficial soil bacteria and fungi. Ecotoxicol. Environ. Saf. 2021, 213, 112027. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Shahid, S.; Ayaz, A.; Alkahtani, J.; Elshikh, M.S.; Riaz, T. Phytomolecules-Coated NiO Nanoparticles Synthesis Using Abutilon indicum Leaf Extract: Antioxidant, Antibacterial, and Anticancer Activities. Nanomedicine 2021, 16, 1757–1773. [Google Scholar] [CrossRef]
- Adams, J.; Wright, M.; Wagner, H.; Valiente, J.; Britt, D.; Anderson, A. Cu from dissolution of CuO nanoparticles signals changes in root morphology. Plant Physiol. Biochem. 2017, 110, 108–117. [Google Scholar] [CrossRef]
- AlQuraidi, A.O.; Mosa, K.A.; Ramamoorthy, K. Phytotoxic and Genotoxic Effects of Copper Nanoparticles in Coriander (Coriandrum sativum—Apiaceae). Plants 2019, 8, 19. [Google Scholar] [CrossRef]
- Tanha, E.Y.; Fallah, S.; Rostamnejadi, A.; Lok, P. Particle size and concentration dependent toxicity of copper oxide nanoparticles (CuONPs) on seed yield and antioxidant defense system in soil grown soybean (Glycine max cv. Kowsar). Sci. Total. Environ. 2020, 715, 136994. [Google Scholar] [CrossRef]
- Tsitsuashvili, V.S.; Minkina, T.M.; Nevidomskaya, D.G.; Rajput, V.D.; Mandzhieva, S.S.; Sushkova, S.N.; Bauer, T.V.; Burachevskaya, M.V. The impact of copper nanoparticles on plants and soil microorganisms (literature review). Bull. Agrar. Sci. Don 2017, 3, 93–100. (In Russian) [Google Scholar]
- Korotkova, A.M.; Lebedev, S.V.; Kayumov, F.G.; Sizova, E.A. The influence metal nanoparticles (Fe, Cu, Ni) and their oxides (Fe3O4, CuO, NiO). Sel’skokhozyaistvennaya Biol. 2017, 52, 172–182. [Google Scholar] [CrossRef][Green Version]
- Zotikova, A.P.; Astafurova, T.P.; Burenin, A.A.; Suchkova, S.A.; Morgalev, Y.N. Morphophysiological features of wheat seedlings (Triticum Aestivum L.) When exposed to nickel nanoparticles. Agric. Biol. 2018, 53, 578–586. [Google Scholar] [CrossRef]
- Ghosh, M.; Jana, A.; Sinha, S.; Jothiramajayam, M.; Nag, A.; Chakraborty, A.; Mukherjee, A. Effects of ZnO nanoparticles on plants: Cytotoxicity, genotoxicity, deregulation of antioxidant defenses, and cell-cycle arrest. Mutat. Res. Genet. Toxicol. Environ. Mutagen 2016, 807, 25–32. [Google Scholar] [CrossRef]
- Shen, M.; Liu, W.; Zeb, A.; Lian, J.; Hu, X.; Wu, J. Bioaccumulation and Phytotoxicity of ZnO Nanoparticles in Soil-Grown Brassica chinensis and Potential Risks. 2021. Available online: https://assets.researchsquare.com/files/rs-202101/v1/f3a5e4c2-da2d-4644-aebf-a659cd0475ad.pdf?c=1631876197 (accessed on 30 June 2021).
- Zoufan, P.; Baroonian, M.; Zargar, B. ZnO nanoparticles-induced oxidative stress in Chenopodium murale L, Zn uptake, and accumulation under hydroponic culture. Environ. Sci. Pollut. Res. 2020, 27, 11066–11078. [Google Scholar] [CrossRef]
- Josko, I.; Oleszczuk, P.; Dobrzyńska, J.; Futa, B.; Joniec, J.; Dobrowolski, R. Long-term effect of ZnO and CuO nanoparticles on soil microbial community in different types of soil. Geoderma 2019, 352, 204–212. [Google Scholar] [CrossRef]
- Kim, S.; Sin, H.; Lee, S.; Lee, I. Influence of Metal Oxide Particles on Soil Enzyme Activity and Bioaccumulation of Two Plants. J. Microbiol. Biotechnol. 2013, 23, 1279–1286. [Google Scholar] [CrossRef]
- Zhao, S.; Su, X.; Wang, Y.; Yang, X.; Bi, M.; He, Q.; Chen, Y. Copper oxide nanoparticles inhibited denitrifying enzymes and electron transport system activities to influence soil denitrification and N2O emission. Chemosphere 2020, 245, 125394. [Google Scholar] [CrossRef]
- Avila-Arias, H.; Nies, L.F.; Gray, M.B.; Turco, R.F. Impacts of molybdenum-, nickel-, and lithium-oxide nanomaterials on soil activity and microbial community structure. Sci. Total Environ. 2019, 652, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Galaktionova, L.; Gavrish, I.; Lebedev, S. Bioeffects of Zn and Cu Nanoparticles in Soil Systems. Toxicol. Environ. Health Sci. 2019, 11, 259–270. [Google Scholar] [CrossRef]
- Kumar, A.; Rakshit, R.; Bhowmik, A.; Mandal, N.; Das, A.; Adhikary, S. Tumor-Targeting NIRF NanoGUMBOS with Cyclodextrin-Enhanced Chemo. Photothermal Antitumor Act. ACS Appl. Mater. Interfaces 2019, 11, 27548–27557. [Google Scholar]
- You, T.; Liu, D.; Chen, J.; Yang, Z.; Dou, R.; Gao, X.; Wang, L. Effects of metal oxide nanoparticles on soil enzyme activities and bacterial communities in two different soil types. J. Soils Sediments 2018, 18, 211–221. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Noureen, S.; Anwar, S.; Ali, B.; Naveed, M.; Abd Allah, E.F.; Alqarawi, A.A.; Ahmad, P. Combined use of biochar and zinc oxide nanoparticle foliar spray improved the plant growth and decreased the cadmium accumulation in rice (Oryza sativa L.) plant. Environ. Sci. Pollut. Res. 2019, 26, 11288–11299. [Google Scholar] [CrossRef] [PubMed]
- Ananda, S.; Shobha, G.; Shashidhara, K.S.; Mahadimane, V. Nano-cuprous oxide enhances seed germination and seedling growth in Lycopersicum esculentum plants. J. Drug Deliv. Ther. 2019, 9, 296–302. [Google Scholar] [CrossRef][Green Version]
- Bashir, A.; Rizwan, M.; Ali, S.; Adrees, M.; Rehman, M.Z.; UrQayyum, M.F. Effect of composted organic amendments and zinc oxide nanoparticles on growth and cadmium accumulation by wheat; a life cycle study. Environ. Sci. Pollut. Res. 2020, 27, 23926–23936. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Singh, U.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Zinc oxide nanoparticles alleviate drought-induced alterations in sorghum performance, nutrient acquisition, and grain fortification. Sci. Total. Environ. 2019, 688, 926–934. [Google Scholar] [CrossRef]
- Luying, S.; Fengbin, S.; Xiangnan, L.; Xiancan, Z.; Shengqun, L.; Yang, W.; Xiaoning, Q. Effects of ZnO nanoparticles on seed germination and root carbon metabolism in maize (Zea mays L.). Soils Crop 2020, 9, 40–49. [Google Scholar]
- Ma, X.; Sharifan, H.; Dou, F.; Sun, W. Simultaneous reduction of arsenic (As) and cadmium (Cd) accumulation in rice by zinc oxide nanoparticles. Chem. Eng. J. 2020, 384, 123802. [Google Scholar] [CrossRef]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in sustainable agriculture: An emerging opportunity. J. Control. Release 2021, 329, 1234–1248. [Google Scholar] [CrossRef] [PubMed]
- Venzhik, Y.V.; Moshkov, I.E.; Dykman, L.A. Influence of nanoparticles of metals and their oxides on the photosynthetic apparatus of plants. Biol. Bulliten Russ. Acad. Sci. 2021, 48, 140–155. [Google Scholar] [CrossRef]
- Chang, H.; Jwo, C.; Lo, C.; Tsung, T.; Kao, M.; Lin, H.-M. Rheology of CuO nanoparticle suspension prepared by ASNSS. Rev. Adv. Master Sci. 2004, 10, 128–132. [Google Scholar]
- Osmond-McLeod, M.; McCall, M. Zinc oxide nanoparticles in modern sunscreens: An analysis of potential exposure and hazard. Nanotoxicology 2010, 4, 15–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ren, Y.; He, J.; Zhang, L.; Wang, X.; Cui, Z. Impact of copper oxide nanoparticles on the germination, seedling growth, and physiological responses in Brassica pekinensis L. Environ. Sci. Pollut. Res. 2020, 27, 31505–31515. [Google Scholar] [CrossRef]
- Kolesnikov, S.; Tsepina, N.; Minnikova, T.; Kazeev, K.; Mandzhieva, S.; Sushkova, S.; Minkina, T.; Mazarji, M.; Singh, R.K.; Rajput, V.D. Influence of Silver Nanoparticles on the Biological Indicators of Haplic Chernozem. Plants 2021, 10, 1022. [Google Scholar] [CrossRef]
- Gautama, A.; Raya, A.; Mukherjeea, S.; Dasa, S.; Palb, K.; Dasc, S.; Karmakar, P.; Ray, M.; Ray, S. Immunotoxicity of copper nanoparticle and copper sulfate in a common Indian earthworm. Ecotoxicol. Environ. Saf. 2018, 148, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.I.L.; Murphy, M.; Nielsen, M.T.; Kristiansen, S.M.; Amorim, M.J.B.; Scott-Fordsmand, J.J. Cu-nanoparticles ecotoxicity-explored and explained? Chemosphere 2015, 139, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Joskoa, I.; Oleszczukb, P.; Futa, B. The effect of inorganic nanoparticles (ZnO, Cr2O3, CuO and Ni) and their bulk counterparts on enzyme activities in different soils. Geoderma 2014, 232–234, 528–537. [Google Scholar] [CrossRef]
- Kolesnikov, S.I.; Timoshenko, A.N.; Kazeev, K.S.; Akimenko, Y.V.; Myasnikova, M.A. Ecotoxicity of copper, nickel, and zinc nanoparticles assessment on the basis of biological indicators of chernozems. Eurasian Soil Sci. 2019, 52, 982–987. [Google Scholar] [CrossRef]
- Varduni, V.M.; Kolesnikov, S.I.; Timoshenko, A.N.; Kazeev, K.S.; Akimenko, Y.V. Influence of AL2O3, TIO2, Fe2O3 and SIO2 nanoparticles on the biological state of ordinary chernozem. North Cauc. Region. Ser. Nat. Sci. 2019, 3, 95–100. (In Russian) [Google Scholar] [CrossRef]
- Kolesnikov, S.I.; Kazeev, K.S.; Valkov, V.F. The Effect of Heavy Metal Contamination on the Microbial System in Chernozem. Eurasian Soil Sci. 1999, 4, 459–465. [Google Scholar]
- Paz-Ferreiro, J.; Baez-Bernal, D.; Castro Insúa, J.; García, M.I. Pomar Effects of mussel shell addition on the chemical and biological properties of a Cambisol. Chemosphere 2011, 86, 1117–1121. [Google Scholar] [CrossRef]
- Zakarauskaitė, D.; Vaišvila, Z.; Motuzas, A.; Grigaliūnienė, K.; Buivydaitė, V.; Vaisvalavičius, R.; Butkus, V. The influence of long-term application of mineral fertilizers on the biological activity of Cambisols. Ekologija 2008, 54, 173–178. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2010; Volume 548. [Google Scholar]
- Wylie, E.M.; Colletti, L.M.; Walker, L.F.; Lujan, E.J.; Garduno, K.; Mathew, K.J. Comparison of the Davies and Gray titrimetric method with potassium dichromate and ceric titrants. J. Radioanalitical Nucl. Chem. 2018, 318, 227–233. [Google Scholar] [CrossRef]
- McFeters, G.A.; Yu, F.P.; Pyle, B.H.; Stewart, P.S. Physiological assessment of bacteria using fluorochromes. J. Microbiol. Methods 1995, 21, 1–13. [Google Scholar] [CrossRef]
- Val’kov, V.F.; Kolesnikov, S.I.; Kazeev, K.S.; Tashchiev, S.S. Influence of heavy metal pollution on microscopic fungi and Azotobacter of common chernozem. Russ. J. Ecol. 1997, 28, 345–346. [Google Scholar]
- Martinez, M.; Gutiérrez-Romero, V.; Jannsens, M.; Ortega-Blu, R. Biological soil quality indicators: A review. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010, 1, 319–328. [Google Scholar]
- Galstyan, A.S. Unification of methods for studying the activity of soil enzymes. Eurasian Soil Sci. 1978, 2, 107–114. [Google Scholar]
- Bab’eva, M.A.; Zenova, N.K. Soil Biology; Moscow State University Publishing House: Moscow, Russia, 1989; Volume 336. [Google Scholar]
- Pandey, S.N. Accumulation heavy metals (cadmium, cromium, copper, nickel and zinc) in Raphanus salivus L. and Spinacia olerac L. Plants Irrigated with Industrial Effluents. J. Environ. Biol. 2006, 27, 381–384. [Google Scholar] [PubMed]
- Plekhanova, I.O.; Zolotareva, O.A.; Tarasenko, I.D.; Yakovlev, A.S. Assessment of ecotoxicity of soils contaminated by heavy metals. Eurasian Soil Sci. 2019, 52, 1274–1288. [Google Scholar] [CrossRef]


| No | Biological Indicators | Measure Unit | Methods |
|---|---|---|---|
| 1 | total number of bacteria | 109 bacteria in gram of dry soil weight | luminescent microscopy with the solution of acridine orange, 40× |
| 2 | Azotobacter sp. abundance | % of the mud balls surrounded by Azotobacter mucus | the method of fouling lumps on the Ashby medium |
| 3 | catalase activity | ml O2 per gram of soil dry weight in 1 min. | by the rate of decomposition of hydrogen peroxide |
| 4 | dehydrogenases activity | mg of triphenylformazane per gram of dry soil weight for hour | according to the rate of conversion of triphenyltetrazolium chloride (TPC) to triphenylformazane (TPF) |
| 5 | the germination rate of radish seeds | % of germination seeds of control | germination of radish (Raphanus sativus L.) after 7 days of the experiment |
| 6 | the length of the radish roots | millimeters | of length of the roots in radish (Raphanus sativus L.) after 7 days of the experiment |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolesnikov, S.; Timoshenko, A.; Minnikova, T.; Tsepina, N.; Kazeev, K.; Akimenko, Y.; Zhadobin, A.; Shuvaeva, V.; Rajput, V.D.; Mandzhieva, S.; et al. Impact of Metal-Based Nanoparticles on Cambisol Microbial Functionality, Enzyme Activity, and Plant Growth. Plants 2021, 10, 2080. https://doi.org/10.3390/plants10102080
Kolesnikov S, Timoshenko A, Minnikova T, Tsepina N, Kazeev K, Akimenko Y, Zhadobin A, Shuvaeva V, Rajput VD, Mandzhieva S, et al. Impact of Metal-Based Nanoparticles on Cambisol Microbial Functionality, Enzyme Activity, and Plant Growth. Plants. 2021; 10(10):2080. https://doi.org/10.3390/plants10102080
Chicago/Turabian StyleKolesnikov, Sergey, Alena Timoshenko, Tatiana Minnikova, Natalia Tsepina, Kamil Kazeev, Yulia Akimenko, Alexander Zhadobin, Victoria Shuvaeva, Vishnu D. Rajput, Saglara Mandzhieva, and et al. 2021. "Impact of Metal-Based Nanoparticles on Cambisol Microbial Functionality, Enzyme Activity, and Plant Growth" Plants 10, no. 10: 2080. https://doi.org/10.3390/plants10102080
APA StyleKolesnikov, S., Timoshenko, A., Minnikova, T., Tsepina, N., Kazeev, K., Akimenko, Y., Zhadobin, A., Shuvaeva, V., Rajput, V. D., Mandzhieva, S., Sushkova, S., Minkina, T., Dudnikova, T., Mazarji, M., Alamri, S., Siddiqui, M. H., & Singh, R. K. (2021). Impact of Metal-Based Nanoparticles on Cambisol Microbial Functionality, Enzyme Activity, and Plant Growth. Plants, 10(10), 2080. https://doi.org/10.3390/plants10102080
















