Effect of ZnO Nanoparticles on Growth and Biochemical Responses of Wheat and Maize
Abstract
:1. Introduction
2. Materials and Methods
2.1. Expterimental Setup, Seed Germination, and Enzyme Assay Analyses
2.2. Zinc Quantification in Plant Tissues
2.3. Plant Biomass and Photosynthetic Pigments Measurement
- A663, A645, A510 and A480 = Absorbance at 663, 645,510 and 480 nm
- V = Final extract volume (mL)
- W = Weight of the sample (g).
2.4. Lipid Peroxidation and Assay of Antioxidant Enzymes
2.5. Characterization of Used Nanoparticles
2.6. Statistical Analysis
3. Results and Discussion
3.1. Seed Germination and Enzyme Activities
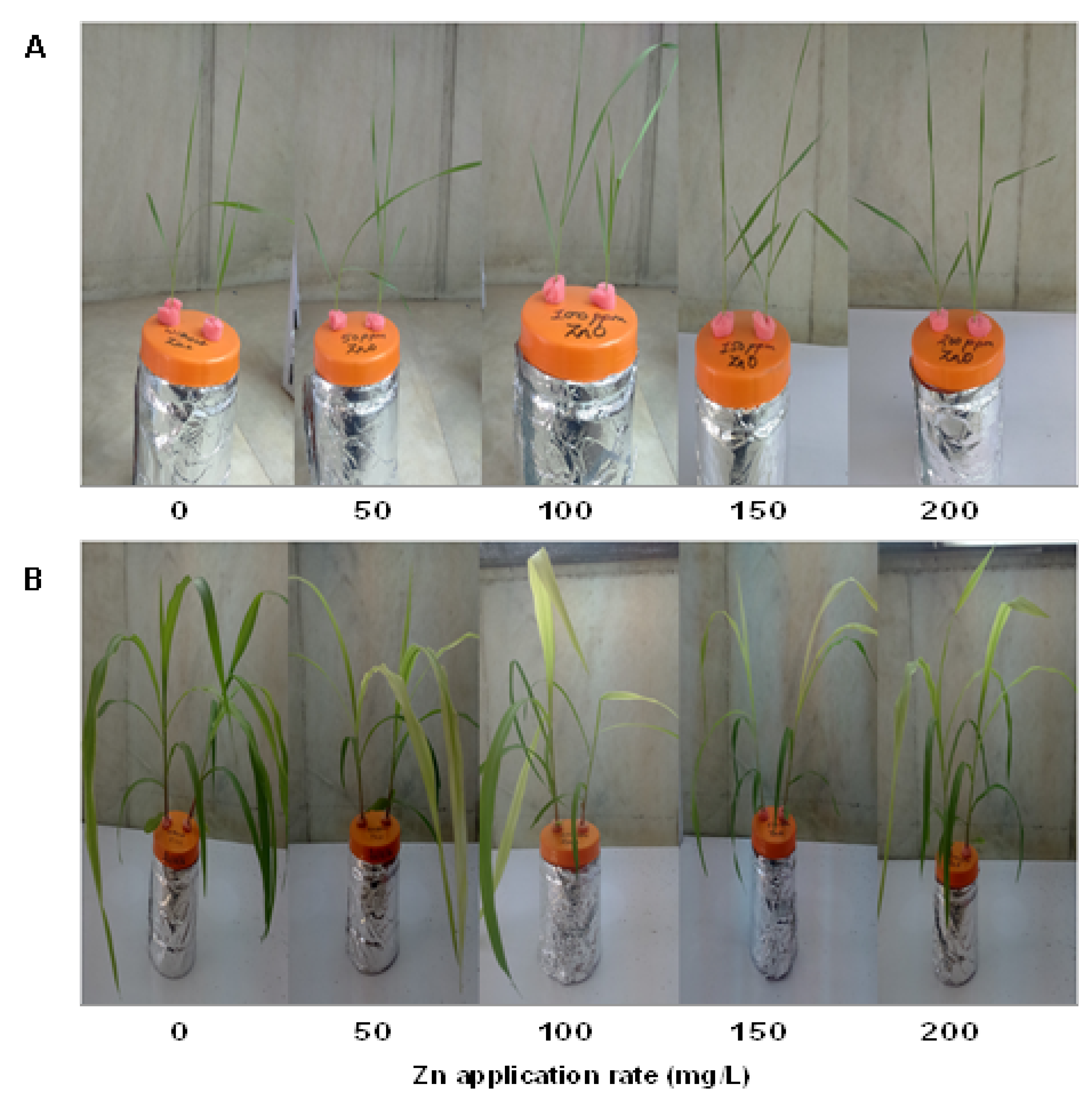
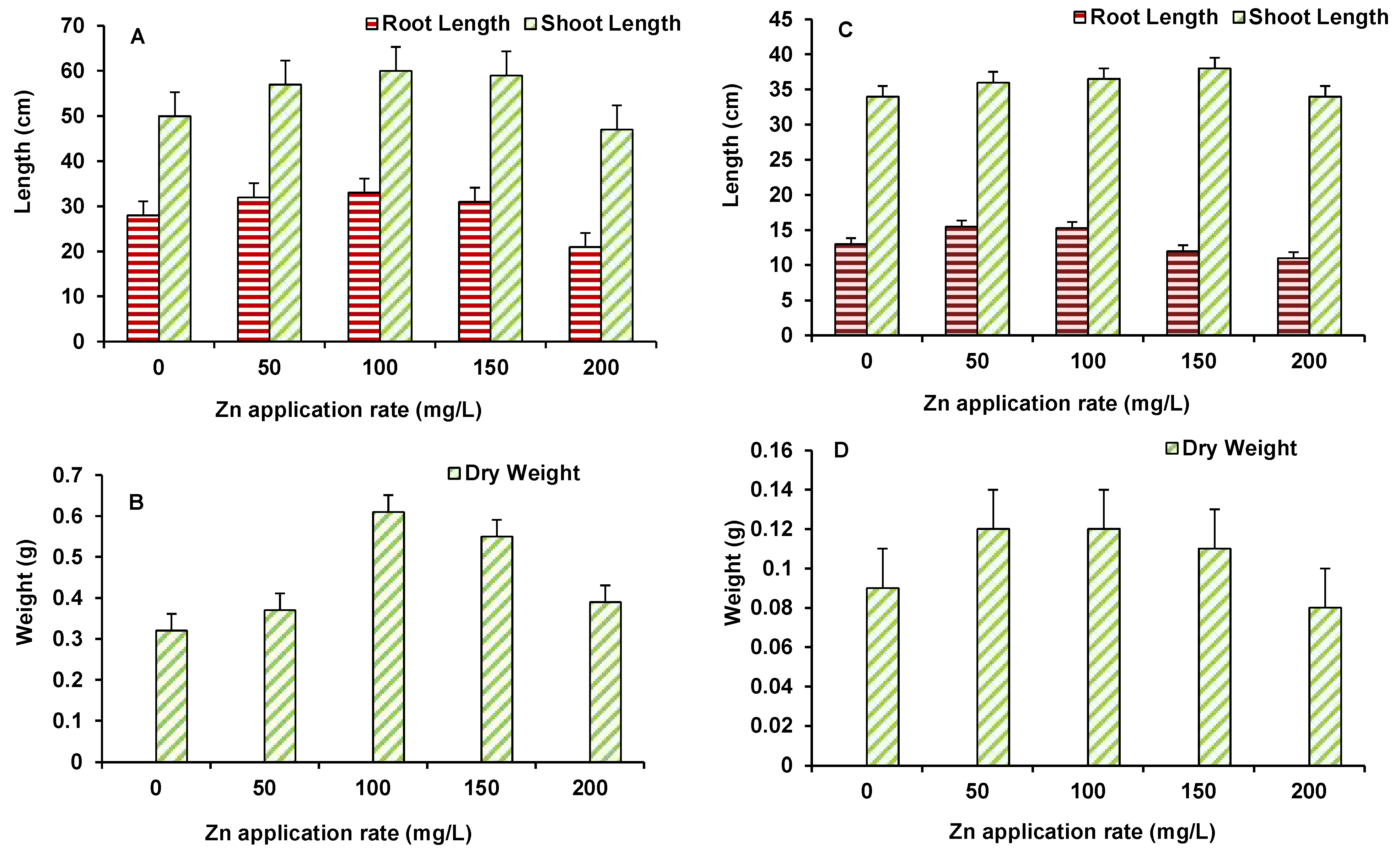
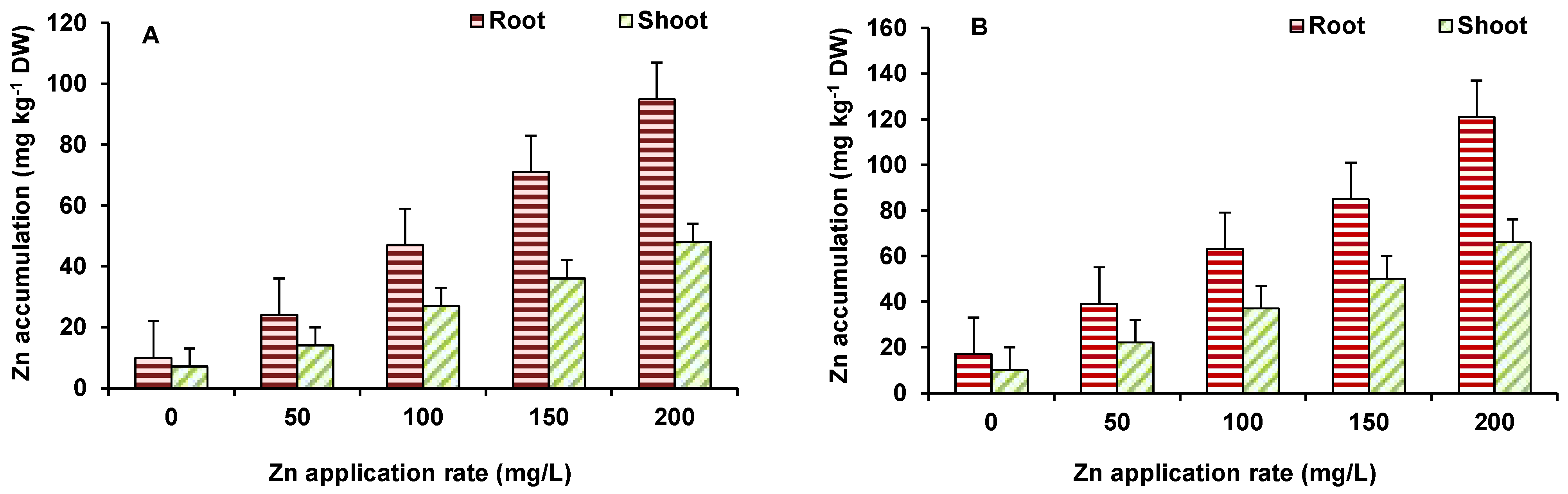
3.2. Accumulation of Zinc in Different Plant Parts and Effect on Growth of Plants
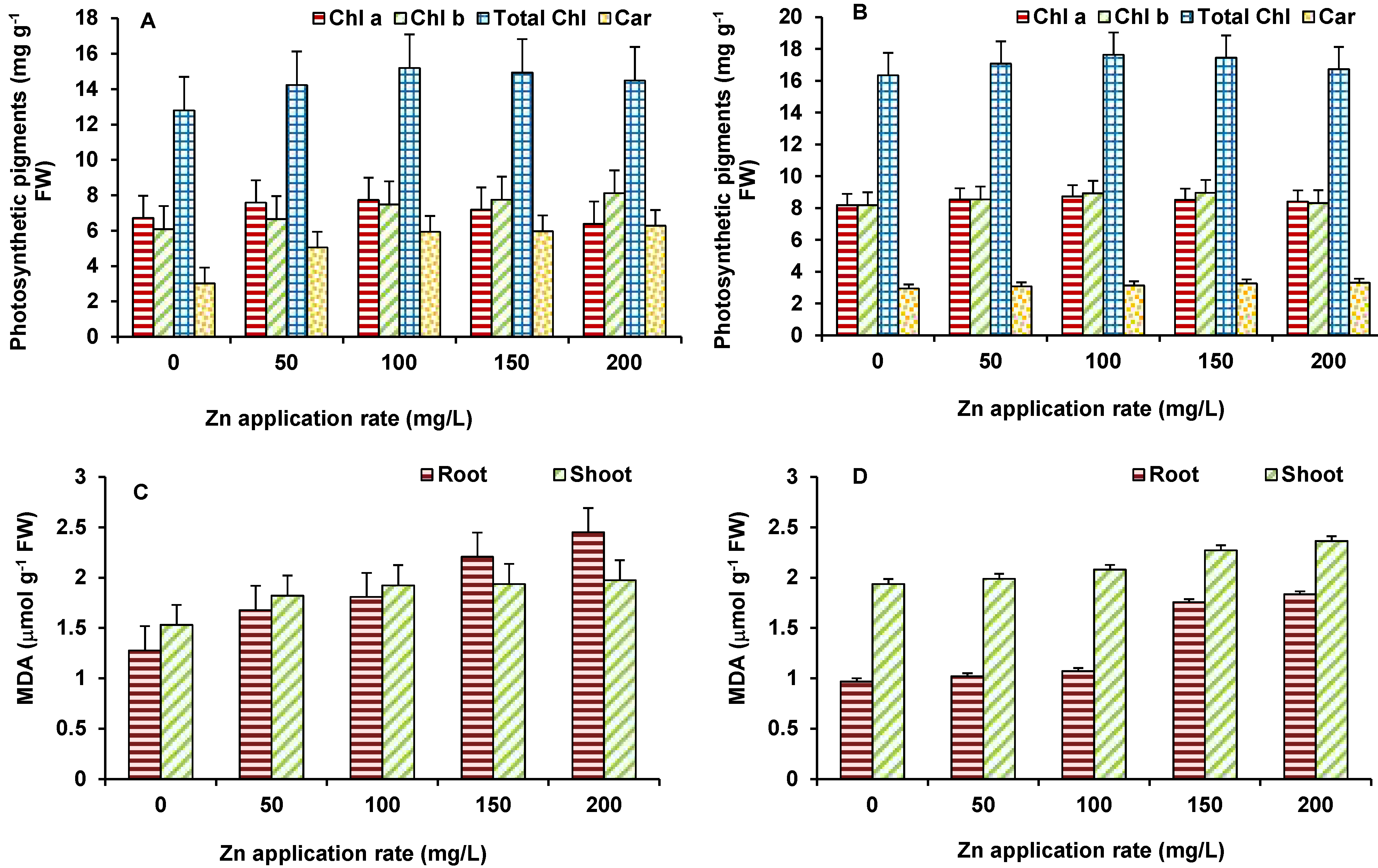
3.3. Effect of Photosynthetic Pigments
3.4. Effect on Lipid Peroxidation and Antioxidant Enzymes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nowack, B. The behaviour and effects of nanoparticles in the environment. Environ. Pollut. 2009, 157, 1063–1185. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, J.; Dou, R.; Gao, X.; Mao, C.; Wang, L. Assessment of the phytotoxicity of metal oxide nanoparticles on two crop plants, maize (Zea mays L.) and rice (Oryza sativa L.). Int. J. Environ. Res. Public Health 2015, 12, 14963. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, P.C.; Rawat, D.; Pachauri, S.P.; Shrivastava, M. Strategies for enhancing zinc efficiency in crop plants. In Nutrient Use Efficiency: From Basics to Advances; Rakshit, A., Singh, H.B., Sen, A., Eds.; Springer: New Delhi, India, 2015; pp. 87–101. [Google Scholar]
- Stietiya, M.H.; Wang, J.J. Zinc and cadmium adsorption to aluminum oxide nanoparticles affected by naturally occurring ligands. J. Environ. Qual. 2014, 43, 498–506. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Subin, M.P.; Steffy, F. Phytotoxic effects of cadmium on seed germination, early seedling growth and antioxidant enzyme activities in Cucurbita maxima Duchesne. Int. Res. J. Biol. Sci. 2013, 2, 40–47. [Google Scholar]
- Kumar, S.A.; Chen, S.M. Nanostructured zinc oxide particles in chemically modified electrodes for biosensor applications. Anal. Lett. 2008, 41, 141–158. [Google Scholar] [CrossRef]
- Gogos, A.; Knauer, K.; Bucheli, T.D. Nanomaterials in plant protection and fertilization: Current state, foreseen applications, and research priorities. J. Agric. Food Chem. 2012, 60, 9781–9792. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Kumari, A.; Harish; Singh, V.K.; Verma, K.K.; Mandzhieva, S.; Sushkova, S.; Srivastava, S.; Keswani, C. Coping with the challenges of abiotic stress in plants: New dimensions in the field application of nanoparticles. Plants 2021, 10, 1221. [Google Scholar] [CrossRef]
- Maynard, A.D.; Aitken, R.J.; Butz, T.; Colvin, V.; Donaldson, K.; Oberdörster, G.; Warheit, D.B. Safe handling of nanotechnology. Nature 2006, 444, 267–269. [Google Scholar] [CrossRef]
- Waalewijn-Kool, P.L.; Ortiz, M.D.; van Straalen, N.M.; van Gestel, C.A.M. Sorption, dissolution and pH determine the long-term equilibration and toxicity of coated and uncoated ZnO nanoparticles in soil. Environ. Pollut. 2013, 178, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Kořenková, L.; Šebesta, M.; Urík, M.; Kolenčík, M.; Kratošová, G.; Bujdoš, M.; Dobročka, E. Physiological response of culture media-grown barley (Hordeum vulgare L.) to titanium oxide nanoparticles. Acta Agric. Scand. Sect. B 2017, 67, 285–291. [Google Scholar] [CrossRef]
- Curtis, T.; Halford, N.G. Food security: The challenge of increasing wheat yield and the importance of not compromising food safety. Ann. Appl. Biol. 2014, 164, 354–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, T.R.; Prasad, K.; Kumar, P. Maize–A potential source of human nutrition and health: A review. Cogent Food Agric. 2016, 2, 1166995. [Google Scholar]
- Yaldagard, M.; Mortazavi, S.A.; Tabatabaie, F. Influence of ultrasonic stimulation on the germination of barley seed and its alpha-amylase activity. Afr. J. Biotechnol. 2008, 7, 2465–2471. [Google Scholar]
- Białecka, B.; Kępczyński, J. Germination, α-, β-amylase and total dehydrogenase activities of Amaranthus caudatus seeds under water stress in the presence of ethephon or gibberellin A3. Acta Biol. Cracov. Ser. Bot. 2010, 52, 7–12. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzyme in isolated chloroplast polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Duxbury, A.C.; Yentsch, C.S. Plantkton pigment monograph. J. Mar. Res. 1956, 15, 93–101. [Google Scholar]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Singh, D.P.; Singh, V.; Gupta, V.K.; Shukla, R.; Prabha, R.; Sarma, B.K.; Patel, J.S. Microbial inoculation in rice regulates antioxidative reactions and defense related genes to mitigate drought stress. Sci. Rep. 2020, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hoshmand, A.R. Experimental Research Design and Analysis: A Practical Approach for Agricultural and Natural Sciences; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Srivastava, S.; Akkarakaran, J.J.; Suprasanna, P.; D’Souza, S.F. Response of adenine and pyridine metabolism during germination and early seedling growth under arsenic stress in Brassica juncea. Acta Physiol. Plant. 2013, 35, 1081–1091. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef]
- Oaikhena, E.E.; Ajibade, G.A.; Appah, J.; Bello, M. Dehydrogenase enzyme activities in germinating cowpea (Vigna unguiculata (L) Walp). J. Biol. Agric. Healthc. 2013, 3, 32–36. [Google Scholar]
- Hafeez, B.M.; Khanif, Y.M.; Saleem, M. Role of zinc in plant nutrition-a review. Am. J. Exp. Agric. 2013, 3, 374–391. [Google Scholar] [CrossRef]
- Stampoulis, D.; Sinha, S.K.; White, J.C. Assay-dependent phytotoxicity of nanoparticles to plants. Environ. Sci. Technol. 2009, 43, 9473–9479. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Faraz, A.; Yusuf, M.; Khan, S.T.; Hayat, S. Zinc oxide nanoparticle-mediated changes in photosynthetic efficiency and antioxidant system of tomato plants. Photosynthetica 2018, 56, 678–686. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Zabaleta, E.; Martin, M.V.; Braun, H.P. A basal carbon concentrating mechanism in plants? Plant Sci. 2012, 187, 97–104. [Google Scholar] [CrossRef]
- Hu, H.; Boisson-Dernier, A.; Israelsson-Nordström, M.; Böhmer, M.; Xue, S.; Ries, A.; Schroeder, J.I. Carbonic anhydrases are upstream regulators of CO 2-controlled stomatal movements in guard cells. Nat. Cell Biol. 2010, 12, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedi, P.; Kaur, A. An overview on uses of zinc oxide nanoparticles. World J. Pharm. Pharm. Sci. 2015, 4, 1177–1196. [Google Scholar]
- Cakmak, I. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef]
- Faizan, M.; Hayat, S.; Pichtel, J. Effects of zinc oxide nanoparticles on crop plants: A perspective analysis. Sustain. Agric. Rev. 2020, 41, 83–99. [Google Scholar]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Ahmed, B.; Khan, M.S.; Musarrat, J. Toxicity assessment of metal oxide nano-pollutants on tomato (Solanum lycopersicon): A study on growth dynamics and plant cell death. Environ. Pollut. 2018, 240, 802–816. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X.; Shi, Z.; Tong, R.; Adams, C.A.; Shi, X. Arbuscular mycorrhizae alleviate negative effects of zinc oxide nanoparticle and zinc accumulation in maize plants–a soil microcosm experiment. Chemosphere 2016, 147, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, P.; Priyanka, N.; Manikandan, K.; Ganeshbabu, I.; Indiraarulselvi, P.; Geetha, N.; Sahi, S.V. Enhanced plant growth promoting role of phycomolecules coated zinc oxide nanoparticles with P supplementation in cotton (Gossypium hirsutum L.). Plant Physiol. Biochem. 2017, 110, 118–127. [Google Scholar] [CrossRef]
- Ozyigit, I.I.; Filiz, E.; Vatansever, R.; Kurtoglu, K.Y.; Koc, I.; Öztürk, M.X.; Anjum, N.A. Identification and comparative analysis of H2O2-scavenging enzymes (ascorbate peroxidase and glutathione peroxidase) in selected plants employing bioinformatics approaches. Front. Plant Sci. 2016, 7, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, A.; Sun, Y.; Morelius, E.; Tamez, C.; Bandyopadhyay, S.; Niu, G.; Gardea-Torresdey, J.L. Differential toxicity of bare and hybrid ZnO nanoparticles in green pea (Pisum sativum L.): A life cycle study. Front. Plant Sci. 2016, 6, 1242. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.P.; Li, Q.Q.; Pei, Z.M.; Wang, S.C. Effects of zinc oxide nanoparticles on the growth, photosynthetic traits, and antioxidative enzymes in tomato plants. Biol. Plant. 2018, 62, 801–808. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N.Á.; Afzal, S.; Singh, T.; Hussain, I. Zinc oxide nanoparticles: A review of their biological synthesis, antimicrobial activity, uptake, translocation and biotransformation in plants. J. Mater. Sci. 2018, 53, 185–201. [Google Scholar] [CrossRef]
- Yruela, I. A Handbook of Plant Nutrition, 2nd ed.; Estación Experimental de Aula Dei, Consejo Superior de Investigaciones Científicas (EEAD-CSIC): Zaragoza, Spain, 2015. [Google Scholar]
- Du, W.; Yang, J.; Peng, Q.; Liang, X.; Mao, H. Comparison study of zinc nanoparticles and zinc sulphate on wheat growth: From toxicity and zinc biofortification. Chemosphere 2019, 227, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Impa, S.M.; Morete, M.J.; Ismail, A.M.; Schulin, R.; Johnson-Beebout, S.E. Zn uptake, translocation and grain Zn loading in rice (Oryza sativa L.) genotypes selected for Zn deficiency tolerance and high grain Zn. J. Exp. Bot. 2013, 64, 2739–2751. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; McLean, J.E.; Britt, D.W.; Anderson, A.J. Nano-CuO and interaction with nano-ZnO or soil bacterium provide evidence for the interference of nanoparticles in metal nutrition of plants. Ecotoxicology 2015, 24, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Sehar, S.; Rajput, V.D.; Faraz, A.; Afzal, S.; Minkina, T.; Sushkova, S.; Adil, M.F.; Yu, F.; Alatar, A.A.; et al. Modulation of cellular redox status and antioxidant defense system after synergistic application of zinc oxide nanoparticles and salicylic acid in rice (Oryza sativa) plant under arsenic stress. Plants 2021, 10, 2254. [Google Scholar] [CrossRef]
- Azarin, K.; Usatov, A.; Minkina, T.; Plotnikov, A.; Kasyanova, A.; Fedorenko, A.; Duplii, N.; Vechkanov, E.; Rajput, V.D.; Mandzhieva, S. Effects of ZnO nanoparticles and its bulk form on growth, antioxidant defense system and expression of oxidative stress related genes in Hordeum vulgare L. Chemosphere 2022, 287, 132167. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Minkina, T.; Fedorenko, A.; Chernikova, N.; Hassan, T.; Mandzhieva, S.; Sushkova, S.; Lysenko, V.; Soldatov, M.A.; Burachevskaya, M. Effects of zinc oxide nanoparticles on physiological and anatomical indices in spring barley tissues. Nanomaterials 2021, 30, 1722. [Google Scholar] [CrossRef]
- Prasad, T.N.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Reddy, K.R.; Pradeep, T. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant Nutr. 2012, 35, 905–927. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Andrews, J.; Sanabria, J.; Bindraban, P.S.; Singh, U.; Elmer, W.H.; White, J.C. Interactive effects of drought, organic fertilizer, and zinc oxide nanoscale and bulk particles on wheat performance and grain nutrient accumulation. Sci. Total Environ. 2020, 722, 137808. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastav, A.; Ganjewala, D.; Singhal, R.K.; Rajput, V.D.; Minkina, T.; Voloshina, M.; Srivastava, S.; Shrivastava, M. Effect of ZnO Nanoparticles on Growth and Biochemical Responses of Wheat and Maize. Plants 2021, 10, 2556. https://doi.org/10.3390/plants10122556
Srivastav A, Ganjewala D, Singhal RK, Rajput VD, Minkina T, Voloshina M, Srivastava S, Shrivastava M. Effect of ZnO Nanoparticles on Growth and Biochemical Responses of Wheat and Maize. Plants. 2021; 10(12):2556. https://doi.org/10.3390/plants10122556
Chicago/Turabian StyleSrivastav, Akansha, Deepak Ganjewala, Rakesh Kumar Singhal, Vishnu D. Rajput, Tatiana Minkina, Marina Voloshina, Sudhakar Srivastava, and Manoj Shrivastava. 2021. "Effect of ZnO Nanoparticles on Growth and Biochemical Responses of Wheat and Maize" Plants 10, no. 12: 2556. https://doi.org/10.3390/plants10122556
APA StyleSrivastav, A., Ganjewala, D., Singhal, R. K., Rajput, V. D., Minkina, T., Voloshina, M., Srivastava, S., & Shrivastava, M. (2021). Effect of ZnO Nanoparticles on Growth and Biochemical Responses of Wheat and Maize. Plants, 10(12), 2556. https://doi.org/10.3390/plants10122556







