Association of Oxidative Stress Markers with Cardio-Kidney-Metabolic Parameters and Cardiovascular Disease in Patients with Type 2 Diabetes Mellitus
Abstract
1. Introduction
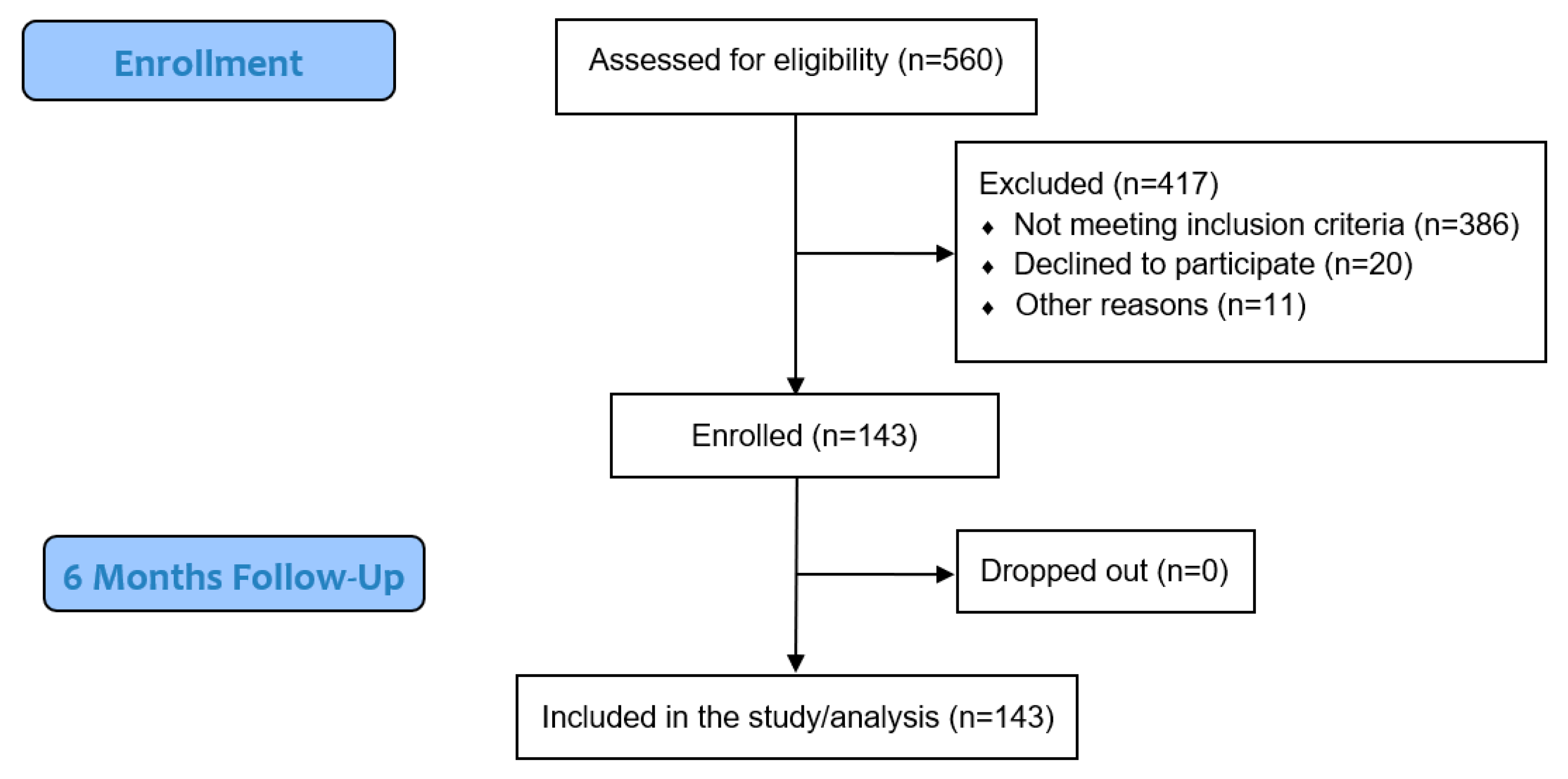
2. Materials and Methods
- Alcoholism;
- Acute inflammatory conditions;
- BMI ≥ 40 kg/m2;
- Chronic autoimmune, degenerative, or inflammatory conditions;
- CKD stages 4–5;
- Dementia;
- Gastrointestinal (malabsorption) disorders;
- Liver cirrhosis;
- Malignant disease;
- Medications affecting vitamin D metabolism;
- Pregnancy and breastfeeding;
- Previous use of vitamin D supplementation;
- Urolithiasis;
- Use of other oral antidiabetic drugs (except metformin), with or without insulin therapy.
2.1. Measurements and Laboratory Analyses
2.1.1. Anthropometric Measurements
2.1.2. Laboratory Analyses
Oxidative Stress Parameters
2.2. Statistics
3. Results
3.1. Correlations and Comparisons Between Groups
3.2. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Xie, J.; Wang, M.; Long, Z.; Ning, H.; Li, J.; Cao, Y.; Liao, Y.; Liu, G.; Wang, F.; Pan, A. Global burden of type 2 diabetes in adolescents and young adults, 1990–2019: Systematic analysis of the Global Burden of Disease Study 2019. BMJ 2022, 379, e072385. [Google Scholar] [CrossRef]
- Gregg, E.W.; Li, Y.; Wang, J.; Rios Burrows, N.; Ali, M.K.; Rolka, D.; Williams, D.E.; Geiss, L. Changes in diabetes-related complications in the United States, 1990–2010. N. Engl. J. Med. 2014, 370, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Gill, P.; Uthman, O.A.; Alam, K. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar]
- Duni, A.; Liakopoulos, V.; Roumeliotis, S.; Peschos, D.; Dounousi, E. Oxidative stress in the pathogenesis and evolution of chronic kidney disease: Untangling Ariadne’s thread. Int. J. Mol. Sci. 2019, 20, 3711. [Google Scholar] [CrossRef]
- Toualbi, L.A.; Mounir, A.; Wafa, B.; Medina, A.; Abderrezak, K.; Chahine, T.; Henni, C.; Abdelghani, B.; Atmane, S. Implications of advanced oxidation protein products and vitamin E in atherosclerosis progression. Arch. Med. Sci.-Atheroscler. Dis. 2021, 6, 135–144. [Google Scholar] [CrossRef]
- Vinereanu, I.-V.; Peride, I.; Niculae, A.; Tiron, A.T.; Caragheorgheopol, A.; Manda, D.; Checherita, I.A. The relationship between advanced oxidation protein products, vascular calcifications and arterial stiffness in predialysis chronic kidney disease patients. Medicina 2021, 57, 452. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Mallamaci, F.; Zoccali, C. Endothelial dysfunction in chronic kidney disease, from biology to clinical outcomes: A 2020 update. J. Clin. Med. 2020, 9, 2359. [Google Scholar] [CrossRef] [PubMed]
- Baylis, C. Nitric oxide deficiency in chronic kidney disease. Am. J. Physiol.-Ren. Physiol. 2008, 294, F1–F9. [Google Scholar] [CrossRef] [PubMed]
- Odobasic, D.; Kitching, A.R.; Holdsworth, S.R. Neutrophil-mediated regulation of innate and adaptive immunity: The role of myeloperoxidase. J. Immunol. Res. 2016, 2016, 2349817. [Google Scholar] [CrossRef]
- Brennan, M.-L.; Penn, M.S.; Van Lente, F.; Nambi, V.; Shishehbor, M.H.; Aviles, R.J.; Goormastic, M.; Pepoy, M.L.; McErlean, E.S.; Topol, E.J. Prognostic value of myeloperoxidase in patients with chest pain. N. Engl. J. Med. 2003, 349, 1595–1604. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Anton, M.I.; Floria, M.; Seritean Isac, P.N.; Hurjui, L.L.; Tarniceriu, C.C.; Costea, C.F.; Ciocoiu, M.; Rezus, C. Oxidative stress and NRF2/KEAP1/ARE pathway in diabetic kidney disease (DKD): New perspectives. Biomolecules 2022, 12, 1227. [Google Scholar] [CrossRef]
- Kurihara, C.; Kerpel-Fronius, S.; Becker, S.; Chan, A.; Nagaty, Y.; Naseem, S.; Schenk, J.; Matsuyama, K.; Baroutsou, V. Declaration of Helsinki: Ethical norm in pursuit of common global goals. Front. Med. 2024, 11, 1360653. [Google Scholar] [CrossRef] [PubMed]
- Grimes, D.A.; Hubacher, D.; Nanda, K.; Schulz, K.F.; Moher, D.; Altman, D.G. The Good Clinical Practice guideline: A bronze standard for clinical research. Lancet 2005, 366, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Cojic, M.; Kocic, R.; Klisic, A.; Cvejanov-Kezunovic, L.; Kavaric, N.; Kocic, G. A novel mechanism of vitamin D anti-inflammatory/antioxidative potential in type 2 diabetic patients on metformin therapy. Arch. Med. Sci. 2020, 16, 1004–1012. [Google Scholar] [CrossRef]
- Cojic, M.; Kocic, R.; Klisic, A.; Kocic, G. The effects of vitamin D supplementation on metabolic and oxidative stress markers in patients with type 2 diabetes: A 6-month follow up randomized controlled study. Front. Endocrinol. 2021, 12, 610893. [Google Scholar] [CrossRef]
- Cojic, M.M.; Klisic, A.; Kocic, R.; Veljkovic, A.; Kocic, G. Data-Driven Cluster Analysis of Oxidative Stress Indexes in relation to Vitamin D Level, Age, and Metabolic Control in Patients with Type 2 Diabetes on Metformin Therapy. Oxidative Med. Cell. Longev. 2021, 2021, 7942716. [Google Scholar] [CrossRef]
- Amer Diabet, A. Standards of medical care in diabetes-2011 American Diabetes Association. Diabetes Care 2011, 34, S11–S61. [Google Scholar]
- Levin, A.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R. Executive summary of the KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease: Known knowns and known unknowns. Kidney Int. 2024, 105, 684–701. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension: Developed by the task force on the management of elevated blood pressure and hypertension of the European Society of Cardiology (ESC) and endorsed by the European Society of Endocrinology (ESE) and the European Stroke Organisation (ESO). Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [PubMed]
- Deurenberg, P.; Weststrate, J.A.; Seidell, J.C. Body mass index as a measure of body fatness: Age-and sex-specific prediction formulas. Br. J. Nutr. 1991, 65, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Khalili, D.; Khayamzadeh, M.; Kohansal, K.; Ahanchi, N.S.; Hasheminia, M.; Hadaegh, F.; Tohidi, M.; Azizi, F.; Habibi-Moeini, A.S. Are HOMA-IR and HOMA-B good predictors for diabetes and pre-diabetes subtypes? BMC Endocr. Disord. 2023, 23, 39. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Dousset, J.-C.; Trouilh, M.; Foglietti, M.-J. Plasma malonaldehyde levels during myocardial infarction. Clin. Chim. Acta 1983, 129, 319–322. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.R.; Friedlander, M.; Khoa, T.N.; Capeillère-Blandin, C.; Nguyen, A.T.; Canteloup, S.; Dayer, J.-M.; Jungers, P.; Drüeke, T.; Descamps-Latscha, B.A. Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure1, 2. J. Immunol. 1998, 161, 2524–2532. [Google Scholar] [CrossRef]
- Pulli, B.; Ali, M.; Forghani, R.; Schob, S.; Hsieh, K.L.; Wojtkiewicz, G.; Linnoila, J.J.; Chen, J.W. Measuring myeloperoxidase activity in biological samples. PLoS ONE 2013, 8, e67976. [Google Scholar] [CrossRef] [PubMed]
- Kizaki, H.; Sakurada, T. Simple micro-assay methods for enzymes of purine metabolism. J. Lab. Clin. Med. 1977, 89, 1135–1144. [Google Scholar]
- Boban, M.; Kocic, G.; Radenkovic, S.; Pavlovic, R.; Cvetkovic, T.; Deljanin-Ilic, M.; Ilic, S.; Bobana, M.D.; Djindjic, B.; Stojanovic, D. Circulating purine compounds, uric acid, and xanthine oxidase/dehydrogenase relationship in essential hypertension and end stage renal disease. Ren. Fail. 2014, 36, 613–618. [Google Scholar] [CrossRef]
- Klisic, A.; Kocic, G.; Kavaric, N.; Pavlovic, R.; Soldatovic, I.; Ninic, A. Nitric oxide products are not associated with metabolic syndrome. J. Med. Biochem. 2019, 38, 361–367. [Google Scholar] [CrossRef]
- Góth, L. Serum catalase: Reversibly formed charge isoform of erythrocyte catalase. Clin. Chem. 1991, 37, 2043–2047. [Google Scholar] [CrossRef]
- Bono, R.; Blanca, M.J.; Arnau, J.; Gómez-Benito, J. Non-normal distributions commonly used in health, education, and social sciences: A systematic review. Front. Psychol. 2017, 8, 1602. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr. Cox proportional hazards regression model. In Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis; Springer: Cham, Switzerland, 2001; pp. 465–507. [Google Scholar]
- Matjuda, E.N.; Engwa, G.A.; Mungamba, M.M.; Sewani-Rusike, C.R.; Nkeh-Chungag, B.N. Oxidative stress is associated with markers of renal dysfunction in children aged 6-9 years old in a South African population. Pan Afr. Med. J. 2022, 42, 35. [Google Scholar]
- Neelofar, K.; Arif, Z.; Arafat, M.Y.; Alam, K.; Ahmad, J. A study on correlation between oxidative stress parameters and inflammatory markers in type 2 diabetic patients with kidney dysfunction in north Indian population. J. Cell. Biochem. 2019, 120, 4892–4902. [Google Scholar] [CrossRef]
- Schnetzer, L.; Leiherer, A.; Festa, A.; Mündlein, A.; Plattner, T.; Mayer, G.; Saely, C.; Drexel, H. Type 2 diabetes and chronic kidney disease as long-term predictors of cardiovascular events in patients with coronary artery disease. Eur. J. Clin. Investig. 2025, 55, e14374. [Google Scholar] [CrossRef]
- De Winter, J.C.; Gosling, S.D.; Potter, J. Comparing the Pearson and Spearman correlation coefficients across distributions and sample sizes: A tutorial using simulations and empirical data. Psychol. Methods 2016, 21, 273–290. [Google Scholar] [CrossRef]
- Kesavulu, M.M.; Rao, B.K.; Giri, R.; Vijaya, J.; Subramanyam, G.; Apparao, C. Lipid peroxidation and antioxidant enzyme status in Type 2 diabetics with coronary heart disease. Diabetes Res. Clin. Pract. 2001, 53, 33–39. [Google Scholar] [CrossRef]
- Ebuehi, O.A.T.; Ajuluchukwu, A.E.; Afolabi, O.T.; Ebuehi, O.M.; Akinwande, A.I. Catalase activity, lipid peroxidation, cholesterol and triglyceride levels in alloxan—Induced diabetes mellitus in female and male rats. Niger. Q. J. Hosp. Med. 2010, 19, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Hishida, A.; Okada, R.; Naito, M.; Morita, E.; Wakai, K.; Hamajima, N.; Hosono, S.; Nanri, H.; Turin, T.C.; Suzuki, S.; et al. Polymorphisms in genes encoding antioxidant enzymes (SOD2, CAT, GPx, TXNRD, SEPP1, SEP15 and SELS) and risk of chronic kidney disease in Japanese—Cross-sectional data from the J-MICC study. J. Clin. Biochem. Nutr. 2013, 53, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Sugiyama, H.; Wang, D.H.; Toda, N.; Maeshima, Y.; Yamasaki, Y.; Masuoka, N.; Yamada, M.; Kira, S.; Makino, H. Catalase deficiency renders remnant kidneys more susceptible to oxidant tissue injury and renal fibrosis in mice. Kidney Int. 2005, 68, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, F.N.; Romero, A.C.; Pedrosa, M.D.S.; Ibuki, F.K.; Bergamaschi, C.T. Oxidative stress and the antioxidant system in salivary glands of rats with experimental chronic kidney disease. Arch. Oral Biol. 2020, 113, 104709. [Google Scholar] [CrossRef] [PubMed]
- Rasool, M.; Ashraf, M.A.; Malik, A.; Waquar, S.; Khan, S.A.; Qazi, M.H.; Ahmad, W.; Asif, M.; Khan, S.U.; Zaheer, A.; et al. Comparative study of extrapolative factors linked with oxidative injury and anti-inflammatory status in chronic kidney disease patients experiencing cardiovascular distress. PLoS ONE 2017, 12, e0171561. [Google Scholar] [CrossRef] [PubMed]
- Kleinbongard, P.; Dejam, A.; Lauer, T.; Jax, T.; Kerber, S.; Gharini, P.; Balzer, J.; Zotz, R.B.; Scharf, R.E.; Willers, R.; et al. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic. Biol. Med. 2006, 40, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Tsinari, A.; Roumeliotis, S.; Neofytou, I.E.; Varouktsi, G.; Veljkovic, A.; Stamou, A.; Leivaditis, K.; Liakopoulos, V. The Clinical Utility and Plausibility of Oxidative and Antioxidant Variables in Chronic and End-Stage Kidney Disease: A Review of the Literature. Int. J. Mol. Sci. 2025, 26, 3376. [Google Scholar] [CrossRef]
- Prabhakar, S.; Starnes, J.; Shi, S.; Lonis, B.; Tran, R. Diabetic nephropathy is associated with oxidative stress and decreased renal nitric oxide production. J. Am. Soc. Nephrol. 2007, 18, 2945–2952. [Google Scholar] [CrossRef]
- Kahveci, A.S.; Barnatan, T.T.; Kahveci, A.; Adrian, A.E.; Arroyo, J.; Eirin, A.; Harris, P.C.; Lerman, A.; Lerman, L.O.; Torres, V.E. Oxidative stress and mitochondrial abnormalities contribute to decreased endothelial nitric oxide synthase expression and renal disease progression in early experimental polycystic kidney disease. Int. J. Mol. Sci. 2020, 21, 1994. [Google Scholar] [CrossRef]
- Izemrane, D.; Benziane, A.; Makrelouf, M.; Hamdis, N.; Rabia, S.H.; Boudjellaba, S.; Baz, A.; Benaziza, D. Living donors kidney transplantation and oxidative stress: Nitric oxide as a predictive marker of graft function. PLoS ONE 2024, 19, e0307824. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Mallamaci, F.; Zoccali, C. Diabetic chronic kidney disease in type 2 diabetes mellitus (albuminuric/non-albuminuric). In Blood Pressure Disorders in Diabetes Mellitus; Springer: Cham, Switzerland, 2023; pp. 243–269. [Google Scholar]
- Mizuno, T.; Matsui, H.; Imamura, A.; Numaguchi, Y.; Sakai, K.; Murohara, T.; Okumura, K. Insulin resistance increases circulating malondialdehyde-modified LDL and impairs endothelial function in healthy young men. Int. J. Cardiol. 2004, 97, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.L.; Al-Dawah, N.K.J.; Al-Barqaawi, M.A. Comparative analysis of antioxidant status in diabetic patients with and without insulin resistance. J. Med. Life 2023, 16, 1321–1326. [Google Scholar] [CrossRef]
- Toualbi, L.A.; Adnane, M.; Abderrezak, K.; Ballouti, W.; Arab, M.; Toualbi, C.; Chader, H.; Tahae, R.; Seba, A. Oxidative stress accelerates the carotid atherosclerosis process in patients with chronic kidney disease. Arch. Med. Sci. Atheroscler. Dis. 2020, 5, e245–e254. [Google Scholar] [CrossRef] [PubMed]
- Papadea, P.; Kalaitzopoulou, E.; Skipitari, M.; Varemmenou, A.; Papasotiriou, M.; Papachristou, E.; Goumenos, D.; Grune, T.; Georgiou, C.D. Novel oxidized LDL-based clinical markers in peritoneal dialysis patients for atherosclerosis risk assessment. Redox Biol. 2023, 64, 102762. [Google Scholar] [CrossRef]
- Hou, J.S.; Wang, C.H.; Lai, Y.H.; Kuo, C.H.; Lin, Y.L.; Hsu, B.G.; Tsai, J.P. Serum Malondialdehyde-Modified Low-Density Lipoprotein Is a Risk Factor for Central Arterial Stiffness in Maintenance Hemodialysis Patients. Nutrients 2020, 12, 2160. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.N.; Hsu, Y.C.; Lu, C.W.; Lin, S.C.; Wu, T.J.; Lin, G.M. Serum Malondialdehyde-Modified Low-Density Lipoprotein as a Risk Marker for Peripheral Arterial Stiffness in Maintenance Hemodialysis Patients. Medicina 2024, 60, 697. [Google Scholar] [CrossRef] [PubMed]
- Liakopoulos, V.; Roumeliotis, S.; Gorny, X.; Dounousi, E.; Mertens, P.R. Oxidative Stress in Hemodialysis Patients: A Review of the Literature. Oxidative Med. Cell. Longev. 2017, 2017, 3081856. [Google Scholar] [CrossRef]
- Xu, H.; Cabezas-Rodriguez, I.; Qureshi, A.R.; Heimburger, O.; Barany, P.; Snaedal, S.; Anderstam, B.; Helin, A.C.; Carrero, J.J.; Stenvinkel, P.; et al. Increased Levels of Modified Advanced Oxidation Protein Products Are Associated with Central and Peripheral Blood Pressure in Peritoneal Dialysis Patients. Perit. Dial. Int. 2015, 35, 460–470. [Google Scholar] [CrossRef]
- Conti, G.; Caccamo, D.; Siligato, R.; Gembillo, G.; Satta, E.; Pazzano, D.; Carucci, N.; Carella, A.; Campo, G.D.; Salvo, A.; et al. Association of Higher Advanced Oxidation Protein Products (AOPPs) Levels in Patients with Diabetic and Hypertensive Nephropathy. Medicina 2019, 55, 675. [Google Scholar] [CrossRef]
- Kutter, D.; Devaquet, P.; Vanderstocken, G.; Paulus, J.-M.; Marchal, V.; Gothot, A. Consequences of total and subtotal myeloperoxidase deficiency: Risk or benefit? Acta Haematol. 2000, 104, 10–15. [Google Scholar] [CrossRef]
- Kutter, D. Prevalence of myeloperoxidase deficiency: Population studies using Bayer-Technicon automated hematology. J. Mol. Med. 1998, 76, 669–675. [Google Scholar] [CrossRef]
- Uchimura, K.; Nagasaka, A.; Hayashi, R.; Makino, M.; Nagata, M.; Kakizawa, H.; Kobayashi, T.; Fujiwara, K.; Kato, T.; Iwase, K. Changes in superoxide dismutase activities and concentrations and myeloperoxidase activities in leukocytes from patients with diabetes mellitus. J. Diabetes Its Complicat. 1999, 13, 264–270. [Google Scholar] [CrossRef]
- Brennan, M.-L.; Gaur, A.; Pahuja, A.; Lusis, A.J.; Reynolds, W.F. Mice lacking myeloperoxidase are more susceptible to experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2001, 112, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Odobasic, D.; Kitching, A.R.; Yang, Y.; O’Sullivan, K.M.; Muljadi, R.C.; Edgtton, K.L.; Tan, D.S.; Summers, S.A.; Morand, E.F.; Holdsworth, S.R. Neutrophil myeloperoxidase regulates T-cell− driven tissue inflammation in mice by inhibiting dendritic cell function. Blood J. Am. Soc. Hematol. 2013, 121, 4195–4204. [Google Scholar] [CrossRef]
- Odobasic, D.; Kitching, A.R.; Semple, T.J.; Holdsworth, S.R. Endogenous myeloperoxidase promotes neutrophil-mediated renal injury, but attenuates T cell immunity inducing crescentic glomerulonephritis. J. Am. Soc. Nephrol. 2007, 18, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Odobasic, D.; Muljadi, R.C.; O’Sullivan, K.M.; Kettle, A.J.; Dickerhof, N.; Summers, S.A.; Kitching, A.R.; Holdsworth, S.R. Suppression of Autoimmunity and Renal Disease in Pristane-Induced Lupus by Myeloperoxidase. Arthritis Rheumatol. 2015, 67, 1868–1880. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, A.; Dunn, J.L.; Rateri, D.L.; Heinecke, J.W. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J. Clin. Investig. 1994, 94, 437–444. [Google Scholar] [CrossRef]
- Hazen, S.L.; Heinecke, J.W. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J. Clin. Investig. 1997, 99, 2075–2081. [Google Scholar] [CrossRef]
- Zhang, R.; Brennan, M.-L.; Fu, X.; Aviles, R.J.; Pearce, G.L.; Penn, M.S.; Topol, E.J.; Sprecher, D.L.; Hazen, S.L. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA 2001, 286, 2136–2142. [Google Scholar] [CrossRef]
- Tang, W.W.; Tong, W.; Troughton, R.W.; Martin, M.G.; Shrestha, K.; Borowski, A.; Jasper, S.; Hazen, S.L.; Klein, A.L. Prognostic value and echocardiographic determinants of plasma myeloperoxidase levels in chronic heart failure. J. Am. Coll. Cardiol. 2007, 49, 2364–2370. [Google Scholar] [CrossRef]
- Karakas, M.; Koenig, W.; Zierer, A.; Herder, C.; Rottbauer, W.; Baumert, J.; Meisinger, C.; Thorand, B. Myeloperoxidase is associated with incident coronary heart disease independently of traditional risk factors: Results from the MONICA/KORA Augsburg study. J. Intern. Med. 2012, 271, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Aratani, Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef]
- Brennan, M.-L.; Anderson, M.M.; Shih, D.M.; Qu, X.-D.; Wang, X.; Mehta, A.C.; Lim, L.L.; Shi, W.; Hazen, S.L.; Jacob, J.S. Increased atherosclerosis in myeloperoxidase-deficient mice. J. Clin. Investig. 2001, 107, 419–430. [Google Scholar] [CrossRef]
- Clark, R.A. Chemotactic factor inactivation by the myeloperoxidase-hydrogen peroxide-halide system: An inflammatory control mechanism. J. Clin. Investig. 1979, 64, 913–920. [Google Scholar] [CrossRef] [PubMed]
- El-Hag, A.; Clark, R. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. J. Immunol. 1987, 139, 2406–2413. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Peppin, G.; Ortiz, X.; Ragsdale, C.; Test, S. Oxidative autoactivation of latent collagenase by human neutrophils. Science 1985, 227, 747–749. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.; Mueller, D.M.; Fabjan, J.S.; Heinecke, J.W. Nitrogen dioxide radical generated by the myeloperoxidase-hydrogen peroxide-nitrite system promotes lipid peroxidation of low density lipoprotein. FEBS Lett. 1999, 455, 243–246. [Google Scholar] [CrossRef]
- Marcinkiewicz, J. Neutrophil chloramines: Missing links between innate and acquired immunity. Immunol. Today 1997, 18, 577–580. [Google Scholar] [CrossRef]
- Shern-Brewer, R.; Santanam, N.; Wetzstein, C.; White-Welkley, J.; Parthasarathy, S. Exercise and cardiovascular disease: A new perspective. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1181–1187. [Google Scholar] [CrossRef]
- Abu-Soud, H.M.; Hazen, S.L. Nitric oxide is a physiological substrate for mammalian peroxidases. J. Biol. Chem. 2000, 275, 37524–37532. [Google Scholar] [CrossRef]
- Eiserich, J.P.; Hristova, M.; Cross, C.E.; Jones, A.D.; Freeman, B.A.; Halliwell, B.; van der Vliet, A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature 1998, 391, 393–397. [Google Scholar] [CrossRef]
- Podrez, E.A.; Schmitt, D.; Hoff, H.F.; Hazen, S.L. Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. J. Clin. Investig. 1999, 103, 1547–1560. [Google Scholar] [CrossRef]
- Hazell, L.J.; Stocker, R. Oxidation of low-density lipoprotein with hypochlorite causes transformation of the lipoprotein into a high-uptake form for macrophages. Biochem. J. 1993, 290, 165–172. [Google Scholar] [CrossRef]
- EA, P. Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J. Clin. Investig. 2000, 105, 1095–1108. [Google Scholar] [PubMed]
- Parchwani, D.N.; Vachhani, U.; Dholariya, S.; Agravatt, A.; Singh, R.; Sonagra, A. Role of Inflammatory and Oxidative Stress Biomarkers with Albuminuria: A Cross Sectional Analysis in Type 2 Diabetes Mellitus Patients. EJIFCC 2025, 36, 284–297. [Google Scholar] [PubMed]
- Ninomiya, T.; Perkovic, V.; De Galan, B.E.; Zoungas, S.; Pillai, A.; Jardine, M.; Patel, A.; Cass, A.; Neal, B.; Poulter, N. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J. Am. Soc. Nephrol. 2009, 20, 1813–1821. [Google Scholar] [CrossRef] [PubMed]



| All Patients | |
|---|---|
| (n = 143) | |
| Age (years) | 61.5 (33, 82) |
| Gender, Male (%) | 47.6 (68) |
| Smoking (%) | 37 (53) |
| History of CV disease (%) | 8.4 (12) |
| CKD stages 1–3 (%) | 84.6 (121) |
| Hypertension (%) | 37.1 (53) |
| Duration of T2DM (years) | 7.1 (1, 14) |
| BMI (kg/m2) | 29.45 (17.3, 46.6) |
| SBP (mm Hg) | 132.5 (87, 198) |
| DBP (mm Hg) | 80 (53, 117) |
| Glucose (mmol/L) | 7.85 (5.3, 21) |
| Vitamin D (nmol/L) | 56.1 (19.1, 127.6) |
| Creatinine (μmol/L) | 82 (47, 157) |
| eGFR (mL/min/1.73 m2) | 82.2 (38, 116) |
| HOMA-IR | 3.8 (0, 25) |
| Albumin (g/L) | 48 (44, 55) |
| Total cholesterol (mmol/L) | 5.28 (3.0, 10.13) |
| LDL-cholesterol (mmol/L) | 3.26 (1.67, 9.92) |
| HDL-cholesterol (mmol/L) | 1.31 ± 0.3 |
| Triglycerides (mmol/L) | 1.69 (0.46, 26) |
| HbA1c (%) | 6.74 (4.9, 10) |
| CRP (mg/L) | 1.61 (0.3, 22) |
| Characteristic | Non-CKD | Stage 1 | Stage 2 | Stage 3 | p |
|---|---|---|---|---|---|
| N | 22 | 24 | 84 | 13 | |
| Age (years) | 55.50 [45, 72] | 61.5 [33, 74] | 63 [42, 78] | 67 [50, 82] | 0.001 |
| Gender, Male (%) | 16 (72.2) | 10 (41.7) | 38 (45.2) | 4 (30.8) | 0.050 |
| Smoking (%) | 13 (59.1) | 15 (62.5) | 48 (57.1) | 8 (61.5) | 0.978 |
| Hypertension (%) | 10 (45) | 7 (29.1) | 28 (33.3) | 8 (61.5) | 0.161 |
| Combined CV event (%) | 4 (18.2) | 5 (20.8) | 12 (14.3) | 3 (23.1) | 0.687 |
| Duration of T2DM (years) | 6 [1, 12] | 6 [1, 13.7] | 7.5 [1, 12.5] | 7 [4, 12] | 0.651 |
| BMI (kg/m2) | 29.07 [21.82, 35.83] | 28.25 [17.73, 37.58] | 29.68 [17.30, 46.60] | 30.66 [24.58, 34.52] | 0.569 |
| SBP (mm Hg) | 132.25 [109, 170.5] | 128.5 [87, 198.5] | 133.75 [90, 185] | 141.0 [13, 173.5] | 0.077 |
| DBP (mm Hg) | 83.5 [67.5, 106.5] | 79 [53.5, 110] | 79 [60, 117.5] | 82.5 [64.5, 100.5] | 0.256 |
| Glucose (mmol/L) | 7.55 [5.3, 15.5] | 8.25 [6.4, 21] | 7.75 [5.3, 13.1] | 8.00 [5.9, 9.1] | 0.295 |
| Vitamin D | 44.84 [19.1, 127.6] | 51.56 [19.48, 110.8] | 58.36 [22.02, 120.1] | 53.85 [22.81, 84.49] | 0.167 |
| HOMA-IR | 3.01 [0.73, 11.82] | 3.98 [0.89, 22.56] | 3.90 [0.0, 25.04] | 4.23 [1.32, 11.41] | 0.619 |
| Albumin (g/L) | 48.50 [45, 55] | 48.36 [46, 52] | 48.00 [44, 55] | 48.00 [44, 53] | 0.774 |
| Total cholesterol (mmol/L) | 6.24 [3.50, 10.13] | 5.42 [4.06, 7.73] | 5.20 [3.09, 9.67] | 4.90 [3.79, 6.39] | 0.015 |
| LDL-cholesterol (mmol/L) | 3.87 [1.67, 7.16] | 3.20 [1.98, 9.92] | 3.26 [1.36, 6.25] | 2.60 [1.99, 4.42] | 0.050 |
| HDL-cholesterol (mmol/L) | 1.29 [0.86, 2.32] | 1.39 [0.80, 2.04] | 1.33 [0.78, 2.17] | 1.21 [0.70, 1.71] | 0.777 |
| Triglycerides (mmol/L) | 1.65 [0.71, 26.02] | 1.95 [0.66, 9.26] | 1.67 [-0.46, 5.40] | 1.80 [1.12, 5.36] | 0.361 |
| HbA1c (%) | 6.45 [5.1, 9.4] | 6.98 [6.0, 10.1] | 6.47 [4.9, 8.8] | 6.50 [5.4, 7.8] | 0.040 |
| CRP (mg/L) | 1.67 [0.30, 22.07] | 1.00 [0.31, 8.68] | 1.73 [0.30, 15.46] | 1.40 [0.30, 10.54] | 0.342 |
| AOPP (µmol/L) | 117.90 [63.11, 578.23] | 117.17 [66.53, 646.72] | 122.54 [43.05, 724.26] | 115.45 [57.24, 465.72] | 0.573 |
| MDA (μΜ/L) | 3.47 [2.15, 8.87] | 3.32 [2.28, 9.31] | 3.10 [1.67, 7.18] | 3.31 [1.97, 11.28] | 0.229 |
| MPO (ng/mL) | 114.28 [53.44, 338.08] | 111.27 [54.90, 399.30] | 97.70 [22.24, 338.37] | 139.31 [49.10, 201.34] | 0.420 |
| NOx (μmol/L) | 55.13 [39.36, 94.02] | 50.69 [20.69, 186.91] | 53.02 [21.36, 238.70] | 67.13 [40.47, 203.58] | 0.031 |
| Catalase (U/mL) | 0.86 [0.72, 0.96] | 0.85 [0.73, 1.06] | 0.85 [0.61, 1.84] | 0.83 [0.76, 1.06] | 0.225 |
| Xanthine Oxidase (U/L) | 27.0 [9, 59] | 25.5 [15, 42] | 29 [4, 50] | 33 [13, 50] | 0.786 |
| r | p | |||||
|---|---|---|---|---|---|---|
| MPO | - | HbA1c | 0.167 | * | 0.046 | |
| MPO | - | Serum albumin | 0.211 | * | 0.011 | |
| MPO | - | CRP | −0.180 | * | 0.032 | |
| NOx | - | MDA | 0.168 | * | 0.045 | |
| NOx | - | AOPP | −0.333 | *** | <0.001 | |
| NOx | - | Creatinine | 0.173 | * | 0.038 | |
| NOx | - | Serum albumin | 0.189 | * | 0.024 | |
| MDA | - | NOx | 0.168 | * | 0.045 | |
| MDA | - | AOPP | −0.255 | ** | 0.002 | |
| MDA | - | HOMA-IR | 0.190 | * | 0.023 | |
| MDA | - | Cholesterol | 0.220 | ** | 0.008 | |
| MDA | - | Triglycerides | 0.320 | *** | <0.001 | |
| Catalase | - | XO | 0.165 | * | 0.049 | |
| Catalase | - | eGFR | 0.187 | * | 0.026 | |
| Catalase | - | Cholesterol | 0.196 | * | 0.019 | |
| Catalase | - | Triglycerides | 0.230 | ** | 0.006 | |
| Catalase | - | LDL-C | 0.188 | * | 0.024 | |
| AOPP | - | NOx | −0.333 | *** | <0.001 | |
| AOPP | - | MDA | −0.255 | ** | 0.002 | |
| AOPP | - | Glucose | 0.165 | * | 0.049 | |
| AOPP | - | Systolic BP | −0.194 | * | 0.020 | |
| AOPP | - | Serum albumin | −0.227 | ** | 0.006 | |
| XO | - | Catalase | 0.165 | * | 0.049 | |
| CRP | - | Albumin | −0.3 | *** | <0.0001 | |
| CRP | - | BMI | 0.3 | *** | <0.0001 | |
| CRP | - | HOMAIR | 0.22 | ** | 0.01 | |
| CRP | - | - | HDL cholesterol | −0.25 | ** | 0.002 |
| Vitamin D | - | - | Diabetes duration | 0.203 | * | 0.015 |
| Vitamin D | - | - | Triglycerides | −0.268 | ** | 0.001 |
| Vitamin D | - | Diastolic BP | −0.17 | * | 0.043 |
| No CV History (n = 131) | CV History (n = 12) | |||
|---|---|---|---|---|
| Variable | Median (Min, Max) | Median (Min, Max) | p-Value | Sig. |
| HbA1c (%) | 6.6 (4.9, 10.10) | 6.1 (5.4, 7.4) | 0.047 | * |
| Creatinine (μmol/L) | 80.00 (47.00, 157.00) | 92.70 (68.00, 127.00) | 0.02 | * |
| GFR (mL/min/1.73 m2) | 84.00 (38.00, 116.00) | 74.00 (42.00, 89.00) | 0.01 | * |
| GFR > 60 mL/min (n = 130) | GFR < 60 mL/min (n = 13) | |||
| Variable | Median (min, max) | Median (min, max) | p-value | Sig. |
| Age (years) | 62.00 (33.00, 78.00) | 67.00 (50.00, 82.00) | 0.014 | * |
| Catalase (U/mL) | 0.86 (0.64, 1.84) | 0.83 (0.61, 1.06) | 0.023 | * |
| NOx (μmol/L) | 52.91 (20.69, 238.70) | 67.13 (40.47, 206.91) | 0.001 | ** |
| Low MPO (n = 71) | High MPO (n = 72) | |||
| Variable | Median (min, max) | Median (min, max) | p-value | Sig. |
| CRP (mg/L) | 1.79 (0.30, 22.07) | 1.18 (0.30, 15.34) | 0.047 | * |
| Albumin (g/L) | 47.64 (44.00, 54.00) | 49.00 (44.00, 55.00) | 0.006 | ** |
| Catalase (U/mL) | 0.87 (0.64, 1.84) | 0.85 (0.61, 1.06) | 0.04 | * |
| Females (n = 68) | Males (n = 75) | |||
| Variable | Median (min, max) | Median (min, max) | p-value | Sig. |
| Vitamin D (ng/mL) | 47.70 (19.10, 127.60) | 63.44 (29.41, 120.10) | 0.0006 | *** |
| Creatinine (μmol/L) | 72.00 (47.00, 123.00) | 90.00 (57.00, 157.00) | 0.0000 | *** |
| Albumin (g/L) | 47.45 (44.00, 53.00) | 49.00 (44.00, 55.00) | 0.0007 | *** |
| B | Std Error | p | 95% CI for B | |
|---|---|---|---|---|
| Model 1 | ||||
| Age | −0.65 | 0.14 | <0.0001 | −0.94 to −0.37 |
| Model 2 | ||||
| Age | −0.60 | 0.13 | <0.0001 | −0.86 to −0.33 |
| Total Cholesterol | 2.67 | 0.90 | 0.005 | 0.89–4.46 |
| History of CVD | −9.4 | 4.3 | 0.03 | −17.9 to −0.95 |
| NOx | −0.097 | 0.04 | 0.006 | −0.17 to −0.03 |
| B | Std Error | p | 95% CI for B | |
|---|---|---|---|---|
| Model 1 | ||||
| Serum Albumin | 7.79 | 2.7 | 0.004 | 2.46–13.12 |
| Model 2 | ||||
| Serum Albumin | 7.50 | 2.66 | 0.005 | 2.25–12.75 |
| HbA1c | 14.6 | 0.19 | 0.019 | 2.4–26.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Roumeliotis, S.; Neofytou, I.E.; Roumeliotis, A.; Veljkovic, A.; Cojic, M.; Kocic, G. Association of Oxidative Stress Markers with Cardio-Kidney-Metabolic Parameters and Cardiovascular Disease in Patients with Type 2 Diabetes Mellitus. Biomolecules 2026, 16, 42. https://doi.org/10.3390/biom16010042
Roumeliotis S, Neofytou IE, Roumeliotis A, Veljkovic A, Cojic M, Kocic G. Association of Oxidative Stress Markers with Cardio-Kidney-Metabolic Parameters and Cardiovascular Disease in Patients with Type 2 Diabetes Mellitus. Biomolecules. 2026; 16(1):42. https://doi.org/10.3390/biom16010042
Chicago/Turabian StyleRoumeliotis, Stefanos, Ioannis E. Neofytou, Athanasios Roumeliotis, Andrej Veljkovic, Milena Cojic, and Gordana Kocic. 2026. "Association of Oxidative Stress Markers with Cardio-Kidney-Metabolic Parameters and Cardiovascular Disease in Patients with Type 2 Diabetes Mellitus" Biomolecules 16, no. 1: 42. https://doi.org/10.3390/biom16010042
APA StyleRoumeliotis, S., Neofytou, I. E., Roumeliotis, A., Veljkovic, A., Cojic, M., & Kocic, G. (2026). Association of Oxidative Stress Markers with Cardio-Kidney-Metabolic Parameters and Cardiovascular Disease in Patients with Type 2 Diabetes Mellitus. Biomolecules, 16(1), 42. https://doi.org/10.3390/biom16010042








