Creatine as a Candidate to Prevent Statin Myopathy
Abstract
:1. Introduction
2. Common Hypothesis on Pathogenesis
3. Statins Decrease Creatine Synthesis
4. Functions of Creatine in the Muscle
5. Decreasing Creatine Content Harms Muscular Function
- Decreased levels of phosphocreatine near cytoplasmic ATPase, which is therefore limiting the substrate (ATP) that is readily available for their function.
- Decreased differentiation of myoblasts into myocytes.
- Lack of sufficient creatine to take up the phosphate from ATP in the mitochondria. This may lead to reduced ATP turnover in the mitochondria, which in turn might be the cause of the mitochondrial dysfunction that was often hypothesized to be the cause of statin myopathy (Table 1).
6. Statins Reduce Synthesis of ATP in the Muscle
7. Creatine Administration Prevents Statin Myopathy
8. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kruth, H.S. Lipoprotein cholesterol and atherosclerosis. Curr. Mol. Med. 2001, 1, 633–653. [Google Scholar] [CrossRef] [PubMed]
- Stancu, C.; Sima, A. Statins: Mechanism of action and effects. J. Cell. Mol. Med. 2001, 5, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Davignon, J. Pleiotropic effects of pitavastatin. Br. J. Clin. Pharmacol. 2012, 73, 518–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amarenco, P.; Labreuche, J. Lipid management in the prevention of stroke: Review and updated meta-analysis of statins for stroke prevention. Lancet Neurol. 2009, 8, 453–463. [Google Scholar] [CrossRef]
- Mills, E.J.; Wu, P.; Chong, G.; Ghement, I.; Singh, S.; Akl, E.A.; Eyawo, O.; Guyatt, G.; Berwanger, O.; Briel, M. Efficacy and safety of statin treatment for cardiovascular disease: A network meta-analysis of 170,255 patients from 76 randomized trials. QJM 2011, 104, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Dana, T.; Blazina, I.; Daeges, M.; Jeanne, T.L. Statins for Prevention of Cardiovascular Disease in Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2016, 316, 2008–2024. [Google Scholar] [CrossRef]
- Krumholz, H.M. Treatment of Cholesterol in 2017. JAMA 2017, 318, 417–418. [Google Scholar] [CrossRef]
- Collins, R.; Reith, C.; Emberson, J.; Armitage, J.; Baigent, C.; Blackwell, L.; Blumenthal, R.; Danesh, J.; Smith, G.D.; DeMets, D.; et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016, 388, 2532–2561. [Google Scholar] [CrossRef] [Green Version]
- Valentino, M.; Al, D.; Panakos, A.; Ragupathi, L.; Duffy, D.; Whellan, D. Impact of the 2013 American College of Cardiology/American Heart Association cholesterol guidelines on the prescription of high-intensity statins in patients hospitalized for acute coronary syndrome or stroke. Am. Heart J. 2016, 181, 130–136. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Kent, S.T.; Brown, T.M.; Farkouh, M.E.; Levitan, E.B.; Yun, H.; Sharma, P.; Safford, M.M.; Kilgore, M.; Muntner, P.; et al. Underutilization of high-intensity statin therapy after hospitalization for coronary heart disease. J. Am. Coll. Cardiol. 2015, 65, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Miller, D. Fear of statins? CMAJ 2009, 181, 399. [Google Scholar] [CrossRef] [PubMed]
- Stroes, E.S.; Thompson, P.D.; Corsini, A.; Vladutiu, G.D.; Raal, F.J.; Ray, K.K.; Roden, M.; Stein, E.; Tokgözoğlu, L.; Nordestgaard, B.G.; et al. Statin-associated muscle symptoms: Impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur. Heart J. 2015, 36, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.S.; Lintott, C.J.; Wilson, M.J. Simvastatin and side effects. N. Z. Med. J. 1991, 104, 493–495. [Google Scholar] [PubMed]
- Bruckert, E.; Hayem, G.; Dejager, S.; Yau, C.; Bégaud, B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc. Drugs Ther. 2005, 19, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Serban, M.-C.; Colantonio, L.D.; Manthripragada, A.D.; Monda, K.L.; Bittner, V.A.; Banach, M.; Chen, L.; Huang, L.; Dent, R.; Kent, S.T.; et al. Statin Intolerance and Risk of Coronary Heart Events and All-Cause Mortality Following Myocardial Infarction. J. Am. Coll. Cardiol. 2017, 69, 1386–1395. [Google Scholar] [CrossRef]
- Christopher-Stine, L.; Basharat, P. Statin-associated immune-mediated myopathy: Biology and clinical implications. Curr. Opin. Lipidol. 2017, 28, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, P.; Oddis, C.V.; Aggarwal, R. Immune-mediated statin myopathy. Expert Rev. Clin. Immunol. 2016, 12, 33–38. [Google Scholar] [CrossRef]
- Mammen, A.L. Statin-Associated Autoimmune Myopathy. N. Engl. J. Med. 2016, 374, 664–669. [Google Scholar] [CrossRef]
- Mohassel, P.; Mammen, A.L. Anti-HMGCR Myopathy. J. Neuromuscul. Dis. 2018, 5, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Natalie, C.W.; Gerald, F.W.; Robert, H.E. Statin Toxicity. Circ. Res. 2019, 124, 328–350. [Google Scholar]
- Ramachandran, R.; Wierzbicki, A.S. Statins, Muscle Disease and Mitochondria. J. Clin. Med. 2017, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Naderi, S.; Cho, L. Statin intolerance: Diagnosis, treatment and alternative therapies. Clin. Lipidol. 2014, 9, 355–367. [Google Scholar] [CrossRef]
- Tomaszewski, M.; Stępień, K.M.; Tomaszewska, J.; Czuczwar, S.J. Statin-induced myopathies. Pharmacol. Rep. 2011, 63, 859–866. [Google Scholar] [CrossRef]
- Vrablik, M.; Zlatohlavek, L.; Stulc, T.; Adamkova, V.; Prusikova, M.; Schwarzova, L.; Hubacek, J.A.; Ceska, R. Statin-associated myopathy: From genetic predisposition to clinical management. Physiol. Res. 2014, 63, S327–S334. [Google Scholar] [PubMed]
- Apostolopoulou, M.; Corsini, A.; Roden, M. The role of mitochondria in statin-induced myopathy. Eur. J. Clin. Investig. 2015, 45, 745–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laufs, U.; Scharnagl, H.; März, W. Statin intolerance. Curr. Opin. Lipidol. 2015, 26, 492–501. [Google Scholar] [CrossRef]
- Muntean, D.M.; Thompson, P.D.; Catapano, A.L.; Stasiolek, M.; Fabis, J.; Muntner, P.; Serban, M.-C.; Banach, M. Statin-associated myopathy and the quest for biomarkers: Can we effectively predict statin-associated muscle symptoms? Drug Discov. Today 2017, 22, 85–96. [Google Scholar] [CrossRef]
- Du Souich, P.; Roederer, G.; Dufour, R. Myotoxicity of statins: Mechanism of action. Pharmacol. Ther. 2017, 175, 1–16. [Google Scholar] [CrossRef]
- Selva-O’Callaghan, A.; Alvarado-Cardenas, M.; Pinal-Fernández, I.; Trallero-Araguás, E.; Milisenda, J.C.; Martínez, M.Á.; Marín, A.; Labrador-Horrillo, M.; Juárez, C.; Grau-Junyent, J.M. Statin-induced myalgia and myositis: An update on pathogenesis and clinical recommendations. Expert Rev. Clin. Immunol. 2018, 14, 215–224. [Google Scholar] [CrossRef]
- Wyss, M.; Wallimann, T. Creatine metabolism and the consequences of creatine depletion in muscle. Mol. Cell Biochem. 1994, 133, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.P.; Whitehair, C.K.; Carrow, R.E.; Heusner, W.W.; Van Huss, W.D. Skeletal muscle function and structure after depletion of creatine. Lab. Investig. 1975, 33, 151–158. [Google Scholar] [PubMed]
- Matisone, D.; Skards, J.; Paeglitis, A.; Dzerve, V. Phosphocreatine as an Energy Store and Energy Shuttle in Human Skeletal Muscles. In The Physiology and Pathophysiology of Exercise Tolerance; Steinacker, J.M., Ward, S.A., Eds.; Springer US: Boston, MA, USA, 1996; pp. 75–80. ISBN 978-1-4615-5887-3. [Google Scholar]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef] [PubMed]
- Stromberger, C.; Bodamer, O.A.; Stöckler-Ipsiroglu, S. Clinical characteristics and diagnostic clues in inborn errors of creatine metabolism. J. Inherit. Metab. Dis. 2003, 26, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, M.; Adriano, E. Beyond sports: Efficacy and safety of creatine supplementation in pathological or paraphysiological conditions of brain and muscle. Med. Res. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, M.E.; Brosnan, J.T. The role of dietary creatine. Amino Acids 2016, 48, 1785–1791. [Google Scholar] [CrossRef]
- Shewmon, D.A.; Craig, J.M. Creatine supplementation prevents statin-induced muscle toxicity. Ann. Internal Med. 2010, 153, 690–692. [Google Scholar] [CrossRef]
- Phulukdaree, A.; Moodley, D.; Khan, S.; Chuturgoon, A.A. Atorvastatin increases miR-124a expression: A mechanism of Gamt modulation in liver cells. J. Cell. Biochem. 2015, 116, 2620–2627. [Google Scholar] [CrossRef]
- Mangravite, L.M.; Engelhardt, B.E.; Medina, M.W.; Smith, J.D.; Brown, C.D.; Chasman, D.I.; Mecham, B.H.; Howie, B.; Shim, H.; Naidoo, D.; et al. A statin-dependent QTL for GATM expression is associated with statin-induced myopathy. Nature 2013, 502, 377–380. [Google Scholar] [CrossRef] [Green Version]
- Norata, G.D.; Tibolla, G.; Catapano, A.L. Statins and skeletal muscles toxicity: From clinical trials to everyday practice. Pharmacol. Res. 2014, 88, 107–113. [Google Scholar] [CrossRef]
- Carr, D.F.; Alfirevic, A.; Johnson, R.; Chinoy, H.; van Staa, T.; Pirmohamed, M. GATM gene variants and statin myopathy risk. Nature 2014, 513, E1. [Google Scholar] [CrossRef] [PubMed]
- Floyd, J.S.; Bis, J.C.; Brody, J.A.; Heckbert, S.R.; Rice, K.; Psaty, B.M. GATM locus does not replicate in rhabdomyolysis study. Nature 2014, 513, E1–E3. [Google Scholar] [CrossRef] [PubMed]
- Mangravite, L.M.; Engelhardt, B.E.; Stephens, M.; Krauss, R.M. Mangravite et al. reply. Nature 2014, 513, E3. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.H. Biochemistry of Na, K-ATPase. Annu. Rev. Biochem. 2002, 71, 511–535. [Google Scholar] [CrossRef] [PubMed]
- Rayment, I. The Structural Basis of the Myosin ATPase Activity. J. Biol. Chem. 1996, 271, 15850–15853. [Google Scholar] [CrossRef] [Green Version]
- Primeau, J.O.; Armanious, G.P.; Fisher, M.E.; Young, H.S. The SarcoEndoplasmic Reticulum Calcium ATPase. Subcell. Biochem. 2018, 87, 229–258. [Google Scholar] [PubMed]
- Wallimann, T.; Wyss, M.; Brdiczka, D.; Nicolay, K.; Eppenberger, H.M. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: The “phosphocreatine circuit” for cellular energy homeostasis. Biochem. J. 1992, 281 Pt 1, 21–40. [Google Scholar] [CrossRef]
- Guimarães-Ferreira, L. Role of the phosphocreatine system on energetic homeostasis in skeletal and cardiac muscles. Einstein (Sao Paulo) 2014, 12, 126–131. [Google Scholar] [CrossRef] [Green Version]
- Wan, J.; Qin, Z.; Wang, P.; Sun, Y.; Liu, X. Muscle fatigue: General understanding and treatment. Exp. Mol. Med. 2017, 49, e384. [Google Scholar] [CrossRef]
- Deldicque, L.; Theisen, D.; Bertrand, L.; Hespel, P.; Hue, L.; Francaux, M. Creatine enhances differentiation of myogenic C2C12 cells by activating both p38 and Akt/PKB pathways. Am. J. Physiol. Cell Physiol. 2007, 293, C1263–C1271. [Google Scholar] [CrossRef]
- Sestili, P.; Barbieri, E.; Stocchi, V. Effects of Creatine in Skeletal Muscle Cells and in Myoblasts Differentiating Under Normal or Oxidatively Stressing Conditions. Mini Rev. Med. Chem. 2016, 16, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Kley, R.A.; Tarnopolsky, M.A.; Vorgerd, M. Creatine for treating muscle disorders. Cochrane Database Syst. Rev. 2013, CD004760. [Google Scholar] [CrossRef] [PubMed]
- D’Antona, G.; Nabavi, S.M.; Micheletti, P.; Di Lorenzo, A.; Aquilani, R.; Nisoli, E.; Rondanelli, M.; Daglia, M. Creatine, L-carnitine, and ω3 polyunsaturated fatty acid supplementation from healthy to diseased skeletal muscle. BioMed Res. Int. 2014, 2014, 613890. [Google Scholar] [CrossRef] [PubMed]
- Nabuurs, C.I.; Choe, C.U.; Veltien, A.; Kan, H.E.; van Loon, L.J.C.; Rodenburg, R.J.T.; Matschke, J.; Wieringa, B.; Kemp, G.J.; Isbrandt, D.; et al. Disturbed energy metabolism and muscular dystrophy caused by pure creatine deficiency are reversible by creatine intake. J. Physiol. (Lond.) 2013, 591, 571–592. [Google Scholar] [CrossRef] [PubMed]
- Petrofsky, J.S.; Fitch, C.D. Contractile characteristics of skeletal muscles depleted of phosphocreatine. Pflug. Arch. 1980, 384, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.A. A linear model of muscle respiration explains monoexponential phosphocreatine changes. Am. J. Physiol.-Cell Physiol. 1988, 254, C548–C553. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.-J.; Xia, L.; Han, Y.-R. Mitochondrion and Apoptosis. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao 2001, 33, 7–12. [Google Scholar]
- Kroemer, G. Mitochondrial control of apoptosis: An overview. Biochem. Soc. Symp. 1999, 66, 1–15. [Google Scholar] [CrossRef]
- Ježek, J.; Cooper, K.F.; Strich, R. Reactive Oxygen Species and Mitochondrial Dynamics: The Yin and Yang of Mitochondrial Dysfunction and Cancer Progression. Antioxidants (Basel) 2018, 7, 13. [Google Scholar] [CrossRef]
- Nickel, A.; Kohlhaas, M.; Maack, C. Mitochondrial reactive oxygen species production and elimination. J. Mol. Cell. Cardiol. 2014, 73, 26–33. [Google Scholar] [CrossRef]
- Schirris, T.J.J.; Renkema, G.H.; Ritschel, T.; Voermans, N.C.; Bilos, A.; van Engelen, B.G.M.; Brandt, U.; Koopman, W.J.H.; Beyrath, J.D.; Rodenburg, R.J.; et al. Statin-Induced Myopathy Is Associated with Mitochondrial Complex III Inhibition. Cell Metab. 2015, 22, 399–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
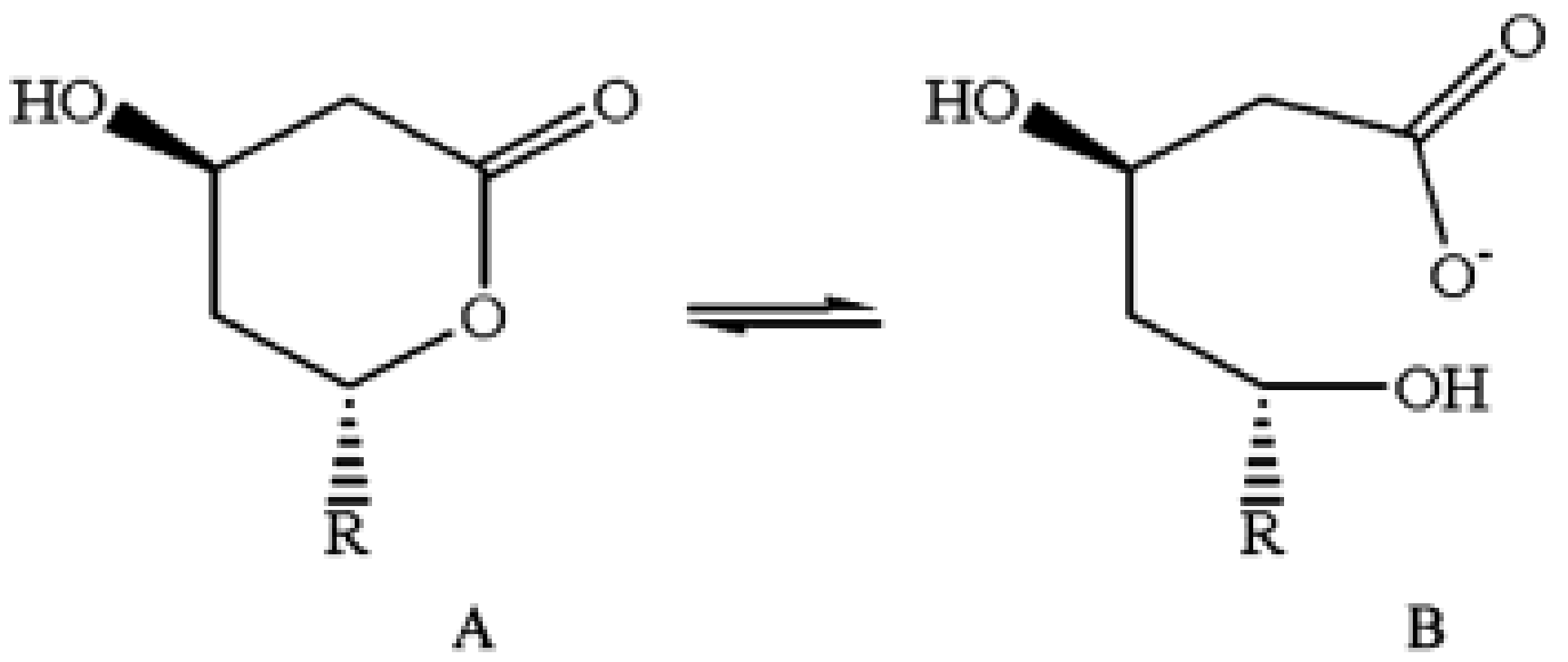
- Kearney, A.S.; Crawford, L.F.; Mehta, S.C.; Radebaugh, G.W. The Interconversion Kinetics, Equilibrium, and Solubilities of the Lactone and Hydroxyacid Forms of the HMG-CoA Reductase Inhibitor, CI-981. Pharm. Res. Off. J. Am. Assoc. Pharm. Sci. 1993, 10, 1461–1465. [Google Scholar]
- Patil, R.H.; Patil, M.P.; Maheshwari, V.L. Rapid Chromatographic Determination and Structural Confirmation of β-Hydroxy Acid Form of Lovastatin in the Fermentation Broth of Aspergillus Terreus PM03. Pharm. Chem. J. 2015, 49, 419–424. [Google Scholar] [CrossRef]
- Skottheim, I.B.; Gedde-Dahl, A.; Hejazifar, S.; Hoel, K.; Åsberg, A. Statin induced myotoxicity: The lactone forms are more potent than the acid forms in human skeletal muscle cells in vitro. Eur. J. Pharm. Sci. 2008, 33, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio, A.; Mullen, P.J.; Mityko, I.S.; Navegantes, L.C.; Bouitbir, J.; Krähenbühl, S. Simvastatin induces mitochondrial dysfunction and increased atrogin-1 expression in H9c2 cardiomyocytes and mice in vivo. Arch. Toxicol. 2016, 90, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Busanello, E.N.B.; Marques, A.C.; Lander, N.; de Oliveira, D.N.; Catharino, R.R.; Oliveira, H.C.F.; Vercesi, A.E. Pravastatin Chronic Treatment Sensitizes Hypercholesterolemic Mice Muscle to Mitochondrial Permeability Transition: Protection by Creatine or Coenzyme Q10. Front. Pharmacol. 2017, 8, 185. [Google Scholar] [CrossRef] [PubMed]
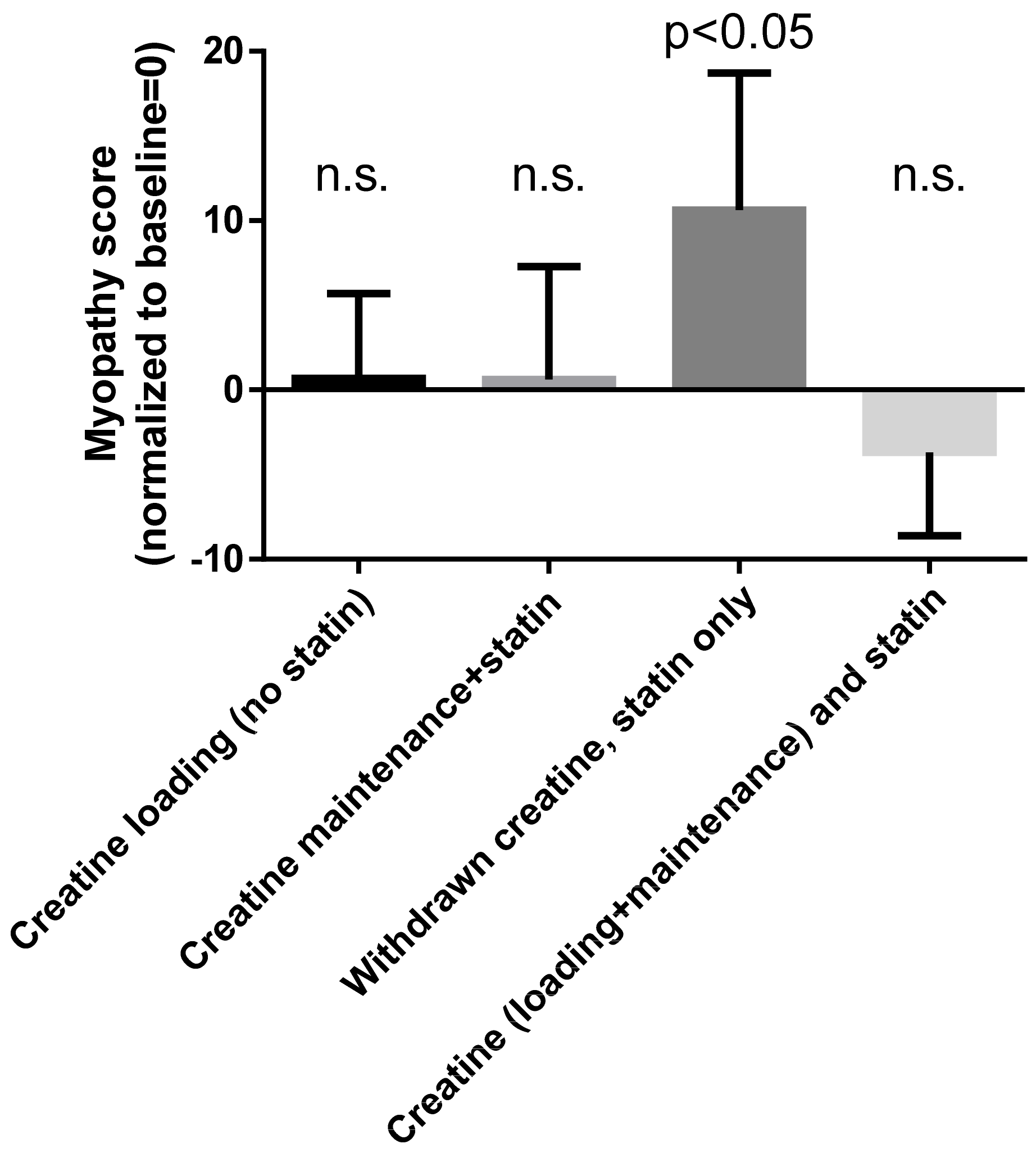
- Balestrino, M.; Adriano, E. Statin-induced myopathy prevented by creatine administration. BMJ Case Rep. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.; Klopstock, T. Creatine for neuroprotection in neurodegenerative disease: End of story? Amino Acids 2016, 48, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Writing Group for the NINDS Exploratory Trials in Parkinson Disease (NET-PD) Investigators; Kieburtz, K.; Tilley, B.C.; Elm, J.J.; Babcock, D.; Hauser, R.; Ross, G.W.; Augustine, A.H.; Augustine, E.U.; Aminoff, M.J.; et al. Effect of creatine monohydrate on clinical progression in patients with Parkinson disease: A randomized clinical trial. JAMA 2015, 313, 584–593. [Google Scholar] [CrossRef] [PubMed]


| Paper | Mechanisms Proposed |
|---|---|
| Tomaszewski et al., 2011 [24] | Altered membrane function due to lower cholesterol content. Altered mitochondrial function due to decreased muscle coenzyme Q10 (CoQ10). Impairment of calcium homeostasis. Induction of apoptosis. Genetic determinants. |
| Vrablik et al., 2014 [25] | Decreased intracellular concentrations of cholesterol. Reduced production of coenzyme Q10 and related ubiquinones. Decreased production of prenylated proteins. Increased uptake of cholesterol from the extracellular space. Increased uptake of phytosterols. Disruption of calcium metabolism in myocytes. Decreased renewal of damaged muscle cells via the ubiquitin pathway. Inhibition of selenoprotein synthesis. Genetic factors 1. Unmasking of pre-existing muscular disorders |
| Apostolopoulou et al., 2015 [26] | Impairment of mitochondrial function. Decreased muscle coenzyme Q10 (CoQ10). Genetic susceptibility. |
| Laufs et al., 2015 [27] | Reduction of cholesterol/isoprenoid concentrations in specific cellular and subcellular compartments. Reduced sarcolemmal and/or sarcoplasmic reticular cholesterol. Alterations of myocellular fat and/or sterol concentration. Increased catabolism of muscular proteins or decreased catabolism of damaged proteins. Failure to repair damaged muscle. Leakage of sarcolemmal calcium into the cytoplasm. Impairment of mitochondrial function 2. |
| Muntean et al., 2017 [28] | Increased fatty acid synthesis and induced triacylglycerol and phospholipid accumulation in lipid droplets 3. Inhibition of the mevalonate pathway and subsequent decrease in availability of isoprenoid intermediates, leading to decreased synthesis of cholesterol, ubiquinone and dolichols, and to impaired prenylation of structural proteins. Calcium release from sarcoplasmic reticulum and mitochondria. Impairment of oxidative phosphorylation. Decrease in mitochondria density and biogenesis. Apoptosis and calpain-mediated cell death. Impairment of muscle regeneration and the remodeling of cytoskeletal architecture. |
| du Souich et al., 2017 [29] | Increased statin accumulation in the myocyte, resulting from the reduced function of the transporters carrying statins into cells or their metabolites out of them. Altered mitochondrial function causing reduced production of ATP, excess production of reactive oxygen species (ROS) and apoptosis. Reduced ubiquinone levels. Toxic effect of statins on mitochondrial function. Direct effect of statins on sarcoplasma chloride and lactate. |
| Selva-O’Callaghan et al., 2018 [30] | Mitochondrial dysfunction. Oxidative stress. Impaired mevalonate metabolism. Isoprenylation of small G-proteins. Genetic susceptibility (polymorphisms of the SLCO1B1 gene 4, alterations in genes coding for plasma membrane calcium transporting ATPase and alterations of the CoQ2 gene 5) 6. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balestrino, M.; Adriano, E. Creatine as a Candidate to Prevent Statin Myopathy. Biomolecules 2019, 9, 496. https://doi.org/10.3390/biom9090496
Balestrino M, Adriano E. Creatine as a Candidate to Prevent Statin Myopathy. Biomolecules. 2019; 9(9):496. https://doi.org/10.3390/biom9090496
Chicago/Turabian StyleBalestrino, Maurizio, and Enrico Adriano. 2019. "Creatine as a Candidate to Prevent Statin Myopathy" Biomolecules 9, no. 9: 496. https://doi.org/10.3390/biom9090496





