Creatine for the Treatment of Depression
Abstract
1. Introduction
2. Methods
2.1. Creatine Biochemistry as it Pertains to Depression
2.2. Animal Studies of Depression-Like Behavior
2.3. Altered Brain Bioenergetics in Human Depression
2.4. Biochemical Effects of Creatine Supplementation
2.5. Clinical Studies in Conditions Related to Depression
2.6. Clinical Trials of Creatine for Depression
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR* D report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Nemeroff, C.B. Prevalence and management of treatment-resistant depression. J. Clin. Psychiatry 2007, 68, 17. [Google Scholar] [PubMed]
- Sackeim, H.A. Modern electroconvulsive therapy: Vastly improved yet greatly underused. JAMA Psychiatry 2017, 74, 779–780. [Google Scholar] [CrossRef] [PubMed]
- Caddy, C.; Amit, B.H.; McCloud, T.L.; Rendell, J.M.; Furukawa, T.A.; McShane, R.; Hawton, K.; Cipriani, A. Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database Syst. Rev. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Newport, D.J.; Carpenter, L.L.; McDonald, W.M.; Potash, J.B.; Tohen, M.; Nemeroff, C.B. Ketamine and Other NMDA Antagonists: Early Clinical Trials and Possible Mechanisms in Depression. Am. J. Psychiatry 2015, 172, 950–966. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.J. Creatine metabolism and psychiatric disorders: Does creatine supplementation have therapeutic value? Neurosci. Biobehav. Rev. 2012, 36, 1442–1462. [Google Scholar] [CrossRef] [PubMed]
- Pazini, F.L.; Cunha, M.P.; Rodrigues, A.L.S. The possible beneficial effects of creatine for the management of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 89, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Andres, R.H.; Ducray, A.D.; Schlattner, U.; Wallimann, T.; Widmer, H.R. Functions and effects of creatine in the central nervous system. Brain Res. Bull. 2008, 76, 329–343. [Google Scholar] [CrossRef]
- Hahn, K.A.; Salomons, G.S.; Tackels-Horne, D.; Wood, T.C.; Taylor, H.A.; Schroer, R.J.; Lubs, H.A.; Jakobs, C.; Olson, R.L.; Holden, K.R. X-linked mental retardation with seizures and carrier manifestations is caused by a mutation in the creatine-transporter gene (SLC6A8) located in Xq28. Am. J. Hum. Genet. 2002, 70, 1349–1356. [Google Scholar] [CrossRef][Green Version]
- Salomons, G.S.; van Dooren, S.J.; Verhoeven, N.M.; Cecil, K.M.; Ball, W.S.; Degrauw, T.J.; Jakobs, C. X-linked creatine-transporter gene (SLC6A8) defect: A new creatine-deficiency syndrome. Am. J. Hum. Genet. 2001, 68, 1497–1500. [Google Scholar] [CrossRef]
- Attwell, D.; Laughlin, S.B. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 2001, 21, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, D.F.; Brown, G.C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997, 77, 731–758. [Google Scholar] [CrossRef]
- Sahlin, K.; Harris, R.C. The creatine kinase reaction: A simple reaction with functional complexity. Amino Acids 2011, 40, 1363–1367. [Google Scholar] [CrossRef]
- Vendelin, M.; Eimre, M.; Seppet, E.; Peet, N.; Andrienko, T.; Lemba, M.; Engelbrecht, J.; Seppet, E.K.; Saks, V.A. Intracellular diffusion of adenosine phosphates is locally restricted in cardiac muscle. Mol. Cell. Biochem. 2004, 256, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Kaldis, P.; Hemmer, W.; Zanolla, E.; Holtzman, D.; Wallimann, T. ‘Hot spots’ of creatine kinase localization in brain: Cerebellum, hippocampus and choroid plexus. Dev. Neurosci. 1996, 18, 542–554. [Google Scholar] [CrossRef]
- Jost, C.R.; Van der Zee, C.E.; In ‘t Zandt, H.J.; Oerlemans, F.; Verheij, M.; Streijger, F.; Fransen, J.; Heerschap, A.; Cools, A.R.; Wieringa, B. Creatine kinase B-driven energy transfer in the brain is important for habituation and spatial learning behaviour, mossy fibre field size and determination of seizure susceptibility. Eur. J. Neurosci. 2002, 15, 1692–1706. [Google Scholar] [CrossRef] [PubMed]
- In ‘t Zandt, H.J.; Renema, W.K.; Streijger, F.; Jost, C.; Klomp, D.W.; Oerlemans, F.; Van der Zee, C.E.; Wieringa, B.; Heerschap, A. Cerebral creatine kinase deficiency influences metabolite levels and morphology in the mouse brain: A quantitative in vivo 1H and 31P magnetic resonance study. J. Neurochem. 2004, 90, 1321–1330. [Google Scholar] [CrossRef]
- Allen, P.J.; D’Anci, K.E.; Kanarek, R.B.; Renshaw, P.F. Chronic creatine supplementation alters depression-like behavior in rodents in a sex-dependent manner. Neuropsychopharmacology 2009, 35, 534–546. [Google Scholar] [CrossRef]
- Allen, P.J.; D′Anci, K.E.; Kanarek, R.B.; Renshaw, P.F. Sex-specific antidepressant effects of dietary creatine with and without sub-acute fluoxetine in rats. Pharmacol. Biochem. Behav. 2012, 101, 588–601. [Google Scholar] [CrossRef]
- Allen, P.J.; DeBold, J.F.; Rios, M.; Kanarek, R.B. Chronic high-dose creatine has opposing effects on depression-related gene expression and behavior in intact and sex hormone-treated gonadectomized male and female rats. Pharmacol. Biochem. Behav. 2015, 130, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Lee, Y.J.; Kim, H.; Lee, D.W.; Woo, D.C.; Choi, C.B.; Chae, J.H.; Choe, B.Y. Desipramine attenuates forced swim test-induced behavioral and neurochemical alterations in mice: An in vivo 1H-MRS study at 9.4T. Brain Res. 2010, 1348, 105–113. [Google Scholar] [CrossRef]
- Lim, S.I.; Song, K.H.; Yoo, C.H.; Woo, D.C.; Choe, B.Y. Decreased glutamatergic activity in the frontal cortex of single prolonged stress model: In vivo and ex vivo proton MR spectroscopy. Neurochem. Res. 2017, 42, 2218–2229. [Google Scholar] [CrossRef] [PubMed]
- Knox, D.; Perrine, S.A.; George, S.A.; Galloway, M.P.; Liberzon, I. Single prolonged stress decreases glutamate, glutamine, and creatine concentrations in the rat medial prefrontal cortex. Neurosci. Lett. 2010, 480, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Yan, G.; Xuan, Y.; Peng, H.; Huang, Q.J.; Wu, R.; Xu, H. Chronic social isolation decreases glutamate and glutamine levels and induces oxidative stress in the rat hippocampus. Behav. Brain Res. 2015, 282, 201–208. [Google Scholar] [CrossRef]
- Czeh, B.; Michaelis, T.; Watanabe, T.; Frahm, J.; de Biurrun, G.; van Kampen, M.; Bartolomucci, A.; Fuchs, E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc. Natl. Acad. Sci. USA 2001, 98, 12796–12801. [Google Scholar] [CrossRef] [PubMed]
- Michael-Titus, A.T.; Albert, M.; Michael, G.J.; Michaelis, T.; Watanabe, T.; Frahm, J.; Pudovkina, O.; van der Hart, M.G.; Hesselink, M.B.; Fuchs, E.; et al. SONU20176289, a compound combining partial dopamine D(2) receptor agonism with specific serotonin reuptake inhibitor activity, affects neuroplasticity in an animal model for depression. Eur. J. Pharmacol. 2008, 598, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Van der Hart, M.G.; Czeh, B.; de Biurrun, G.; Michaelis, T.; Watanabe, T.; Natt, O.; Frahm, J.; Fuchs, E. Substance P receptor antagonist and clomipramine prevent stress-induced alterations in cerebral metabolites, cytogenesis in the dentate gyrus and hippocampal volume. Mol. Psychiatry 2002, 7, 933–941. [Google Scholar] [CrossRef][Green Version]
- Fuchs, E. Social stress in tree shrews as an animal model of depression: An example of a behavioral model of a CNS disorder. CNS Spectr. 2005, 10, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E.; Flugge, G. Social stress in tree shrews: Effects on physiology, brain function, and behavior of subordinate individuals. Pharmcol. Biochem. Behav. 2002, 73, 247–258. [Google Scholar] [CrossRef]
- Almeida, L.S.; Salomons, G.S.; Hogenboom, F.; Jakobs, C.; Schoffelmeer, A.N. Exocytotic release of creatine in rat brain. Synapse 2006, 60, 118–123. [Google Scholar] [CrossRef]
- Cunha, M.P.; Budni, J.; Pazini, F.L.; Oliveira, Á.; Rosa, J.M.; Lopes, M.W.; Leal, R.B.; Rodrigues, A.L.S. Involvement of PKA, PKC, CAMK-II and MEK1/2 in the acute antidepressant-like effect of creatine in mice. Pharmacol. Rep. 2014, 66, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.P.; Budni, J.; Ludka, F.K.; Pazini, F.L.; Rosa, J.M.; Oliveira, A.; Lopes, M.W.; Tasca, C.I.; Leal, R.B.; Rodrigues, A.L.S. Involvement of PI3K/Akt signaling pathway and its downstream intracellular targets in the antidepressant-like effect of creatine. Mol. Neurobiol. 2016, 53, 2954–2968. [Google Scholar] [CrossRef] [PubMed]
- Pazini, F.L.; Cunha, M.P.; Rosa, J.M.; Colla, A.R.; Lieberknecht, V.; Oliveira, A.; Rodrigues, A.L. Creatine, similar to ketamine, counteracts depressive-like behavior induced by corticosterone via PI3K/Akt/mTOR Ppthway. Mol. Neurobiol. 2016, 53, 6818–6834. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.P.; Pazini, F.L.; Lieberknecht, V.; Rodrigues, A.L.S. Subchronic administration of creatine produces antidepressant-like effect by modulating hippocampal signaling pathway mediated by FNDC5/BDNF/Akt in mice. J. Psychiatr. Res. 2018, 104, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.L.; Wauson, E.; Duric, V. Kinase-mediated signaling cascades in mood disorders and antidepressant treatment. J. Neurogenet. 2016, 30, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Abelaira, H.M.; Reus, G.Z.; Neotti, M.V.; Quevedo, J. The role of mTOR in depression and antidepressant responses. Life Sci. 2014, 101, 10–14. [Google Scholar] [CrossRef]
- Bjorkholm, C.; Monteggia, L.M. BDNF—A key transducer of antidepressant effects. Neuropharmacology 2016, 102, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.P.; Pazini, F.L.; Oliveira, A.; Machado, D.G.; Rodrigues, A.L. Evidence for the involvement of 5-HT1A receptor in the acute antidepressant-like effect of creatine in mice. Brain Res. Bull. 2013, 95, 61–69. [Google Scholar] [CrossRef]
- Cunha, M.P.; Machado, D.G.; Capra, J.C.; Jacinto, J.; Bettio, L.E.; Rodrigues, A.L. Antidepressant-like effect of creatine in mice involves dopaminergic activation. J. Psychopharmacol. 2012, 26, 1489–1501. [Google Scholar] [CrossRef]
- Cunha, M.P.; Pazini, F.L.; Rosa, J.M.; Ramos-Hryb, A.B.; Oliveira, A.; Kaster, M.P.; Rodrigues, A.L. Creatine, similarly to ketamine, affords antidepressant-like effects in the tail suspension test via adenosine A1 and A2A receptor activation. Purinergic Signal. 2015, 11, 215–227. [Google Scholar] [CrossRef]
- Cunha, M.P.; Pazini, F.L.; Oliveira, A.; Bettio, L.E.; Rosa, J.M.; Machado, D.G.; Rodrigues, A.L. The activation of alpha1-adrenoceptors is implicated in the antidepressant-like effect of creatine in the tail suspension test. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 44, 39–50. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El Yacoubi, M.; Costentin, J.; Vaugeois, J.M. Adenosine A2A receptors and depression. Neurology 2003, 61, S82–S87. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.P.; Lieberknecht, V.; Ramos-Hryb, A.B.; Olescowicz, G.; Ludka, F.K.; Tasca, C.I.; Gabilan, N.H.; Rodrigues, A.L. Creatine affords protection against glutamate-induced nitrosative and oxidative stress. Neurochem. Int. 2016, 95, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Pouwer, F. Depression: A common and burdensome complication of diabetes that warrants the continued attention of clinicians, researchers and healthcare policy makers. Diabetologia 2017, 60, 30–34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Herder, C.; Furstos, J.F.; Nowotny, B.; Begun, A.; Strassburger, K.; Mussig, K.; Szendroedi, J.; Icks, A.; Roden, M.; Group, G.D.S. Associations between inflammation-related biomarkers and depressive symptoms in individuals with recently diagnosed type 1 and type 2 diabetes. Brain Behav. Immun. 2017, 61, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Roy, T.; Lloyd, C.E. Epidemiology of depression and diabetes: A systematic review. J. Affect. Disord. 2012, 142, S8–S21. [Google Scholar] [CrossRef]
- Sivitz, W.I.; Yorek, M.A. Mitochondrial dysfunction in diabetes: From molecular mechanisms to functional significance and therapeutic opportunities. Antioxid. Redox Signal. 2010, 12, 537–577. [Google Scholar] [CrossRef]
- Bischof, M.G.; Mlynarik, V.; Brehm, A.; Bernroider, E.; Krssak, M.; Bauer, E.; Madl, C.; Bayerle-Eder, M.; Waldhäusl, W.; Roden, M. Brain energy metabolism during hypoglycaemia in healthy and type 1 diabetic subjects. Diabetologia 2004, 47, 648–651. [Google Scholar]
- Metzler, B.; Schocke, M.F.; Steinboeck, P.; Wolf, C.; Judmaier, W.; Lechleitner, M.; Lukas, P.; Pachinger, O. Decreased high-energy phosphate ratios in the myocardium of men with diabetes mellitus type I. J. Cardiovasc. Magn. Reson. 2002, 4, 493–502. [Google Scholar] [CrossRef]
- Scheuermann-Freestone, M.; Madsen, P.L.; Manners, D.; Blamire, A.M.; Buckingham, R.E.; Styles, P.; Radda, G.K.; Neubauer, S.; Clarke, K. Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation 2003, 107, 3040–3046. [Google Scholar] [CrossRef]
- Shivu, G.N.; Phan, T.T.; Abozguia, K.; Ahmed, I.; Wagenmakers, A.; Henning, A.; Narendran, P.; Stevens, M.; Frenneaux, M. Relationship between coronary microvascular dysfunction and cardiac energetics impairment in type 1 diabetes mellitus. Circulation 2010, 121, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Cao, H.J.; Xie, S.Y.; Li, K.C.; Tao, F.B.; Yang, L.S.; Zhang, J.Q.; Bao, Y.S. Adhering to a vegetarian diet may create a greater risk of depressive symptoms in the elderly male Chinese population. J. Affect. Disord. 2019, 243, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Matta, J.; Czernichow, S.; Kesse-Guyot, E.; Hoertel, N.; Limosin, F.; Goldberg, M.; Zins, M.; Lemogne, C. Depressive symptoms and vegetarian diets: Results from the Constances cohort. Nutrients 2018, 10, 1695. [Google Scholar] [CrossRef] [PubMed]
- Larsson, C.L.; Klock, K.S.; Nordrehaug Åstrøm, A.; Haugejorden, O.; Johansson, G. Lifestyle-related characteristics of young low-meat consumers and omnivores in Sweden and Norway. J. Adol. Health 2002, 31, 190–198. [Google Scholar] [CrossRef]
- Michalak, J.; Zhang, X.C.; Jacobi, F. Vegetarian diet and mental disorders: Results from a representative community survey. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Hibbeln, J.R.; Northstone, K.; Evans, J.; Golding, J. Vegetarian diets and depressive symptoms among men. J. Affect. Disord. 2018, 225, 13–17. [Google Scholar] [CrossRef]
- Northstone, K.; Joinson, C.; Emmett, P. Dietary patterns and depressive symptoms in a UK cohort of men and women: A longitudinal study. Public Health Nutr. 2018, 21, 831–837. [Google Scholar] [CrossRef]
- Jin, Y.; Kandula, N.R.; Kanaya, A.M.; Talegawkar, S.A. Vegetarian diet is inversely associated with prevalence of depression in middle-older aged South Asians in the United States. Ethn. Health 2019, 1–8. [Google Scholar] [CrossRef]
- Beezhold, B.L.; Johnston, C.S.; Daigle, D.R. Vegetarian diets are associated with healthy mood states: A cross-sectional study in Seventh Day Adventist adults. Nutr. J. 2010, 9, 26. [Google Scholar] [CrossRef]
- Beezhold, B.; Radnitz, C.; Rinne, A.; DiMatteo, J. Vegans report less stress and anxiety than omnivores. Nutr. Neurosci. 2015, 18, 289–296. [Google Scholar] [CrossRef]
- Sanchez-Villegas, A.; Henriquez-Sanchez, P.; Ruiz-Canela, M.; Lahortiga, F.; Molero, P.; Toledo, E.; Martinez-Gonzalez, M.A. A longitudinal analysis of diet quality scores and the risk of incident depression in the SUN Project. BMC Med. 2015, 13, 197. [Google Scholar] [CrossRef] [PubMed]
- Velten, J.; Bieda, A.; Scholten, S.; Wannemuller, A.; Margraf, J. Lifestyle choices and mental health: A longitudinal survey with German and Chinese students. BMC Public Health 2018, 18, 632. [Google Scholar] [CrossRef] [PubMed]
- Rae, C.; Digney, A.L.; McEwan, S.R.; Bates, T.C. Oral creatine monohydrate supplementation improves brain performance: A double–blind, placebo–controlled, cross–over trial. Proc. Biol. Sci. 2003, 270, 2147–2150. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, R.D.; Demmer, R.T.; Galea, S.; Lemeshow, A.R.; Ortega, A.N.; Beautrais, A.L. Asthma and suicide behaviors: Results from the Third National Health and Nutrition Examination Survey (NHANES III). J. Psychiatr. Res. 2012, 46, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.J.; Chen, V.C.H.; Lee, W.C.; Chen, W.J.; Ferri, C.P.; Stewart, R.; Lai, T.J.; Chen, C.C.; Wang, T.N.; Ko, Y.C. Asthma and suicide mortality in young people: A 12-year follow-up study. Am. J. Psychiatry 2010, 167, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, R.D.; Lavoie, K.L.; Lemeshow, A.R.; Jenkins, E.; Brown, E.S.; Fedoronko, D.A. Depression, anxiety, and COPD: The unexamined role of nicotine dependence. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2011, 14, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Webb, R.T.; Kontopantelis, E.; Doran, T.; Qin, P.; Creed, F.; Kapur, N. Suicide risk in primary care patients with major physical diseases: A case-control study. Arch. Gen. Psychiatry 2012, 69, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Van den Bemt, L.; Schermer, T.; Bor, H.; Smink, R.; Van Weel-Baumgarten, E.; Lucassen, P.J.; Van Weel, C. The risk for depression comorbidity in patients with COPD. Chest 2009, 135, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.A.; Goodson, W.H.; Hopf, H.W.; Hunt, T.K. Cigarette smoking decreases tissue oxygen. Arch. Surg. 1991, 126, 1131–1134. [Google Scholar] [CrossRef]
- Aubin, H.J.; Berlin, I.; Reynaud, M. Current smoking, hypoxia, and suicide. Am. J. Psychiatry 2011, 168, 326–327. [Google Scholar] [CrossRef]
- Chaiton, M.O.; Cohen, J.E.; O′Loughlin, J.; Rehm, J. A systematic review of longitudinal studies on the association between depression and smoking in adolescents. BMC Public Health 2009, 9, 356. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.R. Smoking and suicide: A brief overview. Drug Alcohol Depend. 2008, 98, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Covey, L.S.; Berlin, I.; Hu, M.C.; Hakes, J.K. Smoking and suicidal behaviours in a sample of US adults with low mood: A retrospective analysis of longitudinal data. BMJ Open 2012, 2, e000876. [Google Scholar] [CrossRef] [PubMed]
- Young, S.N. Elevated incidence of suicide in people living at altitude, smokers and patients with chronic obstructive pulmonary disease and asthma: Possible role of hypoxia causing decreased serotonin synthesis. J. Psychiatry Neurosci. 2013, 38, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Sabic, H.; Kious, B.; Boxer, D.; Fitzgerald, C.; Riley, C.; Scholl, L.; McGlade, E.; Yurgelun-Todd, D.; Renshaw, P.F.; Kondo, D.G. Effect of altitude on suicide rates among U.S. military veterans. High Alt. Med. Biol. 2018, 20, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Kious, B.M.; Kondo, D.G.; Renshaw, P.F. Living high and feeling low: Altitude, suicide, and depression. Harv. Rev. Psychiatry 2018, 26, 43–56. [Google Scholar] [CrossRef]
- Kious, B.M.; Bakian, A.V.; Zhao, J.; Mickey, B.; Guille, C.; Renshaw, P.; Sen, S. Altitude and risk of depression and anxiety: Findings from the Intern Health Study. Int. Rev. Psychiatry 2019, 14, 1–9. [Google Scholar] [CrossRef]
- Kim, N.; Mickelson, J.B.; Brenner, B.E.; Haws, C.A.; Yurgelun-Todd, D.A.; Renshaw, P.F. Altitude, gun ownership, rural areas, and suicide. Am. J. Psychiatry 2011, 168, 49–54. [Google Scholar] [CrossRef]
- Kim, J.; Choi, N.; Lee, Y.J.; An, H.; Kim, N.; Yoon, H.K.; Lee, H.J. High altitude remains associated with elevated suicide rates after adjusting for socioeconomic status: A study from South Korea. Psychiatry Investig. 2014, 11, 492–494. [Google Scholar] [CrossRef][Green Version]
- Gamboa, J.L.; Caceda, R.; Arregui, A. Is depression the link between suicide and high altitude? High Alt. Med. Biol. 2011, 12, 403–405. [Google Scholar] [CrossRef]
- Cheng, D.C.; Mendenhall, T.I.; Brenner, B.E. Suicide rates strongly correlate with altitude. Acad. Emerg. Med. 2005, 12, 141. [Google Scholar] [CrossRef]
- Cheng, D.; Yakobi, R.; Dobbins, W.N.; Neuman, K.; Brenner, B. Moderate altitude increases suicide deaths. Ann. Emerg. Med. 2002, 40, S55. [Google Scholar]
- Riblet, N.B.; Gottlieb, D.J.; Watts, B.V.; Cornelius, S.L.; Fan, V.S.; Shi, X.; Shiner, B. Hypoxia-related risk factors for death by suicide in a national clinical sample. Psychiatry Res. 2019, 273, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.; Cheng, D.; Clark, S.; Camargo, C.A. Positive association between altitude and suicide in 2584 U.S. counties. High Alt. Med. Biol. 2011, 12, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Betz, M.E.; Valley, M.A.; Lowenstein, S.R.; Hedegaard, H.; Thomas, D.; Stallones, L.; Honigman, B. Elevated suicide rates at high altitude: Sociodemographic and health issues may be to blame. Suicide Life Threat. Behav. 2011, 41, 562–573. [Google Scholar] [CrossRef]
- Huber, R.S.; Coon, H.; Kim, N.; Renshaw, P.F.; Kondo, D.G. Altitude is a risk factor for completed suicide in bipolar disorder. Med. Hypotheses 2014, 82, 377–381. [Google Scholar] [CrossRef]
- Ha, H.; Tu, W. An ecological study on the spatially varying relationship between county-level suicide rates and altitude in the United States. Int. J. Environ. Res. Public Health 2018, 15, 671. [Google Scholar] [CrossRef]
- Brenner, B.E.; Cheng, D.; Muller, E.; Clark, S.; Camargo, C.A. Suicide rates strongly correlate with altitude: A study of 3060 U.S. counties. Acad. Emerg. Med. 2006, 13, S195. [Google Scholar] [CrossRef]
- Kanekar, S.; Bogdanova, O.V.; Olson, P.R.; Sung, Y.H.; D’Anci, K.E.; Renshaw, P.F. Hypobaric hypoxia induces depression-like behavior in female Sprague-Dawley rats, but not in males. High Alt. Med. Biol. 2015, 16, 52–60. [Google Scholar] [CrossRef]
- Bogdanova, O.V.; Abdullah, O.; Kanekar, S.; Bogdanov, V.B.; Prescot, A.P.; Renshaw, P.F. Neurochemical alterations in frontal cortex of the rat after one week of hypobaric hypoxia. Behav. Brain Res. 2014, 263, 203–209. [Google Scholar] [CrossRef]
- Sheth, C.; Ombach, H.; Olson, P.; Renshaw, P.F.; Kanekar, S. Increased anxiety and anhedonia in female rats following exposure to altitude. High Alt. Med. Biol. 2018, 19, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Kanekar, S.; Sheth, C.S.; Ombach, H.J.; Olson, P.R.; Bogdanova, O.V.; Petersen, M.; Renshaw, C.E.; Sung, Y.H.; D′Anci, K.E.; Renshaw, P.F. Hypobaric hypoxia exposure in rats differentially alters antidepressant efficacy of the selective serotonin reuptake inhibitors fluoxetine, paroxetine, escitalopram and sertraline. Pharm. Biochem. Behav. 2018, 170, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.F.; Carlson, P.J.; Kim, T.S.; Sung, Y.H.; Hellem, T.L.; Fiedler, K.K.; Kim, S.E.; Glaeser, B.; Wang, K.; Zuo, C.S. Effect of altitude on brain intracellular pH and inorganic phosphate levels. Psychiatry Res. Neuroimaging 2014, 222, 149–156. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Renshaw, P.F.; Prescot, A.; Ongur, D.; Huber, R.; Yurgelun-Todd, D. Suicide and brain chemical changes with altitude. In Proceedings of the 6th Biennial Congress of The International Society of Affective Disorders, London, UK, 19–22 June 2012. [Google Scholar]
- Shao, L.; Martin, M.V.; Watson, S.J.; Schatzberg, A.; Akil, H.; Myers, R.M.; Jones, E.G.; Bunney, W.E.; Vawter, M.P. Mitochondrial involvement in psychiatric disorders. Ann. Med. 2008, 40, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Torrell, H.; Montana, E.; Abasolo, N.; Roig, B.; Gaviria, A.M.; Vilella, E.; Martorell, L. Mitochondrial DNA (mtDNA) in brain samples from patients with major psychiatric disorders: Gene expression profiles, mtDNA content and presence of the mtDNA common deletion. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2013, 162, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Rollins, B.; Martin, M.V.; Sequeira, P.A.; Moon, E.A.; Morgan, L.Z.; Watson, S.J.; Schatzberg, A.; Akil, H.; Myers, R.M.; Jones, E.G.; et al. Mitochondrial variants in schizophrenia, bipolar disorder, and major depressive disorder. PLoS ONE 2009, 4, e4913. [Google Scholar] [CrossRef]
- Stine, O.C.; Luu, S.U.; Zito, M.; Casanova, M. The possible association between affective disorder and partially deleted mitochondrial DNA. Biol. Psychiatry 1993, 33, 141–142. [Google Scholar] [CrossRef]
- Bansal, Y.; Kuhad, A. Mitochondrial dysfunction in depression. Curr. Neuropharmacol. 2016, 14, 610–618. [Google Scholar] [CrossRef]
- Fattal, O.; Link, J.; Quinn, K.; Cohen, B.H.; Franco, K. Psychiatric comorbidity in 36 adults with mitochondrial cytopathies. CNS Spectr. 2007, 12, 429–438. [Google Scholar] [CrossRef]
- Koene, S.; Kozicz, T.L.; Rodenburg, R.J.; Verhaak, C.M.; de Vries, M.C.; Wortmann, S.; van de Heuvel, L.; Smeitink, J.A.; Morava, E. Major depression in adolescent children consecutively diagnosed with mitochondrial disorder. J. Affect. Disord. 2009, 114, 327–332. [Google Scholar] [CrossRef]
- Boles, R.G.; Burnett, B.B.; Gleditsch, K.; Wong, S.; Guedalia, A.; Kaariainen, A.; Eloed, J.; Stern, A.; Brumm, V. A high predisposition to depression and anxiety in mothers and other matrilineal relatives of children with presumed maternally inherited mitochondrial disorders. Am. J. Med. Genet. B Neuropsychiatr. Genet 2005, 137, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.; Johansson, A.; Wibom, R.; Nennesmo, I.; von Döbeln, U.; Hagenfeldt, L.; Hällström, T. Alterations of mitochondrial function and correlations with personality traits in selected major depressive disorder patients. J. Affect. Disord. 2003, 76, 55–68. [Google Scholar] [CrossRef]
- Agren, H.; Niklasson, F. Creatinine and creatine in CSF: Indices of brain energy metabolism in depression. Short note. J Neural Transm. 1988, 74, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Niklasson, F.; Agren, H. Brain energy metabolism and blood-brain barrier permeability in depressive patients: Analyses of creatine, creatinine, urate, and albumin in CSF and blood. Biol. Psychiatry 1984, 19, 1183–1206. [Google Scholar] [PubMed]
- Segal, M.; Avital, A.; Drobot, M.; Lukanin, A.; Derevenski, A.; Sandbank, S.; Weizman, A. Serum creatine kinase level in unmedicated nonpsychotic, psychotic, bipolar and schizoaffective depressed patients. Eur. Neuropsychopharmacol. 2007, 17, 194–198. [Google Scholar] [CrossRef]
- Buchsbaum, M.S.; Wu, J.; DeLisi, L.E.; Holcomb, H.; Kessler, R.; Johnson, J.; King, A.C.; Hazlett, E.; Langston, K.; Post, R.M. Frontal cortex and basal ganglia metabolic rates assessed by positron emission tomography with [18F] 2-deoxyglucose in affective illness. J. Affect. Disord. 1986, 10, 137–152. [Google Scholar] [CrossRef]
- Baxter, L.R.; Schwartz, J.M.; Phelps, M.E.; Mazziotta, J.C.; Guze, B.H.; Selin, C.E.; Gerner, R.H.; Sumida, R.M. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch. Gen. Psychiatry 1989, 46, 243–250. [Google Scholar] [CrossRef]
- Martinot, J.L.; Hardy, P.; Feline, A.; Huret, J.D.; Mazoyer, B.; Attar-Levy, D.; Pappata, S.; Syrota, A. Left prefrontal glucose hypometabolism in the depressed state: A confirmation. Am. J. Psychiatry 1990, 147, 1313–1317. [Google Scholar] [CrossRef]
- Ho, A.P.; Gillin, J.C.; Buchsbaum, M.S.; Wu, J.C.; Abel, L.; Bunney, W.E., Jr. Brain glucose metabolism during non-rapid eye movement sleep in major depression. A positron emission tomography study. Arch. Gen. Psychiatry 1996, 53, 645–652. [Google Scholar] [CrossRef]
- Mayberg, H.S.; Liotti, M.; Brannan, S.K.; McGinnis, S.; Mahurin, R.K.; Jerabek, P.A.; Silva, J.A.; Tekell, J.L.; Martin, C.C.; Lancaster, J.L.; et al. Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. Am. J. Psychiatry 1999, 156, 675–682. [Google Scholar] [CrossRef]
- Hosokawa, T.; Momose, T.; Kasai, K. Brain glucose metabolism difference between bipolar and unipolar mood disorders in depressed and euthymic states. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Auer, D.P.; Pütz, B.; Kraft, E.; Lipinski, B.; Schill, J.; Holsboer, F. Reduced glutamate in the anterior cingulate cortex in depression: An in vivo proton magnetic resonance spectroscopy study. Biol. Psychiatry 2000, 47, 305–313. [Google Scholar] [CrossRef]
- Farchione, T.R.; Moore, G.J.; Rosenberg, D.R. Proton magnetic resonance spectroscopic imaging in pediatric major depression. Biol. Psychiatry 2002, 52, 86–92. [Google Scholar] [CrossRef]
- Kumar, A.; Thomas, A.; Lavretsky, H.; Yue, K.; Huda, A.; Curran, J.; Venkatraman, T.; Estanol, L.; Mintz, J.; Mega, M.; et al. Frontal white matter biochemical abnormalities in late-life major depression detected with proton magnetic resonance spectroscopy. Am. J. Psychiatry 2002, 159, 630–636. [Google Scholar] [CrossRef] [PubMed]
- McEwen, A.M.; Burgess, D.T.; Hanstock, C.C.; Seres, P.; Khalili, P.; Newman, S.C.; Baker, G.B.; Mitchell, N.D.; Khudabux-Der, J.; Allen, P.S.; et al. Increased glutamate levels in the medial prefrontal cortex in patients with postpartum depression. Neuropsychopharmacology 2012, 37, 2428–2435. [Google Scholar] [CrossRef] [PubMed]
- Pfleiderer, B.; Michael, N.; Erfurth, A.; Ohrmann, P.; Hohmann, U.; Wolgast, M.; Fiebich, M.; Arolt, V.; Heindel, W. Effective electroconvulsive therapy reverses glutamate/glutamine deficit in the left anterior cingulum of unipolar depressed patients. Psychiatry Res. Neuroimaging 2003, 122, 185–192. [Google Scholar] [CrossRef]
- Portella, M.J.; de Diego-Adelino, J.; Gomez-Anson, B.; Morgan-Ferrando, R.; Vives, Y.; Puigdemont, D.; Perez-Egea, R.; Ruscalleda, J.; Enric, A.; Perez, V. Ventromedial prefrontal spectroscopic abnormalities over the course of depression: A comparison among first episode, remitted recurrent and chronic patients. J. Psychiatric Res. 2011, 45, 427–434. [Google Scholar] [CrossRef]
- Rosa, C.E.; Soares, J.C.; Figueiredo, F.P.; Cavalli, R.C.; Barbieri, M.A.; Schaufelberger, M.S.; Salmon, C.E.G.; Del-Ben, C.M.; Santos, A.C. Glutamatergic and neural dysfunction in postpartum depression using magnetic resonance spectroscopy. Psychiatry Res. Neuroimaging 2017, 265, 18–25. [Google Scholar] [CrossRef]
- Mirza, Y.; O’Neill, J.; Smith, E.A.; Russell, A.; Smith, J.M.; Banerjee, S.P.; Bhandari, R.; Boyd, C.; Rose, M.; Ivey, J.; et al. Increased medial thalamic creatine-phosphocreatine found by proton magnetic resonance spectroscopy in children with obsessive-compulsive disorder versus major depression and healthy controls. J. Child Neurol. 2006, 21, 106–111. [Google Scholar] [CrossRef]
- Bradley, K.A.; Mao, X.; Case, J.A.; Kang, G.; Shungu, D.C.; Gabbay, V. Increased ventricular cerebrospinal fluid lactate in depressed adolescents. Eur. Psychiatry 2016, 32, 1–8. [Google Scholar] [CrossRef]
- Michael, N.; Erfurth, A.; Ohrmann, P.; Arolt, V.; Heindel, W.; Pfleiderer, B. Neurotrophic effects of electroconvulsive therapy: A proton magnetic resonance study of the left amygdalar region in patients with treatment-resistant depression. Neuropsychopharmacology 2003, 28, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Gruber, S.; Frey, R.; Mlynárik, V.; Stadlbauer, A.; Heiden, A.; Kasper, S.; Kemp, G.J.; Moser, E. Quantification of metabolic differences in the frontal brain of depressive patients and controls obtained by 1H-MRS at 3 Tesla. Investig. Radiol. 2003, 38, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Gabbay, V.; Hess, D.A.; Liu, S.; Babb, J.S.; Klein, R.G.; Gonen, O. Lateralized caudate metabolic abnormalities in adolescent major depressive disorder: A proton MR spectroscopy study. Am. J. Psychiatry 2007, 164, 1881–1889. [Google Scholar] [CrossRef]
- Nery, F.G.; Stanley, J.A.; Chen, H.H.; Hatch, J.P.; Nicoletti, M.A.; Monkul, E.S.; Matsuo, K.; Caetano, S.C.; Peluso, M.A.; Najt, P.; et al. Normal metabolite levels in the left dorsolateral prefrontal cortex of unmedicated major depressive disorder patients: A single voxel 1H spectroscopy study. Psychiatry Res. 2009, 174, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, H.; Zhang, Y.; Guan, J.; Zhang, J.; Xu, C.; Shen, Z.; Xiao, B.; Liang, C.; Chen, K.; et al. Differential neurometabolite alterations in brains of medication-free individuals with bipolar disorder and those with unipolar depression: A two-dimensional proton magnetic resonance spectroscopy study. Bipolar Disord. 2016, 18, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Njau, S.; Joshi, S.H.; Espinoza, R.; Leaver, A.M.; Vasavada, M.; Marquina, A.; Woods, R.P.; Narr, K.L. Neurochemical correlates of rapid treatment response to electroconvulsive therapy in patients with major depression. J. Psychiatry Neurosci. 2017, 42, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, T.N.; Krishnan, R.R.; Steffens, D.C.; Song, A.W.; Taylor, W.D. Biochemical abnormalities of the medial temporal lobe and medial prefrontal cortex in late-life depression. Psychiatry Res. 2009, 172, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Takahashi, S.; Shioiri, T.; Inubushi, T. Brain phosphorous metabolism in depressive disorders detected by phosphorus-31 magnetic resonance spectroscopy. J. Affect. Disord. 1992, 26, 223–230. [Google Scholar] [CrossRef]
- Moore, C.M.; Christensen, J.D.; Lafer, B.; Fava, M.; Renshaw, P.F. Lower levels of nucleoside triphosphate in the basal ganglia of depressed subjects: A phosphorous-31 magnetic resonance spectroscopy study. Am. J. Psychiatry 1997, 154, 116–118. [Google Scholar] [CrossRef]
- Volz, H.P.; Rzanny, R.; Riehemann, S.; May, S.; Hegewald, H.; Preussler, B.; Hubner, G.; Kaiser, W.A.; Sauer, H. 31P magnetic resonance spectroscopy in the frontal lobe of major depressed patients. Eur. Arch. Psychiatry Clin. Neurosci. 1998, 248, 289–295. [Google Scholar] [CrossRef]
- Renshaw, P.F.; Parow, A.M.; Hirashima, F.; Ke, Y.; Moore, C.M.; Frederick, B.; Fava, M.; Hennen, J.; Cohen, B.M. Multinuclear magnetic resonance spectroscopy studies of brain purines in major depression. Am. J. Psychiatry 2001, 158, 2048–2055. [Google Scholar] [CrossRef] [PubMed]
- Kondo, D.G.; Sung, Y.H.; Hellem, T.L.; Fiedler, K.K.; Shi, X.F.; Jeong, E.K.; Renshaw, P.F. Open-label adjunctive creatine for female adolescents with SSRI-resistant major depressive disorder: A 31-phosphorus magnetic resonance spectroscopy study. J. Affect. Disord. 2011, 135, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Iosifescu, D.V.; Bolo, N.R.; Nierenberg, A.A.; Jensen, J.E.; Fava, M.; Renshaw, P.F. Brain bioenergetics and response to triiodothyronine augmentation in major depressive disorder. Biol. Psychiatry 2008, 63, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Iosifescu, D.V.; Renshaw, P.F. 31P-Magnetic resonance spectroscopy and thyroid hormones in major depressive disorder: Toward a bioenergetic mechanism in depression? Harv. Rev. Psychiatry 2003, 11, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Forester, B.P.; Harper, D.G.; Jensen, J.E.; Ravichandran, C.; Jordan, B.; Renshaw, P.F.; Cohen, B.M. 31Phosphorus magnetic resonance spectroscopy study of tissue specific changes in high energy phosphates before and after sertraline treatment of geriatric depression. Int. J. Geriatr. Psychiatry 2009, 24, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.G.; Joe, E.B.; Jensen, J.E.; Ravichandran, C.; Forester, B.P. Brain levels of high-energy phosphate metabolites and executive function in geriatric depression. Int. J. Geriatr. Psychiatry 2016, 31, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.G.; Jensen, J.E.; Ravichandran, C.; Perlis, R.H.; Fava, M.; Renshaw, P.F.; Iosifescu, D.V. Tissue type-specific bioenergetic abnormalities in adults with major depression. Neuropsychopharmacology 2017, 42, 876–885. [Google Scholar] [CrossRef]
- Pettegrew, J.W.; Levine, J.; Gershon, S.; Stanley, J.A.; Servan-Schreiber, D.; Panchalingam, K.; McClure, R.J. 31P-MRS study of acetyl-l-carnitine treatment in geriatric depression: Preliminary results. Bipolar Disord. 2002, 4, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Stork, C.; Renshaw, P.F. Mitochondrial dysfunction in bipolar disorder: Evidence from magnetic resonance spectroscopy research. Mol. Psychiatry 2005, 10, 900–919. [Google Scholar] [CrossRef] [PubMed]
- Hamakawa, H.; Kato, T.; Shioiri, T.; Inubushi, T.; Kato, N. Quantitative proton magnetic resonance spectroscopy of the bilateral frontal lobes in patients with bipolar disorder. Psychol. Med. 1999, 29, 639–644. [Google Scholar] [CrossRef]
- Cecil, K.M.; Delbello, M.P.; Sellars, M.C.; Strakowski, S.M. Proton magnetic resonance spectroscopy of the frontal lobe and cerebellar vermis in children with a mood disorder and a familial risk for bipolar disorders. J. Am. Acad. Child Adolesc. Psychiatry 2003, 13, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Deicken, R.F.; Pegues, M.P.; Anzalone, S.; Feiwell, R.; Soher, B. Lower concentration of hippocampal N-acetylaspartate in familial bipolar I disorder. Am. J. Psychiatry 2003, 160, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Port, J.D.; Unal, S.S.; Mrazek, D.A.; Marcus, S.M. Metabolic alterations in medication-free patients with bipolar disorder: A 3T CSF-corrected magnetic resonance spectroscopic imaging study. Psychiatry Res. Neuroimaging 2008, 162, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Özdel, O.; Kalayci, D.; Sözeri-Varma, G.; Kiroğlu, Y.; Tümkaya, S.; Toker-Uğurlu, T. Neurochemical metabolites in the medial prefrontal cortex in bipolar disorder: A proton magnetic resonance spectroscopy study. Neural Regen. Res. 2012, 7, 2929–2936. [Google Scholar] [CrossRef] [PubMed]
- Caetano, S.C.; Olvera, R.L.; Hatch, J.P.; Sanches, M.; Chen, H.H.; Nicoletti, M.; Stanley, J.A.; Fonseca, M.; Hunter, K.; Lafer, B.; et al. Lower n-acetyl-aspartate levels in prefrontal cortices in pediatric bipolar disorder: A 1H magnetic resonance spectroscopy study. J. Am. Acad. Child Adolesc. Psychiatry 2011, 50, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Dager, S.R.; Friedman, S.D.; Parow, A.; Demopulos, C.; Stoll, A.L.; Lyoo, I.K.; Dunner, D.L.; Renshaw, P.F. Brain metabolic alterations in medication-free patients with bipolardisorder. Arch. Gen. Psychiatry 2004, 61, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Frye, M.A.; Watzl, J.; Banakar, S.; O’Neill, J.; Mintz, J.; Davanzo, P.; Fischer, J.; Chirichigno, J.W.; Ventura, J.; Elman, S.; et al. Increased anterior cingulate/medial prefrontal cortical glutamate and creatine in bipolar depression. Neuropsychopharmacology 2007, 32, 2490–2499. [Google Scholar] [CrossRef]
- Patel, N.C.; Cecil, K.M.; Strakowski, S.M.; Adler, C.M.; DelBello, M.P. Neurochemical alterations in adolescent bipolar depression: A proton magnetic resonance spectroscopy pilot study of the prefrontal cortex. J. Child Adolesc. Psychopharmacol. 2008, 18, 623–627. [Google Scholar] [CrossRef]
- Brambilla, P.; Stanley, J.A.; Nicoletti, M.A.; Sassi, R.B.; Mallinger, A.G.; Frank, E.; Kupfer, D.J.; Keshavan, M.S.; Soares, J.C. 1H magnetic resonance spectroscopy investigation of the dorsolateral prefrontal cortex in bipolar disorder patients. J. Affect. Disord. 2005, 86, 61–67. [Google Scholar] [CrossRef]
- Olvera, R.L.; Caetano, S.C.; Fonseca, M.; Nicoletti, M.; Stanley, J.A.; Chen, H.H.; Hatch, J.P.; Hunter, K.; Pliszka, S.R.; Soares, J.C. Low levels of n-acetyl aspartate in the left dorsolateral prefrontal cortex of pediatric bipolar patients. J. Child Adolesc. Psychopharmacol. 2007, 17, 461–473. [Google Scholar] [CrossRef]
- Moore, C.M.; Frazier, J.A.; Glod, C.A.; Breeze, J.L.; Dieterich, M.; Finn, C.T.; Frederick, B.D.; Renshaw, P.F. Glutamine and glutamate levels in children and adolescents with bipolar disorder. J. Am. Acad. Child Adolesc. Psychiatry 2007, 46, 524–534. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Öngür, D.; Prescot, A.P.; Jensen, J.E.; Cohen, B.M.; Renshaw, P.F. Creatine abnormalities in schizophrenia and bipolar disorder. Psychiatry Res. Neuroimaging 2009, 172, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Takahashi, S.; Shioiri, T.; Murashita, J.; Hamakawa, H.; Inubushi, T. Reduction of brain phosphocreatine in bipolar II disorder detected by phosphorus-31 magnetic resonance spectroscopy. J. Affect. Disord. 1994, 31, 125–133. [Google Scholar] [CrossRef]
- Weber, W.A.; Dudley, J.; Lee, J.H.; Strakowski, S.M.; Adler, C.M.; DelBello, M.P. A pilot study of alterations in high energy phosphoryl compounds and intracellular pH in unmedicated adolescents with bipolar disorder. J. Affect. Disord. 2013, 150, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Dudley, J.; DelBello, M.P.; Weber, W.A.; Adler, C.M.; Strakowski, S.M.; Lee, J.H. Tissue-dependent cerebral energy metabolism in adolescents with bipolar disorder. J. Affect. Disord. 2016, 191, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Brennan, B.P.; Jensen, J.E.; Hudson, J.I.; Coit, C.E.; Beaulieu, A.; Pope, H.G., Jr.; Renshaw, P.F.; Cohen, B.M. A placebo-controlled trial of acetyl-l-carnitine and alpha-lipoic acid in the treatment of bipolar depression. J. Clin. Psychopharmacol. 2013, 33, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Deicken, R.F.; Fein, G.; Weiner, M.W. Abnormal frontal lobe phosphorous metabolism in bipolar disorder. Am. J. Psychiatry 1995, 152, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Yuksel, C.; Chouinard, V.A.; Huynh, P.; Ryan, K.; Cohen, B.M.; Öngür, D. Abnormalities in high-energy phosphate metabolism in first-episode bipolar disorder measured using 31P-magnetic resonance spectroscopy. Biol. Psychiatry 2017, 84, 797–802. [Google Scholar] [CrossRef]
- Jensen, J.E.; Daniels, M.; Haws, C.; Bolo, N.R.; Lyoo, I.K.; Yoon, S.J.; Cohen, B.M.; Stoll, A.L.; Rusche, J.R.; Renshaw, P.F. Triacetyluridine (TAU) decreases depressive symptoms and increases brain pH in bipolar patients. Exp. Clin. Psychopharmacol. 2008, 16, 199–206. [Google Scholar] [CrossRef]
- Murashita, J.; Kato, T.; Shioiri, T.; Inubushi, T.; Kato, N. Altered brain energy metabolism in lithium-resistant bipolar disorder detected by photic stimulated 31P-MR spectroscopy. Psychol. Med. 2000, 30, 107–115. [Google Scholar] [CrossRef]
- Shi, X.F.; Carlson, P.J.; Sung, Y.H.; Fiedler, K.K.; Forrest, L.N.; Hellem, T.L.; Huber, R.S.; Kim, S.E.; Zuo, C.; Jeong, E.K.; et al. Decreased brain PME/PDE ratio in bipolar disorder: A preliminary 31P magnetic resonance spectroscopy study. Bipolar Disord. 2015, 17, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, C.; Du, F.; Ravichandran, C.; Goldbach, J.R.; Thida, T.; Lin, P.; Dora, B.; Gelda, J.; O’Connor, L.; Sehovic, S.; et al. Abnormal high-energy phosphate molecule metabolism during regional brain activation in patients with bipolar disorder. Mol. Psychiatry 2015, 20, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Sikoglu, E.M.; Jensen, J.E.; Vitaliano, G.; Liso Navarro, A.A.; Renshaw, P.F.; Frazier, J.A.; Moore, C.M. Bioenergetic measurements in children with bipolar disorder: A pilot 31P magnetic resonance spectroscopy study. PLoS ONE 2013, 8, e54536. [Google Scholar] [CrossRef] [PubMed]
- Dechent, P.; Pouwels, P.J.; Wilken, B.; Hanefeld, F.; Frahm, J. Increase of total creatine in human brain after oral supplementation of creatine-monohydrate. Am. J. Physiol. 1999, 277, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Lyoo, I.K.; Kong, S.W.; Sung, S.M.; Hirashima, F.; Parow, A.; Hennen, J.; Cohen, B.M.; Renshaw, P.F. Multinuclear magnetic resonance spectroscopy of high-energy phosphate metabolites in human brain following oral supplementation of creatine-monohydrate. Psychiatry Res. 2003, 123, 87–100. [Google Scholar] [CrossRef]
- Ipsiroglu, O.S.; Stromberger, C.; Ilas, J.; Hoger, H.; Muhl, A.; Stockler-Ipsiroglu, S. Changes of tissue creatine concentrations upon oral supplementation of creatine-monohydrate in various animal species. Life Sci. 2001, 69, 1805–1815. [Google Scholar] [CrossRef]
- Brault, J.J.; Towse, T.F.; Slade, J.M.; Meyer, R.A. Parallel increases in phosphocreatine and total creatine in human vastus lateralis muscle during creatine supplementation. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 624–634. [Google Scholar] [CrossRef]
- Jones, A.M.; Wilkerson, D.P.; Fulford, J. Influence of dietary creatine supplementation on muscle phosphocreatine kinetics during knee-extensor exercise in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, 1078–1087. [Google Scholar] [CrossRef]
- Kondo, D.G.; Forrest, L.N.; Shi, X.; Sung, Y.H.; Hellem, T.L.; Huber, R.S.; Renshaw, P.F. Creatine target engagement with brain bioenergetics: A dose-ranging phosphorus-31 magnetic resonance spectroscopy study of adolescent females with SSRI-resistant depression. Amino Acids 2016, 1941–1954. [Google Scholar] [CrossRef]
- Bender, A.; Koch, W.; Elstner, M.; Schombacher, Y.; Bender, J.; Moeschl, M.; Gekeler, F.; Muller-Myhsok, B.; Gasser, T.; Tatsch, K.; et al. Creatine supplementation in Parkinson disease: A placebo-controlled randomized pilot trial. Neurology 2006, 67, 1262–1264. [Google Scholar] [CrossRef]
- Kieburtz, K.; Tilley, B.C.; Elm, J.J.; Babcock, D.; Hauser, R.; Ross, G.W.; Augustine, A.H.; Augustine, E.U.; Aminoff, M.J.; Bodis-Wollner, I.G. Effect of creatine monohydrate on clinical progression in patients with Parkinson disease: A randomized clinical trial. JAMA 2015, 313, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.M. Understanding fatigue in major depressive disorder and other medical disorders. Psychosomatics 2008, 49, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.M.; Zhang, L.; Damatarca, C.; Grady, M. Brain circuits determine destiny in depression: A novel approach to the psychopharmacology of wakefulness, fatigue, and executive dysfunction in major depressive disorder. J. Clin. Psychiatry 2003, 64, 6–17. [Google Scholar] [PubMed]
- Kato, T.; Murashita, J.; Shioiri, T.; Inubushi, T.; Kato, N. Relationship of energy metabolism detected by 31P-MRS in the human brain with mental fatigue. Neuropsychobiology 1999, 39, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Kato, N.; Kato, T. Effects of creatine on mental fatigue and cerebral hemoglobin oxygenation. Neurosci. Res. 2002, 42, 279–285. [Google Scholar] [CrossRef]
- McMorris, T.; Harris, R.C.; Swain, J.; Corbett, J.; Collard, K.; Dyson, R.J.; Dye, L.; Hodgson, C.; Draper, N. Effect of creatine supplementation and sleep deprivation, with mild exercise, on cognitive and psychomotor performance, mood state, and plasma concentrations of catecholamines and cortisol. Psychopharmacology 2006, 185, 93–103. [Google Scholar] [CrossRef]
- McMorris, T.; Harris, R.C.; Howard, A.N.; Langridge, G.; Hall, B.; Corbett, J.; Dicks, M.; Hodgson, C. Creatine supplementation, sleep deprivation, cortisol, melatonin and behavior. Physiol. Behav. 2007, 90, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Rawson, E.S.; Lieberman, H.R.; Walsh, T.M.; Zuber, S.M.; Harhart, J.M.; Matthews, T.C. Creatine supplementation does not improve cognitive function in young adults. Physiol. Behav. 2008, 95, 130–134. [Google Scholar] [CrossRef]
- McMorris, T.; Mielcarz, G.; Harris, R.C.; Swain, J.P.; Howard, A. Creatine supplementation and cognitive performance in elderly individuals. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2007, 14, 517–528. [Google Scholar] [CrossRef]
- Kaptsan, A.; Odessky, A.; Osher, Y.; Levine, J. Lack of efficacy of 5 grams daily of creatine in schizophrenia: A randomized, double-blind, placebo-controlled trial. J. Clin. Psychiatry 2007, 68, 881–884. [Google Scholar] [CrossRef]
- Amital, D.; Vishne, T.; Roitman, S.; Kotler, M.; Levine, J. Open study of creatine monohydrate in treatment-resistant posttraumatic stress disorder. J. Clin. Psychiatry 2006, 67, 836–837. [Google Scholar] [CrossRef] [PubMed]
- Thieme, K.; Turk, D.C.; Flor, H. Comorbid depression and anxiety in fibromyalgia syndrome: Relationship to somatic and psychosocial variables. Psychosom. Med. 2004, 66, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Leader, A.; Amital, D.; Rubinow, A.; Amital, H. An open-label study adding creatine monohydrate to ongoing medical regimens in patients with the fibromyalgia syndrome. Ann. N. Y. Acad. Sci. 2009, 1173, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Amital, D.; Vishne, T.; Rubinow, A.; Levine, J. Observed effects of creatine monohydrate in a patient with depression and fibromyalgia. Am. J. Psychiatry 2006, 163, 1840–1841. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.R.; Santiago, B.M.; Lima, F.R.; Otaduy, M.C.; Calich, A.L.; Tritto, A.C.; de Sa Pinto, A.L.; Roschel, H.; Leite, C.C.; Benatti, F.B.; et al. Creatine supplementation in fibromyalgia: A randomized, double-blind, placebo-controlled trial. Arthritis Care Res. 2013, 65, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Roitman, S.; Green, T.; Osher, Y.; Karni, N.; Levine, J. Creatine monohydrate in resistant depression: A preliminary study. Bipolar Disord. 2007, 9, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Lyoo, I.K.; Yoon, S.; Kim, T.S.; Hwang, J.; Kim, J.E.; Won, W.; Bae, S.; Renshaw, P.F. A randomized, double-blind placebo-controlled trial of oral creatine monohydrate augmentation for enhanced response to a selective serotonin reuptake inhibitor in women with major depressive disorder. Am. J. Psychiatry 2012, 169, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Nemets, B.; Levine, J. A pilot dose-finding clinical trial of creatine monohydrate augmentation to SSRIs/SNRIs/NASA antidepressant treatment in major depression. Int. Clin. Psychopharmacol. 2013, 28, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Kious, B.M.; Sabic, H.; Sung, Y.H.; Kondo, D.G.; Renshaw, P. An open-label pilot study of combined augmentation with creatine monohydrate and 5-hydroxytryptophan for selective serotonin reuptake inhibitor- or serotonin-norepinephrine reuptake inhibitor-resistant depression in adult women. J. Clin. Psychopharmacol. 2017, 37, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Toniolo, R.A.; Fernandes, F.B.F.; Silva, M.; Dias, R.S.; Lafer, B. Cognitive effects of creatine monohydrate adjunctive therapy in patients with bipolar depression: Results from a randomized, double-blind, placebo-controlled trial. J. Affect. Disord. 2017, 224, 69–75. [Google Scholar] [CrossRef]
- Toniolo, R.A.; Silva, M.; Fernandes, F.B.F.; Amaral, J.; Dias, R.D.S.; Lafer, B. A randomized, double-blind, placebo-controlled, proof-of-concept trial of creatine monohydrate as adjunctive treatment for bipolar depression. J. Neural Transm. 2018, 125, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Hellem, T.L.; Sung, Y.H.; Shi, X.F.; Pett, M.A.; Latendresse, G.; Morgan, J.; Huber, R.S.; Kuykendall, D.; Lundberg, K.J.; Renshaw, P.F. Creatine as a novel treatment for depression in females using methamphetamine: A pilot study. J. Dual Diagn. 2015, 11, 189–202. [Google Scholar] [CrossRef] [PubMed]
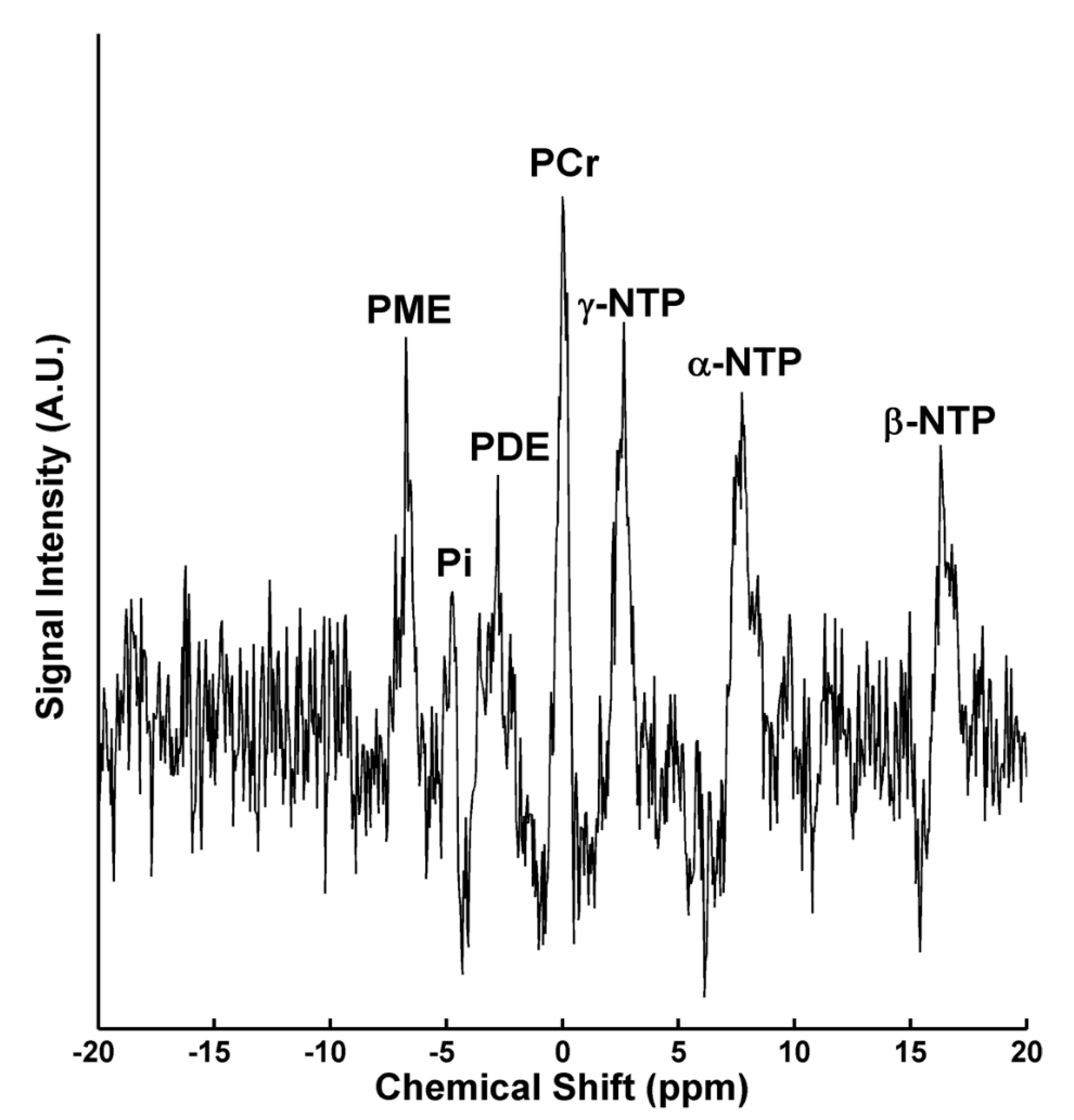
| Study | Condition/Control | Brain Region | Change Compared to Controls |
|---|---|---|---|
| 31P-MRS Studies reporting phosphocreatine levels in MDD and Bipolar Disorder | |||
| Kato 1992 [129] | MDD-D/MDD-E | 30mm frontal axial slice | None |
| Kato 1994 [154] | BDII/HC | 30mm frontal axial slice | ↓ |
| BDI/HC | 30mm F axial slice | None | |
| Murashita 2000 [161] | BD/HC (+ PS) | OCC | ↓ after PS in BD except in lithium responders |
| Pettegrew 2002 [139] | MDD/HC | PFC | ↑ after treatment associated with AD response |
| Iosifescu 2008 [134] | MDD/HC | 20 mm-thick axial slice | None overall but ↓ baseline PCr in those who responded to T3 |
| Sikoglu 2013 [164] | BD/HC | FL | None |
| Weber 2013 [155] | BD-E/HC | L VLP FC | ↓ |
| Yuksel 2015 [163] | BD/HC (+ PS) | OCC | ↓ after PS in HC but not BD; no difference in PCr at baseline |
| Dudley 2016 [156] | BD/HC | WB WM | ↓ |
| Harper 2016 [137] | MDD/HC | WB WM, WB GM | ↑ |
| Harper 2017 [138] | MDD/HC | WB WM, WB GM | ↑ in GM, ↓ in WM |
| 1H-MRS Studies Reporting total creatine levels in MDD and Bipolar Disorder | |||
| Hamakawa 1999 [141] | BD-D/BD-E | L FL | ↓ |
| Auer 2000 [113] | MDD/HC | B A CC | None |
| Farchione 2000 [114] | MDD/HC | B DL PFC | None |
| Kumar 2002 [115] | MDD/HC | DL WM, ACC | None |
| Cecil 2003 [142] | BD-D/HC | CV | ↓ |
| Deicken 2003 [143] | BD- E/HC | B Hippo | ↓ |
| Gruber 2003 [123] | MDD/HC | L PF WM | ↑ |
| Michael 2003 [122] | MDD/HC | L AMG | None |
| Pfleiderer 2003 [117] | MDD/HC | L A CC | None |
| Dager 2004 [147] | BD/HC | FL WM, BG, Thal | tCr inversely correlated with depression severity |
| Brambilla 2005 [150] | BD/HC | L DL PFC | None |
| Mirza 2006 [120] | MDD/HC | B Thal | None |
| Frye 2007 [150] | BD-D/HC | A CC, M CC, M PFC | ↑ |
| Gabbay 2007 [124] | MDD/HC | L CN | ↑ |
| Moore 2007 [152] | BD/HC | B ACC | None |
| Olvera 2007 [151] | BD/HC | L DLPFC | None |
| Patel 2008 [149] | BD-D/HC | B VL PFC | ↑ |
| Port 2008 [144] | BD-D/HC | R CN | ↓ |
| Nery 2009 [125] | MDD/HC | L DL PFC | ↑ in women ↓ in men |
| Öngür 2009 [153] | BD-M/HC | B ACC and POC | None |
| Venkatraman 2009 [128] | MDD/HC | M PFC | ↓ |
| Caetano 2011 [147] | BD/HC | R M PFC, L DL PFC WM | ↓ |
| Portella 2011 [118] | MDD/HC | B VM PFC | None |
| McEwen 2012 [116] | PPD/HC | B M PFC | None |
| Özdel 2012 [145] | BD-E/HC | B M PFC | ↓ |
| Bradley 2016 [121] | MDD/HC | B CN, B Put, B Thal | None |
| Li 2016 [126] | MDD/HC | P CC | ↓ |
| Njau 2017 [127] | MDD/HC | SG ACC, D ACC | ↑ |
| Rosa 2017 [119] | PPD/HC | B A CC, L DL PFC | None |
| Study | Population (n) | Design | Creatine Dose | Duration | Effect | Significant Adverse Effects Related to Creatine |
|---|---|---|---|---|---|---|
| Roitman 2007 [186] | MDD-D (n = 8); BD-D (n = 2) | Open-label, adjunctive | 3–5 g/day | 4 weeks | Average HAM-D scores declined from 23.1 at baseline to 12.6 at week 4 | Both bipolar subjects developed hypomania/mania |
| Kondo 2011 [133] | Adolescent girls with MDD-D (n = 5) | Open-label, adjunctive | 4 g/day | 8 weeks | The mean CDRS-R score fell by 50.6% | None |
| Kondo 2016 [170] | Adolescent and young-adult women with MDD-D (n = 34) | Open-label, adjunctive, dose-ranging | 2 g, 4 g, or 10 g/day | 8 weeks | Creatine increased frontal cortical phosphocreatine levels in a fashion associated with lower depression ratings | None |
| Lyoo 2012 [188] | Women with MDD-D (n = 52) | Randomized, double-blind, placebo-controlled, adjunctive | 3 g/day × 1 week then 5 g/day × 7 weeks | 8 weeks | HAM-D scores in the creatine group fell by 79.7% by week 8, compared to 62.5% in the placebo group | None |
| Nemets 2013 [189] | MDD-D (n = 18) | Randomized, double-blind, placebo-controlled, adjunctive | 5 g/day or 10 g/day | 4 weeks | No significant difference between creatine and placebo in HAM-D scores | None |
| Hellem 2015 [193] | Methamphetamine dependence with depression (n = 14) | Open-label, monotherapy | 5 g/day | 8 weeks | Mean HAM-D scores fell to 10.4 by week 2, representing response | Gastrointestinal symptoms (n = 5) and muscle cramps (n = 2) |
| Kious 2017 [190] | Women with MDD-D (n = 15) | Open-label, adjunctive | 5 g/day (with 5-HTP 200 mg twice daily) | 8 weeks | HAM-D scores improved by ~60% by week 8 | None |
| Toniolo 2017 [191] | BD-D (n = 18) | Randomized, double-blind, placebo-controlled, adjunctive | 6 g/day | 6 weeks | Significant improvement in verbal fluency but no significant changes in other measures reported | None |
| Toniolo 2018 [192] | BD-D (n = 53) | Randomized, double-blind, placebo-controlled, adjunctive | 6 g/day | 6 weeks | No significant difference in MADRS scores between groups, but MADRS remission rate was significantly greater in creatine group (52.9% vs. 11.1%) | Two participants in creatine group developed hypomania/mania |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kious, B.M.; Kondo, D.G.; Renshaw, P.F. Creatine for the Treatment of Depression. Biomolecules 2019, 9, 406. https://doi.org/10.3390/biom9090406
Kious BM, Kondo DG, Renshaw PF. Creatine for the Treatment of Depression. Biomolecules. 2019; 9(9):406. https://doi.org/10.3390/biom9090406
Chicago/Turabian StyleKious, Brent M., Douglas G. Kondo, and Perry F. Renshaw. 2019. "Creatine for the Treatment of Depression" Biomolecules 9, no. 9: 406. https://doi.org/10.3390/biom9090406
APA StyleKious, B. M., Kondo, D. G., & Renshaw, P. F. (2019). Creatine for the Treatment of Depression. Biomolecules, 9(9), 406. https://doi.org/10.3390/biom9090406





