Biodistribution and Biosafety of a Poly(Phosphorhydrazone) Dendrimer, an Anti-Inflammatory Drug-Candidate
Abstract
:1. Introduction
2. Materials and Methods
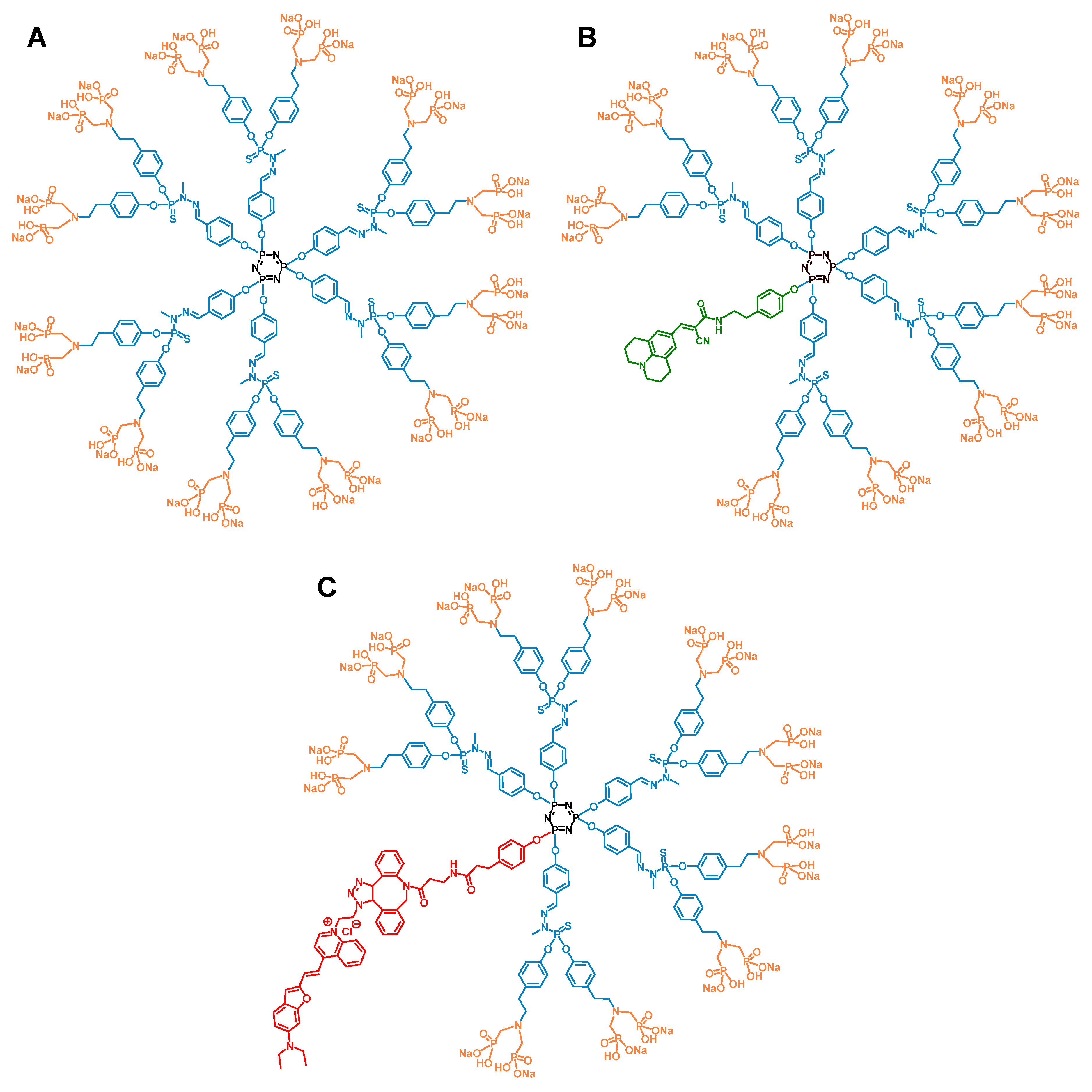
2.1. Dendrimers
Zeta Potential of the Dendrimers
2.2. Biodistribution
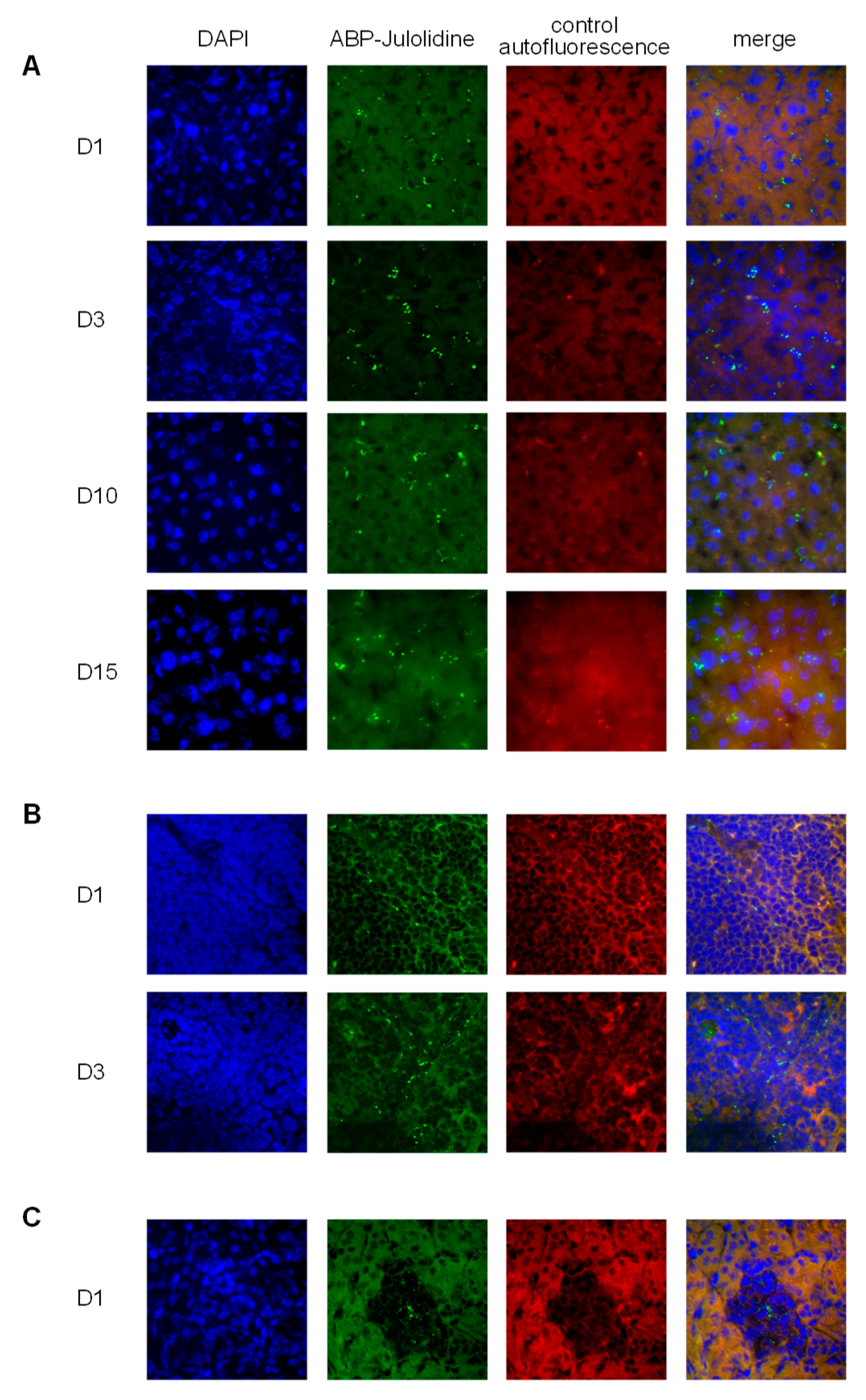
2.2.1. Biodistribution Using the ABP-Julolidine Dendrimer
2.2.2. Biodistribution Using the ABP-NIR Dendrimer
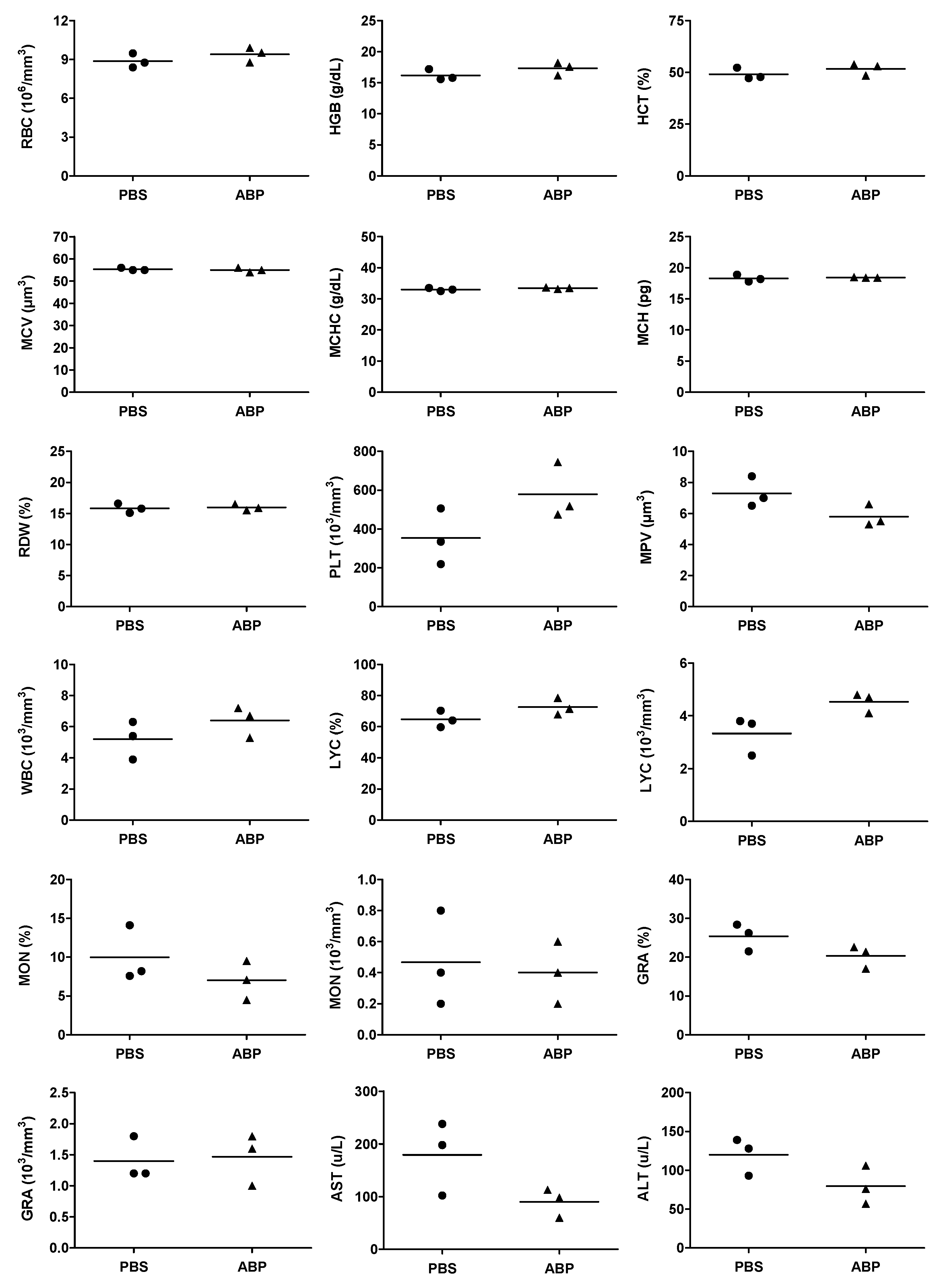
2.3. Hematological Safety
2.4. Genotoxicity
2.4.1. BN Ames’ Test
2.4.2. In Vitro Micronucleus Test
2.5. Maximal Tolerated Dose (MTD)
2.5.1. Single Injection in Mice and Rats
2.5.2. Repeated Injections in Rats
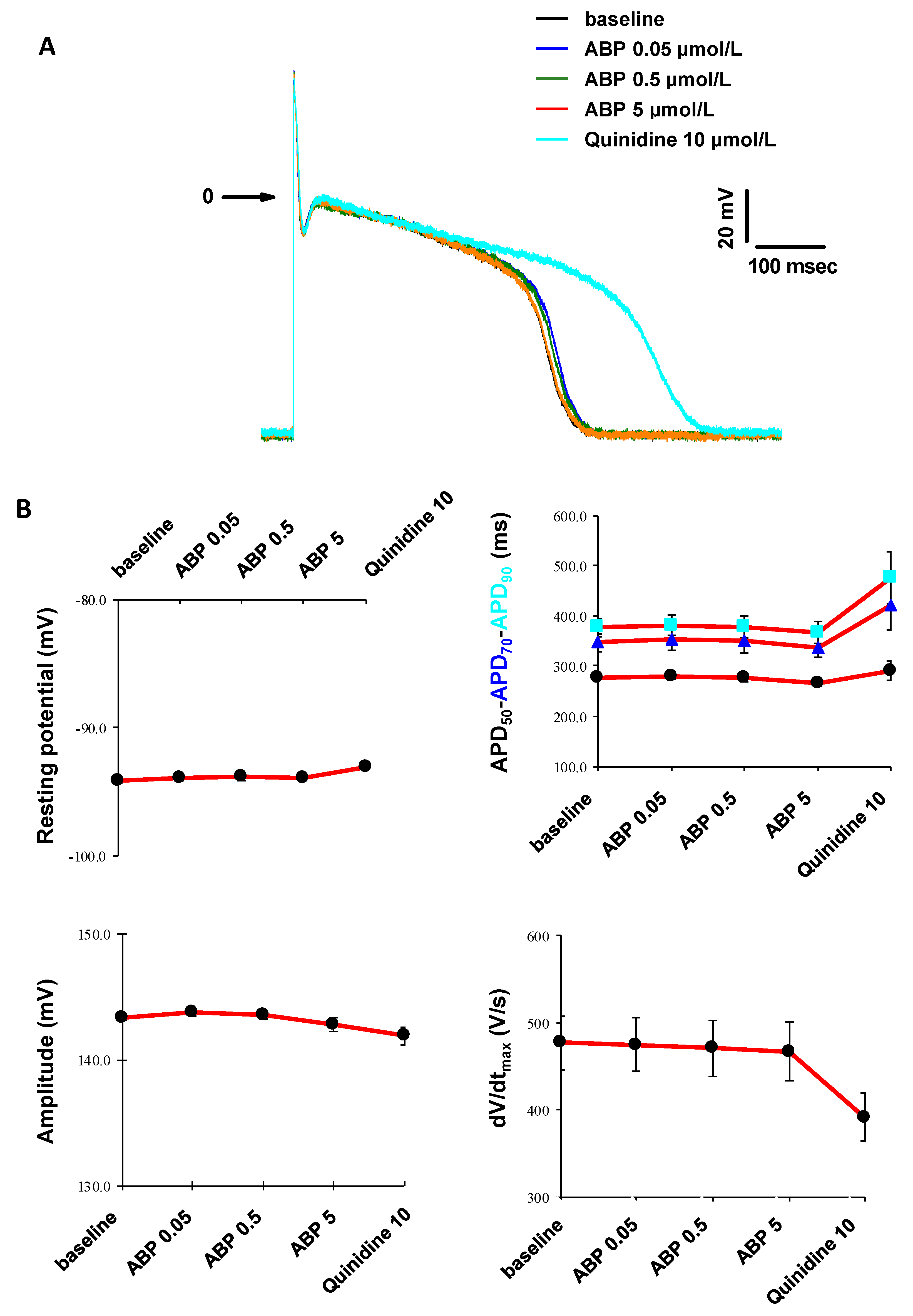
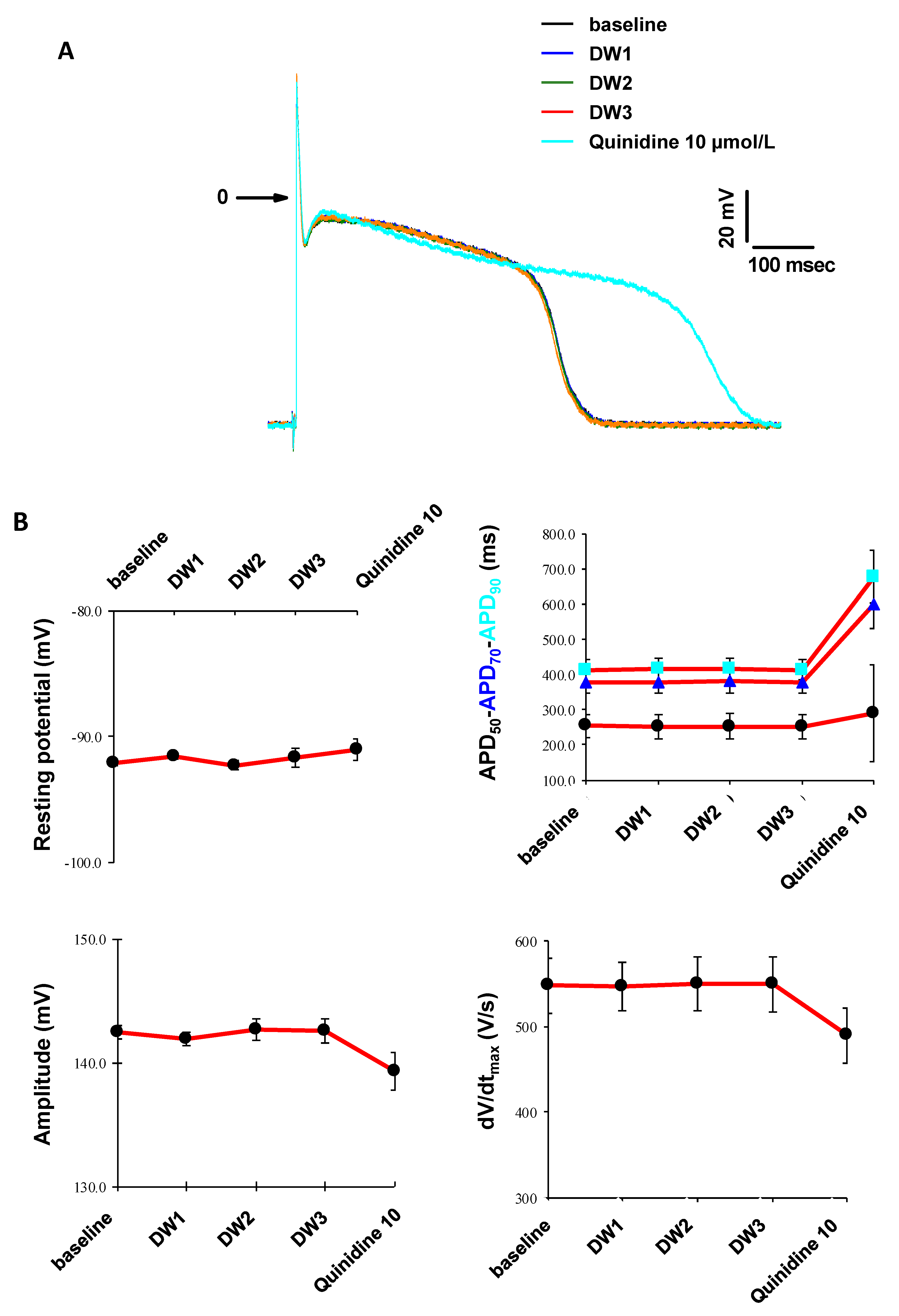
2.6. In Vitro Cardiac Safety
2.7. Statistical Analyses
2.7.1. Biodistribution Using the ABP-NIR Dendrimer
2.7.2. Hematological Safety
2.7.3. Genotoxicity
3. Results
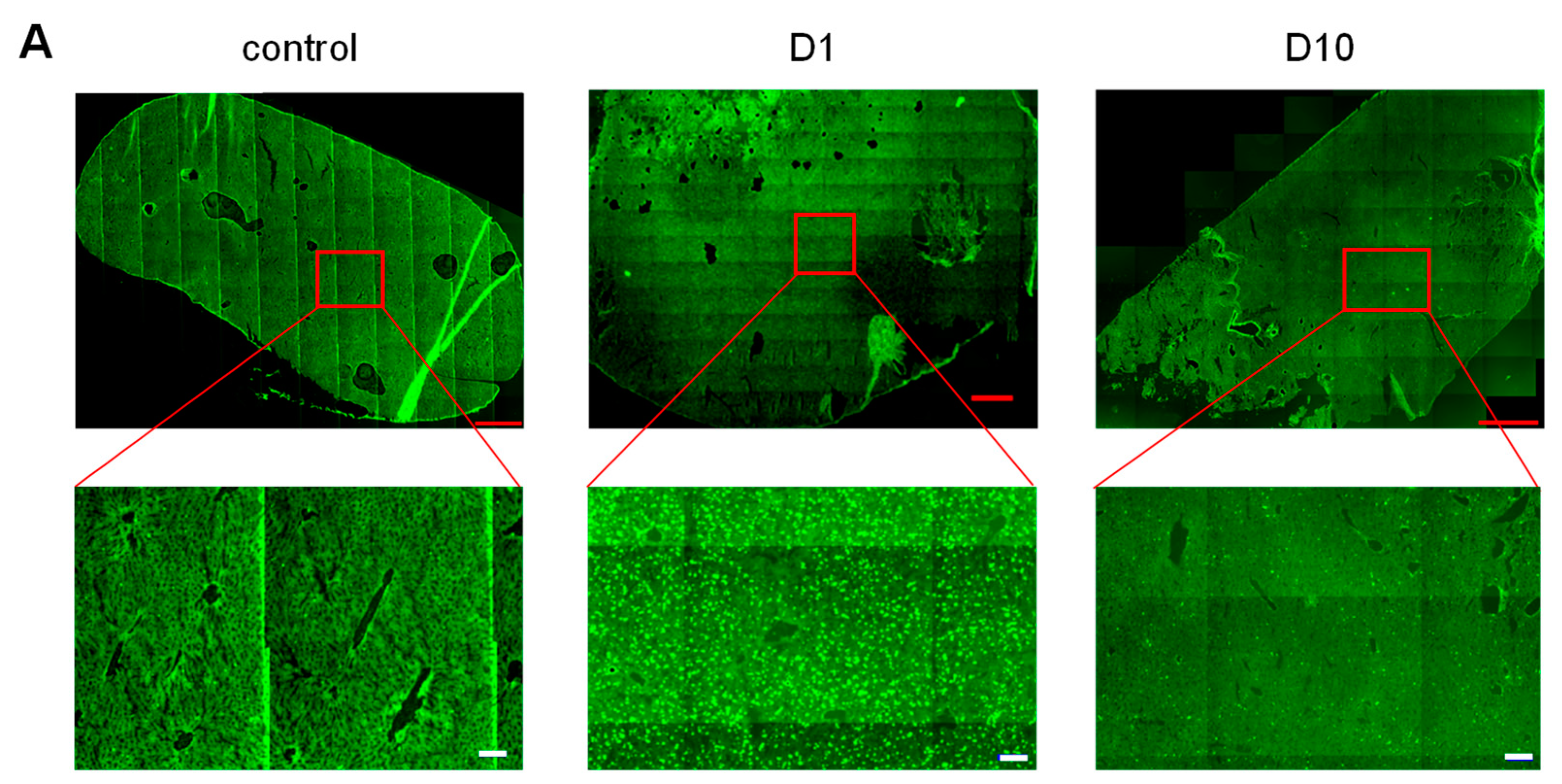
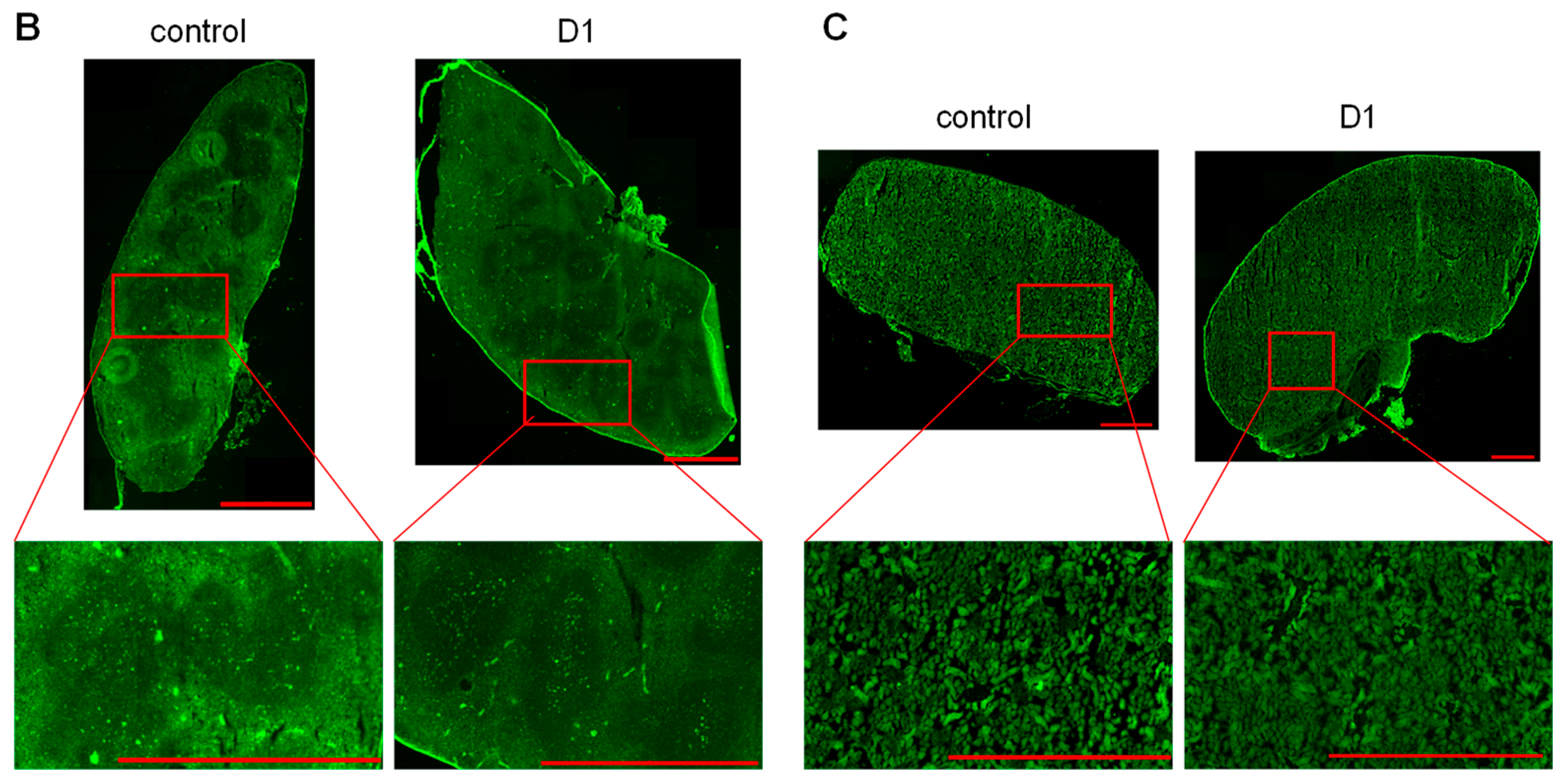
3.1. Biodistribution
3.2. Hematological Safety
3.3. Genotoxicity
3.3.1. BN Ames’ Test
- for the strain TA 1537, a compound that induces a dose-dependent increase in the number of reverse mutations along at least three consecutive doses, with the greatest increase being greater than or equal to three times that of the untreated control (which gives the number of spontaneous reverse mutations) is considered positive to the test;
- for the strains TA 100 and TA 98, a compound that induces a dose-dependent increase in the number of reverse mutations along at least three consecutive doses, with the greatest increase being greater than or equal to twice that of the untreated control (which gives the number of spontaneous reverse mutations) is considered positive to the test.
3.3.2. In Vitro Micronucleus Test
3.4. Maximal Tolerated Dose (MTD)
3.4.1. Single Injection in Mice and Rats
3.4.2. Repeated Injections in Rat
3.5. In Vitro Cardiac Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fruchon, S.; Poupot, R. Pro-inflammatory versus anti-inflammatory effects of dendrimers: The two faces of immuno-modulatory nanoparticles. Nanomaterials 2017, 7, 251. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.W.J.; Wilson, O.M.; Crooks, R.M. Synthesis, characterization, and applications of dendrimer-encapsulated nanoparticles. J. Phys. Chem. B 2005, 109, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Slany, M.; Bardaji, M.; Casanove, M.J.; Caminade, A.M.; Majoral, J.P.; Chaudret, B. Dendrimer surface chemistry. An easy access to poly phosphines and their gold complexes. J. Am. Chem. Soc. 1995, 117, 9764–9765. [Google Scholar] [CrossRef]
- Fruchon, S.; Poupot, M.; Martinet, L.; Turrin, C.O.; Majoral, J.P.; Fournié, J.J.; Caminade, A.M.; Poupot, R. Anti-inflammatory and immuno-suppressive activation of human monocytes by a bio-active dendrimer. J. Leukoc. Biol. 2009, 85, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Portevin, D.; Poupot, M.; Rolland, O.; Turrin, C.O.; Fournié, J.J.; Majoral, J.P.; Caminade, A.M.; Poupot, R. Regulatory activity of azabisphosphonate-capped dendrimers on human CD4+ T cell proliferation enhances ex-vivo expansion of NK cells from PBMCs for immunotherapy. J. Transl. Med. 2009, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Degboé, Y.; Fruchon, S.; Baron, M.; Nigon, D.; Turrin, C.O.; Caminade, A.M.; Poupot, R.; Cantagrel, A.; Davignon, J.L. Modulation of pro-inflammatory activation of monocytes and dendritic cells by aza-bis-phosphonate dendrimer as an experimental therapeutic agent. Arthritis Res. Ther. 2014, 16, R98. [Google Scholar] [CrossRef] [PubMed]
- Poupot, M.; Turrin, C.O.; Caminade, A.M.; Fournié, J.J.; Attal, M.; Poupot, R.; Fruchon, S. Poly(phosphorhydrazone) dendrimers: Yin and yang of monocyte activation for human NK cell amplification applied to immunotherapy against Multiple Myeloma. Nanomedicine 2016, 12, 2321–2330. [Google Scholar] [CrossRef] [PubMed]
- Hayder, M.; Poupot, M.; Baron, M.; Nigon, D.; Turrin, C.O.; Caminade, A.M.; Majoral, J.P.; Eisenberg, R.A.; Fournié, J.J.; Cantagrel, A.; et al. A phosphorus-based dendrimer targets inflammation and osteoclastogenesis in experimental arthritis. Sci. Transl. Med. 2011, 3, 81ra35. [Google Scholar] [CrossRef] [PubMed]
- Hayder, M.; Garzoni, M.; Bochicchio, D.; Caminade, A.M.; Couderc, F.; Ong-Meang, V.; Davignon, J.L.; Turrin, C.O.; Pavan, G.M.; Poupot, R. Three-dimensional directionality is a pivotal structural feature for the bioactivity of azabisphosphonate-capped poly(phosphorhydrazone) nanodrug dendrimers. Biomacromolecules 2018, 19, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Fruchon, S.; Caminade, A.M.; Abadie, C.; Davignon, J.L.; Combette, J.M.; Turrin, C.O.; Poupot, R. An azabisphosphonate-capped poly(phosphorhydrazone) dendrimer for the treatment of endotoxin-induced uveitis. Molecules 2013, 18, 9305–9316. [Google Scholar] [CrossRef] [PubMed]
- Hayder, M.; Varilh, M.; Turrin, C.O.; Saoudi, A.; Caminade, A.M.; Poupot, R.; Liblau, R.S. Phosphorus-based dendrimer ABP treats neuroinflammation by promoting IL-10-producing CD4+ T cells. Biomacromolecules 2015, 16, 3425–3433. [Google Scholar] [CrossRef] [PubMed]
- Fruchon, S.; Poupot, R. The ABP dendrimer, a drug-candidate against inflammatory diseases that triggers the activation of interleukin-10 producing immune cells. Molecules 2018, 23, 1272. [Google Scholar] [CrossRef] [PubMed]
- Colvin, V.L. The potential environmental impact of engineered nanomaterials. Nat. Biotechnol. 2003, 21, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Hoet, P.H.M.; Nemmar, A.; Nemery, B. Health impact of nanomaterials? Nat. Biotechnol. 2004, 22, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Germolec, D.R.; Weaver, J.L. Evaluation of nanoparticle immunotoxicity. Nat. Nanotechnol. 2009, 4, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Fruchon, S.; Mouriot, S.; Thiollier, T.; Grandin, C.; Caminade, A.M.; Turrin, C.O.; Contamin, H.; Poupot, R. Repeated intravenous injections in non-human primates demonstrate preclinical safety of an anti-inflammatory phosphorus-based dendrimer. Nanotoxicology 2015, 9, 933–941. [Google Scholar] [CrossRef]
- Poupot, M.; Griffe, L.; Marchand, P.; Maraval, A.; Rolland, O.; Martinet, L.; L’Faqihi-Olive, F.E.; Turrin, C.O.; Caminade, A.M.; Fournié, J.J.; et al. Design of phosphorylated dendritic architectures to promote human monocyte activation. FASEB J. 2006, 20, 2339–2351. [Google Scholar] [CrossRef]
- Rolland, O.; Griffe, L.; Poupot, M.; Maraval, A.; Ouali, A.; Coppel, Y.; Fournié, J.J.; Bacquet, G.; Turrin, C.O.; Caminade, A.M.; et al. Tailored control and optimization of the number of phosphonic acid termini on phosphorus-containing dendrimers for the ex-vivo activation of human monocytes. Chem. Eur. J. 2008, 14, 4836–4850. [Google Scholar] [CrossRef]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983, 113, 173–215. [Google Scholar] [CrossRef]
- Kirkland, D.J.; Marshall, R.R.; McEnaney, S.; Bilgoog, J.; Rutter, A.; Mullineux, S. Aroclor-1254-induced rat-liver causes chromosomal aberrations in CHO cells but not human lymphocytes: A role for active oxygen? Mutat. Res. 1989, 214, 115–122. [Google Scholar] [CrossRef]
- Nesslany, F.; Marzin, D. A micromethod for in vitro micronucleus assay. Mutagenesis 1999, 14, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxic assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Borenfreud, E.; Babich, H.; Martin-Alguacil, N. Comparison of two in vitro cytotoxicity assays: The neutral red (NR) and tetrazolium MTT tests. Toxicol. In Vitro 1988, 2, 1–6. [Google Scholar] [CrossRef]
- Miller, B.M.; Pujadas, E.; Gocke, E. Evaluation of the micronucleus test in vitro using Chinese hamster cells: Results of four chemicals weakly positive in the in vivo micronucleus test. Environ. Mol. Mutagen. 1995, 26, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Corvi, R.; Madia, F. In Vitro genotoxicity testing–Can the performance be enhanced? Food Chem. Toxicol. 2017, 106 Pt B, 600–608. [Google Scholar] [CrossRef]
- Ames, B.N.; McCann, J.; Yamasaki, E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat. Res. 1975, 31, 347–364. [Google Scholar] [CrossRef]
- Kirsch-Volders, M.; Elhajouji, A.; Cundari, E.; Van Hummelen, P. The in vitro micronucleus test: A multi-endpoint assay to detect simultaneously mitotic delay, apoptosis, chromosome breakage, chromosome loss and non-disjunction. Mutat. Res. 1997, 392, 19–30. [Google Scholar] [CrossRef]
- Soubret, A.; Helmlinger, G.; Dumotier, B.; Bibas, R.; Georgevia, A. Modeling and simulation of preclinical cardiac safety: Towards an integrative framework. Drug Metab. Pharmacokinet. 2009, 24, 76–90. [Google Scholar] [CrossRef]
- Dumotier, B.M.; Adamantidis, M.M.; Puisieux, F.L.; Bastide, M.M.; Dupuis, B.A. Repercussions of pharmacologic reduction in ionic currents on action potential configuration in rabbit Purkinje fibers: Are they indicative of proarrhythmic potential? Drug Dev. Res. 1999, 47, 63–76. [Google Scholar] [CrossRef]
- Shaunak, S. Perspective: Dendrimer drugs for infection and inflammation. Biochem. Biophys. Res. Commun. 2015, 468, 435–441. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Lo, S.T.; Stern, S.; Clogston, J.D.; Zheng, J.; Adiseshaiah, P.P.; Dobrovolskaia, M.; Lim, J.; Patri, A.K.; Sun, X.; Simanek, E.E. Biological assessment of triazine dendrimer: Toxicological profiles, solution behavior, biodistribution, drug release and efficacy in a PEGylated, paclitaxel construct. Mol. Pharm. 2010, 74, 993–1006. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Porterfield, J.E.; Smith, E.; Sharma, R.; Kannan, S.; Kannan, R.M. Effect of mannose targeting of hydroxyl PAMAM dendrimers on cellular and organ biodistribution in a neonatal brain injury model. J. Control. Release 2018, 283, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, G.; Greish, K.; Ghandehari, H. Charge affects the oral toxicity of poly(amidoamine) dendrimers. Eur. J. Pharm. Biopharm. 2013, 84, 330–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enciso, A.E.; Neun, B.; Rodriguez, J.; Ranjan, A.P.; Dobrovolskaia, M.A.; Simanek, E.E. Nanoparticle effects on human platelets in vitro: A comparison between PAMAM and triazine dendrimers. Molecules 2016, 21, 428. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Kesharwani, P.; Gupta, U.; Jain, N.K. Dendrimer toxicity: Let’s meet the challenge. Int. J. Pharm. 2010, 394, 122–142. [Google Scholar] [CrossRef]
- Caminade, A.M.; Fruchon, S.; Turrin, C.O.; Poupot, M.; Ouali, A.; Maraval, A.; Garzoni, M.; Maly, M.; Furer, V.; Kovalenko, V.; et al. The key role of the scaffold on the efficiency of dendrimer nanodrugs. Nat. Commun. 2015, 6, 7722. [Google Scholar] [CrossRef]
- Svenson, S. The dendrimer paradox—High medical expectations but poor clinical translation. Chem. Soc. Rev. 2015, 44, 4131–4144. [Google Scholar] [CrossRef]
- Ledall, J.; Fruchon, S.; Garzoni, M.; Pavan, G.M.; Caminade, A.M.; Turrin, C.O.; Blanzat, M.; Poupot, R. Interaction studies reveal specific recognition of an anti-inflammatory polyphosphorhydrazone dendrimer by human monocytes. Nanoscale 2015, 7, 17672–17684. [Google Scholar] [CrossRef]


| TA 1537 | TA 100 | TA 98 | |||||
|---|---|---|---|---|---|---|---|
| Dose (µg/Dish) | Mutants/Dish | Ratio | Mutants/Dish | Ratio | Mutants/Dish | Ratio | |
| without metabolic activation | untreated | 6.5 | 111.5 | 24 | |||
| control + 1 | 681.7 | 105 ** | 2380.3 | 21.3 | 636.3 | 26.5 ** | |
| 0.39 | 7 | 1.1 | 110.3 | 1 | 21.3 | 0.9 | |
| 0.78 | 9.7 | 1.5 | 128.7 | 1.2 | 26 | 1.1 | |
| 1.56 | 7 | 1.1 | 133 | 1.2 | 26.7 | 1.1 | |
| 3.13 | 7 | 1.1 | 132.3 | 1.2 | 32 | 1.3 | |
| 6.25 | 6.7 | 1 | 119.3 | 1.1 | 27.7 | 1.2 | |
| 12.5 | 9 | 1.4 | 126 | 1.1 | 28 | 1.2 | |
| 25 | 9.3 | 1.4 | 124.3 | 1.1 | 29.3 | 1.2 | |
| 50 | 7.7 | 1.2 | 115.7 | 1 | 33 | 1.4 | |
| 100 | 7 | 1.1 | 114.3 | 1 | 27.7 | 1.2 | |
| 200 | 11.3 | 1.7 | 123.3 | 1.1 | 37.3 | 1.6 ** | |
| with metabolic activation (S9 mix) | untreated | 7.8 | 106.3 | 28.4 | |||
| control + 2 | 177.3 | 22.7 ** | 1498.3 | 14.1 ** | 1686.7 | 59.4 ** | |
| 0.39 | 7.7 | 1 | 125.7 | 1.2 | 28.7 | 1 | |
| 0.78 | 7 | 0.9 | 105.3 | 1 | 35.3 | 1.2 | |
| 1.56 | 7.7 | 1 | 118.3 | 1.1 | 32.3 | 1.1 | |
| 3.13 | 7 | 0.9 | 113.3 | 1.1 | 33 | 1.2 | |
| 6.25 | 7.7 | 1 | 138.3 | 1.3 ** | 30.3 | 1.1 | |
| 12.5 | 5.3 | 0.7 | 123.3 | 1.2 | 31.3 | 1.1 | |
| 25 | 8 | 1 | 111.3 | 1 | 35.7 | 1.3 | |
| 50 | 5.3 | 0.7 | 113.7 | 1.1 | 38 | 1.3 | |
| 100 | 6.7 | 0.9 | 115.7 | 1.1 | 33 | 1.2 | |
| 200 | 4.3 | 0.6 | 127.7 | 1.2 | 26.7 | 0.9 | |
| Dose (µM) | Relative % of Survival | Total Number of Micronuclei for 2000 Mononuclear Cells | Relative % of Survival | Total Number of Micronuclei for 2000 Mononuclear Cells | |
|---|---|---|---|---|---|
| without recovery | with recovery | ||||
| without metabolic activation | untreated | 100 | 3 | 100 | 3 |
| control + 1 | 88.9 | 40 *** | 90.8 | 75 *** | |
| 430 | 31.1 | nd 2 | 51.2 | 17 ** | |
| 215 | 79.2 | 9 * | 99.9 | 12 * | |
| 107 | 69.8 | 4 | 96.2 | 4 | |
| 53.7 | 80.3 | 3 | 111 | nd 2 | |
| with metabolic activation (S9 mix) | untreated | 100 | 3 | ||
| control + 3 | 85.1 | 239 *** | |||
| 773 | 127 | 4 | |||
| 387 | 145 | 2 | |||
| 193 | 119 | 3 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fruchon, S.; Bellard, E.; Beton, N.; Goursat, C.; Oukhrib, A.; Caminade, A.-M.; Blanzat, M.; Turrin, C.-O.; Golzio, M.; Poupot, R. Biodistribution and Biosafety of a Poly(Phosphorhydrazone) Dendrimer, an Anti-Inflammatory Drug-Candidate. Biomolecules 2019, 9, 475. https://doi.org/10.3390/biom9090475
Fruchon S, Bellard E, Beton N, Goursat C, Oukhrib A, Caminade A-M, Blanzat M, Turrin C-O, Golzio M, Poupot R. Biodistribution and Biosafety of a Poly(Phosphorhydrazone) Dendrimer, an Anti-Inflammatory Drug-Candidate. Biomolecules. 2019; 9(9):475. https://doi.org/10.3390/biom9090475
Chicago/Turabian StyleFruchon, Séverine, Elisabeth Bellard, Nicolas Beton, Cécile Goursat, Abdelouahd Oukhrib, Anne-Marie Caminade, Muriel Blanzat, Cédric-Olivier Turrin, Muriel Golzio, and Rémy Poupot. 2019. "Biodistribution and Biosafety of a Poly(Phosphorhydrazone) Dendrimer, an Anti-Inflammatory Drug-Candidate" Biomolecules 9, no. 9: 475. https://doi.org/10.3390/biom9090475
APA StyleFruchon, S., Bellard, E., Beton, N., Goursat, C., Oukhrib, A., Caminade, A.-M., Blanzat, M., Turrin, C.-O., Golzio, M., & Poupot, R. (2019). Biodistribution and Biosafety of a Poly(Phosphorhydrazone) Dendrimer, an Anti-Inflammatory Drug-Candidate. Biomolecules, 9(9), 475. https://doi.org/10.3390/biom9090475







