Connexin43 is Dispensable for Early Stage Human Mesenchymal Stem Cell Adipogenic Differentiation But is Protective against Cell Senescence
Abstract
1. Introduction
2. Materials and Methods
2.1. Human iPSC Cultures
2.2. MSC Differentiation and Culture
2.3. CRISPR-Cas9 Gene Ablation
2.4. Flow Cytometry
2.5. Adipogenic Differentiation of MSCs
2.6. Immunocytochemistry Labeling and LipidTox Green Neutral Lipid Stain Analysis
2.7. Scrape Loading Dye Transfer
2.8. Western Blot Analysis
2.9. Senescence-Associated-β-Galactosidase Assay
2.10. Statistics
3. Results
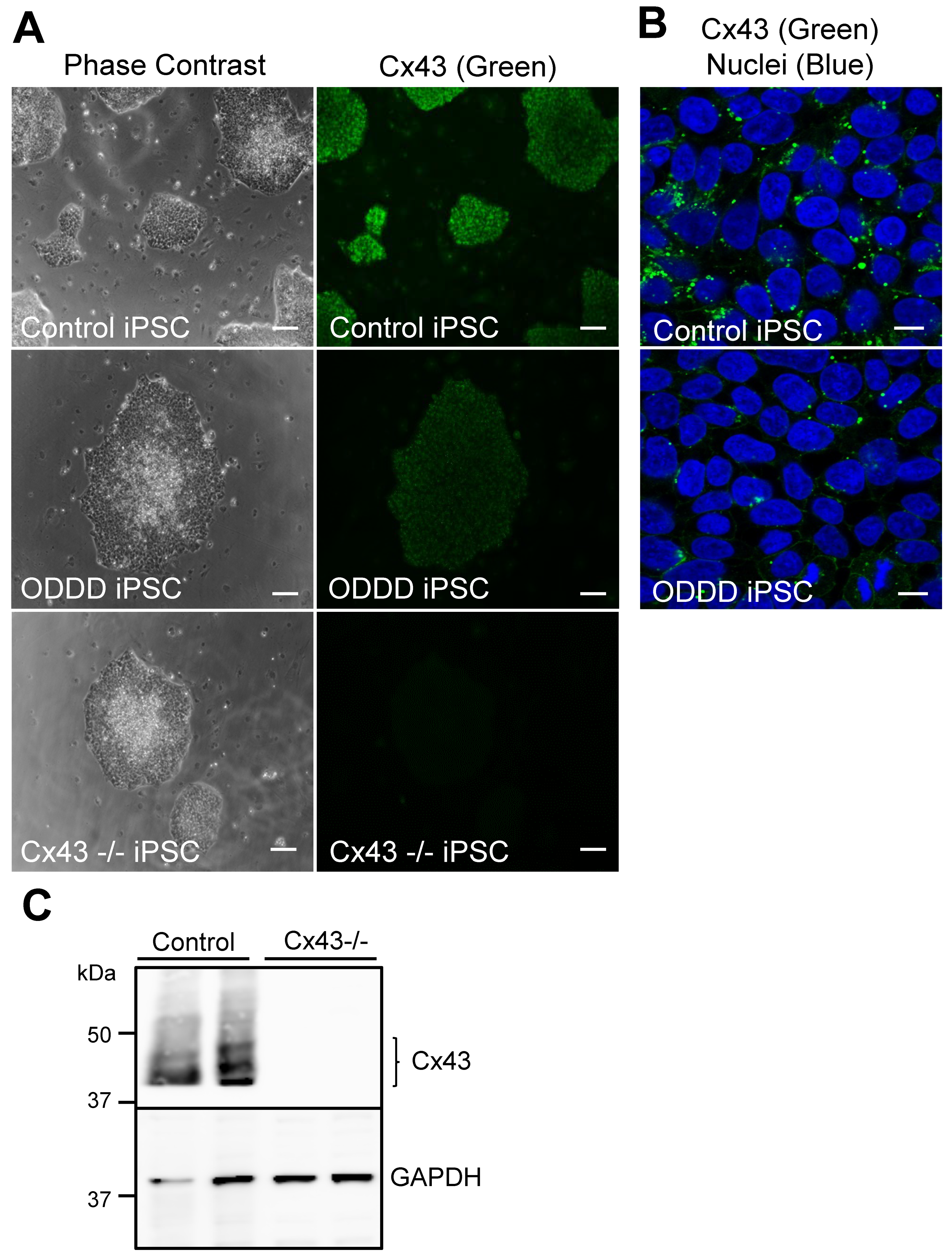
3.1. Differentiation of iPSCs into MSCs Occurs Independent of Cx43
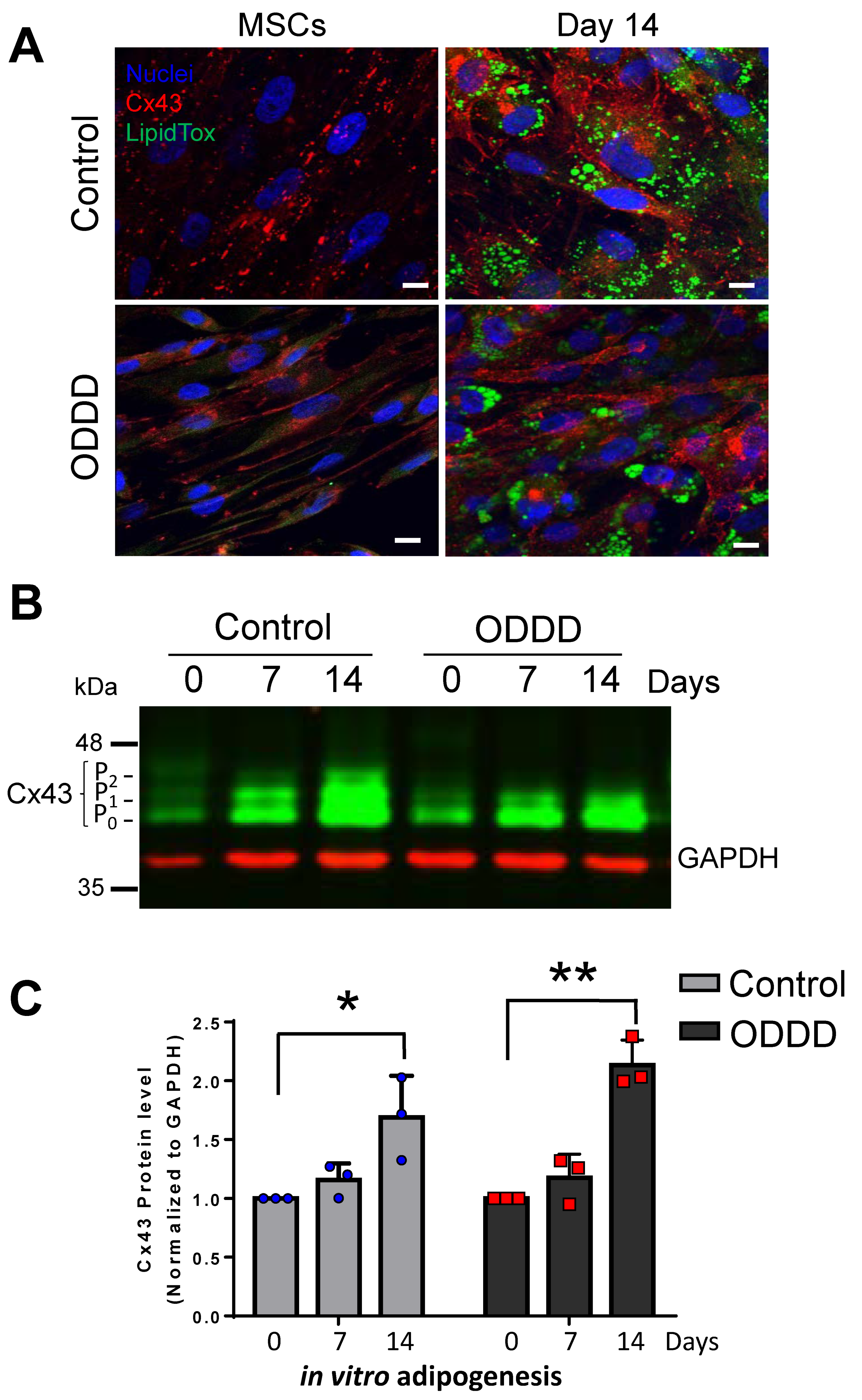
3.2. Cx43 is Upregulated during in vitro Adipogenic Differentiation of MSCs
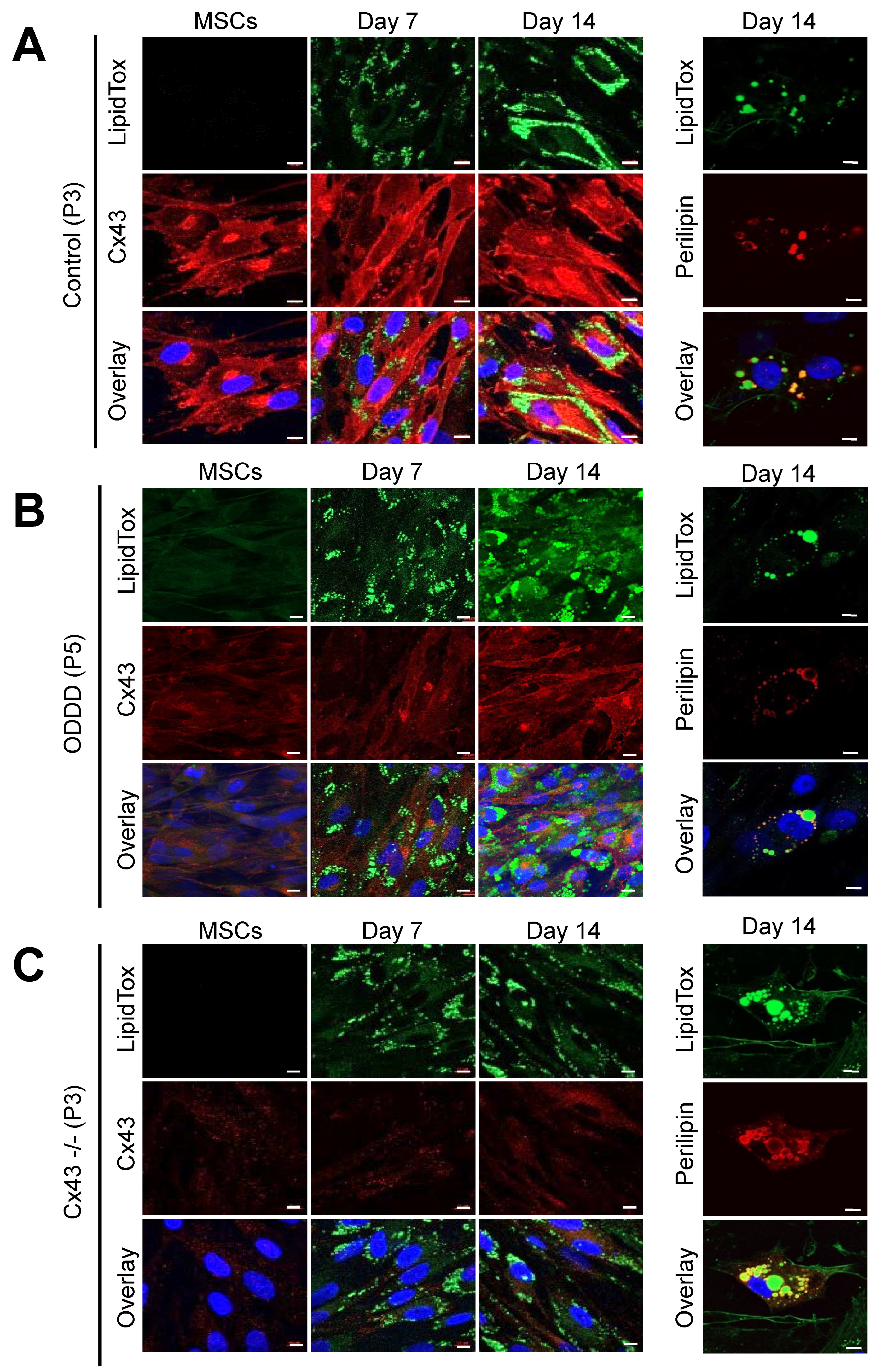
3.3. MSCs Expressing Cx43, a Cx43 Mutant or Lacking Cx43 Retain Adipogenic Potential
3.4. Cx43-Deficient MSCs Undergo Premature Cellular Senescence and Loss the Ability to Differentiate
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GJIC | gap junctional intercellular communication |
| Cx43 | connexin43 |
| iPSC | induced pluripotent stem cells |
| MSC | mesenchymal stem cells |
| ODDD | oculodentodigital dysplasia |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| qPCR | quantitative polymerase chain reaction |
References
- Gerace, D.; Martiniello-Wilks, R.; Nassif, N.T.; Lal, S.; Steptoe, R.; Simpson, A.M. CRISPR-targeted genome editing of mesenchymal stem cell-derived therapies for type 1 diabetes: A path to clinical success? Stem Cell Res. Ther. 2017, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Marofi, F.; Vahedi, G.; Biglari, A.; Esmaeilzadeh, A.; Athari, S.S. Mesenchymal Stromal/Stem Cells: A New Era in the Cell-Based Targeted Gene Therapy of Cancer. Front. Immunol. 2017, 8, 1770. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G. Biochemical heterogeneity of mesenchymal stem cell populations: Clues to their therapeutic efficacy. Cell Cycle 2007, 6, 2884–2889. [Google Scholar] [CrossRef] [PubMed]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical Trials with Mesenchymal Stem Cells: An Update. Cell Transpl. 2016, 25, 829–848. [Google Scholar] [CrossRef] [PubMed]
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Hass, R.; Kasper, C.; Bohm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011, 9, 12. [Google Scholar] [CrossRef]
- Wang, H.; Xi, Y.; Zheng, Y.; Wang, X.; Cooney, A.J. Generation of electrophysiologically functional cardiomyocytes from mouse induced pluripotent stem cells. Stem Cell Res 2016, 16, 522–530. [Google Scholar] [CrossRef]
- Worsdorfer, P.; Bosen, F.; Gebhardt, M.; Russ, N.; Zimmermann, K.; Kessie, D.K.; Sekaran, T.; Egert, A.; Ergun, S.; Schorle, H.; et al. Abrogation of Gap Junctional Communication in ES Cells Results in a Disruption of Primitive Endoderm Formation in Embryoid Bodies. Stem Cells 2017, 35, 859–871. [Google Scholar] [CrossRef]
- Esseltine, J.L.; Shao, Q.; Brooks, C.; Sampson, J.; Betts, D.H.; Seguin, C.A.; Laird, D.W. Connexin43 Mutant Patient-Derived Induced Pluripotent Stem Cells Exhibit Altered Differentiation Potential. J. Bone Miner. Res. 2017, 32, 1368–1385. [Google Scholar] [CrossRef]
- Laird, D.W.; Lampe, P.D. Therapeutic strategies targeting connexins. Nat. Rev. Drug Discov. 2018, 12, 905–921. [Google Scholar] [CrossRef]
- Leybaert, L.; Lampe, P.D.; Dhein, S.; Kwak, B.R.; Ferdinandy, P.; Beyer, E.C.; Laird, D.W.; Naus, C.C.; Green, C.R.; Schulz, R. Connexins in Cardiovascular and Neurovascular Health and Disease: Pharmacological Implications. Pharmacol. Rev. 2017, 69, 396–478. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.K.; Bliss, S.A.; Patel, S.A.; Taborga, M.; Dave, M.A.; Gregory, L.A.; Greco, S.J.; Bryan, M.; Patel, P.S.; Rameshwar, P. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 2011, 71, 1550–1560. [Google Scholar] [CrossRef] [PubMed]
- Schajnovitz, A.; Itkin, T.; D’Uva, G.; Kalinkovich, A.; Golan, K.; Ludin, A.; Cohen, D.; Shulman, Z.; Avigdor, A.; Nagler, A.; et al. CXCL12 secretion by bone marrow stromal cells is dependent on cell contact and mediated by connexin-43 and connexin-45 gap junctions. Nat. Immunol. 2011, 12, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, M.; Berberich, O.; Hoefner, C.; Blunk, T.; Bauer-Kreisel, P. Gap junctional intercellular communication in adipose-derived stromal/stem cells is cell density-dependent and positively impacts adipogenic differentiation. J. Cell. Physiol. 2018, 233, 3315–3329. [Google Scholar] [CrossRef] [PubMed]
- Campbell, F.R. Gap junctions between cells of bone marrow: An ultrastructural study using tannic acid. Anat. Rec. 1980, 196, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Valiunas, V.; Doronin, S.; Valiuniene, L.; Potapova, I.; Zuckerman, J.; Walcott, B.; Robinson, R.B.; Rosen, M.R.; Brink, P.R.; Cohen, I.S. Human mesenchymal stem cells make cardiac connexins and form functional gap junctions. J. Physiol. 2004, 555, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, B. Assembly and disassembly of gap junctions during mesenchymal cell condensation and early chondrogenesis in limb buds of mouse embryos. J. Anat. 1984, 138, 351–363. [Google Scholar]
- Paznekas, W.A.; Boyadjiev, S.A.; Shapiro, R.E.; Daniels, O.; Wollnik, B.; Keegan, C.E.; Innis, J.W.; Dinulos, M.B.; Christian, C.; Hannibal, M.C.; et al. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am. J. Hum. Genet. 2003, 72, 408–418. [Google Scholar] [CrossRef]
- Paznekas, W.A.; Karczeski, B.; Vermeer, S.; Lowry, R.B.; Delatycki, M.; Laurence, F.; Koivisto, P.A.; Van Maldergem, L.; Boyadjiev, S.A.; Bodurtha, J.N.; et al. GJA1 mutations, variants, and connexin 43 dysfunction as it relates to the oculodentodigital dysplasia phenotype. Hum. Mutat. 2009, 30, 724–733. [Google Scholar] [CrossRef]
- Buo, A.M.; Stains, J.P. Gap junctional regulation of signal transduction in bone cells. FEBS Lett. 2014, 588, 1315–1321. [Google Scholar] [CrossRef]
- Plotkin, L.I.; Laird, D.W.; Amedee, J. Role of connexins and pannexins during ontogeny, regeneration, and pathologies of bone. BMC Cell Biol. 2016, 17 (Suppl. 1), 19. [Google Scholar] [CrossRef]
- Stains, J.P.; Civitelli, R. Connexins in the skeleton. Semin. Cell Dev. Biol. 2016, 50, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.N.; Kwon, H.J.; Im, S.W.; Son, Y.H.; Akindehin, S.; Jung, Y.S.; Lee, S.J.; Rhyu, I.J.; Kim, I.Y.; Seong, J.K.; et al. Connexin 43 is required for the maintenance of mitochondrial integrity in brown adipose tissue. Sci. Rep. 2017, 7, 7159. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gao, Y.; Tao, C.; Shao, M.; Zhao, S.; Huang, W.; Yao, T.; Johnson, J.A.; Liu, T.; Cypess, A.M.; et al. Connexin 43 Mediates White Adipose Tissue Beiging by Facilitating the Propagation of Sympathetic Neuronal Signals. Cell Metab. 2016, 24, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.; Nagajyothi, F.; Thi, M.M.; Hanani, M.; Scherer, P.E.; Tanowitz, H.B.; Spray, D.C. Adipocytes in both brown and white adipose tissue of adult mice are functionally connected via gap junctions: Implications for Chagas disease. Microbes Infect. 2014, 16, 893–901. [Google Scholar] [CrossRef]
- Revel, J.P.; Yee, A.G.; Hudspeth, A.J. Gap junctions between electrotonically coupled cells in tissue culture and in brown fat. Proc. Natl. Acad. Sci. USA 1971, 68, 2924–2927. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Picard, G.; Carpentier, J.L.; Girardier, L. Quantitative evaluation of gap junctions in rat brown adipose tissue after cold acclimation. J. Membr. Biol. 1984, 78, 85–89. [Google Scholar] [CrossRef]
- Statuto, M.; Bianchi, C.; Perego, R.; Del Monte, U. Drop of connexin 43 in replicative senescence of human fibroblasts HEL-299 as a possible biomarker of senescence. Exp. Gerontol. 2002, 37, 1113–1120. [Google Scholar] [CrossRef]
- Yeh, H.I.; Chang, H.M.; Lu, W.W.; Lee, Y.N.; Ko, Y.S.; Severs, N.J.; Tsai, C.H. Age-related alteration of gap junction distribution and connexin expression in rat aortic endothelium. J. Histochem. Cytochem. 2000, 48, 1377–1389. [Google Scholar] [CrossRef]
- Ishikawa, E.T.; Gonzalez-Nieto, D.; Ghiaur, G.; Dunn, S.K.; Ficker, A.M.; Murali, B.; Madhu, M.; Gutstein, D.E.; Fishman, G.I.; Barrio, L.C.; et al. Connexin-43 prevents hematopoietic stem cell senescence through transfer of reactive oxygen species to bone marrow stromal cells. Proc. Natl. Acad. Sci. USA 2012, 109, 9071–9076. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; van de Sluis, B.; Kirkland, J.L.; van Deursen, J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Molofsky, A.V.; He, S.; Bydon, M.; Morrison, S.J.; Pardal, R. Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev. 2005, 19, 1432–1437. [Google Scholar] [CrossRef] [PubMed]
- Coppe, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Munoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.Y.; Campisi, J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008, 6, 2853–2868. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Lin, A.W.; McCurrach, M.E.; Beach, D.; Lowe, S.W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997, 88, 593–602. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, J.; Xiong, M.; Petersen, A.J.; Dong, Y.; Tao, Y.; Huang, C.T.; Du, Z.; Zhang, S.C. Engineering Human Stem Cell Lines with Inducible Gene Knockout using CRISPR/Cas9. Cell Stem Cell 2015, 17, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Beers, J.; Gulbranson, D.R.; George, N.; Siniscalchi, L.I.; Jones, J.; Thomson, J.A.; Chen, G. Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions. Nat. Protoc. 2012, 7, 2029–2040. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Esseltine, J.L.; Brooks, C.R.; Edwards, N.A.; Subasri, M.; Sampson, J.; Seguin, C.; Betts, D.H.; Laird, D.W. Dynamic regulation of connexins in stem cell pluripotency. Stem Cells 2019, in press. [Google Scholar]
- Brasaemle, D.L. Thematic review series: Adipocyte biology. The perilipin family of structural lipid droplet proteins: Stabilization of lipid droplets and control of lipolysis. J. Lipid Res. 2007, 48, 2547–2559. [Google Scholar] [CrossRef] [PubMed]
- Turinetto, V.; Vitale, E.; Giachino, C. Senescence in Human Mesenchymal Stem Cells: Functional Changes and Implications in Stem Cell-Based Therapy. Int. J. Mol. Sci. 2016, 17, 1164. [Google Scholar] [CrossRef] [PubMed]
- Rodier, F.; Campisi, J. Four faces of cellular senescence. J. Cell Biol. 2011, 192, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Civitelli, R. Cell-cell communication in the osteoblast/osteocyte lineage. Arch. Biochem. Biophys. 2008, 473, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Yeganeh, A.; Stelmack, G.L.; Fandrich, R.R.; Halayko, A.J.; Kardami, E.; Zahradka, P. Connexin 43 phosphorylation and degradation are required for adipogenesis. Biochim. Biophys. Acta 2012, 1823, 1731–1744. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, S.Y.; Park, S.Y.; Kim, Y.M.; Kim, J.M.; Lee, M.H.; Ryu, H.M. Mesenchymal progenitor cells in the human umbilical cord. Ann. Hematol. 2004, 83, 733–738. [Google Scholar] [CrossRef]
- Lo, C.W.; Gilula, N.B. Gap junctional communication in the preimplantation mouse embryo. Cell 1979, 18, 399–409. [Google Scholar] [CrossRef]
- Beckmann, A.; Schubert, M.; Hainz, N.; Haase, A.; Martin, U.; Tschernig, T.; Meier, C. Ultrastructural demonstration of Cx43 gap junctions in induced pluripotent stem cells from human cord blood. Histochem. Cell Biol. 2016, 146, 529–537. [Google Scholar] [CrossRef]
- Ke, Q.; Li, L.; Cai, B.; Liu, C.; Yang, Y.; Gao, Y.; Huang, W.; Yuan, X.; Wang, T.; Zhang, Q.; et al. Connexin 43 is involved in the generation of human-induced pluripotent stem cells. Hum. Mol. Genet. 2013, 22, 2221–2233. [Google Scholar] [CrossRef]
- Gabashvili, A.N.; Baklaushev, V.P.; Grinenko, N.F.; Levinskii, A.B.; Mel’nikov, P.A.; Cherepanov, S.A.; Chekhonin, V.P. Functionally Active Gap Junctions between Connexin 43-Positive Mesenchymal Stem Cells and Glioma Cells. Bull. Exp. Biol. Med. 2015, 159, 173–179. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Y.; Wang, Z.; Huang, Z.; Li, B.; Fu, J. Up-regulation of connexin-43 expression in bone marrow mesenchymal stem cells plays a crucial role in adhesion and migration of multiple myeloma cells. Leuk. Lymphoma 2015, 56, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Mureli, S.; Gans, C.P.; Bare, D.J.; Geenen, D.L.; Kumar, N.M.; Banach, K. Mesenchymal stem cells improve cardiac conduction by upregulation of connexin 43 through paracrine signaling. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H600–H609. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.G.; Zhang, F.X.; Chen, M.L.; Zhu, H.J.; Yang, B.; Cao, K.J. Cx43 in mesenchymal stem cells promotes angiogenesis of the infarcted heart independent of gap junctions. Mol. Med. Rep. 2014, 9, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Niger, C.; Koh, E.Y.; Stains, J.P. Connexin43 Mediated Delivery of ADAMTS5 Targeting siRNAs from Mesenchymal Stem Cells to Synovial Fibroblasts. PLoS ONE 2015, 10, e0129999. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Zhou, Y.; Tan, S.; Zhou, G.; Aagaard, L.; Xie, L.; Bunger, C.; Bolund, L.; Luo, Y. Mesenchymal stem cells derived from human induced pluripotent stem cells retain adequate osteogenicity and chondrogenicity but less adipogenicity. Stem Cell Res. Ther. 2015, 6, 144. [Google Scholar] [CrossRef] [PubMed]
- Mohamed-Ahmed, S.; Fristad, I.; Lie, S.A.; Suliman, S.; Mustafa, K.; Vindenes, H.; Idris, S.B. Adipose-derived and bone marrow mesenchymal stem cells: A donor-matched comparison. Stem Cell Res. Ther. 2018, 9, 168. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Wu, D.; Liu, W.; Wang, J.; Feng, Z.; Cai, G.; Fu, B.; Hong, Q.; Du, J. Downregulation of connexin 43 expression by high glucose induces senescence in glomerular mesangial cells. J. Am. Soc. Nephrol. 2006, 17, 1532–1542. [Google Scholar] [CrossRef]
- Xu, X.; Gao, D.; Wang, P.; Chen, J.; Ruan, J.; Xu, J.; Xia, X. Efficient homology-directed gene editing by CRISPR/Cas9 in human stem and primary cells using tube electroporation. Sci. Rep. 2018, 8, 11649. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Q.; Esseltine, J.L.; Huang, T.; Novielli-Kuntz, N.; Ching, J.E.; Sampson, J.; Laird, D.W. Connexin43 is Dispensable for Early Stage Human Mesenchymal Stem Cell Adipogenic Differentiation But is Protective against Cell Senescence. Biomolecules 2019, 9, 474. https://doi.org/10.3390/biom9090474
Shao Q, Esseltine JL, Huang T, Novielli-Kuntz N, Ching JE, Sampson J, Laird DW. Connexin43 is Dispensable for Early Stage Human Mesenchymal Stem Cell Adipogenic Differentiation But is Protective against Cell Senescence. Biomolecules. 2019; 9(9):474. https://doi.org/10.3390/biom9090474
Chicago/Turabian StyleShao, Qing, Jessica L. Esseltine, Tao Huang, Nicole Novielli-Kuntz, Jamie E. Ching, Jacinda Sampson, and Dale W. Laird. 2019. "Connexin43 is Dispensable for Early Stage Human Mesenchymal Stem Cell Adipogenic Differentiation But is Protective against Cell Senescence" Biomolecules 9, no. 9: 474. https://doi.org/10.3390/biom9090474
APA StyleShao, Q., Esseltine, J. L., Huang, T., Novielli-Kuntz, N., Ching, J. E., Sampson, J., & Laird, D. W. (2019). Connexin43 is Dispensable for Early Stage Human Mesenchymal Stem Cell Adipogenic Differentiation But is Protective against Cell Senescence. Biomolecules, 9(9), 474. https://doi.org/10.3390/biom9090474



