Comparative Analysis of MicroRNA and mRNA Profiles of Sperm with Different Freeze Tolerance Capacities in Boar (Sus scrofa) and Giant Panda (Ailuropoda melanoleuca)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement, Sperm Collection and Cryopreservation
2.2. Total RNA Extraction, Small RNA Library Preparation and Sequencing
2.3. Quality Analysis, Mapping, miRNA Identification and Differential Expression Analysis
2.4. MiRNA Target Prediction, GO and KEGG Enrichment Analyses
2.5. RT-qPCR Validation
2.6. Combined Analysis of Differentially Expressed miRNA-mRNA of Boar and Giant Panda Sperm
2.7. Statistical Analysis
3. Results
3.1. MiRNA Profile of Fresh and Frozen-Thawed Giant Panda Sperm
3.2. Comparison of Quality Parameters in Boar and Giant Panda Sperm Before and After Cryopreservation
3.3. Combined Analysis of mRNA-miRNA Sequencing of Boar and Giant Panda Sperm
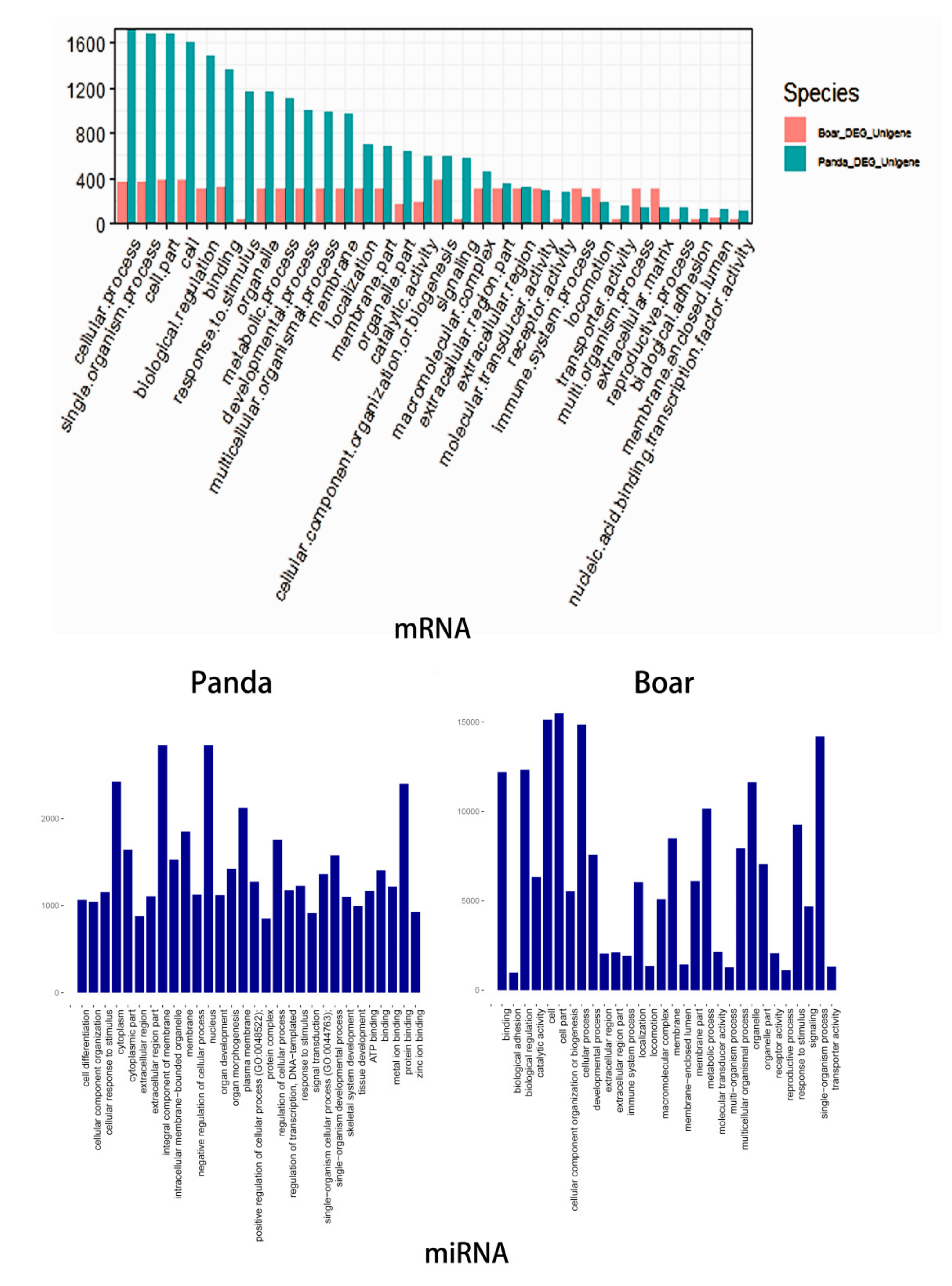
3.4. Functional Analysis of DE mRNAs of DE miRNAs
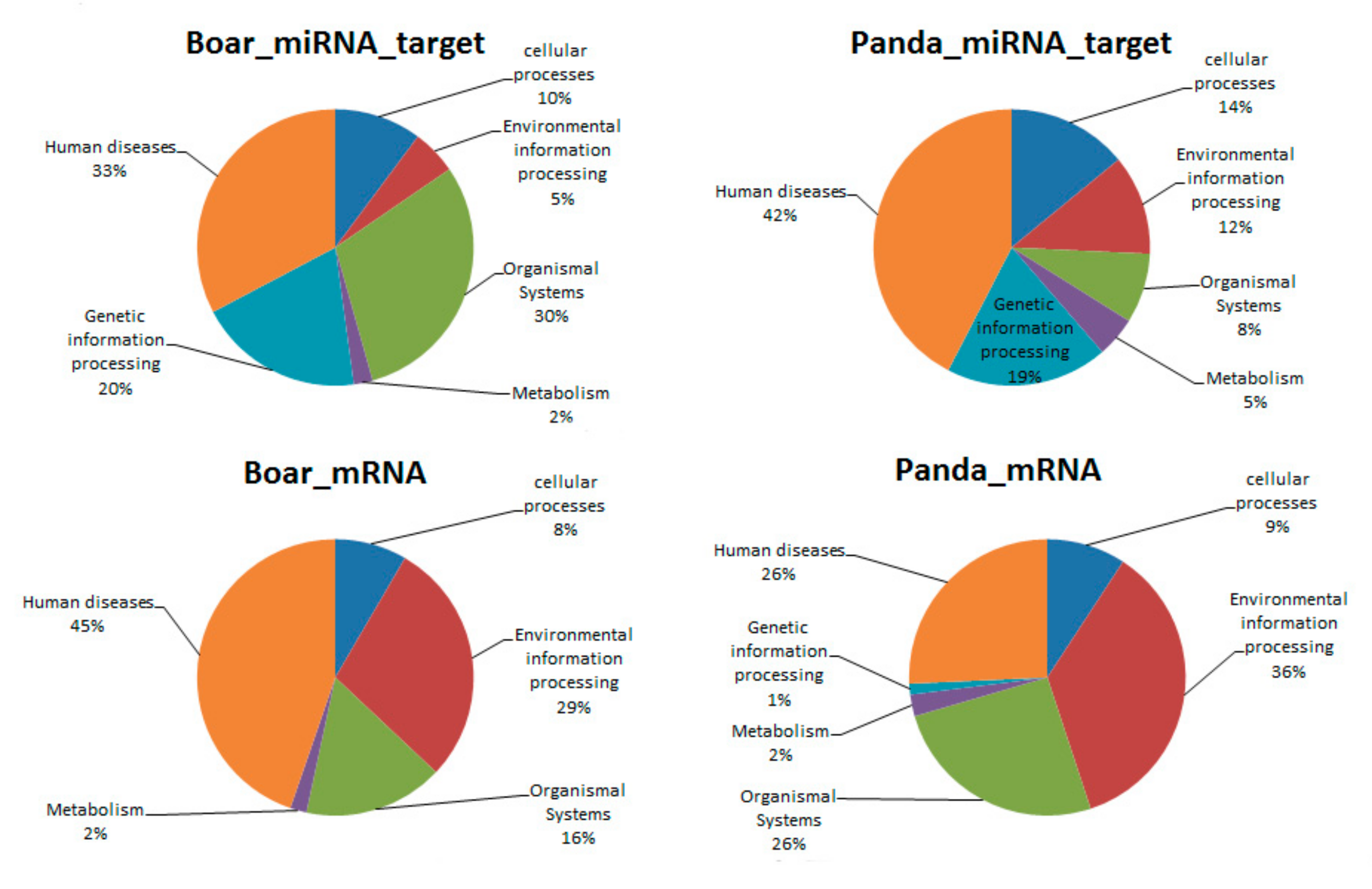
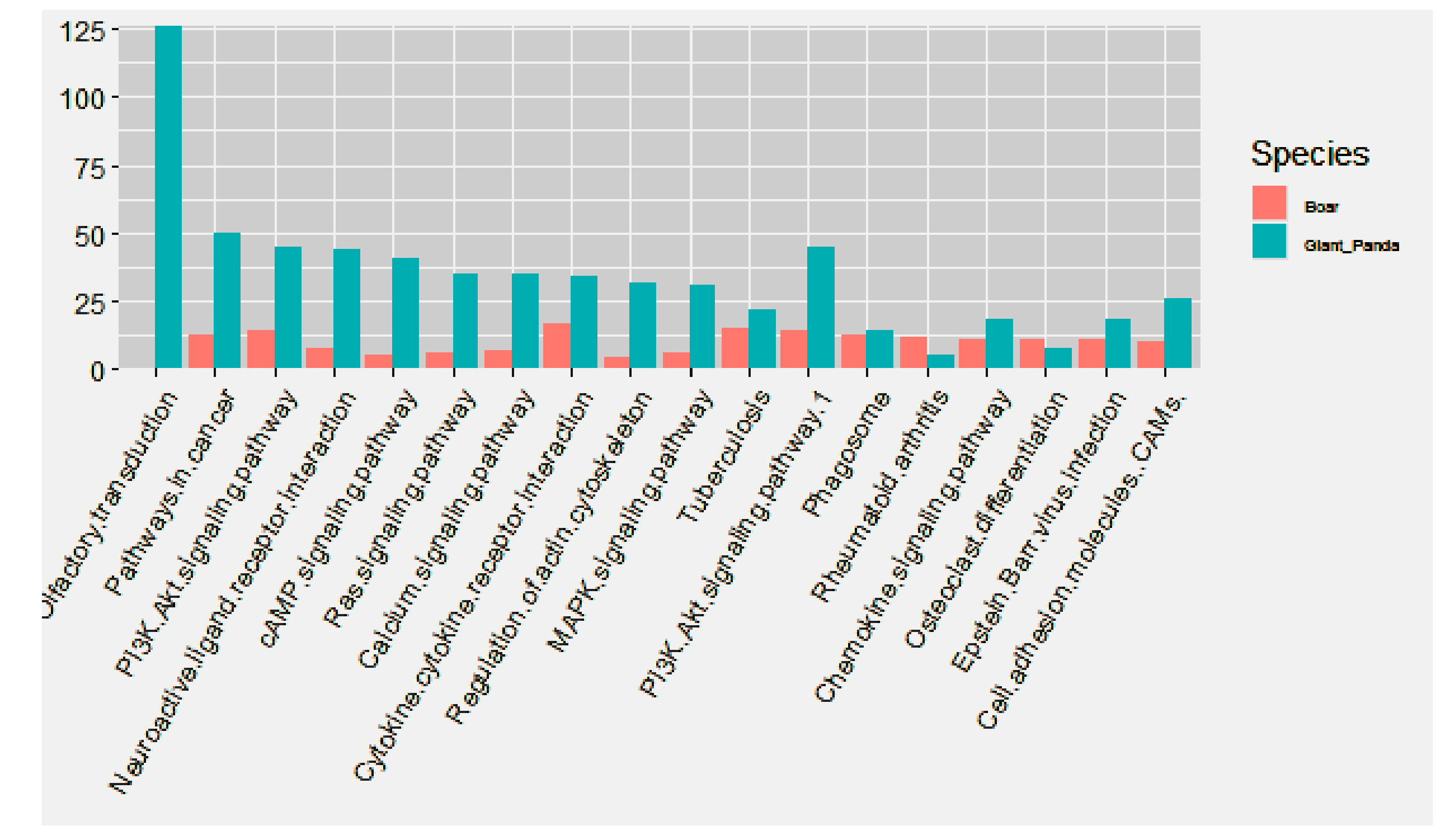
3.5. Comparative GO and KEGG Analysis of DE miRNAs and mRNAs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yeste, M.; Rodriguez-Gil, J.E.; Bonet, S. Artificial insemination with frozen-thawed boar sperm. Mol. Reprod. Dev. 2017, 84, 802–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, J.L.; Bilodeau, J.F.; Cormier, N. Semen cryopreservation in domestic animals: a damaging and capacitating phenomenon minireview. J. Androl. 2000, 21, 1–7. [Google Scholar] [PubMed]
- Saleh, R.A.; Agarwal, A. Oxidative stress and male infertility: From research bench to clinical practice. J. Androl. 2002, 23, 737–752. [Google Scholar]
- Di Santo, M.; Tarozzi, N.; Nadalini, M.; Borini, A. Human sperm cryopreservation: Update on techniques, effect on DNA integrity, and implications for art. Adv. Urol. 2012, 2012, 854837. [Google Scholar] [CrossRef]
- Trzcinska, M.; Bryła, M. Apoptotic-like changes of boar spermatozoa in freezing media supplemented with different antioxidants. Polish J. Vet. Sci. 2015, 18, 473–480. [Google Scholar] [Green Version]
- Yeste, M. Sperm cryopreservation update: Cryodamage, markers, and factors affecting the sperm freezability in pigs. Theriogenology 2016, 85, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.H.; Qazi, I.H.; Ran, M.X.; Liang, K.; Zhang, Y.; Zhang, M.; Zhou, G.B.; Angel, C.; Zeng, C.J. Exploration of miRNA and mRNA profiles in fresh and frozen-thawed boar sperm by transcriptome and small RNA sequencing. Int. J. Mol. Sci. 2019, 20, 802. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dai, D.H.; Chang, Y.; Li, Y.; Zhang, M.; Zhou, G.B.; Peng, Z.H.; Zeng, C.J. Cryopreservation of boar sperm induces differential microRNAs expression. Cryobiology 2017, 76, 24–33. [Google Scholar]
- Guimarães, D.B.; Barros, T.B.; van Tilburg, M.F.; Martins, J.A.M.; Moura, A.A.; Moreno, F.B.; Monteiro-Moreira, A.C.; Moreira, R.A.; Toniolli, R. Sperm membrane proteins associated with the boar semen cryopreservation. Anim. Reprod. Sci. 2017, 183, 27–38. [Google Scholar] [CrossRef]
- Johnson, L.A.; Weitze, K.F.; Fiser, P.; Maxwell, W.M. Storage of boar semen. Anim. Reprod. Sci. 2000, 62, 143–172. [Google Scholar]
- Chen, X.L.; Zhu, H.B.; Hao, H.S.; Du, W.H.; Zhao, X.M.; Sun, Y.Y.; Hu, C.H.; Wang, D. Progress on the cryodamage of frozen-thawed boar spermatozoa. China Biotechnol. 2010, 30, 86–91. [Google Scholar]
- Guthrie, H.D.; Welch, G.R. Effects of reactive oxygen species on sperm function. Theriogenology 2012, 78, 1700–1708. [Google Scholar] [PubMed]
- Kim, J.C.; Li, Y.; Lee, S.; Yi, Y.J.; Park, C.S.; Woo, S.H. Effects of cryopreservation on Ca2+ signals induced by membrane depolarization, caffeine, thapsigargin and progesterone in boar spermatozoa. Mol. Cells 2008, 26, 558–565. [Google Scholar] [PubMed]
- Albrizio, M.; Moramarco, A.M.; Nicassio, M.; Micera, E.; Zarrilli, A.; Lacalandra, G.M. Localization and functional modification of L-type voltage gated calcium channels in equine spermatozoa from fresh and frozen semen. Theriogenology 2015, 83, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Moreno, J.; Esteso, M.C.; Pradiee, J.; Castano, C.; Toledano-Diaz, A.; O’Brien, E.; Lopez-Sebastian, A.; Martinez-Nevado, E.; Delclaux, M.; Fernandez-Moran, J.; et al. Giant panda (Ailuropoda melanoleuca) sperm morphometry and function after repeated freezing and thawing. Andrologia 2016, 48, 470–474. [Google Scholar]
- Spindler, R.E.; Huang, Y.; Howard, J.G.; Wang, P.; Zhang, H.; Zhang, G.; Wildt, D.E. Acrosomal integrity and capacitation are not influenced by sperm cryopreservation in the giant panda. Reproduction 2004, 127, 547–556. [Google Scholar] [CrossRef]
- Spindler, R.E.; Huang, Y.; Howard, J.G.; Wang, P.Y.; Zhang, H.M.; Zhang, G.Q.; Wildt, D.E. Giant panda (Ailuropoda melanoleuca) spermatozoon decondensation in vitro is not compromised by cryopreservation. Reprod. Fertil. Dev. 2006, 18, 767–775. [Google Scholar] [CrossRef]
- Ran, M.X.; Li, Y.; Zhang, Y.; Liang, K.; Ren, Y.N.; Zhang, M.; Zhou, G.B.; Zhou, Y.M.; Wu, K.; Wang, C.D.; et al. Transcriptome sequencing reveals the differentially expressed lncRNAs and mRNAs involved in cryoinjuries in frozen-thawed giant panda (Ailuropoda melanoleuca) sperm. Int. J. Mol. Sci. 2018, 19, 3066. [Google Scholar] [CrossRef]
- Capra, E.; Turri, F.; Lazzari, B.; Cremonesi, P.; Gliozzi, T.M.; Fojadelli, I.; Stella, A.; Pizzi, F. Small RNA sequencing of cryopreserved semen from single bull revealed altered miRNAs and piRNAs expression between high-and low-motile sperm populations. BMC Genom. 2017, 18, 14. [Google Scholar]
- Niu, Z.; Goodyear, S.M.; Rao, S.; Wu, X.; Tobias, J.W.; Avarbock, M.R.; Brinster, R.L. MicroRNA-21 regulates the self-renewal of mouse spermatogonial stem cells. PNAS 2011, 108, 12740–12745. [Google Scholar] [CrossRef] [Green Version]
- Maatouk, D.M.; Loveland, K.L.; McManus, M.T.; Moore, K.; Harfe, B.D. Dicer1 is required for differentiation of the mouse male germline. Biol. Reprod. 2008, 79, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, S.D.; Shcherbata, H.R.; Fischer, K.A.; Nakahara, K.; Carthew, R.W.; Ruohola-Baker, H. Stem cell division is regulated by the microRNA pathway. Nature 2005, 435, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Govindaraju, A.; Dogan, S.; Rodriguez-Osorio, N.; Grant, K.; Kaya, A.; Memili, E. Delivering value from sperm proteomics for fertility. Cell Tissue Res. 2012, 349, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, D.; Zhou, Y.; Zhou, Q.; Li, R.; Wang, C.; Huang, Z.; Hull, V.; Zhang, H. Factors affecting the outcome of artificial insemination using cryopreserved spermatozoa in the giant panda (Ailuropoda melanoleuca). Zoo Biol. 2012, 31, 561–573. [Google Scholar] [PubMed]
- Zeng, C.; He, L.; Peng, W.; Ding, L.; Tang, K.; Fang, D.; Zhang, Y. Selection of optimal reference genes for quantitative RT-PCR studies of boar spermatozoa cryopreservation. Cryobiology 2014, 68, 113–121. [Google Scholar] [CrossRef]
- Friedlander, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. MiRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012, 40, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The gene ontology consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Fahlgren, N.; Howell, M.D.; Kasschau, K.D.; Chapman, E.J.; Sullivan, C.M.; Cumbie, J.S.; Givan, S.A.; Law, T.F.; Grant, S.R.; Dangl, J.L.; et al. High-throughput sequencing of arabidopsis microRNAs: Evidence for frequent birth and death of miRNA genes. PloS ONE 2007, 2, e219. [Google Scholar]
- Betel, D.; Wilson, M.; Gabow, A.; Marks, D.S.; Sander, C. The microRNA.org resource: Targets and expression. Nucleic Acids Res. 2008, 36, 149–153. [Google Scholar] [CrossRef]
- Rehmsmeier, M.; Steffen, P.; Hochsmann, M.; Giegerich, R. Fast and effective prediction of microRNA/target duplexes. RNA 2004, 10, 1507–1517. [Google Scholar] [CrossRef] [Green Version]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T)) method. Methods 2001, 25, 402–408. [Google Scholar] [PubMed]
- Garcia-Herreros, M.; Baron, F.J.; Aparicio, I.M.; Santos, A.J.; Garcia-Marin, L.J.; Gil, M.C. Morphometric changes in boar spermatozoa induced by cryopreservation. Int. J. Androl. 2008, 31, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, Y.J.; Kim, Y.J. Changes in sperm membrane and ros following cryopreservation of liquid boar semen stored at 15 °C. Anim. Reprod. Sci. 2011, 124, 118–124. [Google Scholar] [CrossRef]
- Garde, J.J.; Soler, A.J.; Cassinello, J.; Crespo, C.; Malo, A.F.; Espeso, G.; Gomendio, M.; Roldan, E.R. Sperm cryopreservation in three species of endangered gazelles (Gazella cuvieri, G. Dama mhorr, and G. Dorcas neglecta). Biol. Reprod. 2003, 69, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Zhao, F.; Xie, P.; Zhong, L.; Li, D.; Gai, Q.; Li, L.; Wei, H.; Zhang, L.; An, W. Mechanism of the effect of glycosyltransferase GLT8D2 on fatty liver. Lipids Health Dis. 2015, 14, 43. [Google Scholar] [CrossRef]
- Wei, H.S.; Wei, H.L.; Zhao, F.; Zhong, L.P.; Zhan, Y.T. Glycosyltransferase GLT8D2 positively regulates ApoB100 protein expression in hepatocytes. Int. J. Mol. Sci. 2013, 14, 21435–21446. [Google Scholar]
- Yoshida, T.; Kim, J.H.; Carver, K.; Su, Y.; Weremowicz, S.; Mulvey, L.; Yamamoto, S.; Brennan, C.; Mei, S.; Long, H.; et al. CLK2 is an oncogenic kinase and splicing regulator in breast cancer. Cancer Res. 2015, 75, 1516–1526. [Google Scholar]
- Cantó, C.; Auwerx, J. Clking on PGC-1alpha to inhibit gluconeogenesis. Cell Metab. 2010, 11, 6–7. [Google Scholar] [CrossRef]
- Tabata, M.; Rodgers, J.T.; Hall, J.A.; Lee, Y.; Jedrychowski, M.P.; Gygi, S.P.; Puigserver, P. Cdc2-like kinase 2 suppresses hepatic fatty acid oxidation and ketogenesis through disruption of the PGC-1α and MED1 complex. Diabetes 2014, 63, 1519–1532. [Google Scholar] [PubMed]
- Rodgers, J.T.; Wilhelm, H.; Gygi, S.P.; Pere, P. Cdc2-like kinase 2 is an insulin-regulated suppressor of hepatic gluconeogenesis. Cell Metab. 2010, 11, 23–34. [Google Scholar] [PubMed]
- Itoh, A.; Uchiyama, A.; Taniguchi, S.; Sagara, J. Phactr3/scapinin, a member of protein phosphatase 1 and actin regulator (phactr) family, interacts with the plasma membrane via basic and hydrophobic residues in the N-terminus. PloS ONE 2014, 9, e113289. [Google Scholar] [CrossRef] [PubMed]
- Junji, S.; Toshiaki, A.; Shunichiro, T. Scapinin, the protein phosphatase 1 binding protein, enhances cell spreading and motility by interacting with the actin cytoskeleton. PloS ONE 2009, 4, e4247. [Google Scholar]
- Liu, J.; Han, C.; Xie, B.; Wu, Y.; Liu, S.; Chen, K.; Xia, M.; Zhang, Y.; Song, L.; Li, Z.; et al. Rhbdd3 controls autoimmunity by suppressing the production of IL-6 by dendritic cells via K27-linked ubiquitination of the regulator NEMO. Nat. Immunol. 2014, 15, 612–622. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Xia, M.; Xu, S.; Wang, C.; Bao, Y.; Jiang, M.; Wu, Y.; Xu, T.; Cao, X. Rhomboid domain-containing protein 3 is a negative regulator of TLR3-triggered natural killer cell activation. PNAS 2013, 110, 7814–7819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nekooki-Machida, Y.; Nakakura, T.; Nishijima, Y.; Tanaka, H.; Arisawa, K.; Kiuchi, Y.; Miyashita, T.; Hagiwara, H. Dynamic localization of α-tubulin acetyltransferase ATAT1 through the cell cycle in human fibroblastic KD cells. Med. Mol. Morphol. 2018, 51, 217–226. [Google Scholar] [CrossRef]
- Lin, S.; Liu, Q.; Lelyveld, V.S.; Choe, J.; Szostak, J.W.; Gregory, R.I. Mettl1/wdr4-mediated m7G tRNA methylome is required for normal mRNA translation and embryonic stem cell self-renewal and differentiation. Mol. Cell 2018, 71, 244–255. [Google Scholar] [CrossRef]
- Chen, J.; Ren, R.; Yu, F.; Wang, C.; Zhang, X.; Li, W.; Tan, S.; Jiang, S.; Liu, S.; Li, L. A degraded fragment of HIV-1 gp120 in rat hepatocytes forms fibrils and enhances HIV-1 infection. Biophys. J. 2017, 113, 1425–1439. [Google Scholar] [CrossRef]
- Kusano, S.; Shiimura, Y.; Eizuru, Y. I-mfa domain proteins specifically interact with SERTA domain proteins and repress their transactivating functions. Biochimie 2011, 93, 1555–1564. [Google Scholar]
- Gu, Z.Y.; Zhao, X.L.; Yan, N.; Wang, L.; Wang, F.Y.; Wang, L.L.; Gao, C.J.; Hematology, D.O. Influence ofS1PR5 defect on the lymphocyte distribution in mice. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2016, 24, 1168–1172. [Google Scholar] [PubMed]
- Emilie, D.; Katia, M.; Vincent, B.; Cécile, D.; Mercedes Gomez, D.A.; Morgan, T.; Nicolas, D.; Lilia, B.; Thomas, H.; Bertrand, D. S1PR5 is pivotal for the homeostasis of patrolling monocytes. Eur. J. Immunol. 2013, 43, 1667–1675. [Google Scholar] [Green Version]
- Sivik, T.; Vikingsson, S.; Gréen, H.; Jansson, A. Expression patterns of 17β-hydroxysteroid dehydrogenase 14 in human tissues. Horm. Metab. Res. 2012, 44, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ye, D.; Xie, W.; Hua, F.; Yang, Y.; Wu, J.; Gu, A.; Ren, Y.; Mao, K. Defect of branched-chain amino acid metabolism promotes the development of alzheimer’s disease by targeting the mtor signaling. Biosci. Rep. 2018, 38, BSR20180127. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.J.; Chang, C.F.; Ko, H.H.; Liu, Y.C.; Peng, H.H.; Wang, H.J.; Lin, H.S.; Chiang, C.P. Hypermethylated ZNF582 and PAX1 genes in oral scrapings collected from cancer-adjacent normal oral mucosal sites are associated with aggressive progression and poor prognosis of oral cancer. Oral Oncol. 2017, 75, 169–177. [Google Scholar] [CrossRef]
- Anunciado-Koza, R.P.; Jingying, Z.; Jozef, U.; Sudip, B.; Koza, R.A.; Rogers, R.C.; Cefalu, W.T.; Mynatt, R.L.; Kozak, L.P. Inactivation of the mitochondrial carrier SLC25A25 (ATP-Mg2+/Pi transporter) reduces physical endurance and metabolic efficiency in mice. J. Biol. Chem. 2011, 286, 11659–11671. [Google Scholar] [CrossRef] [PubMed]
- Nakao, R.; Shimba, S.; Oishi, K. Ketogenic diet induces expression of the muscle circadian gene SLC25A25 via neural pathway that might be involved in muscle thermogenesis. Sci. Rep. 2017, 7, 2885. [Google Scholar] [CrossRef]
- Van den Hurk, W.H.; Martens, G.J.; Geurts van Kessel, A.; van Groningen, J.J. Isolation and characterization of the xenopus laevis orthologs of the human papillary renal cell carcinoma-associated genes PRCC and MAD2L2 (MAD2B). Cytogenet. Genome Res. 2004, 106, 68–73. [Google Scholar] [CrossRef]
- Song, Z.; Feng, C.; Lu, Y.; Gao, Y.; Lin, Y.; Dong, C. Overexpression and biological function of MEF2D in human pancreatic cancer. Am. J. Transl. Res. 2017, 9, 4836–4847. [Google Scholar]
- Gao, L.; She, H.; Li, W.; Zhu, J.; Jones, D.P.; Mao, Z.; Gao, G.; Yang, Q. Oxidation of survival factor MEF2D in neuronal death and Parkinson’s disease. Antioxid. Redox Signal. 2013, 20, 2936–2948. [Google Scholar] [CrossRef]
- Liu, J.; Guo, Q.; Chen, B.; Yu, Y.; Lu, H.; Li, Y.Y. Cathepsin B and its interacting proteins, bikunin and TSRC1, correlate with TNF-induced apoptosis of ovarian cancer cells OV-90. FEBS Lett. 2006, 580, 245–250. [Google Scholar] [PubMed]
- Uemura, T.; Takasaka, T.; Igarashi, K.; Ikegaya, H. Spermine oxidase promotes bile canalicular lumen formation through acrolein production. Sci. Rep. 2017, 7, 14841. [Google Scholar] [PubMed]
- Nita, I.I.; Michal, H.; Daniel, F.; Eyal, O.; Rutter, G.A.; Sensi, S.L.; Daniel, K.; Lewis, E.C.; Israel, S. The mitochondrial Na+/Ca2+ exchanger upregulates glucose dependent Ca2+ signalling linked to insulin secretion. PloS ONE 2012, 7, e46649. [Google Scholar] [CrossRef] [PubMed]
- Komori, T.; Morikawa, Y. Oncostatin m in the development of metabolic syndrome and its potential as a novel therapeutic target. Anat. Sci. Int. 2017, 93, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Stephens, J.M.; Elks, C.M. Oncostatin M: Potential implications for malignancy and metabolism. Curr. Pharm. Des. 2017, 23, 3645–3657. [Google Scholar] [CrossRef] [PubMed]
- Narni-Mancinelli, E.; Gauthier, L.; Baratin, M.; Guia, S.; Fenis, A.; Deghmane, A.E.; Rossi, B.; Fourquet, P.; Escalière, B.; Kerdiles, Y.M. Complement factor P is a ligand for the natural killer cell-activating receptor NPK46. Sci. Immunol. 2017, 2, eaam9628. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.K.; Kim, S.H.; Kim, Y.H.; Lee, D.G.; Oh, H.S.; Han, C.; Kim, Y.I.; Jeon, Y.; Lee, H.; Kwon, B.S. RELT negatively regulates the early phase of the T-cell response in mice. Eur. J. Immunol. 2018, 48, 1739–1749. [Google Scholar] [CrossRef] [Green Version]
- Moua, P.; Checketts, M.; Xu, L.G.; Shu, H.B.; Reyland, M.E.; Cusick, J. RELT family members activate p38 and induce apoptosis by a mechanism distinct from TNFR1. Biochem. Biophys. Res. Commun. 2017, 491, 25–32. [Google Scholar]
- Skibinska, I.; Andrusiewicz, M.; Soin, M.; Jendraszak, M.; Urbaniak, P.; Jedrzejczak, P.; Kotwicka, M. Increased expression of PELP1 in human sperm is correlated with decreased semen quality. Asian J. Androl. 2018, 20, 425–431. [Google Scholar] [CrossRef]
- Thakkar, R.; Sareddy, G.R.; Zhang, Q.; Wang, R.; Vadlamudi, R.K.; Brann, D. PELP1: A key mediator of oestrogen signalling and actions in the brain. J. Neuroendocrinol. 2018, 30. [Google Scholar] [CrossRef]
- Tanteles, G.A.; Dixit, A.; Dhar, S.; Suri, M. Two somali half-siblings with CHST3-related chondrodysplasia illustrating the phenotypic spectrum and intrafamilial variability. Am. J. Med. Genet. Part A 2013, 161A, 2588–2593. [Google Scholar] [PubMed]
- Moog-Lutz, C.; Peterson, E.J.; Lutz, P.G.; Eliason, S.; Cavé-Riant, F.; Singer, A.; Di Gioia, Y.; Dmowski, S.; Kamens, J.; Cayre, Y.E.; et al. PRAM-1 is a novel adaptor protein regulated by retinoic acid (RA) and promyelocytic leukemia (PML)-RA receptor alpha in acute promyelocytic leukemia cells. J. Biol. Chem. 2001, 276, 22375–22381. [Google Scholar] [PubMed]
- Denis, F.M.; Arndt, B.; Yolande, D.G.; Touw, I.P.; Cayre, Y.E.; Lutz, P.G. PRAM-1 potentiates arsenic trioxide-induced JNK activation. J. Biol. Chem. 2005, 280, 9043–9048. [Google Scholar] [CrossRef] [PubMed]
- Govindaraju, A.; Uzun, A.; Robertson, L.; Atli, M.O.; Kaya, A.; Topper, E.; Crate, E.A.; Padbury, J.; Perkins, A.; Memili, E. Dynamics of microRNAs in bull spermatozoa. Reprod. Biol. Endocrinol. 2012, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Valcarce, D.G.; Cartón-García, F.; Herráez, M.P.; Robles, V. Effect of cryopreservation on human sperm messenger RNAs crucial for fertilization and early embryo development. Cryobiology 2013, 67, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Hezavehei, M.; Sharafi, M.; Kouchesfahani, H.M.; Henkel, R.; Agarwal, A.; Esmaeili, V.; Shahverdi, A. Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches. Reprod. Biomed. Online 2018, 37, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ao, L.; Yan, Y.; Jiang, J.; Chen, B.; Duan, Y.; Shen, F.; Chen, J.; Inglis, B.; Ni, R.; et al. Differential motility parameters and identification of proteomic profiles of human sperm cryopreserved with cryostraw and cryovial. Clin. Proteom. 2019, 16, 24. [Google Scholar]
- Lusignan, M.F.; Li, X.; Herrero, B.; Delbes, G.; Chan, P.T.K. Effects of different cryopreservation methods on DNA integrity and sperm chromatin quality in men. Andrology 2018, 6, 829–835. [Google Scholar] [Green Version]
- Said, T.M.; Gaglani, A.; Agarwal, A. Implication of apoptosis in sperm cryoinjury. Reprod. Biomed. Online 2010, 21, 456–462. [Google Scholar] [Green Version]
- Kadirvel, G.; Periasamy, S.; Kumar, S. Effect of cryopreservation on apoptotic-like events and its relationship with cryocapacitation of buffalo (Bubalus bubalis) sperm. Reprod. Domest. Anim. Zuchthyg. 2012, 47, 143–150. [Google Scholar] [CrossRef]
- Ortega-Ferrusola, C.; Sotillo-Galan, Y.; Varela-Fernandez, E.; Gallardo-Bolanos, J.M.; Muriel, A.; Gonzalez-Fernandez, L.; Tapia, J.A.; Pena, F.J. Detection of “apoptosis-like” changes during the cryopreservation process in equine sperm. J. Androl. 2008, 29, 213–221. [Google Scholar] [PubMed]
- Trzcinska, M.; Bryla, M.; Gajda, B.; Gogol, P. Fertility of boar semen cryopreserved in extender supplemented with butylated hydroxytoluene. Theriogenology 2015, 83, 307–313. [Google Scholar] [PubMed]
- Khan, D.R.; Ahmad, N.; Anzar, M.; Channa, A.A. Apoptosis in fresh and cryopreserved buffalo sperm. Theriogenology 2009, 71, 872–876. [Google Scholar] [PubMed]
- Karabulut, S.; Demiroglu-Zergeroglu, A.; Yilmaz, E.; Kutlu, P.; Keskin, I. Effects of human sperm cryopreservation on apoptotic markers in normozoospermic and non-normozoospermic patients. Zygote 2018, 26, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Dias, T.R.; Alves, M.G.; Silva, B.M.; Oliveira, P.F. Sperm glucose transport and metabolism in diabetic individuals. Mol. Cell. Endocrinol. 2014, 396, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, S.S.; Agarwal, A.; Mohanty, G.; van der Linde, M. Oxidative phosphorylation versus glycolysis: What fuel do spermatozoa use? Asian J. Androl. 2015, 17, 230–235. [Google Scholar] [PubMed]
- Turri, F.; Capra, E.; Gliozzi, T.M.; Cremonesi, P.; Lazzari, B.; Stella, A.; Pizzi, F. Bull sperm miRNAs profiling in motile and low motile cell populations. In Proceedings of the 6th International Symposium on Animal Functional Genomics, Piacenza, Italy, July 2015. [Google Scholar]
- Liu, T.; Cheng, W.; Gao, Y.; Wang, H.; Liu, Z. Microarray analysis of microRNA expression patterns in the semen of infertile men with semen abnormalities. Mol. Med. Rep. 2012, 6, 535–542. [Google Scholar] [CrossRef]
- Cerolini, S.; Maldjian, A.; Pizzi, F.; Gliozzi, T.M. Changes in sperm quality and lipid composition during cryopreservation of boar semen. Reproduction 2001, 121, 395–401. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, C.J.; He, L.; Ding, L.; Tang, K.Y.; Peng, W.P. Selection of endogenous reference microRNA genes for quantitative reverse transcription polymerase chain reaction studies of boar spermatozoa cryopreservation. Theriogenology 2015, 83, 634–641. [Google Scholar]
- Galindo, B.E.; Neill, A.T.; Vacquier, V.D. A new hyperpolarization-activated, cyclic nucleotide-gated channel from sea urchin sperm flagella. Biochem. Biophys. Res. Commun. 2005, 334, 96–101. [Google Scholar] [CrossRef]
- Cisneros-Mejorado, A.; Sanchez Herrera, D.P. cGMP and cyclic nucleotide-gated channels participate in mouse sperm capacitation. FEBS Lett. 2012, 586, 149–153. [Google Scholar] [PubMed]
- Michalakis, S.; Becirovic, E.; Biel, M. Retinal cyclic nucleotide-gated channels: From pathophysiology to therapy. Int. J. Mol. Sci. 2018, 19, 749. [Google Scholar]
- Fraser, L.R. The “switching on” of mammalian spermatozoa: Molecular events involved in promotion and regulation of capacitation. Mol. Reprod. Dev. 2010, 77, 197–208. [Google Scholar] [PubMed]
- Kobori, H.; Miyazaki, S.; Kuwabara, Y. Characterization of intracellular Ca (2+) increase in response to progesterone and cyclic nucleotides in mouse spermatozoa. Biol. Reprod. 2000, 63, 113–120. [Google Scholar] [PubMed]
- Yamashiro, H.; Toyomizu, M.; Toyama, N.; Aono, N.; Sakurai, M.; Hiradate, Y.; Yokoo, M.; Moisyadi, S.; Sato, E. Extracellular ATP and dibutyryl cAMP enhance the freezability of rat epididymal sperm. J. Am. Assoc. Lab. Anim. Sci. 2010, 49, 167–172. [Google Scholar] [PubMed]
- Loveland, K.L.; Klein, B.; Pueschl, D.; Indumathy, S.; Bergmann, M.; Loveland, B.E.; Hedger, M.P.; Schuppe, H.C. Cytokines in male fertility and reproductive pathologies: Immunoregulation and beyond. Front. Endocrinol. 2017, 8, 307. [Google Scholar]
- Qian, L.; Zhou, Y.; Du, C.; Wen, J.; Teng, S.; Teng, Z. IL-18 levels in the semen of male infertility: Semen analysis. Int. J. Biol. Macromol. 2014, 64, 190–192. [Google Scholar] [PubMed]
- Qian, L.; Bian, G.R.; Li, H.B.; Zhou, Y.; Dong, S.D.; Wang, W.J.; Song, J. Effects of ureaplasma urealyticum infection on sperm quality and concentrations of nitric oxide and cytokine in the semen of infertile males. Am. J. Reprod. Immunol. 2016, 75, 605–608. [Google Scholar] [CrossRef]
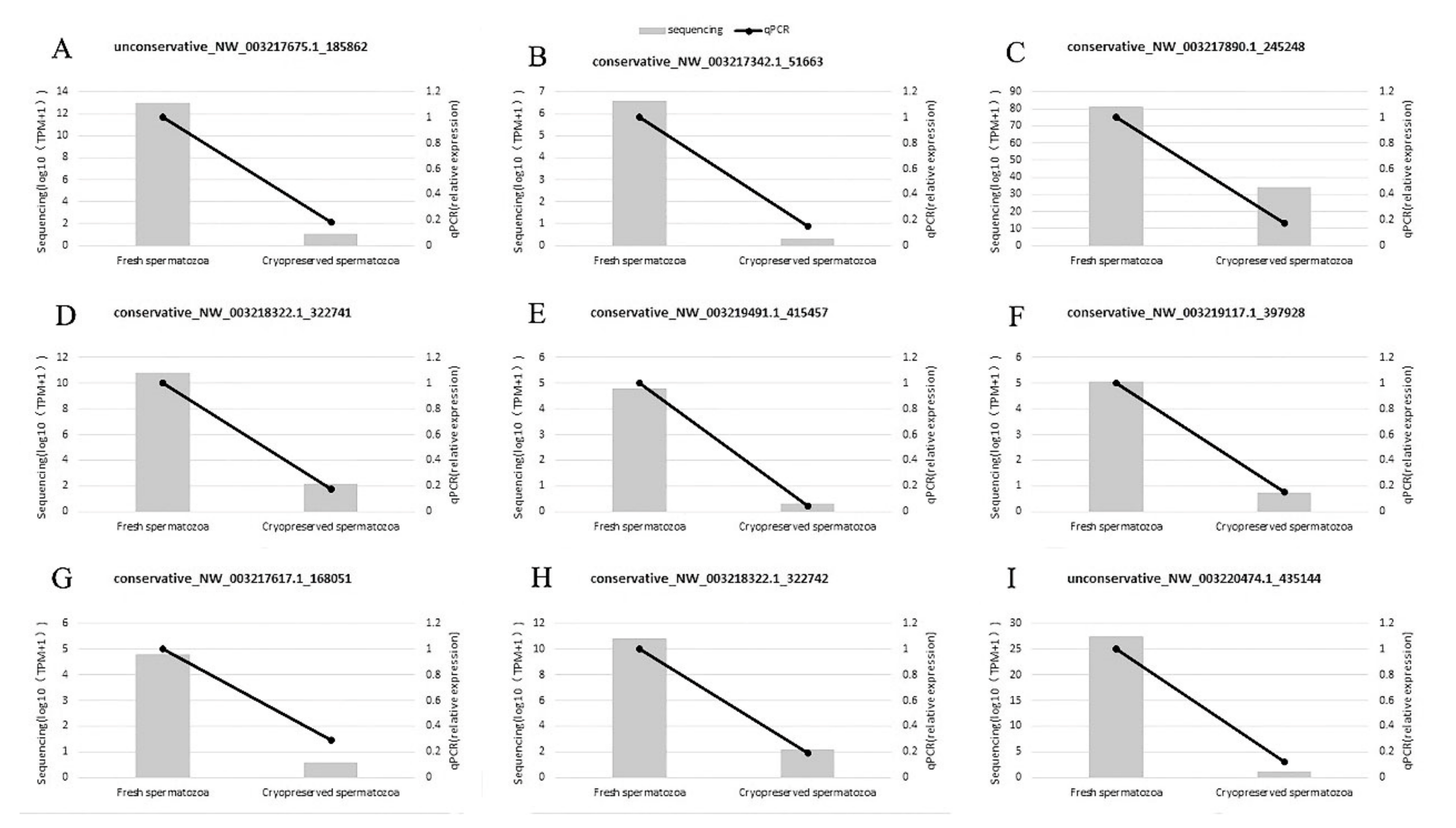

| Gene | Primer (5′–3′) |
|---|---|
| U6 | F: TTATGGGTCCTAGCCTGAC |
| R: CACTATTGCGGGTCTGC | |
| unconservative_NW_003217675.1_185862 | CCTGGTCGGTGATGCTCTGC |
| conservative_NW_003217342.1_51663 | CCTGGTTCTGGAGGCTGGAAGTCTG |
| conservative_NW_003217890.1_245248 | CTGCCCTGGCCCGAGGGACCGACT |
| conservative_NW_003218322.1_322741 | GAGACTGTCTGGAGCCCTGGG |
| conservative_NW_003219491.1_415457 | CCCTGGGTCGGCCGGGCTGGGGAG |
| conservative_NW_003219117.1_397928 | CCGCCTGGACCTGGGCTACTCAA |
| conservative_NW_003217617.1_168051 | CTGGGAGTCAGACTGTGAGG |
| conservative_NW_003218322.1_322742 | GAGACTGTCTGGAGCCCTGGG |
| unconservative_NW_003220474.1_435144 | TACGGGGCTGCATCAACTCTGAGGA |
| Sperm Quality Parameters | Boar | Giant Panda | |||
|---|---|---|---|---|---|
| Fresh | Post-Thawed | Fresh | Post-Thawed | ||
| Motility (%) | 92.00 ± 2.12 | 41.80 ± 1.78 | 71.70 ± 6.00 | 56.10 ± 3.90 | |
| Viability (%) | 94.03 ± 0.68 | 45.19 ± 3.15 | 72.05 ± 6.00 | 63.00 ± 7.00 | |
| Acrosome integrity (%) | 79.37 ± 1.43 | 56.26 ± 2.15 | 93.00 ± 1.70 | 81.70 ± 4.70 | |
| Sperm head | Length (µm) | 8.12 ± 0.13 | 8.01 ± 0.12 | 4.7 ± 0.3 | 4.7 ± 0.2 |
| Width (µm) | 4.07 ± 0.05 | 3.98 ± 0.04 | 3.6 ± 0.2 | 3.7 ± 0.1 | |
| Area (μm2) | 28.48 ± 0.26 | 27.28 ± 0.46 | 14.3 ± 1.4 | 14.7 ± 0.9 | |
| Perimeter length (µm) | 22.37 ± 0.16 | 21.69 ± 0.21 | 14.1 ± 0.8 | 14.2 ± 0.4 | |
| mRNA | Boar | Giant Panda | Functions |
|---|---|---|---|
| GLT8D2 (Glycosyltransferase 8 Domain Containing 2) | up | up | Participates in nonalcoholic fatty liver disease (NAFLD) pathogenesis [37,38]. |
| CLK2 (CDC Like Kinase 2) | down | down | Acts as a hepatic gluconeogenesis and glucose output suppressor that inhibits transcriptional activity of PPARGC1A on gluconeogenic genes via its phosphorylation [39,40,41,42]. |
| PHACTR3 (Phosphatase and Actin Regulator 3) | up | up | Regulates cell morphogenesis, enhances cell spreading and motility through direct interaction with actin [43,44]. |
| RHBDD3 (Rhomboid Domain Containing 3) | up | up | Acts as a critical regulator of dendritic cell activation [45,46]. |
| ATAT1 (Alpha Tubulin Acetyltransferase 1) | up | up | Affects intracellular transport, cell motility, cilia formation, and neuronal signaling [47]. |
| METTL1 (Methyltransferase Like 1) | down | down | Mediates tRNA Methylome [48]. |
| DPF1 (Double PHD Fingers 1) | up | up | Promotes the formation of semen-derived virus infection enhancer and semenogelin fibrils [49]. |
| SERTAD4 (SERTA Domain Containing 4) | down | down | Interacts with I-mfa domain proteins and negatively regulates transcriptional activity of SERTA domain proteins [50]. |
| S1PR5 (Sphingosine-1-Phosphate Receptor 5) | up | up | Regulates trafficking of monocytes and influences natural killer (NK) cell distribution [51,52]. |
| mRNA | Boar | Giant Panda | Functions |
|---|---|---|---|
| HSD17B14 (Hydroxysteroid 17-Beta Dehydrogenase 14) | down | up | Acts as a stereo-specific oxidation/reduction catalyst at carbon 17β of androgens and estrogens [53]. |
| BCAT2 (Branched Chain Amino Acid Transaminase 2) | down | up | Serves as a transporter of branched chain alpha-keto acids that catalyzes the first reaction in the catabolism of the essential branched chain amino acids leucine, isoleucine, and valine [54]. |
| ZNF582 (Zinc Finger Protein 582) | down | up | Involved in the aggressive progression and poor prognosis of oral squamous cell carcinoma [55]. |
| SLC25A25 (Solute Carrier Family 25 Member 25) | down | up | Controls adenosine triphosphate (ATP) homeostasis and contributes to the maintenance of body temperature during cold stress in mice, and is potentially involved in muscle thermogenesis under ketogenic diet (KD)-induced hypothermia in mammals [56,57]. |
| PRCC (Proline Rich Mitotic Checkpoint Control Factor) | down | up | Interacts with the cell cycle control protein Mad2B, and translocates to the nucleus [58]. |
| MEF2D (Myocyte Enhancer Factor 2D) | down | up | Regulates myogenesis, neuronal death and cancer cell proliferation, migration and invasion [59,60]. |
| ADAMTSL4 (ADAMTS Like 4) | down | up | Positively regulates apoptosis and facilitates FBN1 microfibril biogenesis [61]. |
| SMOX (Spermine oxidase) | down | up | Catalyzes oxidation of spermine to generate spermidine, H2O2 and 3-aminopropanal [62]. |
| SLC8B1 (Solute Carrier Family 8 Member B1) | down | up | Mediates sodium-dependent calcium efflux from mitochondrion [63]. |
| OSM (Oncostatin M) | down | up | Involved in inflammatory responses [64,65]. |
| CFP (Complement Factor Properdin) | down | up | Acts as a positive regulator of the alternative complement pathway [66]. |
| RELT (RELT TNF Receptor) | down | up | Controls the early phase of T-cell activation, probably by promoting T-cell apoptosis. Induces apoptosis in human epithelial cells [67,68]. |
| PELP1 (Proline, Glutamate and Leucine Rich Protein 1) | down | up | Involves in chromatin remodeling and DNA repair. PELP1-positive cells are shown to be significantly decreased in males with normal semen [69,70]. |
| CHST3 (carbohydrate sulfotransferase 3) | down | up | Associated with severe chondrodysplasia and progressive spinal involvement [71]. |
| PRAM1 (PML-RARA Regulated Adaptor Molecule 1) | down | up | Mediates retinoic acid effects in leukemia cells and stimulates the activity of HPK-1 and c-Jun N-terminal kinase (JNK) [72,73]. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ran, M.-X.; Zhou, Y.-M.; Liang, K.; Wang, W.-C.; Zhang, Y.; Zhang, M.; Yang, J.-D.; Zhou, G.-B.; Wu, K.; Wang, C.-D.; et al. Comparative Analysis of MicroRNA and mRNA Profiles of Sperm with Different Freeze Tolerance Capacities in Boar (Sus scrofa) and Giant Panda (Ailuropoda melanoleuca). Biomolecules 2019, 9, 432. https://doi.org/10.3390/biom9090432
Ran M-X, Zhou Y-M, Liang K, Wang W-C, Zhang Y, Zhang M, Yang J-D, Zhou G-B, Wu K, Wang C-D, et al. Comparative Analysis of MicroRNA and mRNA Profiles of Sperm with Different Freeze Tolerance Capacities in Boar (Sus scrofa) and Giant Panda (Ailuropoda melanoleuca). Biomolecules. 2019; 9(9):432. https://doi.org/10.3390/biom9090432
Chicago/Turabian StyleRan, Ming-Xia, Ying-Min Zhou, Kai Liang, Wen-Can Wang, Yan Zhang, Ming Zhang, Jian-Dong Yang, Guang-Bin Zhou, Kai Wu, Cheng-Dong Wang, and et al. 2019. "Comparative Analysis of MicroRNA and mRNA Profiles of Sperm with Different Freeze Tolerance Capacities in Boar (Sus scrofa) and Giant Panda (Ailuropoda melanoleuca)" Biomolecules 9, no. 9: 432. https://doi.org/10.3390/biom9090432







