Extraction, Structural Characterization, and Biological Functions of Lycium Barbarum Polysaccharides: A Review
Abstract
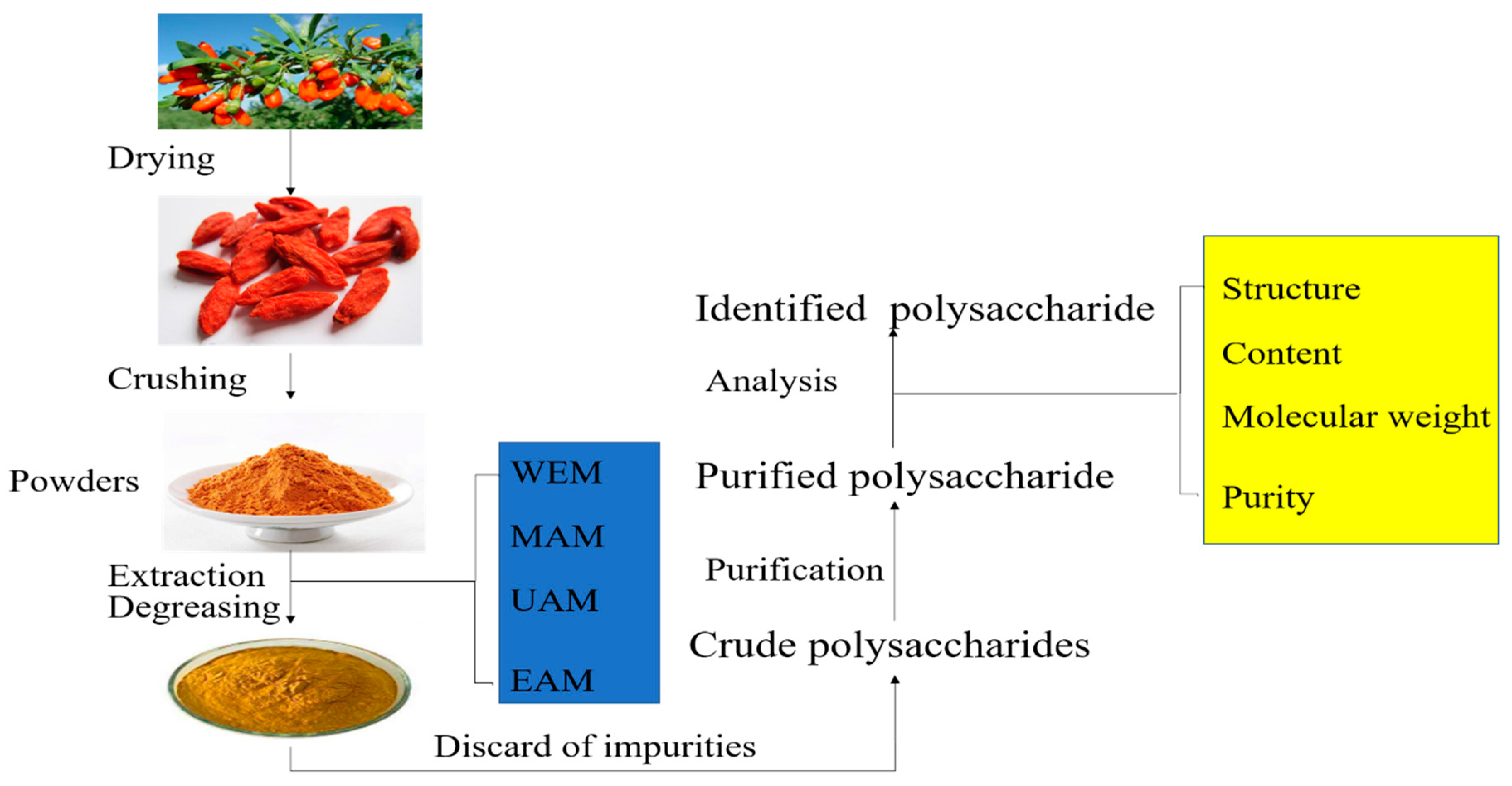
:1. Introduction
2. Extraction Methods of LBPs
3. Structure and Composition of LBPs
4. Biological Function of LBPs
4.1. Antioxidant Function
4.2. Immune Regulation
4.3. Antitumor Activity of LBPs
4.4. Neuroprotective Effects of LBPs
4.5. Other Biological Activities
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| LBPs | Lycium barbarum polysaccharides |
| WEM | water extraction method |
| EAM | enzyme-assisted extraction method |
| MAM | microwave-assisted extraction method |
| UAM | ultrasonic-assisted extraction method |
| SEC | size exclusive chromatography |
| ESI-MS | electrospray ionization mass spectrometry |
| GC–MS | gas chromatography–mass spectrometry |
| NMR | nuclear magnetic resonance |
| HPGPC | high performance gel permeation chromatography |
References
- Li, X.M. Protective effect of Lycium barbarum polysaccharides on streptozotocin-induced oxidative stress in rats. Int. J. Biol. Macromol. 2007, 40, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Amagase, H.; Farnsworth, N.R. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji). Food Res. Int. 2011, 44, 1702–1717. [Google Scholar] [CrossRef]
- Wu, D.-T.; Guo, H.; Lin, S.; Lam, S.-C.; Zhao, L.; Lin, D.-R.; Qin, W. Review of the structural characterization, quality evaluation, and industrial application of Lycium barbarum polysaccharides. Trends Food Sci. Technol. 2018, 79, 171–183. [Google Scholar] [CrossRef]
- Potterat, O. Goji (Lycium barbarum and L. chinense): Phytochemistry, Pharmacology and Safety in the Perspective of Traditional Uses and Recent Popularity. Planta Med. 2010, 76, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, J.; Tao, W.; Zhang, X.; Gao, X.; Yong, J.; Zhao, J.; Zhang, L.; Li, Y.; Duan, J.-A. Lycium ruthenicum studies: Molecular biology, Phytochemistry and pharmacology. Food Chem. 2018, 240, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Liu, Y.; Wu, M.; Liu, X.; Shen, X.; Liu, C.; Wang, Y. Identification and validation of reference genes for quantitative real-time PCR normalization and its applications in lyceum. PLoS ONE 2014, 9, e97039. [Google Scholar]
- Wawruszak, A.; Czerwonka, A.; Okła, K.; Rzeski, W. Anticancer effect of ethanol Lycium barbarum (Goji berry) extract on human breast cancer T47D cell line. Nat. Prod. Res. 2015, 30, 1. [Google Scholar]
- Tang, W.-M.; Chan, E.; Kwok, C.-Y.; Lee, Y.-K.; Wu, J.-H.; Wan, C.-W.; Chan, R.Y.-K.; Yu, P.H.-F.; Chan, S.-W. A review of the anticancer and immunomodulatory effects of Lycium barbarum fruit. Inflammopharmacology 2012, 20, 307–314. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Chen, W.W.; Zhao, J.H.; Xi, W.P. Functional constituents and antioxidant activities of eight Chinese native goji genotypes. Food Chem. 2016, 200, 230–236. [Google Scholar] [CrossRef]
- Yao, R.; Heinrich, M.; Weckerle, C.S. The genus Lycium as food and medicine: A botanical, ethnobotanical and historical review. J. Ethnopharmacol. 2018, 212, 50–66. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Chen, S.; Zhang, Z. Determination of Polysaccharide Contents in Fructus Lycii. Chin. Herbal Med. 1991, 22, 67–69. [Google Scholar]
- Lu, S.-P.; Zhao, P.-T. Chemical characterization of Lycium barbarum polysaccharides and their reducing myocardial injury in ischemia/reperfusion of rat heart. Int. J. Biol. Macromol. 2010, 47, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.-F.; Wan, L.-L.; Peng, J.-L.; Guo, C. Alleviation of the acute doxorubicin-induced cardiotoxicity by Lycium barbarum polysaccharides through the suppression of oxidative stress. Food Chem. Toxicol. 2011, 49, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.M.; Wang, R.; Zhang, B.K.; Wang, C.L.; Guo, H.; Zhang, S.J. Impact of Lycium Barbarum Polysaccharide and Danshensu on vascular endothelial growth factor in the process of retinal neovascularization of rabbit. Int. J. Ophthalmol. 2013, 6, 59–61. [Google Scholar] [PubMed]
- Gong, H.; Shen, P.; Jin, L.; Xing, C.; Tang, F. Therapeutic effects of Lycium barbarum polysaccharide (LBP) on irradiation or chemotherapy-induced myelosuppressive mice. Cancer Biother. Radiopharm. 2005, 20, 155–162. [Google Scholar] [CrossRef]
- Chang, R.C.-C.; So, K.-F. Use of anti-aging herbal medicine, Lycium barbarum, against aging-associated diseases. What do we know so far? Cell. Mol. Neurobiol. 2008, 28, 643–652. [Google Scholar] [CrossRef]
- He, N.; Yang, X.; Jiao, Y.; Tian, L.; Zhao, Y. Characterisation of antioxidant and antiproliferative acidic polysaccharides from Chinese wolfberry fruits. Food Chem. 2012, 133, 978–989. [Google Scholar] [CrossRef]
- Xiao, J.; Liong, E.C.; Ching, Y.P.; Chang, R.C.; So, K.F.; Fung, M.L.; Tipoe, G.L. Lycium barbarum polysaccharides protect mice liver from carbon tetrachloride-induced oxidative stress and necroinflammation. J. Ethnopharmacol. 2012, 139, 462–470. [Google Scholar] [CrossRef]
- Zhou, Z.Q.; Fan, H.X.; He, R.R.; Xiao, J.; Tsoi, B.; Lan, K.H.; Kurihara, H.; So, K.F.; Yao, X.S.; Gao, H. Lycibarbarspermidines A–O, New Dicaffeoylspermidine Derivatives from Wolfberry, with Activities against Alzheimer’s Disease and Oxidation. J. Agric. Food Chem. 2016, 64, 2223. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, S.W.; Cheung, P.C.K.; Wang, Q. Antitumor polysaccharides from mushrooms: a review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci. Technol. 2007, 18, 4–19. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, F.; Ming, K.; Wang, D.; Hu, Y.; Liu, J.J.M. Polysaccharides from Traditional Chinese Medicines: Extraction, Purification, Modification, and Biological Activity. Molecules 2016, 21, 1705. [Google Scholar] [CrossRef] [PubMed]
- Masci, A.; Carradori, S.; Casadei, M.A.; Paolicelli, P.; Petralito, S.; Ragno, R.; Cesa, S. Lycium barbarum polysaccharides: Extraction, purification, structural characterisation and evidence about hypoglycaemic and hypolipidaemic effects. A review. Food Chem. 2018, 254, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Skenderidis, P.; Petrotos, K.; Giavasis, I.; Hadjichristodoulou, C.; Tsakalof, A. Optimization of ultrasound assisted extraction of of goji berry (Lycium barbarum) fruits and evaluation of extracts’ bioactivity. J. Food Process. Eng. 2017, 40, 12. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, R.F.; Qiu, T.Q. Ultrasound-enhanced subcritical water extraction of polysaccharides from Lycium barbarum L. Sep. Purif. Technol. 2013, 120, 141–147. [Google Scholar]
- Zhang, J.; Jia, S.Y.; Liu, Y.; Wu, S.H.; Ran, J.Y. Optimization of enzyme-assisted extraction of the Lycium barbarum polysaccharides using response surface methodology. Carbohydr. Polym. 2011, 86, 1089–1092. [Google Scholar] [CrossRef]
- Liu, Z.G.; Dang, J.; Wang, Q.L.; Yu, M.F.; Jiang, L.; Mei, L.J.; Shao, Y.; Tao, Y.D. Optimization of polysaccharides from Lycium ruthenicum fruit using RSM and its anti-oxidant activity. Int. J. Biol. Macromol. 2013, 61, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E.; de Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Ma, T.; Sun, X.; Tian, C.; Luo, J.; Zheng, C.; Zhan, J. Polysaccharide extraction from Sphallerocarpus gracilis roots by response surface methodology. Int. J. Biol. Macromol. 2016, 88, 162–170. [Google Scholar] [CrossRef]
- Zhong-Qiua, H.U.; Liu, J.D.; Wang, B.L. Research on extraction technology of Lycium barbarum polysaccharides by the alkaline solution. J. Northwest A F Univ. Nat. Sci. Ed. 2008, 36, 173–178. [Google Scholar]
- Luo, Q.; Yan, J.; Zhang, S. Isolation and purification of Lycium barbarum polysaccharides and its antifatigue effect. J. Hyg. Res. 2000, 29, 115–117. [Google Scholar]
- Ji, X.L.; Peng, Q.; Yuan, Y.P.; Liu, F.; Wang, M. Extraction and physicochemical properties of polysaccharides from Ziziphus Jujuba cv. Muzao by ultrasound-assisted aqueous two-phase extraction. Int. J. Biol. Macromol. 2018, 108, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.H.; Dang, Y.L. Optimization of extraction technology of the Lycium barbarum polysaccharides by Box-Behnken statistical design. Carbohydr. Polym. 2008, 74, 603–610. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, G.; Zhang, J.; Jia, S.; Li, F.; Wang, Y.; Wu, S. Response surface optimization of ultrasound-assisted enzymatic extraction polysaccharides from Lycium barbarum. Carbohydr. Polym. 2014, 110, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.-F.; Zhao, C.; Chen, X.; Chan, S.-W.; Wu, J.-Y. Chemical properties and bioactivities of Goji (Lycium barbarum) polysaccharides extracted by different methods. J. Funct. Foods 2015, 17, 903–909. [Google Scholar] [CrossRef]
- Li, X.M.; Li, X.L.; Zhou, A.G. Evaluation of antioxidant activity of the polysaccharides extracted from Lycium barbarum fruits in vitro. Eur. Polym. J. 2007, 43, 488–497. [Google Scholar] [CrossRef]
- Muatasim, R.; Ma, H.L.; Yang, X. Effect of multimode ultrasound assisted extraction on the yield of crude polysaccharides from Lycium Barbarum (Goji). Food Sci. Technol. 2018, 38, 160–166. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Ali, Z.; Khan, I.A. Glycerogalactolipids from the fruit of Lycium barbarum. Phytochemistry 2008, 69, 2856–2861. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.X.; Li, Y.Q.; Xiao, Q.W. Simultaneous determination of monosaccharides and oligosaccharides in Lycium barbarum L. by high performance liquid chromatography. Sichuan Da Xue Xue Bao. Yi Xue Ban = J. Sichuan Univ. Med. Sci. Ed. 2007, 38, 1040. [Google Scholar]
- Yang, J.; Wei, Y.Q.; Ding, J.B.; Li, Y.L.; Ma, J.L.; Liu, J.L. Research and application of Lycii Fructus in medicinal field. Chin. Herb. Med. 2018, 10, 339–352. [Google Scholar] [CrossRef]
- Peng, X.; Tian, G. Structural characterization of the glycan part of glycoconjugate LbGp2 from Lycium barbarum L. Carbohydr. Res. 2001, 331, 95–99. [Google Scholar] [CrossRef]
- Huang, L.J.; Tian, G.Y.; Ji, G.Z. Structure Elucidation of Glycan of Glycoconjugate LbGp3 Isolated from the Fruit of Lycium barbarum L. J. Asian Nat. Prod. Res. 1999, 1, 259–267. [Google Scholar] [CrossRef]
- Peng, X.M.; Huang, L.J.; Qi, C.H.; Zhang, Y.X.; Tian, G.Y. Studies on Chemistry and Immuno-modulating Mechanism of a Glycoconjugate from Lycium barbarum L. Chin. J. Chem. 2001, 19, 1190–1197. [Google Scholar] [CrossRef]
- Zhao, C.; Li, R.; He, Y.; Chui, G. Studies on chemistry of Gouqi polysaccharides. Yie Daxue Xuebao 1997, 29, 231–232. [Google Scholar]
- Zhang, M.; Tang, X.; Wang, F.; Zhang, Q.; Zhang, Z. Characterization of Lycium barbarum polysaccharide and its effect on human hepatoma cells. Int. J. Biol. Macromol. 2013, 61, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.J.; He, Y.Q.; Li, R.Z.; Cui, G.H. Chemistry and pharmacological activity of peptidoglycan from lycium barbaruml. Chin. Chem. Lett. 1996, 7, 1009–1010. [Google Scholar]
- Duan, C.L.; Qiao, S.Y.; Wang, N.L.; Zhao, Y.M.; Qi, C.H.; Yao, X.S. Studies on the active polysaccharides from Lycium barbarum L. Yaoxue Xuebao 2001, 36, 196–199. [Google Scholar]
- Gong, G.P.; Fan, J.B.; Sun, Y.J.; Wu, Y.M.; Liu, Y.; Sun, W.; Zhang, Y.; Wang, Z.F. Isolation, structural characterization, and antioxidativity of polysaccharide LBLP5-A from Lycium barbarum leaves. Process. Biochem. 2016, 51, 314–324. [Google Scholar] [CrossRef]
- Redgwell, R.J.; Curti, D.; Wang, J.; Dobruchowska, J.M.; Gerwig, G.J.; Kamerling, J.P.; Bucheli, P. Cell wall polysaccharides of Chinese Wolfberry (Lycium barbarum): Part 2. Characterisation of arabinogalactan-proteins. Carbohydr. Polym. 2011, 84, 1075–1083. [Google Scholar] [CrossRef]
- Liu, H.; Fan, Y.; Wang, W.; Liu, N.; Zhang, H.; Zhu, Z.; Liu, A. Polysaccharides from Lycium barbarum leaves: Isolation, characterization and splenocyte proliferation activity. Int. J. Biol. Macromol. 2012, 51, 417–422. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Sun, Y.; Mou, Q.; Wang, B.; Zhang, Y.; Huang, L. Structural characterization of LbGp1 from the fruits of Lycium barbarum L. Food Chem. 2014, 159, 137–142. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Y.; Zhu, R.; Yu, J.; Lu, W.; Pan, C.; Yao, W.; Gao, X. Structure characterization, chemical and enzymatic degradation, and chain conformation of an acidic polysaccharide from Lycium barbarum L. Carbohydr. Polym. 2016, 147, 114–124. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, L.; Yue, H.; Ding, K. Structure analysis of a heteropolysaccharide from fruits of Lycium barbarum L. and anti-angiogenic activity of its sulfated derivative. Int. J. Biol. Macromol. 2018, 108, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Lv, X.P.; Xu, Q.S.; Li, Y.; Huang, L.J.; Du, Y.G. Isolation and structural characterization of the polysaccharide LRGP1 from Lycium ruthenicum. Carbohydr. Polym. 2012, 90, 95–101. [Google Scholar] [CrossRef]
- Gil-Chavez, G.J.; Villa, J.A.; Ayala-Zavala, J.F.; Heredia, J.B.; Sepulveda, D.; Yahia, E.M.; Gonzalez-Aguilar, G.A. Technologies for Extraction and Production of Bioactive Compounds to be Used as Nutraceuticals and Food Ingredients: An Overview. Compr. Rev. Food Sci. Food Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
- Gomez-Guillen, M.C.; Gimenez, B.; Lopez-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, N.C.; Turkoglu, S.; Yildirim, N.; Ince, O.K. Antioxidant properties of wild edible mushroom Pleurotus eryngii collected from Tunceli province of Turkey. Dig. J. Nanomater. Biostruct. 2012, 7, 1647–1654. [Google Scholar]
- Ding, Y.; Yan, Y.M.; Peng, Y.J.; Chen, D.; Mi, J.; Lu, L.; Luo, Q.; Li, X.Y.; Zeng, X.X.; Cao, Y.L. In vitro digestion under simulated saliva, gastric and small intestinal conditions and fermentation by human gut microbiota of polysaccharides from the fruits of Lycium barbarum. Int. J. Biol. Macromol. 2019, 125, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Song, N.; Liu, C.Y.; Yang, G.L. The estrogen-like protective effect of Lycium barbarum polysaccharides in reducing oxidative stress on myocardial cells from ovariectomized rats. Mol. Med. Rep. 2019, 19, 2271–2278. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.Z.; Ben, H.J.; Li, H.Q.; Li, X.H.; Dong, T.C.; Xu, S.M.; Yan, Y.L.; Sun, B.K.; Bai, J.W.; Li, Y.S. Lycium barbarum polysaccharide reduces hyperoxic acute lung injury in mice through Nrf2 pathway. Biomed. Pharm. 2019, 111, 733–739. [Google Scholar] [CrossRef]
- Guo, D.J.; Cheng, H.L.; Chan, S.W.; Yu, P.H.F. Antioxidative activities and the total phenolic contents of tonic Chinese Medicinal Herbs. Inflammopharmacology 2008, 16, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Chang, S.C.; Inbaraj, B.S.; Chen, B.H. Isolation of carotenoids, flavonoids and polysaccharides from Lycium barbarum L. and evaluation of antioxidant activity. Food Chem. 2010, 120, 184–192. [Google Scholar] [CrossRef]
- Cheng, D.; Kong, H. The effect of Lycium barbarum polysaccharide on alcohol-induced oxidative stress in rats. Molecules 2011, 16, 2542–2550. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, B.; Wang, S.; Liang, Y.X.; Yang, J.; So, K.F.; Yuan, T.F. Image processing methods to elucidate spatial characteristics of retinal microglia after optic nerve transection. Sci. Rep. 2016, 6, 21816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosio, M.S.; Buratti, S.; Mannino, S.; Benedetti, S. Use of an electrochemical method to evaluate the antioxidant activity of herb extracts from the Labiatae family. Food Chem. 2006, 97, 725–731. [Google Scholar] [CrossRef]
- Liang, B.; Jin, M.; Liu, H. Water-soluble polysaccharide from dried Lycium barbarum fruits: Isolation, structural features and antioxidant activity. Carbohydr. Polym. 2011, 83, 1947–1951. [Google Scholar] [CrossRef]
- Niu, A.J.; Wu, J.M.; Yu, D.H.; Wang, R. Protective effect of Lycium barbarum polysaccharides on oxidative damage in skeletal muscle of exhaustive exercise rats. Int. J. Biol. Macromol. 2008, 42, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Zhou, J.; Ma, T.; Chai, Q. Lycium barbarum Polysaccharides Reduce Exercise-Induced Oxidative Stress. Int. J. Mol. Sci. 2011, 12, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Wang, P.L.; Jiang, R. A study on the preventive effect of Lycium barbarum polysaccharide on the development of alcoholic fatty liver in rats and its possible mechanisms. Zhonghua Gan Zang Bing Za Zhi = Zhonghua Ganzangbing Zazhi = Chin. J. Hepatol. 2007, 15, 204. [Google Scholar]
- Amagase, H.; Sun, B.; Borek, C. Lycium barbarum (goji) juice improves in vivo antioxidant biomarkers in serum of healthy adults. Nutr. Res. 2009, 29, 19–25. [Google Scholar] [CrossRef]
- Gong, G.P.; Dang, T.T.; Deng, Y.N.; Han, J.L.; Zou, Z.H.; Jing, S.; Zhang, Y.; Liu, Q.; Huang, L.J.; Wang, Z.F. Physicochemical properties and biological activities of polysaccharides from Lycium barbarum prepared by fractional precipitation. Int. J. Biol. Macromol. 2018, 109, 611–618. [Google Scholar] [CrossRef]
- Chen, L.; Li, W.; Qi, D.; Wang, D. Lycium barbarum polysaccharide protects against LPS-induced ARDS by inhibiting apoptosis, oxidative stress and inflammation in pulmonary endothelial cells. Free Radic Res. 2018, 52, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lao, W.; Ji, Q.S.; Yang, Z.H.; Yu, G.C.; Zhong, J.X. Lycium barbarum polysaccharides protected human retinal pigment epithelial cells against oxidative stress-induced apoptosis. Int. J. Ophthalmol. 2015, 8, 11. [Google Scholar]
- Wu, H.T.; He, X.J.; Hong, Y.K.; Ma, T.; Xu, Y.P.; Li, H.H. Chemical characterization of lycium barbarum polysaccharides and its inhibition against liver oxidative injury of high-fat mice. Int. J. Biol. Macromol. 2010, 46, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.J.; Bao, S.Y.; Du, Y.; Jian, Z.Y.; Wuliji, A.O.; Ren, X.; Zhang, C.H.; Chu, H.Y.; Kong, L.; Ma, H.Y. Antioxidant effects of Lycium barbarum polysaccharides on photoreceptor degeneration in the light-exposed mouse retina. Biomed. Pharm. 2018, 103, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.J.; Zhang, Z.W.; Li, H.; Pan, X.Y.; Chen, S.S.; Cui, Z.Y.; Ma, J.; Zhou, Z.X.; Xing, B. Lycium barbarum polysaccharides protects H9c2 cells from hypoxia-induced injury by down-regulation of miR-122. Biomed. Pharm. 2019, 110, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.J.; Zheng, J.; Han, X.X.; Jiang, Y.P.; Li, Z.M.; Wu, J.; Chang, Q.; Niu, Y.; Sun, T.; Li, Y.X.; et al. Lycium barbarum polysaccharide attenuates diabetic testicular dysfunction via inhibition of the PI3K/Akt pathway-mediated abnormal autophagy in male mice. Cell Tissue Res. 2018, 374, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Skenderidis, P.; Kerasioti, E.; Karkanta, E.; Stagos, D.; Kouretas, D.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Assessment of the antioxidant and antimutagenic activity of extracts from goji berry of Greek cultivation. Toxicol. Rep. 2018, 5, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.M.; Zhang, J.Q.; Fei, Y.F. Lycium barbarum polysaccharide attenuates chemotherapy-induced ovarian injury by reducing oxidative stress. J. Obs. Gynaecol. Res. 2017, 43, 1621–1628. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhou, W.; Zhang, Y. Immunoregulation and Lycium Barbarum; Springer: Dordrecht, The Netherlands, 2015. [Google Scholar]
- Wang, W.; Liu, M.X.; Wang, Y.; Yang, T.; Li, D.S.; Ding, F.; Sun, H.Z.; Bai, G.; Li, Q. Lycium barbarum Polysaccharide Promotes Maturation of Dendritic Cell via Notch Signaling and Strengthens Dendritic Cell Mediated T Lymphocyte Cytotoxicity on Colon Cancer Cell CT26-WT. Evid. Based Complement. Altern. Med. 2018. [Google Scholar] [CrossRef]
- Liu, Y.L.; Yin, R.Q.; Liang, S.S.; Duan, Y.L.; Yao, J.H.; Duan, Y.L.; Yang, X.J. Effect of dietary Lycium barbarum polysaccharide on growth performance and immune function of broilers. J. Appl. Poult. Res. 2017, 26, 200–208. [Google Scholar]
- Bo, R.N.; Zheng, S.S.; Xing, J.; Luo, L.; Niu, Y.L.; Huang, Y.; Liu, Z.G.; Hu, Y.L.; Liu, J.G.; Wu, Y.; et al. The immunological activity of Lycium barbarum polysaccharides liposome in vitro and adjuvanticity against PCV2 in vivo. Int. J. Biol. Macromol. 2016, 85, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.Y.; Lai, Y.K.; Yu, H.C.; Chen, N.Y.; Chang, C.Y.; Lo, H.C.; Hsu, T.H. Effects of Lycium barbarum extract on production and immunomodulatory activity of the extracellular polysaccharopeptides from submerged fermentation culture of Coriolus versicolor. Food Chem. 2008, 110, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, Y.; Wang, D.; Liu, J.; Zhang, J.; Abula, S.; Zhao, B.; Ruan, S. Sulfated modification can enhance the immune-enhancing activity of lycium barbarum polysaccharides. Cell. Immunol. 2010, 263, 219–223. [Google Scholar] [CrossRef]
- Chen, Z.; Kwong, H.T.B.; Chan, S.H. Activation of T lymphocytes by polysaccharide-protein complex from Lycium barbarum L. Int. Immunopharmacol. 2008, 8, 1663–1671. [Google Scholar] [CrossRef]
- Deng, X.L.; Luo, S.; Luo, X.; Hu, M.H.; Ma, F.L.; Wang, Y.Y.; Lai, X.P.; Zhou, L. Polysaccharides from Chinese Herbal Lycium barbarum Induced Systemic and Local Immune Responses in H22 Tumor-Bearing Mice. J. Immunol. Res. 2018. [Google Scholar] [CrossRef]
- Shen, C.Y.; Zhang, W.L.; Jiang, J.G. Immune-enhancing activity of polysaccharides from Hibiscus sabdariffa Linn. via MAPK and NF-kappa B signaling pathways in RAW264.7 cells. J. Funct. Foods 2017, 34, 118–129. [Google Scholar] [CrossRef]
- Bo, R.N.; Sun, Y.Q.; Zhou, S.Z.; Ou, N.; Gu, P.F.; Liu, Z.G.; Hu, Y.L.; Liu, J.G.; Wang, D.Y. Simple nanoliposomes encapsulating Lycium barbarum polysaccharides as adjuvants improve humoral and cellular immunity in mice. Int. J. Nanomed. 2017, 12, 6289–6301. [Google Scholar] [CrossRef]
- Chen, S.Y.; Liang, L.N.; Wang, Y.; Diao, J.H.; Zhao, C.X.; Chen, G.; He, Y.F.; Luo, C.L.; Wu, X.H.; Zhang, Y. Synergistic immunotherapeutic effects of Lycium barbarum polysaccharide and interferon-alpha 2b on the murine Renca renal cell carcinoma cell line in vitro and in vivo. Mol. Med. Rep. 2015, 12, 6727–6737. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.Y.; Li, Y.; Meng, X.; Zhao, C.N.; Li, S.; Gan, R.Y.; Li, H.B. Dietary natural products and lung cancer: Effects and mechanisms of action. J. Funct. Foods 2019, 52, 316–331. [Google Scholar] [CrossRef]
- Yuan, Q.X.; Zhao, L.Y. The Mulberry (Morus alba L.) Fruit-A Review of Characteristic Components and Health Benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef]
- Khan, T.; Date, A.; Chawda, H.; Patel, K. Polysaccharides as potential anticancer agents-A review of their progress. Carbohydr. Polym. 2019, 210, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, M.; Wang, C.; Yang, Y.; Chen, J.; Ding, J.; Guo, W. Characterization and in vitro antitumor activity of polysaccharides from the mycelium of Sarcodon aspratus. Int. J. Biol. Macromol. 2013, 52, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Kwok, S.S.; Bu, Y.S.; Lo, A.C.Y.; Chan, T.C.Y.; So, K.F.; Lai, J.S.M.; Shih, K.C. A Systematic Review of Potential Therapeutic Use of Lycium Barbarum Polysaccharides in Disease. Biomed. Res. Int. 2019. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.J.; Chiang, S.W.; Wang, C.C.; Tsai, Y.H.; Wu, M.S. Hot water-extracted Lycium barbarum and Rehmannia glutinosa inhibit proliferation and induce apoptosis of hepatocellular carcinoma cells. World J. Gastroenterol. 2006, 12, 4478–4484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, F.; Xiao, B.; Jiang, Z.; Zhao, J.; Huang, X.; Guo, J. Anticancer effect of Lycium barbarum polysaccharides on colon cancer cells involves G0/G1 phase arrest. Med. Oncol. 2011, 28, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Xiao, B.X.; Jiang, Z.; Guo, Y.A.; Mao, F.; Zhao, J.W.; Huang, X.; Guo, J.M. Growth inhibition and cell-cycle arrest of human gastric cancer cells by Lycium barbarum polysaccharide. Med. Oncol. 2010, 27, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zou, Y.; Yi, L.I.; Qiao, W.U.; Zhang, B.; Liu, H.; Zhao, W.; Shen, B.; Neurosurgery, D.O. Lycium barbarum polysaccharide inhibits the growth of rat glioma by regulating the blood-brain barrier. Tumor 2018, 38, 102–110. [Google Scholar]
- Zhang, M.; Chen, H.X.; Huang, J.; Li, Z.; Zhu, C.P.; Zhang, S.H. Effect of lycium barbarum polysaccharide on human hepatoma QGY7703 cells: Inhibition of proliferation and induction of apoptosis. Life Sci. 2005, 76, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Du, G. Lycium barbarum polysaccharide stimulates proliferation of MCF-7 cells by the ERK pathway. Life Sci. 2012, 91, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ran, L.W.; Mi, J.; Yan, Y.M.; Lu, L.; Jin, B.; Li, X.Y.; Cao, Y.L. Isolation, Characterization and Antitumor Effect on DU145 Cells of a Main Polysaccharide in Pollen of Chinese Wolfberry. Molecules 2018, 23, 13. [Google Scholar] [CrossRef]
- Gao, Q.H.; Fu, X.; Zhang, R.; Wang, Z.; Guo, M. Neuroprotective effects of plant polysaccharides: A review of the mechanisms. Int. J. Biol. Macromol. 2017, 106, S0141813017313600. [Google Scholar] [CrossRef]
- Nelson, E.D.; Ramberg, J.E.; Best, T.; Sinnott, R.A.J.N.N. Neurologic effects of exogenous saccharides: A review of controlled human, animal, and in vitro studies. Nutr. Neurosci. 2012, 15, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.S.; Zhu, L.H.; Li, T.T.; Tang, X.Y.; Xiang, Y.H.; Han, X.J.; Xia, L.X.; Zeng, L.; Nie, J.H.; Huang, Y.X.; et al. Neuroprotective Mechanisms of Lycium barbarum Polysaccharides Against Ischemic Insults by Regulating NR2B and NR2A Containing NMDA Receptor Signaling Pathways. Front. Cell. Neurosci. 2017, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Qu, Y.; Chu, Q.; Li, W.; He, J. Investigation of the neuroprotective effects of Lycium barbarum water extract in apoptotic cells and Alzheimer’s disease mice. Mol. Med. Rep. 2018, 17, 3599–3606. [Google Scholar] [PubMed]
- Bie, M.; Lv, Y.; Ren, C.; Xing, F.; Cui, Q.; Xiao, J.; So, K.F. Lycium barbarum polysaccharide improves bipolar pulse current-induced microglia cell injury through modulating autophagy. Cell Transplant. 2015, 24, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Peng, T.; Yang, S.; Wang, W.; Zhang, Y.; Li, H. Lycium barbarum polysaccharide attenuates the cytotoxicity of mutant huntingtin and increases the activity of AKT. Int. J. Dev. Neurosci. 2016, 52, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ding, J.; Zhou, X.; Zhang, X.; Tao, H.; Wang, Y.; Li, G.J.B.R. Effects of lycium barbarum polysaccharides on neuropeptide Y and heat-shock protein 70 expression in rats exposed to heat. Biomed. Rep. 2014, 2, 687. [Google Scholar] [CrossRef]
- Teng, P.; Li, Y.H.; Cheng, W.J.; Zhou, L.; Shen, Y.; Wang, Y. Neuroprotective effects of Lycium barbarum polysaccharides in lipopolysaccharide-induced BV2 microglial cells. Mol. Med. Rep. 2013, 7, 1977–1981. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.S.; Yu, M.S.; Yang, X.F.; So, K.F.; Yuen, W.H.; Chang, R.C.C. Neuroprotective Effects of Polysaccharides from Wolfberry, the Fruits of Lycium barbarum, Against Homocysteine-induced Toxicity in Rat Cortical Neurons. J. Alzheimers Dis. 2010, 19, 813–827. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Pan, X.; Li, T.; Zhang, C.; Shi, N.J.O.M. Cellular Longevity, Lycium barbarum Polysaccharides Protect against Trimethyltin Chloride-Induced Apoptosis via Sonic Hedgehog and PI3K/Akt Signaling Pathways in Mouse Neuro-2a Cells. Oxidative Med. Cell. Longev. 2016, 2016, 9826726. [Google Scholar] [CrossRef]
- Zhao, Z.K.; Yu, H.L.; Liu, B.; Wang, H.; Luo, Q.; Ding, X.G. Antioxidative mechanism of Lycium barbarum polysaccharides promotes repair and regeneration following cavernous nerve injury. Neural Regen. Res. 2016, 11, 1312–1321. [Google Scholar]
- Di, Y.; Suk-Yee, L.; Chung-Man, Y.; Chuen-Chung, C.R.; Kwok-Fai, S.; David, W.; Lo, A.C. Lycium barbarum extracts protect the brain from blood-brain barrier disruption and cerebral edema in experimental stroke. PLoS ONE 2012, 7, e33596. [Google Scholar]
- Olatunji, O.J.; Chen, H.; Zhou, Y.J.N.L. Lycium chinensis Mill attenuates glutamate induced oxidative toxicity in PC12 cells by increasing antioxidant defense enzymes and down regulating ROS and Ca2+ generation. Neurosci. Lett. 2016, 616, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, Y.; Chiu, K.; Yuan, Q.; Lin, B.; Chang, R.C.-C.; So, K.-F. Lycium Barbarum (Wolfberry) Reduces Secondary Degeneration and Oxidative Stress, and Inhibits JNK Pathway in Retina after Partial Optic Nerve Transection. PLoS ONE 2013, 8, e68881. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Y.; Shen, Y.; Zhou, H.; Yu, X. Lycium barbarum polysaccharides induce Toll-like receptor 2- and 4-mediated phenotypic and functional maturation of murine dendritic cells via activation of NF-κB. Mol. Med. Rep. 2013, 8, 1216–1220. [Google Scholar] [CrossRef] [PubMed]
- Pavan, B.; Capuzzo, A.; Forlani, G. High glucose-induced barrier impairment of human retinal pigment epithelium is ameliorated by treatment with Goji berry extracts through modulation of cAMP levels. Exp. Eye Res. 2014, 120, 50–54. [Google Scholar] [CrossRef]
- Tengfei, W.; Yuxiang, L.; Yongsheng, W.; Ru, Z.; Lin, M.; Yinju, H.; Shaoju, J.; Juan, D.; Chengjun, Z.; Tao, S.; et al. Lycium barbarum polysaccharide prevents focal cerebral ischemic injury by inhibiting neuronal apoptosis in mice. PLoS ONE 2014, 9, e90780. [Google Scholar]
- Hynd, M.R.; Scott, H.L.; Dodd, P.R. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 2004, 45, 583–595. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, Q.; Gao, H.; Peng, X.; Wen, Y.; Dai, G. Lycium barbarum polysaccharides attenuates N-methy-N-nitrosourea-induced photoreceptor cell apoptosis in rats through regulation of poly (ADP-ribose) polymerase and caspase expression. J. Ethnopharmacol. 2016, 191, 125–134. [Google Scholar] [CrossRef]
- Yang, D.; So, K.F.; Lo, A.C.Y. Lycium barbarum polysaccharide extracts preserve retinal function and attenuate inner retinal neuronal damage in a mouse model of transient retinal ischaemia. Clin. Exp. Ophthalmol. 2017, 45, 717–729. [Google Scholar] [CrossRef]
- Liang, B.; Peng, L.; Li, R.; Li, H.; Mo, Z.; Dai, X.; Jiang, N.; Liu, Q.; Zhang, E.; Deng, H.J.C.; et al. Lycium barbarum polysaccharide protects HSF cells against ultraviolet-induced damage through the activation of Nrf2. Cell. Mol. Biol. Lett. 2018, 23, 18. [Google Scholar] [CrossRef] [PubMed]
- Chien, K.J.; Horng, C.T.; Huang, Y.S.; Hsieh, Y.H.; Wang, C.J.; Yang, J.S.; Lu, C.C.; Chen, F.A. Effects of Lycium barbarum (goji berry) on dry eye disease in rats. Mol. Med. Rep. 2018, 17, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ju, S.; Yang, B.H.; Yan, C.C.; Cao, X.; Zhang, X.F.; Wang, N.; Lian, X.T. Inhibitory effects and related mechanisms of lycium barbarum polysaccharides on vascular lesions in type 2 diabetes mellitus. Int. J. Clin. Exp. Med. 2018, 11, 10660–10666. [Google Scholar]
- Gan, F.; Liu, Q.; Liu, Y.H.; Huang, D.; Pan, C.L.; Song, S.Q.; Huang, K.H. Lycium barbarum polysaccharides improve CCl4-induced liver fibrosis, inflammatory response and TLRs/NF-kappa B signaling pathway expression in wistar rats. Life Sci. 2018, 192, 205–212. [Google Scholar] [CrossRef]
- Tang, H.L.; Chen, C.; Wang, S.K.; Sun, G.J. Biochemical analysis and hypoglycemic activity of a polysaccharide isolated from the fruit of Lycium barbarum L. Int. J. Biol. Macromol. 2015, 77, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.M.; Du, J.L.; Hei, Q.H. Lycium barbarum polysaccharide protects against neurotoxicity via the Nrf2-HO-1 pathway. Exp. Ther. Med. 2017, 14, 4919–4927. [Google Scholar] [CrossRef] [PubMed]
- Varoni, M.V.; Gadau, S.D.; Pasciu, V.; Baralla, E.; Serra, E.; Palomba, D.; Demontis, M.P. Investigation of the effects of Lycium barbarum polysaccharides against cadmium induced damage in testis. Exp. Mol. Pathol. 2017, 103, 26–32. [Google Scholar] [CrossRef]

| Extraction Methods | Extraction Conditions | Yield (%) | Ref |
|---|---|---|---|
| Water extraction method | The ratio liquid to solid 70:1, pH 10, at 65 °C, extracted in soakage for 3.5 h. | 7.46–7.63% | [30,34,35] |
| Ultrasound-assisted extraction method | Extraction time of 30 min, temperature of 60 °C, solid/liquid ratio of 20 g/600 mL, power density of 300 W/L, ultrasound frequency of 28 kHz. | 2.286–5.701% | [23,36] |
| Enzyme-assisted extraction method | Extraction time of 91 min, extraction temperature of 59.7 °C, pH 5.0. | 6.81 ± 0.10 % | [26] |
| Microwave-assisted extraction method | Ratio of water to raw material of 31.5 mL/g, extraction time of 25.8 min, microwave power of 544.0 W. | 8.25 ± 0.07% | [27] |
| Combination of extraction methods | Temperature of 100 °C, extraction time of 53 min, liquid-to-solid ratio of 26 mL/g, ultrasonic electric power of 160 W. | 5.728% | [24] |
| No | Name | Mw (kDa) | Molar Ratio | Analysis Technique | Possible Structure of Repeat Unit | Ref |
|---|---|---|---|---|---|---|
| 1 | LbGp2 | 68,200 | Ara:Gal = 4:5 | SEC, GC-MS | Backbone composed of (1→6)- β-Gal. Branches composed of (1→3)- β-Ara and (1→3)- β-Gal terminated with (1 → 3)/(1→5)- α-Ara. | [40] |
| 2 | LbGp3 | 92,500 | Ara:Gal = 1:1 | NMR | Backbone composed of (1→4)- β-Gal. Branches composed of (1→3)- β-Ara and (1→3)- α-Gal terminated with (1 → 3)/ (1→5)- α-Ara. | [41] |
| 3 | LbGp4 | 214,800 | Ara:Gal:Rha:Glc = 1.5:2.5:0.43:0.23 | NMR | Backbone composed of (1→4)- β-Gal. Branches composed of (1→3)- β-Gal terminated with (1→3)- α-Ara and (1→3)- β-Rha. | [42] |
| 4 | LBPA3 | 66,000 | Ara:Gal = 1.2:1 | Ion exchange chromatography | Heteropolysaccharide with (1→4), (1→6). | [43] |
| 5 | LBPB1 | 18,000 | Ara:Glc = 1:3.1 | Heteropolysaccharide with (1→4), (1→6) β-glycosidic bond. | ||
| 6 | LBP-a4 | 10,200 | Fuc: gal = 0.41:1 | Ultrafiltration membrane method | [43] | |
| 7 | LBPC2 | 12,000 | Xyl:Rha:Man = 8.8:2.3:1 | Heteropolysaccharide with (1→4), (1→6) β-glycosidic bond. | [44] | |
| 8 | LBPC4 | 10,000 | Glc | IR, GC | Heteropolysaccharide with (1→4), (1→6) α-glycosidic bond. | [45] |
| 9 | LBP1a-1 | 115,000 | Glc | α-(1→6)- D –glucan. | [46] | |
| 10 | LBP1a-2 | 94,000 | Glc | α-(1→6)- D –glucan. | ||
| 11 | LBP3a-1 | 103,000 | GalA composed of a small amount of Gal and Ara | Gel permeation chromatography, NMR | Polygalacturonan with (1→4)- α-glycosidic bond. | |
| 12 | LBP3a-2 | 82,000 | GalA composed of a small amount of Gal and Ara | Polygalacturonan with (1→4)- α-glycosidic bond. | ||
| 13 | LBLP5-A | 113,300 | (1 -> 3)-linked Gal, (1 -> 4)-linked Gal, (1 -> 3)-linked Araf, (1 -> 5)-linked Araf, and (1 -> 2, 4)-linked Rhaf. | [47] | ||
| 14 | WSP | Rha:Fuc:Ara:Xyl:Man:Gal:Glc = 1.6:0.2:51.4:4.8:1.2:25.9:7.3 | NMR, ESI-MS | Backbone composed of (1 → 2)-linked-Rha and (1→4)-linked-Gal. Branches composed of (1→5)-linked-Ara terminated with Ara residues, and (1→4)-linked-Xyl terminated with Man residues. | ||
| 15 | AGP | Rha:Ara:Xyl:Gal:Glc:GalA:GlcA = 3.3:42.9:0.3:44.3:2.4:7.0 | NMR | Backbone composed of linear homogalacturonan fragments and rhamnogalacturonan fragments. Side chains mainly composed of β−1,6- and β−1,4-galactopyranan and α−1,5-arabinofuranan. | [48] | |
| 16 | LBP-IV | 41,800 | Rha:Ara:Xyl:Glc:Gal = 1.61:3.82:3.44: 7.54:1.00 | DEAE-Sephadex, HPGPC, IR, UV | Backbone composed of both α- and β- anomeric configurations of Ara and Glc. Rha was located at terminal of polysaccharide chain. | [49] |
| 17 | LbGp1 | 49,100 | Ara:Gal = 5.6:1 | HPGPC | Backbone composed of (1→6)-Gal. Side chains mainly composed of (1→3)-Gal/(1→4)-Gal and (1→3)-Ara/(1→4)-Ara. Ara was located at terminal of branch. | [50] |
| 18 | p -LBP | 64,000 | Fuc:Rha:Ara:Gal:Glc:Xyl:GalA:GlcA = 1.00:6.44:54.84:22.98:4.05: 2.95:136.98:3.35 | HPAEC-PAD, HPSEC, FT-IR, GC–MS, and NMR | Backbone composed of (1→4)- α-GalA. Side chains mainly composed of α−1,2- and α−1,4-Rha and α−1,5-Ara. | [51] |
| 19 | LBP1B-S-2 | 80,000 | Rha:Ara:Gal:Glu = 3.13: 53.55: 39.37: 3.95 | DEAE Sepharose | Backbone consisted of 1, 3-linked beta-D-Galp, 1, 6-linked beta-D-Galp and branches contained 1, 4-linked beta-D-GlcpA, T-linked beta-D-Galp, 1, 6-linked beta-D-Galp, T-linked alpha-L-Araf, T-linked beta-L-Aral 1, 5-linked alpha-L-Araf and T-linked beta-L-Rhap. | [52] |
| 20 | LRGP1 | 56,200 | Rha:Ara:Xyl:Man:Glu:Gal = 0.65:10.71:0.33:0.67:1:10.41 | HPGPC, ESI-MS | Backbone composed of (1 -> 3)-linked Gal. The branches were composed of (1 -> 5)-linked Ara, (1 -> 2)-linked Ara, (1 -> 6)-linked Gal, (1 -> 3)-linked Gal, (1 -> 4)-linked Gal and (1 -> 2,4)-linked Rha. | [53] |
| Antioxidant Activity | Mechanisms | Dose | Experiment Model | Experiment Type | Ref |
|---|---|---|---|---|---|
| Reduce oxidative stress | Regulating the level of MDA, SOD, GSH | 100, 200, and 400 mg/kg | Rats | In vivo | [71,74] |
| Against hypoxia-induced injury | Down-regulation of miR-122 | 300 mu g/mL | Cells | In vitro | [75] |
| Reduces hyperoxic acute | Induced activation of Nrf2 | 100 mg/kg | Mice | In vivo | [59] |
| Attenuates diabetic testicular dysfunction | Upregulated p-PI3K and p-Akt protein expressions | 40 mg/kg | Mice | In vivo | [76] |
| Radical scavenging | Free radical scavenging | IC 50:1.29–3.00 mg/mL(DPPH) 0.39–1.10 mg/mL (ABTS) | Chemical reagent | In vivo | [77] |
| Regulate the activity of enzymes | Increased activity of antioxidative enzymes | 200–400 mg/kg | Rats | In vivo | [78] |
| Immune Regulation Activity | Mechanism | Experiment Type | Ref |
|---|---|---|---|
| Enhanced macrophage endocytic and phagocytic capacities in vivo | Activate transcription factors NFAT, AP-1, prompt CD25 expression, induce IL-2 and IFN-gamma gene transcription and protein secretion | In vitro | [85] |
| Regulation of immune cells | Maintain high levels of T cells, prevent the increase of Tregs, promote infiltration of CD8+ T cells | In vivo | [86] |
| Induce the phenotypic and functional maturation of DCs | Upregulate the expression of Notch and Jagged and Notch targets Hes1 and Hes5 | In vitro | [80] |
| Promote the proliferation of spleen cells | Increase secretion of INF-alpha and IL-6, mRNA expression of iNOS, IL-beta and IL-6 through activating phosphorylation of ERIC, JNK, p38 and p65 | In vitro | [87] |
| Increased immune organ indexes | Promote blood B and T lymphocyte proliferation | In vivo | [81] |
| Improve immune responses | Stimulate CD4(+) and CD8(+) T cell proliferation | In vitro | [88] |
| Enhance the immune activity | Enhance PCV2-specific IgG antibody responses, promote Th1 cytokines (IFN-gamma and TNF-alpha) and Th2 cytokine (IL-4) secretion | In vitro | [82] |
| Enhance the immune activity | Inhibit cell proliferation, retard cell cycle growth, and promote apoptosis | In vitro, In vivo | [89] |
| Antitumor Activity | Mechanism | Tumor Model | Experiment Type | Ref |
|---|---|---|---|---|
| Reduce cell viability | Inhibit growth of tumor | MCF-7, T47D, SMMC-7721, DU145 | In vitro | [7,44,100] |
| Regulate apoptosis | Induce apoptosis | MCF-7, BIU87 | In vitro | [100] |
| Regulate cell cycle | Arrest the cells at the G1 phase | SW480, Caco-2 cells | In vitro | [101] |
| Regulate immune activity | Enhance immunity | Mice | In vivo | [84,86] |
| Neuroprotection Effects | Molecular Mechanism | Experiment Type | Ref |
|---|---|---|---|
| Improve neurodegenerative diseases | Increase the activity of Akt; regulate the expression of HSP60/HSP70; reduce caspase cascade reaction | In vitro, In vivo | [107,108,109,110,111] |
| Inhibition of oxidative stress | Increase SOD, CAT and GSH-Px; decrease the ROS level, inhibit JNK pathway | In vitro, In vivo | [112,113,114,115] |
| Inhibition of inflammation | Inhibit of NF-κB | In vivo | [116] |
| Inhibit abnormal differentiation of nerve cells | Increase differentiation of hippocampal neuron stem cells and inhibit abnormal differentiation | In vitro | [117] |
| Inhibition of apoptosis | Promotes Bcl-2, inhibits Bax, overexpression of CytC gene | In vivo | [118] |
| Reduce glutamate toxicity | Decrease neurotoxic effects of glutamate on PC12 cells; inhibition of ROS accumulation, LDH release and Ca[2]+ overload | In vivo | [119,120] |
| Inhibit the tube formation of microvascular endothelial cells | No report | In vivo | [52] |
| Neuroprotective agent in ischaemic retinopathies | Enhance immunoreactivity of protein kinase C alpha and attenuated glial fibrillary acidic protein expression | In vivo | [121]. |
| Biological activities | Mechanism | Experiment type | Ref |
|---|---|---|---|
| Attenuates diabetic testicular dysfunction | Inhibition of the PI3K/Akt pathway-mediated abnormal autophagy | In vivo | [76] |
| Inhibit the vascular lesions | Regulating p38MAPK signaling pathways, inhibiting absorption of glucose | In vivo | [124,126] |
| Prevents against ultraviolet-induced damage | Activation of Nrf2 | In vivo | [122] |
| Protect the liver from hepatotoxicity | Regulating oxidative stress | In vivo | [62] |
| Alleviating effects of CCl4-induced liver fibrosis | Inhibition of the TLRs/NF-kappa B signaling pathway expression | In vivo | [125] |
| Alleviating dry-eye disease | Schirmer’s test, tear break-up time (BUT) measurement | In vivo | [123] |
| Protects against neurotoxicity | Upregulating Nrf2/HO-1 signaling | In vitro | [127] |
| Ameliorate Cd testicular damage | Regulate oxidative stress | In vivo | [128] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, X.; Liang, T.; Liu, Y.; Ding, G.; Zhang, F.; Ma, Z. Extraction, Structural Characterization, and Biological Functions of Lycium Barbarum Polysaccharides: A Review. Biomolecules 2019, 9, 389. https://doi.org/10.3390/biom9090389
Tian X, Liang T, Liu Y, Ding G, Zhang F, Ma Z. Extraction, Structural Characterization, and Biological Functions of Lycium Barbarum Polysaccharides: A Review. Biomolecules. 2019; 9(9):389. https://doi.org/10.3390/biom9090389
Chicago/Turabian StyleTian, Xiaojing, Tisong Liang, Yuanlin Liu, Gongtao Ding, Fumei Zhang, and Zhongren Ma. 2019. "Extraction, Structural Characterization, and Biological Functions of Lycium Barbarum Polysaccharides: A Review" Biomolecules 9, no. 9: 389. https://doi.org/10.3390/biom9090389
APA StyleTian, X., Liang, T., Liu, Y., Ding, G., Zhang, F., & Ma, Z. (2019). Extraction, Structural Characterization, and Biological Functions of Lycium Barbarum Polysaccharides: A Review. Biomolecules, 9(9), 389. https://doi.org/10.3390/biom9090389





