Stabilin Receptors: Role as Phosphatidylserine Receptors
Abstract
1. Introduction
2. Apoptotic Cell Clearance
2.1. Phosphatidylserine Externalization in Apoptotic Cells
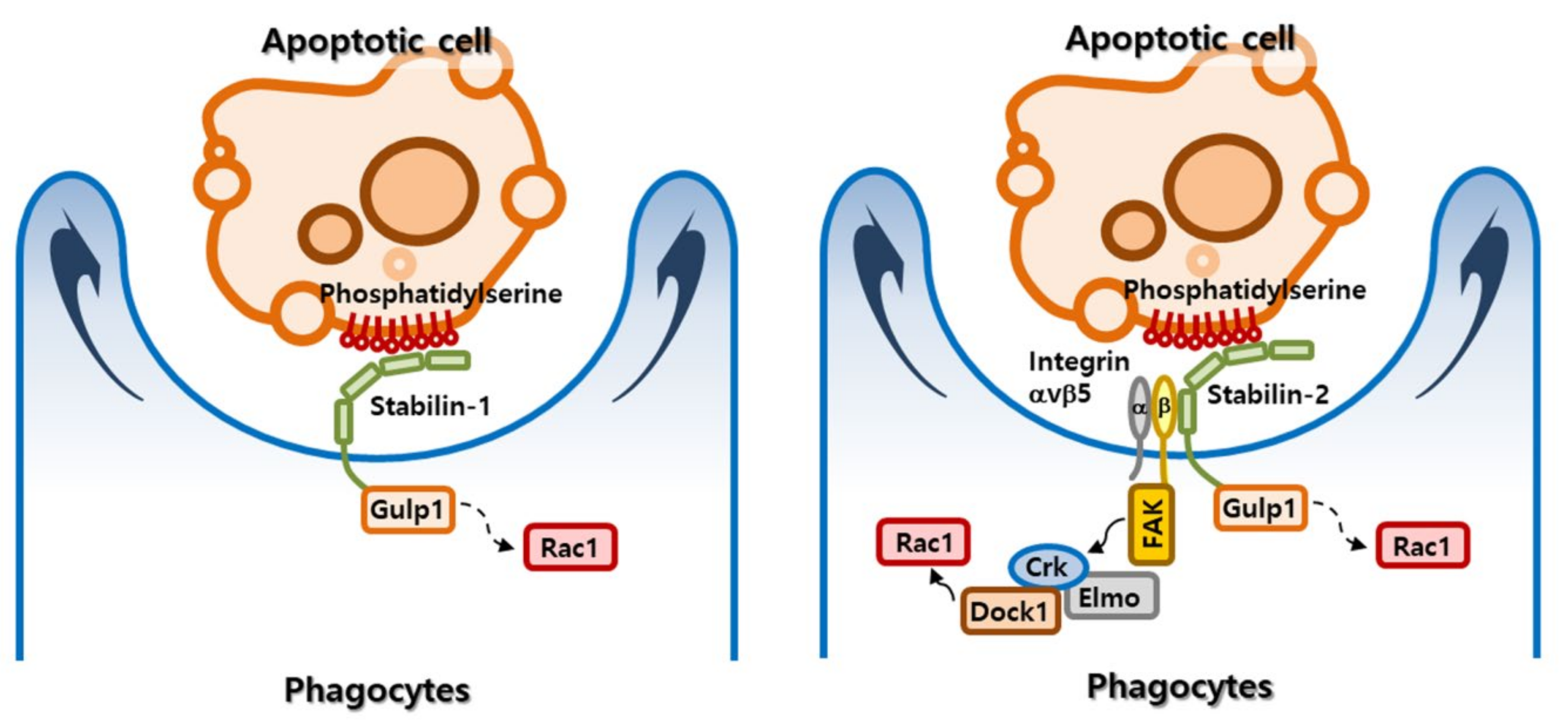
2.2. Apoptotic Cell Clearance by Stabilin Receptors
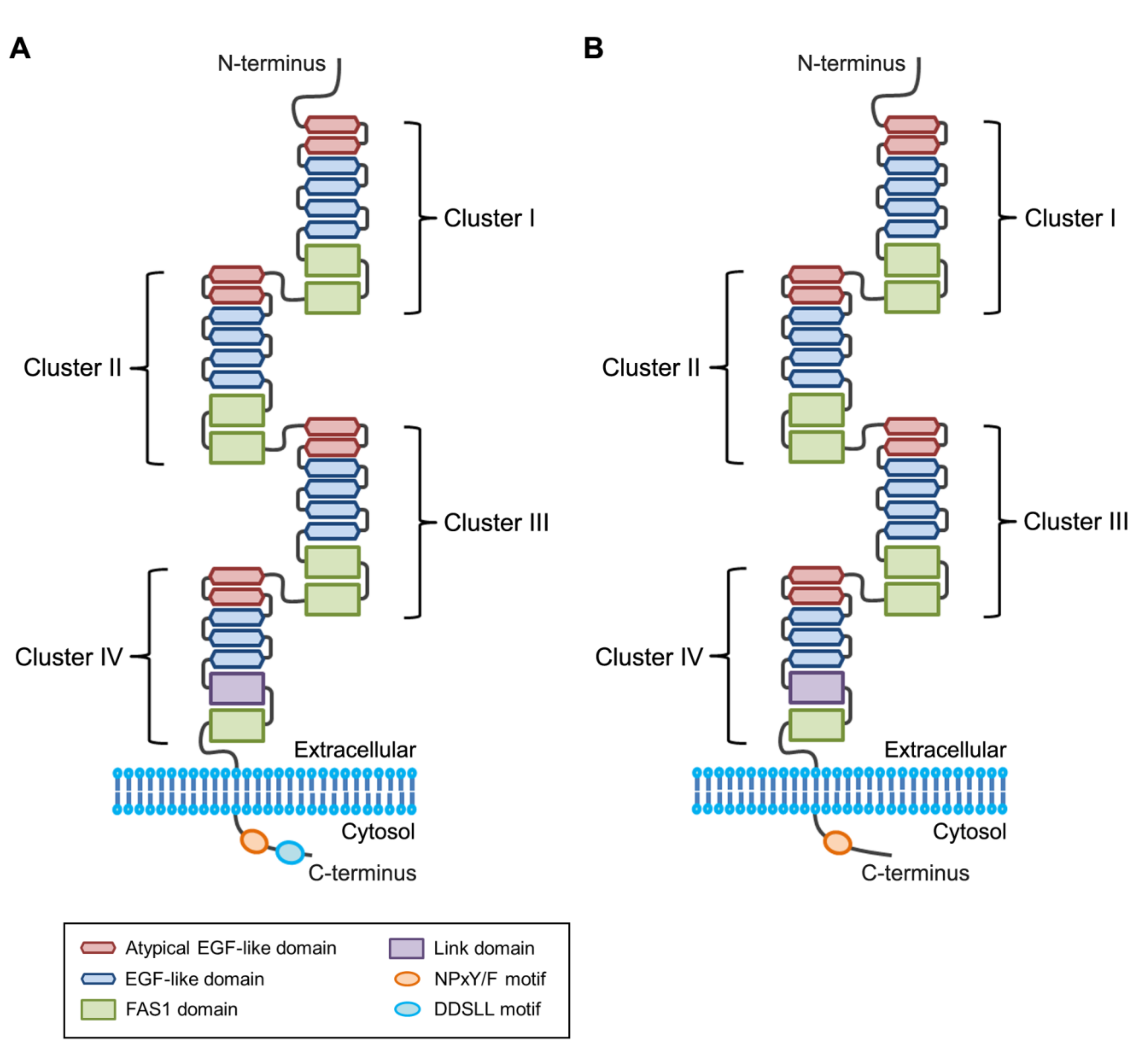
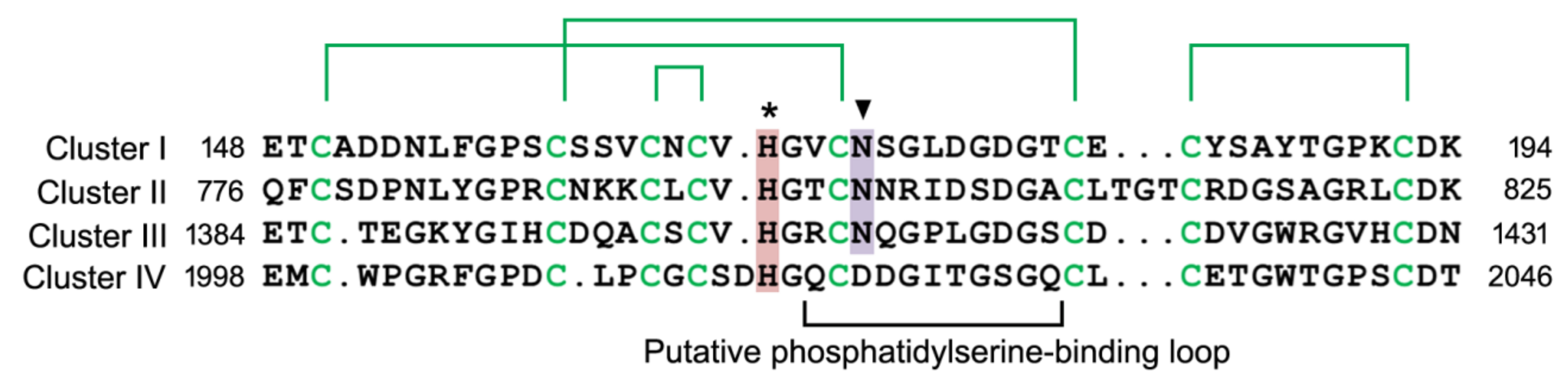
2.3. Phosphatidylserine-binding Domain in Stabilin Receptors
2.4. Signaling Pathway Mediated by Stabilin Receptors
2.5. Regulation of Inflammation Following Efferocytosis
3. Myoblast Fusion
3.1. Phosphatidylserine Externalization during Cell Fusion
3.2. Stabilin-2: A Phosphatidylserine Receptor in Myoblasts
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Heemskerk, J.W.; Bevers, E.M.; Lindhout, T. Platelet activation and blood coagulation. Thromb. Haemost. 2002, 88, 186–193. [Google Scholar] [PubMed]
- Whyte, C.S.; Swieringa, F.; Mastenbroek, T.G.; Lionikiene, A.S.; Lance, M.D.; van der Meijden, P.E.; Heemskerk, J.W.; Mutch, N.J. Plasminogen associates with phosphatidylserine-exposing platelets and contributes to thrombus lysis under flow. Blood 2015, 125, 2568–2578. [Google Scholar] [CrossRef] [PubMed]
- Fadeel, B.; Xue, D. PS externalization: From corpse clearance to drug delivery. Cell Death Differ. 2006, 13, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Imanishi, E.; Nagata, S. Xkr8 phospholipid scrambling complex in apoptotic phosphatidylserine exposure. Proc. Natl. Acad. Sci. USA 2016, 113, 9509–9514. [Google Scholar] [CrossRef]
- Van den Eijnde, S.M.; van den Hoff, M.J.; Reutelingsperger, C.P.; van Heerde, W.L.; Henfling, M.E.; Vermeij-Keers, C.; Schutte, B.; Borgers, M.; Ramaekers, F.C. Transient expression of phosphatidylserine at cell-cell contact areas is required for myotube formation. J. Cell Sci. 2001, 114, 3631–3642. [Google Scholar]
- Whitlock, J.M.; Yu, K.; Cui, Y.Y.; Hartzell, H.C. Anoctamin 5/TMEM16E facilitates muscle precursor cell fusion. J. Gen. Physiol. 2018, 150, 1498–1509. [Google Scholar] [CrossRef]
- Das, M.; Xu, B.; Lin, L.; Chakrabarti, S.; Shivaswamy, V.; Rote, N.S. Phosphatidylserine efflux and intercellular fusion in a BeWo model of human villous cytotrophoblast. Placenta 2004, 25, 396–407. [Google Scholar] [CrossRef]
- Martin, S.; Pombo, I.; Poncet, P.; David, B.; Arock, M.; Blank, U. Immunologic stimulation of mast cells leads to the reversible exposure of phosphatidylserine in the absence of apoptosis. Int. Arch. Allergy Immunol. 2000, 123, 249–258. [Google Scholar] [CrossRef]
- Elliott, J.I.; Surprenant, A.; Marelli-Berg, F.M.; Cooper, J.C.; Cassady-Cain, R.L.; Wooding, C.; Linton, K.; Alexander, D.R.; Higgins, C.F. Membrane phosphatidylserine distribution as a non-apoptotic signalling mechanism in lymphocytes. Nat. Cell Biol. 2005, 7, 808–816. [Google Scholar] [CrossRef]
- Abay, Z.C.; Wong, M.Y.; Teoh, J.S.; Vijayaraghavan, T.; Hilliard, M.A.; Neumann, B. Phosphatidylserine save-me signals drive functional recovery of severed axons in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2017, 114, E10196–E10205. [Google Scholar] [CrossRef]
- Neumann, B.; Coakley, S.; Giordano-Santini, R.; Linton, C.; Lee, E.S.; Nakagawa, A.; Xue, D.; Hilliard, M.A. EFF-1-mediated regenerative axonal fusion requires components of the apoptotic pathway. Nature 2015, 517, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Politz, O.; Gratchev, A.; McCourt, P.A.; Schledzewski, K.; Guillot, P.; Johansson, S.; Svineng, G.; Franke, P.; Kannicht, C.; Kzhyshkowska, J.; et al. Stabilin-1 and -2 constitute a novel family of fasciclin-like hyaluronan receptor homologues. Biochem. J. 2002, 362, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.S.; Weigel, P.H. Hyaluronic acid receptor for endocytosis (HARE)-mediated endocytosis of hyaluronan, heparin, dermatan sulfate, and acetylated low density lipoprotein (AcLDL), but not chondroitin sulfate types A, C, D, or E, activates NF-κB-regulated gene expression. J. Biol. Chem. 2014, 289, 1756–1767. [Google Scholar] [CrossRef] [PubMed]
- Kzhyshkowska, J.; Gratchev, A.; Brundiers, H.; Mamidi, S.; Krusell, L.; Goerdt, S. Phosphatidylinositide 3-kinase activity is required for stabilin-1-mediated endosomal transport of acLDL. Immunobiology 2005, 210, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Adachi, H.; Osuga, J.; Ohashi, K.; Yahagi, N.; Sekiya, M.; Okazaki, H.; Tomita, S.; Iizuka, Y.; Shimano, H.; et al. FEEL-1 and FEEL-2 are endocytic receptors for advanced glycation end products. J. Biol. Chem. 2003, 278, 12613–12617. [Google Scholar] [CrossRef] [PubMed]
- Salmi, M.; Koskinen, K.; Henttinen, T.; Elima, K.; Jalkanen, S. CLEVER-1 mediates lymphocyte transmigration through vascular and lymphatic endothelium. Blood 2004, 104, 3849–3857. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.Y.; Park, S.Y.; Kim, I.S. Stabilin-2 is involved in lymphocyte adhesion to the hepatic sinusoidal endothelium via the interaction with αMβ2 integrin. J. Leukoc. Biol. 2007, 82, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Karikoski, M.; Irjala, H.; Maksimow, M.; Miiluniemi, M.; Granfors, K.; Hernesniemi, S.; Elima, K.; Moldenhauer, G.; Schledzewski, K.; Kzhyshkowska, J.; et al. Clever-1/Stabilin-1 regulates lymphocyte migration within lymphatics and leukocyte entrance to sites of inflammation. Eur. J. Immunol. 2009, 39, 3477–3487. [Google Scholar] [CrossRef]
- Miller, C.M.; Donner, A.J.; Blank, E.E.; Egger, A.W.; Kellar, B.M.; Ostergaard, M.E.; Seth, P.P.; Harris, E.N. Stabilin-1 and Stabilin-2 are specific receptors for the cellular internalization of phosphorothioate-modified antisense oligonucleotides (ASOs) in the liver. Nucleic Acids Res. 2016, 44, 2782–2794. [Google Scholar] [CrossRef]
- Park, S.Y.; Jung, M.Y.; Kim, H.J.; Lee, S.J.; Kim, S.Y.; Lee, B.H.; Kwon, T.H.; Park, R.W.; Kim, I.S. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 2008, 15, 192–201. [Google Scholar] [CrossRef]
- Park, S.Y.; Jung, M.Y.; Lee, S.J.; Kang, K.B.; Gratchev, A.; Riabov, V.; Kzhyshkowska, J.; Kim, I.S. Stabilin-1 mediates phosphatidylserine-dependent clearance of cell corpses in alternatively activated macrophages. J. Cell Sci. 2009, 122, 3365–3373. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S.; Hanayama, R.; Kawane, K. Autoimmunity and the clearance of dead cells. Cell 2010, 140, 619–630. [Google Scholar] [CrossRef] [PubMed]
- McCubbrey, A.L.; Curtis, J.L. Efferocytosis and lung disease. Chest 2013, 143, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Van Vre, E.A.; Ait-Oufella, H.; Tedgui, A.; Mallat, Z. Apoptotic cell death and efferocytosis in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Yun, Y.; Lim, J.S.; Kim, M.J.; Kim, S.Y.; Kim, J.E.; Kim, I.S. Stabilin-2 modulates the efficiency of myoblast fusion during myogenic differentiation and muscle regeneration. Nat. Commun. 2016, 7, 10871. [Google Scholar] [CrossRef] [PubMed]
- Arandjelovic, S.; Ravichandran, K.S. Phagocytosis of apoptotic cells in homeostasis. Nat. Immunol. 2015, 16, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Penberthy, K.K.; Lysiak, J.J.; Ravichandran, K.S. Rethinking Phagocytes: Clues from the Retina and Testes. Trends Cell Biol. 2018, 28, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.R.; Zheng, S.; Park, D.; Woodson, R.I.; Reardon, M.A.; Juncadella, I.J.; Kinchen, J.M.; Zhang, J.; Lysiak, J.J.; Ravichandran, K.S. Unexpected requirement for ELMO1 in clearance of apoptotic germ cells in vivo. Nature 2010, 467, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; Voelker, D.R.; Campbell, P.A.; Cohen, J.J.; Bratton, D.L.; Henson, P.M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992, 148, 2207–2216. [Google Scholar] [PubMed]
- Van den Eijnde, S.M.; Boshart, L.; Baehrecke, E.H.; De Zeeuw, C.I.; Reutelingsperger, C.P.; Vermeij-Keers, C. Cell surface exposure of phosphatidylserine during apoptosis is phylogenetically conserved. Apoptosis Int. J. Program. Cell Death 1998, 3, 9–16. [Google Scholar] [CrossRef]
- Venegas, V.; Zhou, Z. Two alternative mechanisms that regulate the presentation of apoptotic cell engulfment signal in Caenorhabditis elegans. Mol. Biol. Cell 2007, 18, 3180–3192. [Google Scholar] [CrossRef]
- Suzuki, J.; Denning, D.P.; Imanishi, E.; Horvitz, H.R.; Nagata, S. Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science 2013, 341, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Z.; Mapes, J.; Lee, E.S.; Skeen-Gaar, R.R.; Xue, D. Caspase-mediated activation of Caenorhabditis elegans CED-8 promotes apoptosis and phosphatidylserine externalization. Nat. Commun. 2013, 4, 2726. [Google Scholar] [CrossRef] [PubMed]
- Kawano, M.; Nagata, S. Lupus-like autoimmune disease caused by a lack of Xkr8, a caspase-dependent phospholipid scramblase. Proc. Natl. Acad. Sci. USA 2018, 115, 2132–2137. [Google Scholar] [CrossRef] [PubMed]
- Pomorski, T.; Menon, A.K. Lipid flippases and their biological functions. Cell. Mol. Life Sci. 2006, 63, 2908–2921. [Google Scholar] [CrossRef] [PubMed]
- Segawa, K.; Kurata, S.; Yanagihashi, Y.; Brummelkamp, T.R.; Matsuda, F.; Nagata, S. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science 2014, 344, 1164–1168. [Google Scholar] [CrossRef]
- Sakuragi, T.; Kosako, H.; Nagata, S. Phosphorylation-mediated activation of mouse Xkr8 scramblase for phosphatidylserine exposure. Proc. Natl. Acad. Sci. USA 2019, 116, 2907–2912. [Google Scholar] [CrossRef]
- Hochreiter-Hufford, A.; Ravichandran, K.S. Clearing the dead: Apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb. Perspect. Biol. 2013, 5, a008748. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, I.S. Engulfment signals and the phagocytic machinery for apoptotic cell clearance. Exp. Mol. Med. 2017, 49, e331. [Google Scholar] [CrossRef]
- Goerdt, S.; Walsh, L.J.; Murphy, G.F.; Pober, J.S. Identification of a novel high molecular weight protein preferentially expressed by sinusoidal endothelial cells in normal human tissues. J. Cell Biol. 1991, 113, 1425–1437. [Google Scholar] [CrossRef]
- Palani, S.; Maksimow, M.; Miiluniemi, M.; Auvinen, K.; Jalkanen, S.; Salmi, M. Stabilin-1/CLEVER-1, a type 2 macrophage marker, is an adhesion and scavenging molecule on human placental macrophages. Eur. J. Immunol. 2011, 41, 2052–2063. [Google Scholar] [CrossRef] [PubMed]
- Schonhaar, K.; Schledzewski, K.; Michel, J.; Dollt, C.; Gkaniatsou, C.; Geraud, C.; Kzhyshkowska, J.; Goerdt, S.; Schmieder, A. Expression of stabilin-1 in M2 macrophages in human granulomatous disease and melanocytic lesions. Int. J. Clin. Exp. Pathol. 2014, 7, 1625–1634. [Google Scholar] [PubMed]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef]
- Park, S.Y.; Bae, D.J.; Kim, M.J.; Piao, M.L.; Kim, I.S. Extracellular low pH modulates phosphatidylserine-dependent phagocytosis in macrophages by increasing stabilin-1 expression. J. Biol. Chem. 2012, 287, 11261–11271. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Weigel, J.A.; Fauss, L.; Weigel, P.H. Identification of the hyaluronan receptor for endocytosis (HARE). J. Biol. Chem. 2000, 275, 37733–37741. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Johansson, S.; McCourt, P.; Smedsrod, B.; Ekblom, M. Stabilins are expressed in bone marrow sinusoidal endothelial cells and mediate scavenging and cell adhesive functions. Biochem. Biophys. Res. Commun. 2009, 390, 883–886. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, S.; Park, S.Y.; Kim, I.S. Stabilin-2 acts as an engulfment receptor for the phosphatidylserine-dependent clearance of primary necrotic cells. Biochem. Biophys. Res. Commun. 2013, 432, 412–417. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, S.Y.; Jung, M.Y.; Bae, S.M.; Kim, I.S. Mechanism for phosphatidylserine-dependent erythrophagocytosis in mouse liver. Blood 2011, 117, 5215–5223. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, S.Y.; Jung, M.Y.; Bae, D.J.; Kim, I.S. Epidermal growth factor-like domain repeat of stabilin-2 recognizes phosphatidylserine during cell corpse clearance. Mol. Cell. Biol. 2008, 28, 5288–5298. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Bae, D.J.; Hong, M.; Park, S.Y.; Kim, I.S. The conserved histidine in epidermal growth factor-like domains of stabilin-2 modulates pH-dependent recognition of phosphatidylserine in apoptotic cells. Int. J. Biochem. Cell Biol. 2010, 42, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Borisenko, G.G.; Iverson, S.L.; Ahlberg, S.; Kagan, V.E.; Fadeel, B. Milk fat globule epidermal growth factor 8 (MFG-E8) binds to oxidized phosphatidylserine: Implications for macrophage clearance of apoptotic cells. Cell Death Differ. 2004, 11, 943–945. [Google Scholar] [CrossRef] [PubMed]
- Hanayama, R.; Tanaka, M.; Miwa, K.; Shinohara, A.; Iwamatsu, A.; Nagata, S. Identification of a factor that links apoptotic cells to phagocytes. Nature 2002, 417, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Wijeyesakere, S.J.; Bedi, S.K.; Huynh, D.; Raghavan, M. The C-Terminal Acidic Region of Calreticulin Mediates Phosphatidylserine Binding and Apoptotic Cell Phagocytosis. J. Immunol. 2016, 196, 3896–3909. [Google Scholar] [CrossRef] [PubMed]
- Twarda-Clapa, A.; Labuzek, B.; Krzemien, D.; Musielak, B.; Grudnik, P.; Dubin, G.; Holak, T.A. Crystal structure of the FAS1 domain of the hyaluronic acid receptor stabilin-2. Acta Cryst. D Struct. Biol. 2018, 74, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Reddien, P.W.; Horvitz, H.R. The engulfment process of programmed cell death in caenorhabditis elegans. Annu. Rev. Cell Dev. Biol. 2004, 20, 193–221. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.A.; Hengartner, M.O. Human CED-6 encodes a functional homologue of the Caenorhabditis elegans engulfment protein CED-6. Curr. Biol. CB 1999, 9, 1347–1350. [Google Scholar] [CrossRef]
- Hamon, Y.; Trompier, D.; Ma, Z.; Venegas, V.; Pophillat, M.; Mignotte, V.; Zhou, Z.; Chimini, G. Cooperation between engulfment receptors: The case of ABCA1 and MEGF10. PloS ONE 2006, 1, e120. [Google Scholar] [CrossRef] [PubMed]
- Hamon, Y.; Broccardo, C.; Chambenoit, O.; Luciani, M.F.; Toti, F.; Chaslin, S.; Freyssinet, J.M.; Devaux, P.F.; McNeish, J.; Marguet, D.; et al. ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat. Cell Biol. 2000, 2, 399–406. [Google Scholar] [CrossRef]
- Wu, Y.C.; Horvitz, H.R. C. elegans phagocytosis and cell-migration protein CED-5 is similar to human DOCK180. Nature 1998, 392, 501–504. [Google Scholar] [CrossRef]
- Reddien, P.W.; Horvitz, H.R. CED-2/CrkII and CED-10/Rac control phagocytosis and cell migration in Caenorhabditis elegans. Nat. Cell Biol. 2000, 2, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Gumienny, T.L.; Brugnera, E.; Tosello-Trampont, A.C.; Kinchen, J.M.; Haney, L.B.; Nishiwaki, K.; Walk, S.F.; Nemergut, M.E.; Macara, I.G.; Francis, R.; et al. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell 2001, 107, 27–41. [Google Scholar] [CrossRef]
- Park, S.Y.; Kang, K.B.; Thapa, N.; Kim, S.Y.; Lee, S.J.; Kim, I.S. Requirement of adaptor protein GULP during stabilin-2-mediated cell corpse engulfment. J. Biol. Chem. 2008, 283, 10593–10600. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, S.Y.; Kang, K.B.; Kim, I.S. Adaptor protein GULP is involved in stabilin-1-mediated phagocytosis. Biochem. Biophys. Res. Commun. 2010, 398, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Su, H.P.; Nakada-Tsukui, K.; Tosello-Trampont, A.C.; Li, Y.; Bu, G.; Henson, P.M.; Ravichandran, K.S. Interaction of CED-6/GULP, an adapter protein involved in engulfment of apoptotic cells with CED-1 and CD91/low density lipoprotein receptor-related protein (LRP). J. Biol. Chem. 2002, 277, 11772–11779. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, C.S.; Scheib, J.L.; Ma, Z.; Dang, R.P.; Schafer, J.M.; Hickman, F.E.; Brodsky, F.M.; Ravichandran, K.S.; Carter, B.D. The adaptor protein GULP promotes Jedi-1-mediated phagocytosis through a clathrin-dependent mechanism. Mol. Biol. Cell 2014, 25, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Osada, Y.; Sunatani, T.; Kim, I.S.; Nakanishi, Y.; Shiratsuchi, A. Signalling pathway involving GULP, MAPK and Rac1 for SR-BI-induced phagocytosis of apoptotic cells. J. Biochem. 2009, 145, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Kinchen, J.M.; Cabello, J.; Klingele, D.; Wong, K.; Feichtinger, R.; Schnabel, H.; Schnabel, R.; Hengartner, M.O. Two pathways converge at CED-10 to mediate actin rearrangement and corpse removal in C. elegans. Nature 2005, 434, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, S.Y.; Kim, S.Y.; Bae, D.J.; Pyo, J.H.; Hong, M.; Kim, I.S. Cross talk between engulfment receptors stabilin-2 and integrin αvβ5 orchestrates engulfment of phosphatidylserine-exposed erythrocytes. Mol. Cell. Biol. 2012, 32, 2698–2708. [Google Scholar] [CrossRef]
- Park, D.; Tosello-Trampont, A.C.; Elliott, M.R.; Lu, M.; Haney, L.B.; Ma, Z.; Klibanov, A.L.; Mandell, J.W.; Ravichandran, K.S. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 2007, 450, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.L.; Kim, J.I.; Birge, R.B. αvβ5 integrin recruits the CrkII-Dock180-rac1 complex for phagocytosis of apoptotic cells. Nat. Cell Biol. 2000, 2, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Jeong, H.W.; Nam, J.O.; Lee, B.H.; Choi, J.Y.; Park, R.W.; Park, J.Y.; Kim, I.S. Identification of motifs in the fasciclin domains of the transforming growth factor-β-induced matrix protein betaig-h3 that interact with the αvβ5 integrin. J. Biol. Chem. 2002, 277, 46159–46165. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.O.; Kim, J.E.; Jeong, H.W.; Lee, S.J.; Lee, B.H.; Choi, J.Y.; Park, R.W.; Park, J.Y.; Kim, I.S. Identification of the αvβ3 integrin-interacting motif of betaig-h3 and its anti-angiogenic effect. J. Biol. Chem. 2003, 278, 25902–25909. [Google Scholar] [CrossRef] [PubMed]
- Henson, P.M.; Bratton, D.L. Antiinflammatory effects of apoptotic cells. J. Clin. Investig. 2013, 123, 2773–2774. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; Bratton, D.L.; Konowal, A.; Freed, P.W.; Westcott, J.Y.; Henson, P.M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J. Clin. Investig. 1998, 101, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Freire-de-Lima, C.G.; Xiao, Y.Q.; Gardai, S.J.; Bratton, D.L.; Schiemann, W.P.; Henson, P.M. Apoptotic cells, through transforming growth factor-β, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J. Biol. Chem. 2006, 281, 38376–38384. [Google Scholar] [CrossRef] [PubMed]
- Kushwah, R.; Wu, J.; Oliver, J.R.; Jiang, G.; Zhang, J.; Siminovitch, K.A.; Hu, J. Uptake of apoptotic DC converts immature DC into tolerogenic DC that induce differentiation of Foxp3+ Treg. Eur. J. Immunol. 2010, 40, 1022–1035. [Google Scholar] [CrossRef] [PubMed]
- Palani, S.; Elima, K.; Ekholm, E.; Jalkanen, S.; Salmi, M. Monocyte Stabilin-1 suppresses the activation of Th1 lymphocytes. J. Immunol. 2016, 196, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Dunkel, J.; Viitala, M.; Karikoski, M.; Rantakari, P.; Virtakoivu, R.; Elima, K.; Hollmen, M.; Jalkanen, S.; Salmi, M. Enhanced antibody production in clever-1/Stabilin-1-deficient mice. Front. Immunol. 2018, 9, 2257. [Google Scholar] [CrossRef]
- Lee, W.; Park, S.Y.; Yoo, Y.; Kim, S.Y.; Kim, J.E.; Kim, S.W.; Seo, Y.K.; Park, E.K.; Kim, I.S.; Bae, J.S. Macrophagic Stabilin-1 restored disruption of vascular integrity caused by sepsis. Thromb. Haemost. 2018, 118, 1776–1789. [Google Scholar] [CrossRef]
- Qin, S.; Wang, H.; Yuan, R.; Li, H.; Ochani, M.; Ochani, K.; Rosas-Ballina, M.; Czura, C.J.; Huston, J.M.; Miller, E.; et al. Role of HMGB1 in apoptosis-mediated sepsis lethality. J. Exp. Med. 2006, 203, 1637–1642. [Google Scholar] [CrossRef] [PubMed]
- Wakelam, M.J. The fusion of myoblasts. Biochem. J. 1985, 228, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, K.A.; Horwitz, A.F. Tandem events in myoblast fusion. Dev. Biol. 1977, 58, 328–338. [Google Scholar] [CrossRef]
- Kim, J.H.; Ren, Y.; Ng, W.P.; Li, S.; Son, S.; Kee, Y.S.; Zhang, S.; Zhang, G.; Fletcher, D.A.; Robinson, D.N.; et al. Mechanical tension drives cell membrane fusion. Dev. Cell 2015, 32, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Shilagardi, K.; Li, S.; Luo, F.; Marikar, F.; Duan, R.; Jin, P.; Kim, J.H.; Murnen, K.; Chen, E.H. Actin-propelled invasive membrane protrusions promote fusogenic protein engagement during cell-cell fusion. Science 2013, 340, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jin, P.; Duan, R.; Chen, E.H. Mechanisms of myoblast fusion during muscle development. Curr. Opin. Genet. Dev. 2015, 32, 162–170. [Google Scholar] [CrossRef]
- Van den Eijnde, S.M.; Boshart, L.; Reutelingsperger, C.P.; De Zeeuw, C.I.; Vermeij-Keers, C. Phosphatidylserine plasma membrane asymmetry in vivo: A pancellular phenomenon which alters during apoptosis. Cell Death Differ. 1997, 4, 311–316. [Google Scholar] [CrossRef]
- Jeong, J.; Conboy, I.M. Phosphatidylserine directly and positively regulates fusion of myoblasts into myotubes. Biochem. Biophys. Res. Commun. 2011, 414, 9–13. [Google Scholar] [CrossRef]
- Adler, R.R.; Ng, A.K.; Rote, N.S. Monoclonal antiphosphatidylserine antibody inhibits intercellular fusion of the choriocarcinoma line, JAR. Biol. Reprod. 1995, 53, 905–910. [Google Scholar] [CrossRef]
- Verma, S.K.; Leikina, E.; Melikov, K.; Gebert, C.; Kram, V.; Young, M.F.; Uygur, B.; Chernomordik, L.V. Cell-surface phosphatidylserine regulates osteoclast precursor fusion. J. Biol. Chem. 2018, 293, 254–270. [Google Scholar] [CrossRef]
- Helming, L.; Winter, J.; Gordon, S. The scavenger receptor CD36 plays a role in cytokine-induced macrophage fusion. J. Cell Sci. 2009, 122, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Umeda, M.; Sims, P.J.; Nagata, S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature 2010, 468, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Sakata, A.; Nishimura, S.; Eto, K.; Nagata, S. TMEM16F is required for phosphatidylserine exposure and microparticle release in activated mouse platelets. Proc. Natl. Acad. Sci. USA 2015, 112, 12800–12805. [Google Scholar] [CrossRef] [PubMed]
- Falzone, M.E.; Rheinberger, J.; Lee, B.C.; Peyear, T.; Sasset, L.; Raczkowski, A.M.; Eng, E.T.; Di Lorenzo, A.; Andersen, O.S.; Nimigean, C.M.; et al. Structural basis of Ca(2+)-dependent activation and lipid transport by a TMEM16 scramblase. eLife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Alvadia, C.; Lim, N.K.; Clerico Mosina, V.; Oostergetel, G.T.; Dutzler, R.; Paulino, C. Cryo-EM structures and functional characterization of the murine lipid scramblase TMEM16F. eLife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Dang, S.; Han, T.W.; Ye, W.; Jin, P.; Cheng, T.; Li, J.; Jan, Y.N.; Jan, L.Y.; Cheng, Y. Cryo-EM Studies of TMEM16F Calcium-Activated Ion Channel Suggest Features Important for Lipid Scrambling. Cell Rep. 2019, 28, 1385. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Jia, Z.; Le, S.C.; Zhang, Y.; Chen, J.; Yang, H. An inner activation gate controls TMEM16F phospholipid scrambling. Nat. Commun. 2019, 10, 1846. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, K.; Kim, I.; Seong, S.; Kim, S.W.; Kim, N. Role of anoctamin 5, a gene associated with gnathodiaphyseal dysplasia, in osteoblast and osteoclast differentiation. Bone 2019, 120, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Di Zanni, E.; Gradogna, A.; Scholz-Starke, J.; Boccaccio, A. Gain of function of TMEM16E/ANO5 scrambling activity caused by a mutation associated with gnathodiaphyseal dysplasia. Cell Mol. Life Sci. 2018, 75, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Millay, D.P.; O’Rourke, J.R.; Sutherland, L.B.; Bezprozvannaya, S.; Shelton, J.M.; Bassel-Duby, R.; Olson, E.N. Myomaker is a membrane activator of myoblast fusion and muscle formation. Nature 2013, 499, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Bi, P.; Ramirez-Martinez, A.; Li, H.; Cannavino, J.; McAnally, J.R.; Shelton, J.M.; Sanchez-Ortiz, E.; Bassel-Duby, R.; Olson, E.N. Control of muscle formation by the fusogenic micropeptide myomixer. Science 2017, 356, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.E.; Goh, Q.; Kurosaka, M.; Gamage, D.G.; Petrany, M.J.; Prasad, V.; Millay, D.P. Myomerger induces fusion of non-fusogenic cells and is required for skeletal muscle development. Nat. Commun. 2017, 8, 15665. [Google Scholar] [CrossRef] [PubMed]
- Hogan, P.G.; Chen, L.; Nardone, J.; Rao, A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003, 17, 2205–2232. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, V.A.; Rietze, B.A.; Chambers, P.J.; Bellissimo, C.; Bombardier, E.; Quadrilatero, J.; Tupling, A.R. Effects of sarcolipin deletion on skeletal muscle adaptive responses to functional overload and unload. Am. J. Physiol. Cell Physiol. 2017, 313, C154–C161. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, V.A.; Chambers, P.J.; Juracic, E.S.; Rietze, B.A.; Gamu, D.; Bellissimo, C.; Kwon, F.; Quadrilatero, J.; Russell Tupling, A. Sarcolipin deletion in mdx mice impairs calcineurin signalling and worsens dystrophic pathology. Hum. Mol. Genet. 2018, 27, 4094–4102. [Google Scholar] [CrossRef] [PubMed]
- Hochreiter-Hufford, A.E.; Lee, C.S.; Kinchen, J.M.; Sokolowski, J.D.; Arandjelovic, S.; Call, J.A.; Klibanov, A.L.; Yan, Z.; Mandell, J.W.; Ravichandran, K.S. Phosphatidylserine receptor BAI1 and apoptotic cells as new promoters of myoblast fusion. Nature 2013, 497, 263–267. [Google Scholar] [CrossRef]
- Vasyutina, E.; Martarelli, B.; Brakebusch, C.; Wende, H.; Birchmeier, C. The small G-proteins Rac1 and Cdc42 are essential for myoblast fusion in the mouse. Proc. Natl. Acad. Sci. USA 2009, 106, 8935–8940. [Google Scholar] [CrossRef]
- Moore, C.A.; Parkin, C.A.; Bidet, Y.; Ingham, P.W. A role for the myoblast city homologues Dock1 and Dock5 and the adaptor proteins Crk and Crk-like in zebrafish myoblast fusion. Development 2007, 134, 3145–3153. [Google Scholar] [CrossRef]
- Hamoud, N.; Tran, V.; Croteau, L.P.; Kania, A.; Cote, J.F. G-protein coupled receptor BAI3 promotes myoblast fusion in vertebrates. Proc. Natl. Acad. Sci. USA 2014, 111, 3745–3750. [Google Scholar] [CrossRef]
- Hamoud, N.; Tran, V.; Aimi, T.; Kakegawa, W.; Lahaie, S.; Thibault, M.P.; Pelletier, A.; Wong, G.W.; Kim, I.S.; Kania, A.; et al. Spatiotemporal regulation of the GPCR activity of BAI3 by C1qL4 and Stabilin-2 controls myoblast fusion. Nat. Commun. 2018, 9, 4470. [Google Scholar] [CrossRef]
- Miyanishi, M.; Tada, K.; Koike, M.; Uchiyama, Y.; Kitamura, T.; Nagata, S. Identification of Tim4 as a phosphatidylserine receptor. Nature 2007, 450, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Kzhyshkowska, J.; Workman, G.; Cardo-Vila, M.; Arap, W.; Pasqualini, R.; Gratchev, A.; Krusell, L.; Goerdt, S.; Sage, E.H. Novel function of alternatively activated macrophages: Stabilin-1-mediated clearance of SPARC. J. Immunol. 2006, 176, 5825–5832. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Weigel, J.A.; Saxena, A.; Weigel, P.H. Molecular cloning and functional expression of the rat 175-kDa hyaluronan receptor for endocytosis. Mol. Biol. Cell 2002, 13, 2853–2868. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.N.; Weigel, J.A.; Weigel, P.H. The human hyaluronan receptor for endocytosis (HARE/Stabilin-2) is a systemic clearance receptor for heparin. J. Biol. Chem. 2008, 283, 17341–17350. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.N.; Weigel, P.H. The ligand-binding profile of HARE: Hyaluronan and chondroitin sulfates A, C, and D bind to overlapping sites distinct from the sites for heparin, acetylated low-density lipoprotein, dermatan sulfate, and CS-E. Glycobiology 2008, 18, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.; Helenius, A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science 2008, 320, 531–535. [Google Scholar] [CrossRef]
- Carrera Silva, E.A.; Chan, P.Y.; Joannas, L.; Errasti, A.E.; Gagliani, N.; Bosurgi, L.; Jabbour, M.; Perry, A.; Smith-Chakmakova, F.; Mucida, D.; et al. T cell-derived protein S engages TAM receptor signaling in dendritic cells to control the magnitude of the immune response. Immunity 2013, 39, 160–170. [Google Scholar] [CrossRef]
| Receptor | Details and Comments | Ref. |
|---|---|---|
| Stabilin-1 | Stabilin-1 mediates phagocytosis of aged red blood cells (RBCs) and apoptotic cells in alternatively activated macrophages in a phosphatidylserine-dependent manner. Stabilin-1 interacts with phosphatidylserine through its epidermal growth factor (EGF)-like domain repeats. | [21] |
| Gulp1 (phosphotyrosine-binding domain-containing engulfment adaptor protein 1) functions downstream of stabilin-1 receptor to remove aged RBCs. Stabilin-1 binds to phosphotyrosine-binding domain of Gulp1 via its asparagine-proline-x-phenylalanine (NPxF) motif (where x is any amino acid). | [64] | |
| Stabilin-1 enhances phosphatidylserine-dependent erythrophagocytosis through hepatic sequestration of damaged RBC. Knockdown of stabilin-1 and stabilin-2 delays hepatic clearance of damaged RBCs in vivo. | [49] | |
| Acidic pH enhances phagocytosis of aged RBCs through enhancing stabilin-1 expression. Ets-2 (E26 avian leukemia oncogene 2) acts as a positive regulator to regulate stabilin-1 expression. | [45] | |
| Stabilin-1-mediated phagocytosis plays an important role in maintaining vascular integrity during sepsis. Stabilin-1 deficiency promotes disease progression caused by septic shock. | [80] | |
| Stabilin-2 | Stabilin-2 mediates phagocytosis of aged RBCs and apoptotic cells in human monocyte-derived macrophages in a phosphatidylserine-dependent manner. Stabilin-2 activation stimulates transforming growth factor (TGF)-β production. | [20] |
| Gulp1 functions downstream of stabilin-2 receptor to effectively clear aged RBCs. Stabilin-2 binds to phosphotyrosine-binding domain of Gulp1 via its asparagine-proline-x- tyrosine (NPxY) motif (where x is any amino acid). | [63] | |
| Stabilin-2 interacts with phosphatidylserine through its EGF-like domain repeats. Atypical EGF-like domains in Stabilin-2 play an important role in phosphatidylserine binding. | [50] | |
| Extracellular acidic pH enhances stabilin-2-mediated efferocytosis. The conserved histidine in atypical EGF-like domain modulates the phosphatidylserine-binding affinity of stabilin-2. | [51] | |
| Stabilin-2 enhances phosphatidylserine-dependent erythrophagocytosis through hepatic sequestration of damaged RBCs. Knockdown of stabilin-1 and stabilin-2 delays hepatic clearance of damaged RBCs in vivo. | [49] | |
| Stabilin-2 binds to integrin β5 through its fasciclin I (FAS1) domains. Integrin αvβ5 and its signaling pathway are involved in stabilin-2–mediated phagocytosis. | [69] | |
| Stabilin-2 modulates efficiency of myoblast fusion during myogenic differentiation. Stabilin-2–deficient mice display impaired muscle regeneration. | [25] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-Y.; Kim, I.-S. Stabilin Receptors: Role as Phosphatidylserine Receptors. Biomolecules 2019, 9, 387. https://doi.org/10.3390/biom9080387
Park S-Y, Kim I-S. Stabilin Receptors: Role as Phosphatidylserine Receptors. Biomolecules. 2019; 9(8):387. https://doi.org/10.3390/biom9080387
Chicago/Turabian StylePark, Seung-Yoon, and In-San Kim. 2019. "Stabilin Receptors: Role as Phosphatidylserine Receptors" Biomolecules 9, no. 8: 387. https://doi.org/10.3390/biom9080387
APA StylePark, S.-Y., & Kim, I.-S. (2019). Stabilin Receptors: Role as Phosphatidylserine Receptors. Biomolecules, 9(8), 387. https://doi.org/10.3390/biom9080387





