DNA Methylation Status in Cancer Disease: Modulations by Plant-Derived Natural Compounds and Dietary Interventions
Abstract
1. Introduction
1.1. Source of Data
1.2. Aim of the Study
2. The Role of DNA Methylation Patterns as Potential Diagnostic/Prognostic Markers of Carcinogenesis
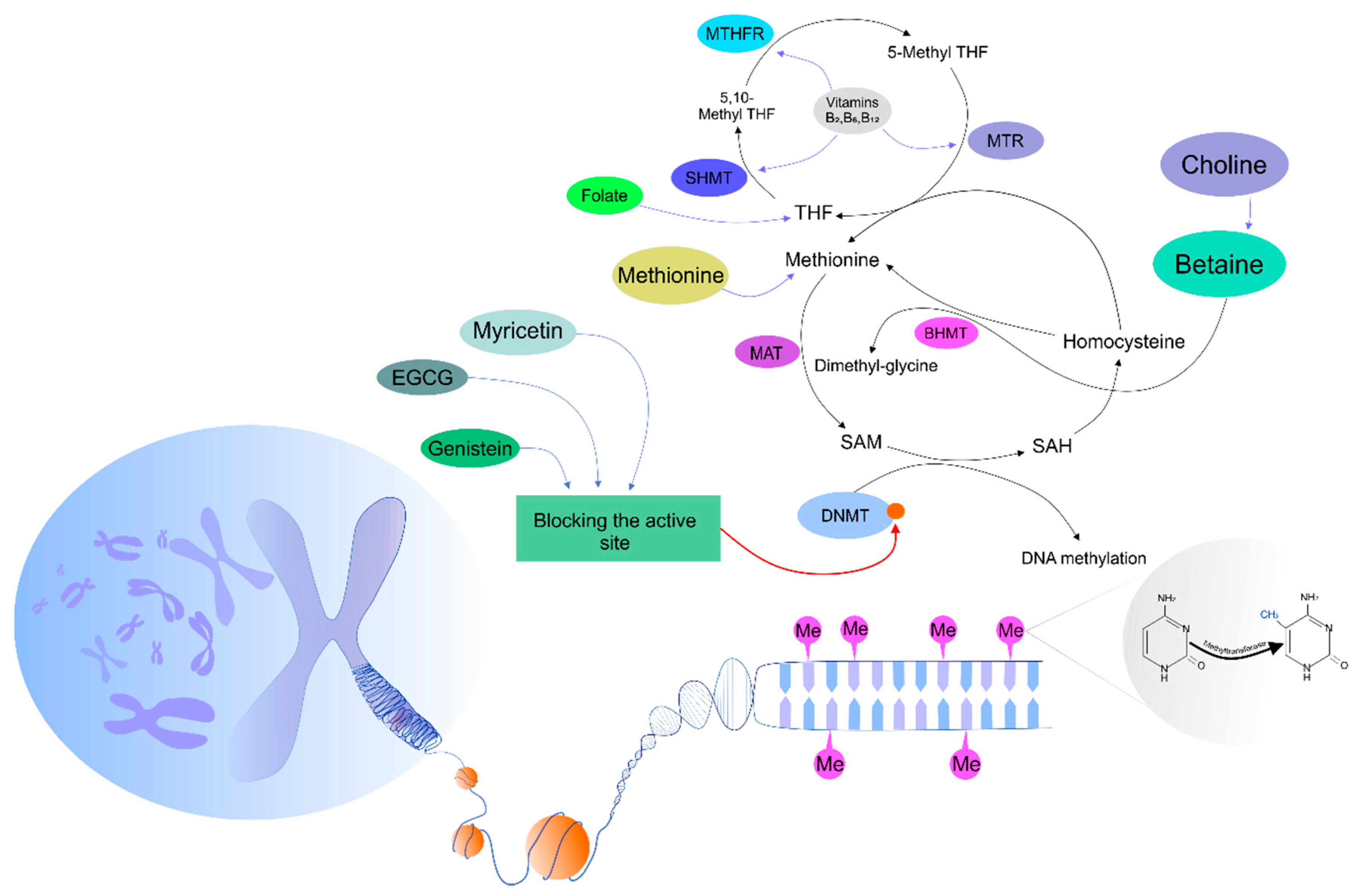
3. Regulatory Mechanisms Controlling DNA Methyltransferase Activity
The Effects of Phytochemicals on DNA Methylation Patterns
4. Preclinical Cancer Research
4.1. In Vitro Studies
4.2. In Vivo Studies
5. Dietary Intervention and DNA Methylation Patterns in Cancer Clinical Research
6. Conclusions and Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Carlos-Reyes, Á.; López-González, J.S.; Meneses-Flores, M.; Gallardo-Rincón, D.; Ruíz-García, E.; Marchat, L.A.; La Vega, H.A.-D.; De La Cruz, O.N.H.; López-Camarillo, C. Dietary Compounds as Epigenetic Modulating Agents in Cancer. Front. Genet. 2019, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Perri, F.; Longo, F.; Giuliano, M.; Sabbatino, F.; Favia, G.; Ionna, F.; Addeo, R.; Scarpati, G.D.V.; Di Lorenzo, G.; Pisconti, S.; et al. Epigenetic control of gene expression: Potential implications for cancer treatment. Crit. Rev. Oncol. 2017, 111, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Uramova, S.; Kubatka, P.; Dankova, Z.; Kapinova, A.; Zolakova, B.; Samec, M.; Zubor, P.; Zulli, A.; Valentova, V.; Kwon, T.K.; et al. Plant natural modulators in breast cancer prevention: Status quo and future perspectives reinforced by predictive, preventive, and personalized medical approach. EPMA J. 2018, 9, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Chen, T. DNA Methylation Reprogramming during Mammalian Development. Genes 2019, 10, 257. [Google Scholar] [CrossRef] [PubMed]
- Gujar, H.; Weisenberger, D.J.; Liang, G. The Roles of Human DNA Methyltransferases and Their Isoforms in Shaping the Epigenome. Genes 2019, 10, 172. [Google Scholar] [CrossRef]
- Liu, P.; Shen, J.K.; Xu, J.; Trahan, C.A.; Hornicek, F.J.; Duan, Z. Aberrant DNA methylations in chondrosarcoma. Epigenomics 2016, 8, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Liu, Y.; Lv, J.; Ding, H.; Zhang, X.A.; Shao, L.; Yang, N.; Cheng, H.; Sun, L.; Zhu, D.; et al. ERα propelled aberrant global DNA hypermethylation by activating the DNMT1 gene to enhance anticancer drug resistance in human breast cancer cells. Oncotarget 2016, 7, 20966–20980. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Z.-Y.; Wang, F. Association between DNA methylation and multidrug resistance in human glioma SHG-44 cells. Mol. Med. Rep. 2015, 11, 43–52. [Google Scholar] [CrossRef][Green Version]
- Ng, J.M.-K.; Yu, J. Promoter Hypermethylation of Tumour Suppressor Genes as Potential Biomarkers in Colorectal Cancer. Int. J. Mol. Sci. 2015, 16, 2472–2496. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, H.; Li, H.; Li, X.; Yang, S. DNA methylation as an early diagnostic marker of cancer (Review). Biomed. Rep. 2014, 2, 326–330. [Google Scholar] [CrossRef]
- Van Tongelen, A.; Loriot, A.; De Smet, C. Oncogenic roles of DNA hypomethylation through the activation of cancer-germline genes. Cancer Lett. 2017, 396, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Cheng, J.; Cao, X.; Surowy, H.; Burwinkel, B. Blood-based DNA methylation as biomarker for breast cancer: A systematic review. Clin. Epigenetics 2016, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Xiong, X.; Zhang, L. Promoter hypermethylation of RARβ2, DAPK, hMLH1, p14, and p15 is associated with progression of breast cancer. Medicine 2018, 97, e13666. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, K.; Yamashita, K.; Watanabe, M. Analysis of DNA Methylation Status in Bodily Fluids for Early Detection of Cancer. Int. J. Mol. Sci. 2017, 18, 735. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.D.; Issa, J.-P.J. The promise of epigenetic therapy: Reprogramming the cancer epigenome. Curr. Opin. Genet. Dev. 2017, 42, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Samec, M.; Liskova, A.; Kubatka, P.; Uramova, S.; Zubor, P.; Samuel, S.M.; Zulli, A.; Pec, M.; Bielik, T.; Biringer, K.; et al. The role of dietary phytochemicals in the carcinogenesis via the modulation of miRNA expression. J. Cancer Res. Clin. Oncol. 2019, 145, 1665–1679. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, G.P. Defining Driver DNA Methylation Changes in Human Cancer. Int. J. Mol. Sci. 2018, 19, 1166. [Google Scholar] [CrossRef]
- Hao, X.; Luo, H.; Krawczyk, M.; Wei, W.; Wang, W.; Wang, J.; Flagg, K.; Hou, J.; Zhang, H.; Yi, S.; et al. DNA methylation markers for diagnosis and prognosis of common cancers. Proc. Natl. Acad. Sci. USA 2017, 114, 7414–7419. [Google Scholar] [CrossRef]
- Witte, T.; Plass, C.; Gerhäuser, C. Pan-cancer patterns of DNA methylation. Genome Med. 2014, 6, 2652. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, H.; Li, J.; Liu, H.; Wang, F.; Wei, Y.; Su, J.; Zhang, D.; Liu, T.; Zhang, Y. The Identification of Specific Methylation Patterns across Different Cancers. PLoS ONE 2015, 10, e0120361. [Google Scholar] [CrossRef]
- Holubeková, V.; Mendelová, A.; Grendár, M.; Meršaková, S.; Kapustová, I.; Jašek, K.; Vaňochová, A.; Danko, J.; Lasabová, Z. Methylation pattern of CDH1 promoter and its association with CDH1 gene expression in cytological cervical specimens. Oncol. Lett. 2016, 12, 2613–2621. [Google Scholar] [CrossRef] [PubMed]
- Jašek, K.; Kasubova, I.; Holubekova, V.; Stanclova, A.; Plank, L.; Lasabová, Z. Epigenetics: An alternative pathway in GISTs tumorigenesis. Neoplasma 2018, 65, 477–493. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guo, J.; Wang, W.; Wang, D. Relationship between MGMT gene expression and treatment effectiveness and prognosis in glioma. Oncol. Lett. 2017, 14, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Barchitta, M.; Quattrocchi, A.; Maugeri, A.; Vinciguerra, M.; Agodi, A. LINE-1 Hypomethylation in Blood and Tissue Samples as an Epigenetic Marker for Cancer Risk: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e109478. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wang, H.; Wang, Y.; Wang, T.; Wang, M.; Ma, M.; Duan, Y.; Meng, X.; Liu, L. Low expression of Bin1, along with high expression of IDO in tumor tissue and draining lymph nodes, are predictors of poor prognosis for esophageal squamous cell cancer patients. Int. J. Cancer 2015, 137, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, J.; Jia, Y.; Wang, Y.; Han, X.; Duan, Y.; Lv, W.; Ma, M.; Liu, L. Methylation decreases the Bin1 tumor suppressor in ESCC and restoration by decitabine inhibits the epithelial mesenchymal transition. Oncotarget 2017, 8, 19661–19673. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Melnikov, A.A.; Levenson, V.; Guerra, E.; Simeone, P.; Alberti, S.; Deng, Y. A seven-gene CpG-island methylation panel predicts breast cancer progression. BMC Cancer 2015, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Julsing, J.R.; Peters, G.J. Methylation of DNA repair genes and the efficacy of DNA targeted anticancer treatment. Oncol. Discov. 2014, 2, 3. [Google Scholar] [CrossRef]
- Wojtczyk-Miaskowska, A.; Presler, M.; Michajlowski, J.; Matuszewski, M.; Schlichtholz, B. Gene Expression, DNA Methylation and Prognostic Significance of DNA Repair Genes in Human Bladder Cancer. Cell. Physiol. Biochem. 2017, 42, 2404–2417. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Herman, J.G.; Guo, M. The clinical value of aberrant epigenetic changes of DNA damage repair genes in human cancer. Oncotarget 2016, 7, 37331–37346. [Google Scholar] [CrossRef] [PubMed]
- Kašubová, I.; Kalman, M.; Jašek, K.; Burjanivová, T.; Malicherová, B.; Vaňochová, A.; Meršaková, S.; Lasabová, Z.; Plank, L. Stratification of patients with colorectal cancer without the recorded family history. Oncol. Lett. 2019, 17, 3649–3656. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Zhang, Y. DNA methylation in mammals. Cold Spring Harb. Perspect. Biol. 2014, 6, a019133. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Daito, T.; Sasaki, Y.; Chung, Y.H.; Xing, X.; Pondugula, S.; Swamidass, S.J.; Wang, T.; Kim, A.H.; Yano, H. Inhibition of DNA Methyltransferases Blocks Mutant Huntingtin-Induced Neurotoxicity. Sci. Rep. 2016, 6, 31022. [Google Scholar] [CrossRef] [PubMed]
- Poh, W.J.; Wee, C.P.P.; Gao, Z. DNA Methyltransferase Activity Assays: Advances and Challenges. Theranostics 2016, 6, 369–391. [Google Scholar] [CrossRef] [PubMed]
- Heiland, D.H.; Ferrarese, R.; Claus, R.; Dai, F.; Masilamani, A.P.; Kling, E.; Weyerbrock, A.; Kling, T.; Nelander, S.; Carro, M.S. c-Jun-N-terminal phosphorylation regulates DNMT1 expression and genome wide methylation in gliomas. Oncotarget 2017, 8, 6940–6954. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.-L.; Yin, Y.-R.; Xie, C.-R.; Zhang, S.; Zhao, W.-X.; Pan, C.; Wang, X.-M.; Yin, Z.-Y. Mechanistic and biological significance of DNA methyltransferase 1 upregulated by growth factors in human hepatocellular carcinoma. Int. J. Oncol. 2015, 46, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-A.; Tsai, Y.-T.; Lin, R.-K.; Hsu, H.-S.; Chen, C.-Y.; Wang, Y.-C. Deregulation of p53 and RB Transcriptional Control Leads to Overexpression of DNA Methyltransferases in Lung Cancer. J. Cancer Res. Pr. 2014, 1, 14–27. [Google Scholar] [CrossRef]
- Xie, S.; Qian, C. The Growing Complexity of UHRF1-Mediated Maintenance DNA Methylation. Genes 2018, 9, 600. [Google Scholar] [CrossRef]
- Kadayifci, F.Z.; Zheng, S.; Pan, Y.-X. Molecular Mechanisms Underlying the Link between Diet and DNA Methylation. Int. J. Mol. Sci. 2018, 19, 4055. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, H.; Wang, H. Long noncoding RNAs in DNA methylation: New players stepping into the old game. Cell Biosci. 2016, 6, 89. [Google Scholar] [CrossRef]
- Jeltsch, A.; Jurkowska, R.Z. Allosteric control of mammalian DNA methyltransferases—A new regulatory paradigm. Nucleic Acids Res. 2016, 44, 8556–8575. [Google Scholar] [CrossRef] [PubMed]
- Yarychkivska, O.; Tavana, O.; Gu, W.; Bestor, T.H. Independent functions of DNMT1 and USP7 at replication foci. Epigenetics Chromatin 2018, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Bai, X.-Y.; Wang, C.-H. Traditional Chinese Medicine: A Treasured Natural Resource of Anticancer Drug Research and Development. Am. J. Chin. Med. 2014, 42, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Meybodi, N.M.; Mortazavian, A.M.; Monfared, A.B.; Sohrabvandi, S. Phytochemicals in Cancer Prevention: A Review of the Evidence. Iran. J. Cancer Prev. 2017, 10, e7219. [Google Scholar]
- Shukla, S.; Meeran, S.M.; Katiyar, S.K. Epigenetic regulation by selected dietary phytochemicals in cancer chemoprevention. Cancer Lett. 2014, 355, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.G.; Merlo, A.; Mao, L.; Lapidus, R.G.; Issa, J.P.; Davidson, N.E.; Sidransky, D.; Baylin, S.B. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 1995, 55, 4525–4530. [Google Scholar] [PubMed]
- Jones, P.A. DNA methylation errors and cancer. Cancer Res. 1996, 56, 2463–2467. [Google Scholar]
- Laird, P.W.; Jaenisch, R. DNA methylation and cancer. Hum. Mol. Genet. 1994, 3, 1487–1495. [Google Scholar] [CrossRef]
- Kurdyukov, S.; Bullock, M. DNA Methylation Analysis: Choosing the Right Method. Biology 2016, 5, 3. [Google Scholar] [CrossRef]
- Shankar, S.; Kumar, D.; Srivastava, R.K. Epigenetic modifications by dietary phytochemicals: Implications for personalized nutrition. Pharmacol. Ther. 2013, 138, 1–17. [Google Scholar] [CrossRef]
- Day, J.K.; Desbordes, C.; Zhuang, Y.; Newton, L.G.; Nehra, V.; Forsee, K.M.; Besch-Williford, C.; Huang, T.H.-M.; Lubahn, D.B.; Bauer, A.M.; et al. Genistein Alters Methylation Patterns in Mice. J. Nutr. 2002, 132, 2419S–2423S. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Zhu, W.; Sauter, E. Resveratrol induced DNA methylation in ER+ breast cancer. Cancer Res. 2005, 65, 647. [Google Scholar]
- Fang, M.Z.; Wang, Y.; Ai, N.; Hou, Z.; Sun, Y.; Lu, H.; Welsh, W.; Yang, C.S. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003, 63, 7563–7570. [Google Scholar] [PubMed]
- Zhang, N. Role of methionine on epigenetic modification of DNA methylation and gene expression in animals. Anim. Nutr. 2018, 4, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Ali, M.M. Methyl Donor Micronutrients that Modify DNA Methylation and Cancer Outcome. Nutrients 2019, 11, 608. [Google Scholar] [CrossRef] [PubMed]
- Llanos, A.A.; Dumitrescu, R.G.; Brasky, T.M.; Liu, Z.; Mason, J.B.; Marian, C.; Makambi, K.H.; Spear, S.L.; Kallakury, B.V.S.; Freudenheim, J.L.; et al. Relationships among folate, alcohol consumption, gene variants in one-carbon metabolism and p16INK4a methylation and expression in healthy breast tissues. Carcinogenesis 2015, 36, 60–67. [Google Scholar] [CrossRef]
- Farkas, S.A.; Befekadu, R.; Hahn-Strömberg, V.; Nilsson, T.K. DNA methylation and expression of the folate transporter genes in colorectal cancer. Tumor Boil. 2015, 36, 5581–5590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Naifeng, Z. Epigenetic modulation of DNA methylation by nutrition and its mechanisms in animals. Anim. Nutr. 2015, 1, 144–151. [Google Scholar] [CrossRef]
- Busch, C.; Burkard, M.; Leischner, C.; Lauer, U.M.; Frank, J.; Venturelli, S. Epigenetic activities of flavonoids in the prevention and treatment of cancer. Clin. Epigenetics 2015, 7, 100. [Google Scholar] [CrossRef]
- Morris, J.; Moseley, V.R.; Cabang, A.B.; Coleman, K.; Wei, W.; Garrett-Mayer, E.; Wargovich, M.J. Reduction in promotor methylation utilizing EGCG (epigallocatechin-3-gallate) restores RXRα expression in human colon cancer cells. Oncotarget 2016, 7, 35313–35326. [Google Scholar] [CrossRef]
- Sanaei, M.; Kavoosi, F.; Roustazadeh, A.; Golestan, F. Effect of Genistein in Comparison with Trichostatin A on Reactivation of DNMTs Genes in Hepatocellular Carcinoma. J. Clin. Transl. Hepatol. 2018, 6, 141–146. [Google Scholar] [CrossRef]
- Romagnolo, D.F.; Donovan, M.G.; Papoutsis, A.J.; Doetschman, T.C.; Selmin, O.I. Genistein Prevents BRCA1 CpG Methylation and Proliferation in Human Breast Cancer Cells with Activated Aromatic Hydrocarbon Receptor. Curr. Dev. Nutr. 2017, 1, e000562. [Google Scholar] [CrossRef]
- Finer, S.; Saravanan, P.; Hitman, G.; Yajnik, C. The role of the one-carbon cycle in the developmental origins of Type 2 diabetes and obesity. Diabet. Med. 2014, 31, 263–272. [Google Scholar] [CrossRef]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef]
- Li, J.; Hao, D.; Wang, L.; Wang, H.; Wang, Y.; Zhao, Z.; Li, P.; Deng, C.; Di, L.-J. Epigenetic targeting drugs potentiate chemotherapeutic effects in solid tumor therapy. Sci. Rep. 2017, 7, 4035. [Google Scholar] [CrossRef]
- Subramaniam, D.; Thombre, R.; Dhar, A.; Anant, S. DNA Methyltransferases: A Novel Target for Prevention and Therapy. Front. Oncol. 2014, 4, 80. [Google Scholar] [CrossRef]
- Gao, Y.; Tollefsbol, T.O. Combinational Proanthocyanidins and Resveratrol Synergistically Inhibit Human Breast Cancer Cells and Impact Epigenetic–Mediating Machinery. Int. J. Mol. Sci. 2018, 19, 2204. [Google Scholar] [CrossRef]
- Naselli, F.; Belshaw, N.J.; Gentile, C.; Livrea, M.A.; CaraDonna, F.; Tutone, M.; Tesoriere, L. Phytochemical Indicaxanthin Inhibits Colon Cancer Cell Growth and Affects the DNA Methylation Status by Influencing Epigenetically Modifying Enzyme Expression and Activity. J. Nutr. Nutr. 2015, 8, 114–127. [Google Scholar] [CrossRef]
- Jiang, F.; Li, Y.; Mu, J.; Hu, C.; Zhou, M.; Wang, X.; Si, L.; Ning, S.; Li, Z. Glabridin inhibits cancer stem cell-like properties of human breast cancer cells: An epigenetic regulation of miR-148a/SMAd2 signaling. Mol. Carcinog. 2016, 55, 929–940. [Google Scholar] [CrossRef]
- Wong, C.P.; Hsu, A.; Buchanan, A.; Palomera-Sanchez, Z.; Beaver, L.M.; Houseman, E.A.; Williams, D.E.; Dashwood, R.H.; Ho, E. Effects of Sulforaphane and 3,3′-Diindolylmethane on Genome-Wide Promoter Methylation in Normal Prostate Epithelial Cells and Prostate Cancer Cells. PLoS ONE 2014, 9, 86787. [Google Scholar] [CrossRef]
- Lewis, K.A.; Jordan, H.R.; Tollefsbol, T.O. Effects of SAHA and EGCG on Growth Potentiation of Triple-Negative Breast Cancer Cells. Cancers 2018, 11, 23. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, Q.; Xiao, Q.; Yang, L.; Hann, S.S. Targeting EP4 downstream c-Jun through ERK1/2-mediated reduction of DNMT1 reveals novel mechanism of solamargine-inhibited growth of lung cancer cells. J. Cell. Mol. Med. 2017, 21, 222–233. [Google Scholar] [CrossRef]
- Szic, K.S.V.; Diddens, J.; Gerhäuser, C.; Declerck, K.; Crans, R.A.J.; Scherf, D.B.; Berghe, W.V. Epigenetic silencing of triple negative breast cancer hallmarks by Withaferin A. Oncotarget 2017, 8, 40434–40453. [Google Scholar]
- Moiseeva, E.P.; Almeida, G.M.; Jones, G.D.D.; Manson, M.M. Extended treatment with physiologic concentrations of dietary phytochemicals results in altered gene expression, reduced growth, and apoptosis of cancer cells. Mol. Cancer Ther. 2007, 6, 3071–3079. [Google Scholar] [CrossRef]
- Li, Y.; Meeran, S.M.; Patel, S.N.; Chen, H.; Hardy, T.M.; Tollefsbol, T.O. Epigenetic reactivation of estrogen receptor-α (ERα) by genistein enhances hormonal therapy sensitivity in ERα-negative breast cancer. Mol. Cancer 2013, 12, 9. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its’ Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Du, L.; Xie, Z.; Wu, L.-C.; Chiu, M.; Lin, J.; Chan, K.K.; Liu, S.; Liu, Z. Reactivation of RASSF1A in Breast Cancer Cells by Curcumin. Nutr. Cancer 2012, 64, 1228–1235. [Google Scholar] [CrossRef]
- Jiang, A.; Wang, X.; Shan, X.; Li, Y.; Wang, P.; Jiang, P.; Feng, Q. Curcumin Reactivates Silenced Tumor Suppressor Gene RARβ by Reducing DNA Methylation. Phytother. Res. 2015, 29, 1237–1245. [Google Scholar] [CrossRef]
- Yu, J.; Peng, Y.; Wu, L.-C.; Xie, Z.; Deng, Y.; Hughes, T.; He, S.; Mo, X.; Chiu, M.; Wang, Q.-E.; et al. Curcumin Down-Regulates DNA Methyltransferase 1 and Plays an Anti-Leukemic Role in Acute Myeloid Leukemia. PLoS ONE 2013, 8, e55934. [Google Scholar] [CrossRef]
- Khor, T.O.; Huang, Y.; Wu, T.-Y.; Shu, L.; Lee, J.H.; Kong, A.-N.T. Pharmacodynamics of curcumin as DNA hypomethylation agent in restoring the expression of Nrf2 via promoter CpGs demethylation. Biochem. Pharmacol. 2011, 82, 1073–1078. [Google Scholar] [CrossRef]
- Hu, P.; Ma, L.; Wang, Y.-G.; Ye, F.; Wang, C.; Zhou, W.-H.; Zhao, X. Genistein, a dietary soy isoflavone, exerts antidepressant-like effects in mice: Involvement of serotonergic system. Neurochem. Int. 2017, 108, 426–435. [Google Scholar] [CrossRef]
- Li, H.; Xu, W.; Huang, Y.; Huang, X.; Xu, L.; Lv, Z. Genistein demethylates the promoter of CHD5 and inhibits neuroblastoma growth in vivo. Int. J. Mol. Med. 2012, 30, 1081–1086. [Google Scholar] [CrossRef]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef]
- Qin, W.; Zhang, K.; Clarke, K.; Weiland, T.; Sauter, E.R. Methylation and miRNA Effects of Resveratrol on Mammary Tumors vs. Normal Tissue. Nutr. Cancer 2014, 66, 270–277. [Google Scholar] [CrossRef]
- Yi, X.; Zuo, J.; Tan, C.; Xian, S.; Luo, C.; Chen, S.; Yu, L.; Luo, Y. Kaempferol, A Flavonoid Compound from Gynura Medica Induced Apoptosis and Growth Inhibition in Mcf-7 Breast Cancer Cell. Afr. J. Tradit. Complement. Altern. Med. AJTCAM 2016, 13, 210–215. [Google Scholar] [CrossRef]
- Qiu, W.; Lin, J.; Zhu, Y.; Zhang, J.; Zeng, L.; Su, M.; Tian, Y. Kaempferol Modulates DNA Methylation and Downregulates DNMT3B in Bladder Cancer. Cell. Physiol. Biochem. 2017, 41, 1325–1335. [Google Scholar] [CrossRef]
- Wang, N.; Wang, Z.; Wang, Y.; Xie, X.; Shen, J.; Peng, C.; You, J.; Peng, F.; Tang, H.; Guan, X.; et al. Dietary compound isoliquiritigenin prevents mammary carcinogenesis by inhibiting breast cancer stem cells through WIF1 demethylation. Oncotarget 2015, 6, 9854–9876. [Google Scholar] [CrossRef]
- Kubatka, P.; Uramova, S.; Kello, M.; Kajo, K.; Samec, M.; Jasek, K.; Zubor, P. Anticancer Activities of Thymus vulgaris L. in Experimental Breast Carcinoma in Vivo and in Vitro. Int. J. Mol. Sci. 2019, 20, 1749. [Google Scholar] [CrossRef]
- Kubatka, P.; Uramova, S.; Kello, M.; Kajo, K.; Kruzliak, P.; Mojzis, J.; Vybohova, D.; Adamkov, M.; Jasek, K.; Lasabová, Z.; et al. Antineoplastic effects of clove buds (Syzygium aromaticum L.) in the model of breast carcinoma. J. Cell. Mol. Med. 2017, 21, 2837–2851. [Google Scholar] [CrossRef]
- Kresty, L.A.; Mallery, S.R.; Stoner, G.D. Black raspberries in cancer clinical trials: Past, present and future. J. Berry Res. 2016, 6, 251–261. [Google Scholar] [CrossRef]
- Wang, L.-S.; Kuo, C.-T.; Huang, T.H.-M.; Yearsley, M.; Oshima, K.; Stoner, G.D.; Yu, J.; Lechner, J.F.; Huang, Y.-W. Black Raspberries Protectively Regulate Methylation of Wnt Pathway Genes in Precancerous Colon Tissue. Cancer Prev. Res. 2013, 6, 1317–1327. [Google Scholar] [CrossRef]
- Huang, Y.-W.; Gu, F.; Dombkowski, A.; Wang, L.-S.; Stoner, G.D. Black raspberries demethylate Sfrp4, a WNT pathway antagonist, in rat esophageal squamous cell papilloma. Mol. Carcinog. 2016, 55, 1867–1875. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, G.; Zhou, F.; Su, B.; Li, Y. DNA methylation profiles in cancer diagnosis and therapeutics. Clin. Exp. Med. 2018, 18, 1–14. [Google Scholar] [CrossRef]
- Bayat, S.; Shekari Khaniani, M.; Choupani, J.; Alivand, M.R.; Mansoori Derakhshan, S. HDACis (class I), cancer stem cell, and phytochemicals: Cancer therapy and prevention implications. Biomed Pharmacother. 2018, 97, 1445–1453. [Google Scholar] [CrossRef]
- Majid, S.; Dar, A.A.; Shahryari, V.; Hirata, H.; Ahmad, A.; Saini, S.; Tanaka, Y.; Dahiya, A.V.; Dahiya, R. Genistein reverses hypermethylation and induces active histone modifications in tumor suppressor gene B-Cell translocation gene 3 in prostate cancer. Cancer 2010, 116, 66–76. [Google Scholar] [CrossRef]
- Qin, W.; Zhu, W.; Shi, H.; Hewett, J.E.; Ruhlen, R.L.; Macdonald, R.S.; Rottinghaus, G.E.; Chen, Y.-C.; Sauter, E.R. Soy Isoflavones Have an Antiestrogenic Effect and Alter Mammary Promoter Hypermethylation in Healthy Premenopausal Women. Nutr. Cancer 2009, 61, 238–244. [Google Scholar] [CrossRef]
- Zhu, W.; Qin, W.; Zhang, K.; Rottinghaus, G.E.; Chen, Y.-C.; Kliethermes, B.; Sauter, E.R. Trans-Resveratrol Alters Mammary Promoter Hypermethylation in Women at Increased Risk for Breast Cancer. Nutr. Cancer 2012, 64, 393–400. [Google Scholar] [CrossRef]
- Liu, J.; Ward, R.L. Folate and One-Carbon Metabolism and Its Impact on Aberrant DNA Methylation in Cancer. Adv. Genet. 2010, 71, 79–121. [Google Scholar]
- Pentieva, K.; Lees-Murdock, D.J.; Walsh, C.P.; Irwin, R.E.; Cassidy, T.; McLaughlin, M.; Prasad, G.; McNulty, H. The interplay between DNA methylation, folate and neurocognitive development. Epigenomics 2016, 8, 863–879. [Google Scholar]
- Lendoiro, E.; Russell, W.; Bestwick, C.; Bermano, G.; Duthie, S. Folate and genomic stability: Differential effect of methylated and oxidised folate on DNA damage and ROS production in human colon fibroblasts. Proc. Nutr. Soc. 2018, 77, 77. [Google Scholar] [CrossRef]
- Coppedè, F.; Migheli, F.; Lopomo, A.; Failli, A.; Legitimo, A.; Consolini, R.; Fontanini, G.; Sensi, E.; Servadio, A.; Seccia, M.; et al. Gene promoter methylation in colorectal cancer and healthy adjacent mucosa specimens: Correlation with physiological and pathological characteristics, and with biomarkers of one-carbon metabolism. Epigenetics 2014, 9, 621–633. [Google Scholar] [CrossRef]
- Pirouzpanah, S.; Taleban, F.-A.; Mehdipour, P.; Atri, M. Association of folate and other one-carbon related nutrients with hypermethylation status and expression of RARB, BRCA1, and RASSF1A genes in breast cancer patients. J. Mol. Med. 2015, 93, 917–934. [Google Scholar] [CrossRef]
- Colacino, J.A.; Arthur, A.E.; Dolinoy, D.C.; Sartor, M.A.; Duffy, S.A.; Chepeha, D.B.; Bradford, C.R.; Walline, H.M.; McHugh, J.B.; D’Silva, N.J.; et al. Pretreatment dietary intake is associated with tumor suppressor DNA methylation in head and neck squamous cell carcinomas. Epigenetics 2012, 7, 883–891. [Google Scholar] [CrossRef]
- Kraunz, K.S.; Hsiung, D.; McClean, M.D.; Liu, M.; Osanyingbemi, J.; Nelson, H.H.; Kelsey, K.T. Dietary folate is associated with p16(INK4A) methylation in head and neck squamous cell carcinoma. Int. J. Cancer 2006, 119, 1553–1557. [Google Scholar] [CrossRef]
- Tao, M.-H.; Mason, J.B.; Marian, C.; McCann, S.E.; Platek, M.E.; Millen, A.; Ambrosone, C.; Edge, S.B.; Krishnan, S.S.; Trevisan, M.; et al. Promoter Methylation of E-Cadherin, p16, and RAR-β2Genes in Breast Tumors and Dietary Intake of Nutrients Important in One-Carbon Metabolism. Nutr. Cancer 2011, 63, 1143–1150. [Google Scholar] [CrossRef]
- Delgado-Cruzata, L.; Zhang, W.; McDonald, J.A.; Tsai, W.Y.; Valdovinos, C.; Falci, L.; Wang, Q.; Crew, K.D.; Santella, R.M.; Hershman, D.L.; et al. Dietary Modifications, Weight Loss, and Changes in Metabolic Markers Affect Global DNA Methylation in Hispanic, African American, and Afro-Caribbean Breast Cancer Survivors12. J. Nutr. 2015, 145, 783–790. [Google Scholar] [CrossRef]
- Piyathilake, C.J.; Johanning, G.L.; Macaluso, M.; Whiteside, M.; Oelschlager, D.K.; Heimburger, D.C.; Grizzle, W.E. Localized Folate and Vitamin B-12 Deficiency in Squamous Cell Lung Cancer Is Associated with Global DNA Hypomethylation. Nutr. Cancer 2000, 37, 99–107. [Google Scholar] [CrossRef]
- O’Reilly, S.L.; McGlynn, A.P.; McNulty, H.; Reynolds, J.; Wasson, G.R.; Molloy, A.M.; Strain, J.; Weir, D.G.; Ward, M.; McKerr, G.; et al. Folic Acid Supplementation in Postpolypectomy Patients in a Randomized Controlled Trial Increases Tissue Folate Concentrations and Reduces Aberrant DNA Biomarkers in Colonic Tissues Adjacent to the Former Polyp Site. J. Nutr. 2016, 146, 933–939. [Google Scholar] [CrossRef]
- Castellano-Castillo, D.; Morcillo, S.; Crujeiras, A.B.; Sánchez-Alcoholado, L.; Clemente-Postigo, M.; Torres, E.; Tinahones, F.J.; Macias-Gonzalez, M. Association between serum 25-hydroxyvitamin D and global DNA methylation in visceral adipose tissue from colorectal cancer patients. BMC Cancer 2019, 19, 93. [Google Scholar] [CrossRef]
- Golubnitschaja, O.; Filep, N.; Yeghiazaryan, K.; Blom, H.J.; Hofmann-Apitius, M.; Kuhn, W. Multi-omic approach decodes paradoxes of the triple-negative breast cancer: Lessons for predictive, preventive and personalised medicine. Amino Acids 2018, 50, 383–395. [Google Scholar] [CrossRef]
- Fröhlich, H.; Patjoshi, S.; Yeghiazaryan, K.; Kehrer, C.; Kuhn, W.; Golubnitschaja, O. Premenopausal breast cancer: Potential clinical utility of a multi-omics based machine learning approach for patient stratification. EPMA J. 2018, 9, 175–186. [Google Scholar] [CrossRef]
- Lu, M.; Zhan, X. The crucial role of multiomic approach in cancer research and clinically relevant outcomes. EPMA J. 2018, 9, 77–102. [Google Scholar] [CrossRef]
- Cheng, T.; Zhan, X. Pattern recognition for predictive, preventive, and personalized medicine in cancer. EPMA J. 2017, 8, 51–60. [Google Scholar] [CrossRef]
- Janssens, J.P.; Schuster, K.; Voss, A. Preventive, predictive, and personalized medicine for effective and affordable cancer care. EPMA J. 2018, 9, 113–123. [Google Scholar] [CrossRef]
| Phytochemicals | Cancer Type | Cell Line | Effects on DNA Methylation and/or Gene Expression | Reference |
|---|---|---|---|---|
| Grape seed proanthocyanidins + trans-resveratrol | Breast | MDA-MB-231, MCF-7 | Decreased DNMT activity | [67] |
| Indicaxanthin | Colon | HT29 | Gene promoter demethylation of p16INK4a, GATA4, ESR1, hypermethylation of SFRP1 and HPP1 | [68] |
| Glabridin | Breast | MDA-MB-231, Hs-578T | Gene promoter demethylation of miR-148a | [69] |
| Sulforaphane and 3,3′-diindolylmethane | Prostate | LnCAP, PC3 | Gene promoter methylation changes in CCR4, TGFBR1, CYR61, and CXCR4 | [70] |
| Epigallocatechin-3-gallate + SAHA | Breast | MDA-MB-231, MDA-MB-157 | Decreased activity of DNMTs, decreased gene expressions of miR-221/222, p27, PTEN, ERα | [71] |
| Solamargine | Lung | H1299, A549 | Inhibited protein expression of DNMT1 via activation of ERK1/2 | [72] |
| Withaferin A | Breast | MDA-MB-231 | Hypermethylation of oncogenes PLAU, ADAM8, TNSF12, GSTM1, and ME3 | [73] |
| Curcumin, 3,3′-diindolylmethane, epigallocatechin gallate, genistein, indole-3-carbinol | Breast | MDA-MB-231 | Changes in DNA methylation/gene expression of CDH11, p21Cip1, PLAU, and IL-6 | [74] |
| Genistein | Breast | MDA-MB-231 | Increase of ERα expression via the regulation of DNMT1-involved transcription | [75] |
| Phytochemicals (Isolated/Mixture) | Type of Cancer | Animal Model | Effects on DNA Methylation and/or Gene Expression | Reference |
|---|---|---|---|---|
| Curcumin | Breast | Female athymic nu/nu mice | Decrease in promoter methylation status of RASSF1 | [76] |
| Lung | BALB/c nude mice | Decrease in promoter methylation status of RARβ | [78] | |
| Acute myeloid leukemia | Female athymic nu/nu mice | Decrease in DNMT1 expression | [79] | |
| Prostate | TRAMP mice | Decrease in promoter methylation status of Nrf2 | [80] | |
| Genistein | Breast | Female immunodeficiency nu/nu mice | Decrease in DNMT1 expression | [75] |
| Neuroblastoma | BALA/c nude mice | Demethylation of CDH5 promoter, decrease in DNMT3 activity | [82] | |
| Trans-resveratrol | Breast | ACI rats | Decrease in DNMT3 expression | [84] |
| Kaempferol | Bladder | BALB/c nude mice | Decrease in DNMT3 expression | [86] |
| Isoliquiritigenin | Breast | MMTV-PyMT mice | Demethylation of WIF1 promoter, decrease in DNMT1 activity | [87] |
| Thymus vulgaris L. | Breast | Rat model | Decrease in methylation status of ATM, RASSF1, PTEN, and TIMP3 promoters | [88] |
| Clove buds | Breast | Rat model | Decrease in methylation status of RASSF1 promoter | [89] |
| Black raspberries | Colon | IL-10 KO mice | Decrease in methylation status of WIF1, SOX17, and GKI promoters | [91] |
| Esophageal | Rat model | Decrease in methylation status of Sfr4 promoter | [92] |
| Dietary Intervention | Dosage | Study Design | Subjects Characteristics (n) | Dietary Intake-Based Methylation Changes | Reference |
|---|---|---|---|---|---|
| Genistein 5Aza-C | Different doses | - | Prostate cancer patients | Genistein and 5-Aza-C treatment: ↓ BTG3 promoter methylation | [95] |
| Isoflavones—circulating genistein | 40 mg/d or 140 mg/d | Prospective, double-blind, randomized trial | Healthy premenopausal women (n = 34) | Lower level of genistein: ↓ RARβ2 and CCND2 methylation Higher level of genistein: ↑ RARβ2 and CCND2 methylation | [96] |
| Trans-resveratrol | 10 mg/d or 100 mg/d or placebo | Prospective, double-blind, and placebo-controlled study | Women with increased risk for breast cancer (n = 39) | ↑ Trans-resveratrol: ↓ RASSF-1α methylation | [97] |
| Folate, B2, B6, B12, methionine | Dietary intake estimated via questionnaire | Prospective case cohort study | Primary breast cancer patients (n = 139) | ↑ Riboflavin and pyridoxine: ↑ RARB promoter methylation; folate and cobalamin: Age-dependent correlation with the methylation status of RARB and BRCA1 | [102] |
| Folate, vitamin B12, Vitamin A, cruciferous vegetables | Dietary intake estimated via questionnaire | Cross-sectional study | First primary head and neck cancer patients (n = 49) | ↑ Folate, vitamin B12, and vitamin A: ↓ Tumor suppressors methylation | [103] |
| Folate | Dietary intake estimated via questionnaire | Population-based study | Head and neck squamous cell cancer patients (n = 169) | ↓ Folate: ↑ p16INK4A methylation | [104] |
| Folate | - | - | Colorectal cancer patients (approx. n = 40) | ↓ Folate: ↑ hMLH1 promoter methylation | [101] |
| Folate, B2, B6, B12, methionine | Dietary intake estimated via questionnaire | Population-based case-control study | Primary breast cancer patients (n = 1170) | No association found | [105] |
| Folate, vitamin B12 deficiency | - | - | Squamous cell lung cancer patients (n = 12) | ↓ Folate, vitamin B12: Global DNA hypomethylation | [107] |
| Folic acid supplementation | 600 μg/d or placebo | Randomized controlled trial | Patients with adenomatous polyps (n = 20) | ↑ Folic acid supplementation: ↓ Global DNA hypomethylation | [108] |
| Dietary modifications (vegetable, proteins, changes in caloric intake) and weight loss | Randomized, crossover, pilot study | Hispanic, African-American, and Afro-Caribbean overweight and sedentary breast cancer survivors (n = 24) | ↑ LINE-1 | [106] | |
| 25-hydroxyvitamin D | - | - | Colorectal cancer patients (n = 55) and control subjects (n = 35) | ↑ 25-hydroxyvitamin D: ↑ LINE-1 | [109] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasek, K.; Kubatka, P.; Samec, M.; Liskova, A.; Smejkal, K.; Vybohova, D.; Bugos, O.; Biskupska-Bodova, K.; Bielik, T.; Zubor, P.; et al. DNA Methylation Status in Cancer Disease: Modulations by Plant-Derived Natural Compounds and Dietary Interventions. Biomolecules 2019, 9, 289. https://doi.org/10.3390/biom9070289
Jasek K, Kubatka P, Samec M, Liskova A, Smejkal K, Vybohova D, Bugos O, Biskupska-Bodova K, Bielik T, Zubor P, et al. DNA Methylation Status in Cancer Disease: Modulations by Plant-Derived Natural Compounds and Dietary Interventions. Biomolecules. 2019; 9(7):289. https://doi.org/10.3390/biom9070289
Chicago/Turabian StyleJasek, Karin, Peter Kubatka, Marek Samec, Alena Liskova, Karel Smejkal, Desanka Vybohova, Ondrej Bugos, Kristina Biskupska-Bodova, Tibor Bielik, Pavol Zubor, and et al. 2019. "DNA Methylation Status in Cancer Disease: Modulations by Plant-Derived Natural Compounds and Dietary Interventions" Biomolecules 9, no. 7: 289. https://doi.org/10.3390/biom9070289
APA StyleJasek, K., Kubatka, P., Samec, M., Liskova, A., Smejkal, K., Vybohova, D., Bugos, O., Biskupska-Bodova, K., Bielik, T., Zubor, P., Danko, J., Adamkov, M., Kwon, T. K., & Büsselberg, D. (2019). DNA Methylation Status in Cancer Disease: Modulations by Plant-Derived Natural Compounds and Dietary Interventions. Biomolecules, 9(7), 289. https://doi.org/10.3390/biom9070289









